Abstract
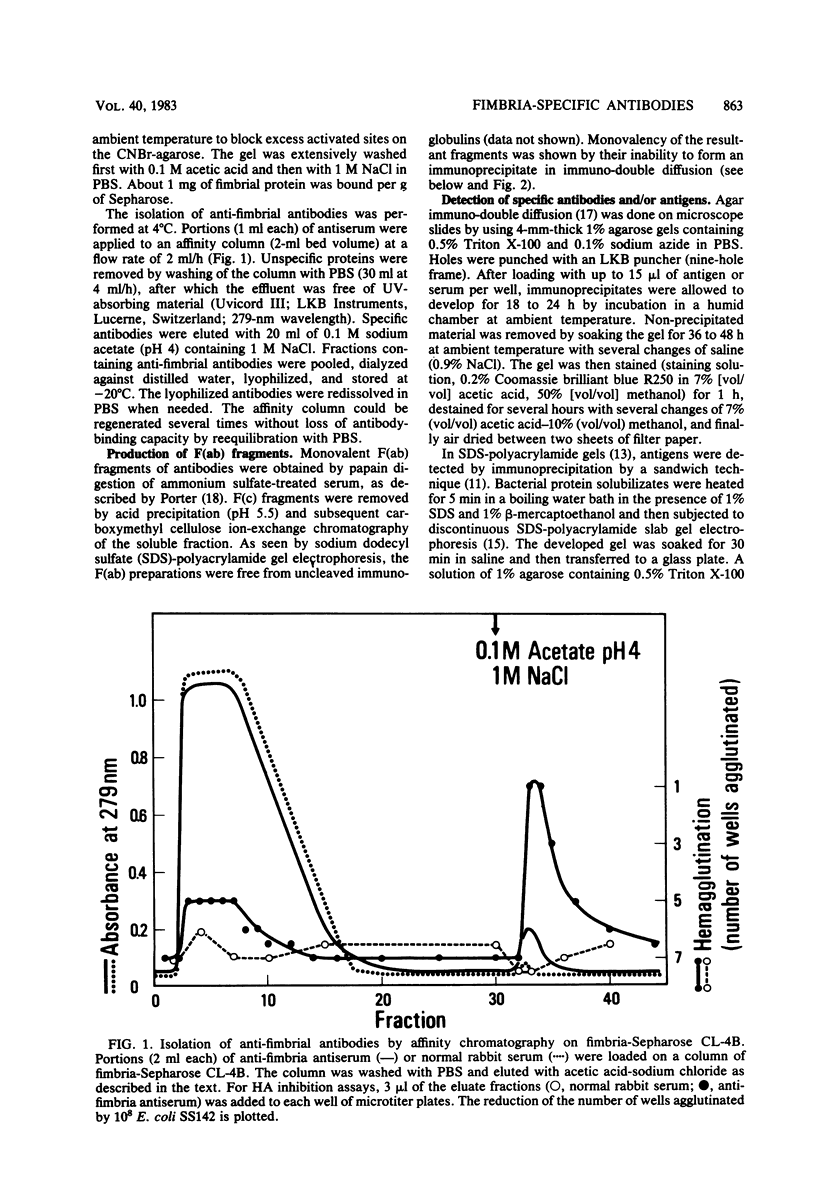
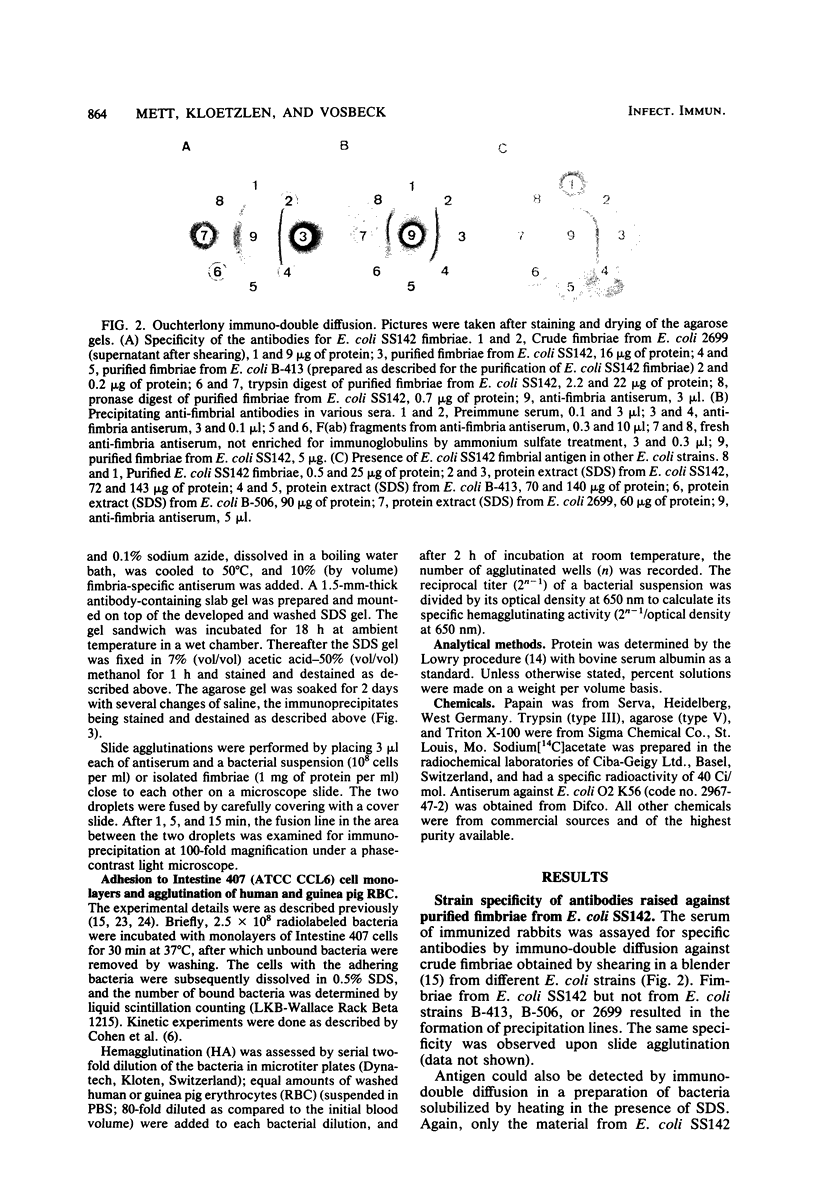
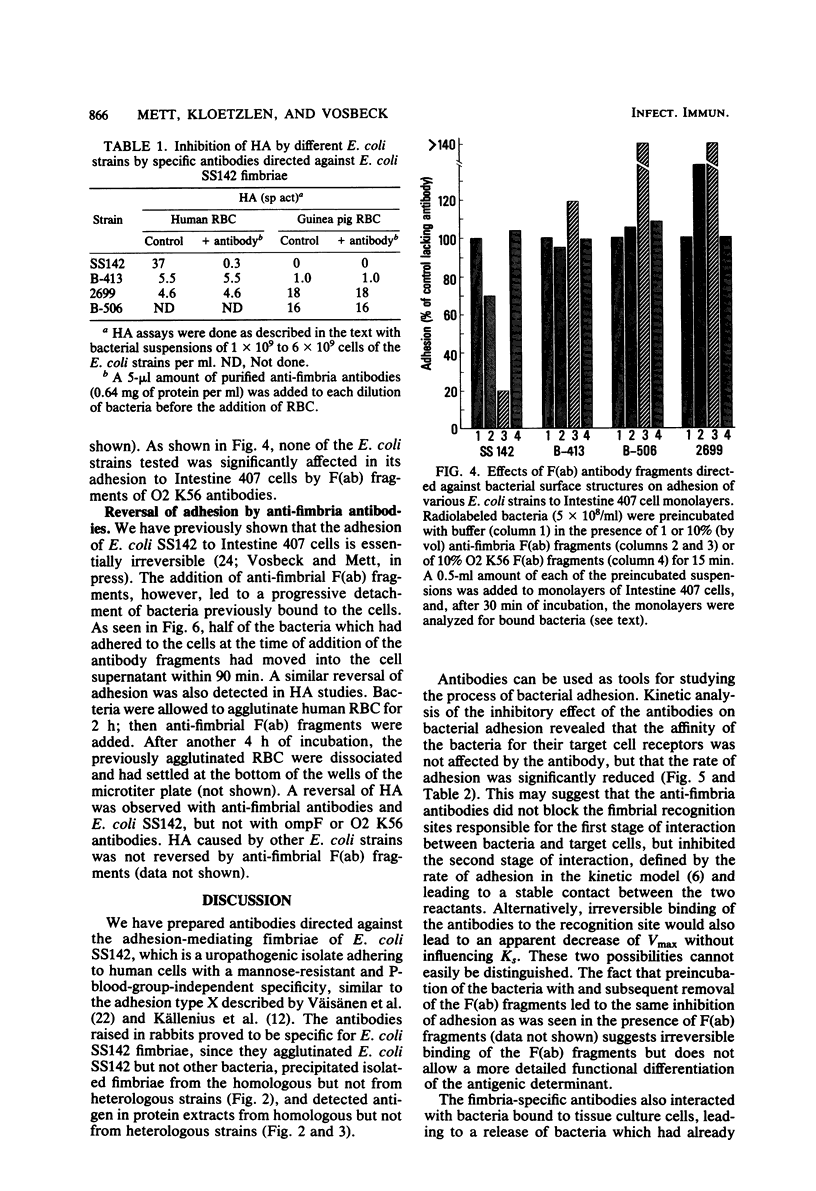
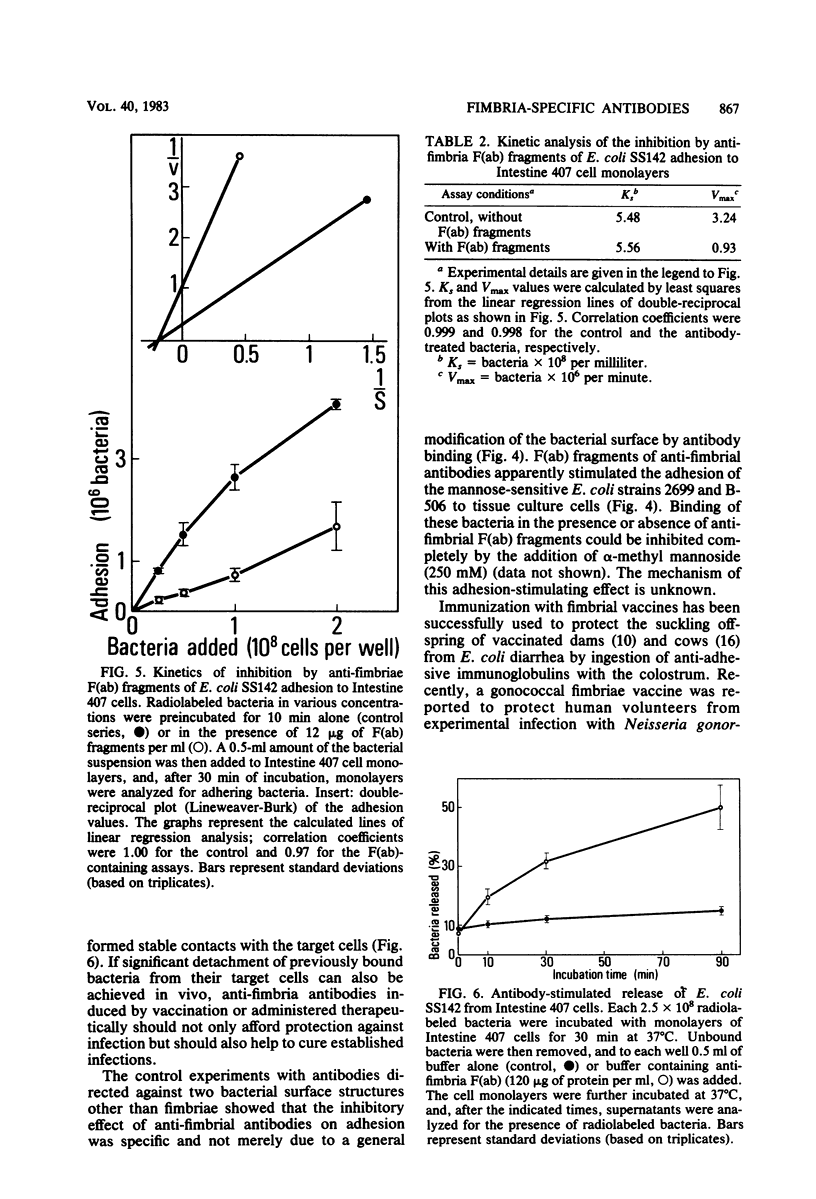
Antibodies obtained from rabbits immunized with purified adhesion-mediating fimbriae of Escherichia coli SS142 were specific for the fimbriae of the homologous strain; they did not cross-react with isolated fimbriae of three different E. coli strains or with protein extracts from nine other adhesive E. coli strains. The antibodies inhibited adhesion to Intestine 407 tissue culture cell monolayers and hemagglutinating activity of E. coli SS142 but not of several other E. coli strains. The antibodies were able not only to prevent but also to reverse the adhesion of E. coli SS142 to Intestine 407 cells or human erythrocytes. Analysis of the kinetics of inhibition suggested that the antibodies did not competitively inhibit adhesion. Such antibodies can be useful for distinguishing different mechanistic steps of bacterial adhesion. Their ability to reverse bacterial adhesion in vitro may be of clinical relevance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Contribution of the K88 antigen of Escherichia coli to enteropathogenicity; protection against disease by neutralizing the adhesive properties of K88 antigen. Am J Clin Nutr. 1974 Dec;27(12):1441–1449. doi: 10.1093/ajcn/27.12.1441. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mett H., Kloetzlen L., Vosbeck K. Properties of pili from Escherichia coli SS142 that mediate mannose-resistant adhesion to mammalian cells. J Bacteriol. 1983 Feb;153(2):1038–1044. doi: 10.1128/jb.153.2.1038-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B. Vaccination of cows with a K99 extract to protect newborn calves against experimental enterotoxic colibacillosis. Infect Immun. 1980 Jan;27(1):21–24. doi: 10.1128/iai.27.1.21-24.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M., Olsen D., Critchlow C., Buchanan T. M. Gonococcal pili: safety and immunogenicity in humans and antibody function in vitro. J Infect Dis. 1982 Mar;145(3):300–310. doi: 10.1093/infdis/145.3.300. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Boslego J. W., Ciak J., McChesney D., Brinton C. C., Wood S., Takafuji E. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. J Clin Invest. 1981 Oct;68(4):881–888. doi: 10.1172/JCI110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosbeck K., Handschin H., Menge E. B., Zak O. Effects of subminimal inhibitory concentrations of antibiotics on adhesiveness of Escherichia coli in vitro. Rev Infect Dis. 1979 Sep-Oct;1(5):845–851. doi: 10.1093/clinids/1.5.845. [DOI] [PubMed] [Google Scholar]
- Vosbeck K., Huber U. An assay for measuring specific adhesion of an Escherichia coli strain to tissue culture cells. Eur J Clin Microbiol. 1982 Feb;1(1):22–28. doi: 10.1007/BF02014136. [DOI] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]





