Background: The hypoxic cartilaginous growth plate is rich in extracellular matrix (ECM).
Results: Expression of the key enzymes in ECM synthesis, the collagen prolyl 4-hydroxylases (C-P4Hs), is induced specifically by hypoxia-inducible factor 1.
Conclusion: Hypoxia inducibility of C-P4Hs ensures sufficient C-P4H activity in hypoxic chondrocytes.
Significance: Quantitative regulation of C-P4H may be a key modality by which hypoxia influences early chondrocyte survival and differentiation.
Keywords: Chondrocytes, Collagen, Extracellular Matrix Proteins, Growth Plate, Hydroxylase, Hydroxyproline, Hypoxia-inducible Factor (HIF), Prolyl 4-Hydroxylase
Abstract
Hypoxia-inducible factors (HIFs) are the master regulators of hypoxia-responsive genes. They play a critical role in the survival, development, and differentiation of chondrocytes in the avascular hypoxic fetal growth plate, which is rich in extracellular matrix (ECM) and in its main component, collagens. Several genes involved in the synthesis, maintenance, and degradation of ECM are regulated by HIFs. Collagen prolyl 4-hydroxylases (C-P4Hs) are key enzymes in collagen synthesis because the resulting 4-hydroxyprolines are necessary for the stability of all collagen molecules. The vertebrate C-P4Hs are α2β2 tetramers with three isoforms of the catalytic α subunit, yielding C-P4Hs of types I–III. C-P4H-I is the main form in most cells, but C-P4H-II is the major form in chondrocytes. We postulated here that post-translational modification of collagens, particularly 4-hydroxylation of proline residues, could be one of the modalities by which HIF regulates the adaptive responses of chondrocytes in fetal growth plates. To address this hypothesis, we used primary epiphyseal growth plate chondrocytes isolated from newborn mice with conditionally inactivated genes for HIF-1α, HIF-2α, or the von Hippel-Lindau protein. The data obtained showed that C-P4H α(I) and α(II) mRNA levels were increased in hypoxic chondrocytes in a manner dependent on HIF-1 but not on HIF-2. Furthermore, the increases in the C-P4H mRNA levels were associated with both increased amounts of the C-P4H tetramers and augmented C-P4H activity in hypoxia. The hypoxia inducibility of the C-P4H isoenzymes is thus likely to ensure sufficient C-P4H activity for collagen synthesis occurring in chondrocytes in a hypoxic environment.
Introduction
The development, maintenance, and function of cartilage all occur in a hypoxic environment, and thus chondrocytes are adapted to functioning at low oxygen concentrations. Hypoxia-inducible factors (HIFs)4 are the master regulators of a complex homeostatic response that allows cells to survive in a hypoxic environment; this response also includes regulation of extracellular matrix (ECM) genes, among numerous others (1–3). HIFs are αβ heterodimers in which the stability and activity of the α subunit is regulated in an oxygen-dependent manner by HIF prolyl 4-hydroxylases (HIF-P4Hs, also known as PHDs) (2, 4). The 4-hydroxyproline residues formed by the HIF-P4Hs are required for the binding of HIF-α to the von Hippel-Lindau (VHL) E3 ubiquitin ligase complex and its rapid subsequent proteasomal degradation in normoxia. Under hypoxic conditions this oxygen-requiring hydroxylation is inhibited, and HIF-α escapes degradation and dimerizes with HIF-β. The dimer is then translocated into the nucleus and becomes bound to the HIF-responsive elements present in a number of hypoxia-regulated genes. HIF-α has three isoforms in the human, mouse, and rat, of which HIF-1α and HIF-2α have been the most extensively studied (5).
Analysis of genetically modified mice has demonstrated that HIF-1α is essential for the survival of chondrocytes in the hypoxic growth plates of developing bone in vivo (6). A lack of HIF-1α in growth plate chondrocytes results in massive cell death, particularly in the center of the developing growth plate, delayed chondrogenesis, and shortening of the long bones (6, 7). Notably, HIF-1α has been shown to be required for the maintenance of anaerobic glycolysis, and thereby ECM synthesis, in the epiphyseal chondrocytes of mice (8). Likewise, conditional inactivation of VHL in chondrocytes causes severe dwarfism, with a reduced chondrocyte proliferation rate, apparent increase in ECM, and the presence of atypical large cells within the resting zone of the growth plates (9). HIF-2α has recently been implicated as a regulator of endochondral ossification during skeletal growth via the induction of chondrocyte hypertrophy, cartilage degradation, and vascular invasion (10). Furthermore, expression of HIF-2α is increased in both human and mouse osteoarthritic cartilage, which is consistent with the notion that this transcription factor is involved in the pathological destruction of cartilage (11). On the other hand, endochondral bone development is only transiently delayed in HIF-2α heterozygous mice and in mice where HIF-2α is conditionally inactivated in the limb bud mesenchyme (10, 12).
The genes for the major cartilage matrix collagens, types II and IX, are not direct HIF targets, but hypoxia appears to up-regulate their expression in a SOX9-dependent manner, at least in human articular chondrocytes (13, 14). Notably, both HIF-1α and HIF-2α have been implicated in the hypoxic induction of SOX9 in human articular chondrocytes, although, based on siRNA experiments, HIF-2α but not HIF-1α seems to be essential for this induction (13–15). Continuing along these lines, inhibition of HIF-P4H-2, the major enzyme targeting HIF-α for proteasomal degradation under normoxic conditions, enhances matrix synthesis by human articular chondrocytes via the stabilization of HIF-2α and induction of SOX9 (16).
The collagen prolyl 4-hydroxylases (C-P4Hs, EC 1.14.11.2) catalyze the formation of 4-hydroxyproline through the hydroxylation of proline residues in -Xaa-Pro-Gly- sequences in collagens and in more than 20 other proteins with collagen-like sequences (17, 18). Thus, they have an essential role in the synthesis of all collagens, as the resulting 4-hydroxyproline residues are necessary for the folding of the newly synthesized collagen polypeptide chains into stable triple-helical molecules (17, 18). The vertebrate C-P4Hs are α2β2 tetramers in which the enzyme and chaperone protein disulfide isomerase (PDI) serves as the β subunit (17, 18). Three isoforms of the catalytic α subunit have been identified and shown to form (α(I))2β2, (α(II))2β2, and (α(III))2β2 tetramers with PDI, yielding the type I, II, and III C-P4Hs, respectively (19–22). The C-P4H α subunit mRNAs are expressed in a variety of human and mouse cell types and tissues, including chondrocytes and cartilage, expression of the α(III) subunit mRNA generally being at a much lower level than that of the α(I) and α(II) mRNAs (19–21). At the protein level, C-P4H-I is the main form in most cells, whereas C-P4H-II is the main form in chondrocytes, osteoblasts, endothelial cells, and certain other cell types (20, 23). Hypoxia has been shown to induce the expression of the mRNAs encoding for C-P4H α(I) and α(II) in various cell types (3), including primary human articular chondrocytes (24).
Regulation of the prolyl 4-hydroxylation of collagens may be one of the modalities by which HIF controls chondrocyte survival and differentiation. To establish the effects of hypoxia on C-P4H expression and activity and their HIF dependence, we made use here of epiphyseal growth plate chondrocytes isolated from newborn mice with conditionally inactivated genes for HIF-1α, HIF-2α, or VHL, respectively. Using this experimental model, we were able to demonstrate that the levels of mRNAs encoding for the C-P4H α(I) and α(II) subunits are increased by approximately 2.5–6-fold in hypoxic newborn growth plate chondrocytes and that this increase is exclusively dependent on HIF-1α. Increased amounts of C-P4H-I and C-P4H-II tetramers are associated with these changes at the mRNA level and with augmented C-P4H activity in hypoxia. The hypoxia inducibility of the C-P4H isoenzymes is thus likely to ensure sufficient C-P4H activity for collagen synthesis occurring in growth plate chondrocytes in a hypoxic environment.
EXPERIMENTAL PROCEDURES
Chondrocyte Isolation and Culture
Chondrocytes were isolated from newborn conditional Vhlf/f, Hif-1af/f, or Hif-2af/f mice, as described previously (8, 9). In brief, the forelimbs and hindlimbs were dissected and the soft tissue carefully removed. The epiphyses were microdissected and placed in Hanks' Balanced Salt Solution (Invitrogen). They were then digested in 0.25% trypsin containing EDTA for 30 min at 37 °C (without Ca2+ and Mg2+) followed by digestion with 195 units/ml collagenase type 2 (Worthington) in Hanks' Balanced Salt Solution. The chondrocytes were plated at a density of 4 × 105 cells/well in 6-well plates and grown in monolayer cultures in high glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin/streptomycin. On day 1 after plating, the adherent chondrocytes were infected with an adenovirus encoding either β-galactosidase (β-gal) or Cre recombinase to create control or VHL, HIF-1α, or HIF-2α knock-down cells, respectively (multiplicity of infection 1:400). These cells were incubated with adenovirus-containing medium for 24 h, and on day 10 they were exposed to 1% (hypoxic) O2 for 8–72 h in a three-gas incubator (Binder) or cultured under normoxic conditions (21% O2) for the same length of time. Biological triplicates or duplicates were used for these experiments, and the medium was changed every other day.
Quantification of Genomic Efficiency of Deletion
Deletion of Vhl, Hif-1a, or Hif-2a was confirmed by quantitative real-time PCR (qPCR) analysis of the genomic DNA, as described previously (8, 9). Loss of the conditional Vhl allele was measured using the forward primer 5′-CTAGGCACCGAGCTTAGAGGTTTGCG-3′ and the reverse primer 5′-CTGACTTCCACTGATGCTTGTCACAG-3′. The Hif-1a gene was amplified as an internal control using the forward primer 5′-TGATGTGGGTGCTGGTGTC-3′ and the reverse primer 5′-TTGTGTTGGGGCAGTACTG-3′. Loss of the conditional Hif-1a alleles was measured using the above primers. The Vhl gene was amplified as an internal control, also using the above primers. Loss of the Hif-2a allele was measured using the forward primer 5′-CAGGCAGTATGCCTGGCTAATTCCAGTT-3′ and the reverse primer 5′-CTTCTTCCATCATCTGGGATCTGGGACT-3′. As above, the Vhl gene was amplified as an internal control. Cycle threshold (Ct) values were calculated, and relative genomic DNA contents were calculated as x = 2−ΔΔCt, in which ΔΔCt = ΔE − ΔC, where ΔE = Ctnull − Ctref gene and ΔC = Ctcontrol − Ctref gene. At least a 4–5-fold difference in the efficiency of amplification was calculated between the mutants and controls, indicating that the efficiency of deletion in the mutant samples was >80%.
Isolation of Total RNA, RT-PCR, and qPCR
Chondrocytes isolated from the growth plates of newborn mice were lysed with RNA-Bee (Tel-Test) directly in 6-well plates. The cell lysate was then transferred to a tube and supplemented with chloroform. The tubes were centrifuged at 14,000 × g for 10 min at 4 °C. After carefully pipetting the aqueous phase, RNA was precipitated with isopropyl alcohol. After a 13,000 × g spin, the RNA pellet was washed in 70% ethanol. The RNA yield was determined spectrophotometrically. A digestion step with DNase I (Qiagen) was introduced to avoid genomic DNA interference with the PCRs. For reverse transcription, the Omniscript First-strand Synthesis System with random nanomer primers was used according to the manufacturer's instructions (Qiagen).
cDNAs from triplicate wells of two or three independent experiments were amplified by standard qPCR using SYBR Green mix (Qiagen) and specific primers (Table 1). 18 S rRNA was amplified as an internal control. The relative amounts of mRNA were normalized to 18 S rRNA and calculated with the software program Microsoft Excel. Relative mRNA contents were calculated as x = 2−ΔΔCt, where ΔΔCt = ΔE − ΔC, ΔE = Ctsample − Ct18S and ΔC = Ctcontrol − Ct18S. The efficiency of deletion of Vhl, Hif-1a, or Hif-2a was confirmed at the mRNA level by qPCR using the primers listed in Table 1. Identity of the PCR products was confirmed by agarose gel electrophoresis and by direct sequencing.
TABLE 1.
Sequences of the qPCR oligonucleotides
| Namea | Sequence |
|---|---|
| m18S F | 5′-AAACGGCTACCACATCCAAG-3′ |
| m18S R | 5′-CCTCCAATGGATCCTCGTTA-3′ |
| Vhl F | 5′-CAGCTACCGAGGTCATCTTTG-3′ |
| Vhl R | 5′-CTGTCCATCGACATTGAGGGA-3′ |
| Hif-1a F | 5′-CTATGGAGGCCAGAAGAGGGTAT-3′ |
| Hif-1a R | 5′-CCCACATCAGGTGGCTCATAA-3′ |
| Hif-2a F | 5′-CTGAGGAAGGAGAAATCCCGT-3′ |
| Hif-2a R | 5′-TGTGTCCGAAGGAAGCTGATG-3′ |
| Vegfa F | 5′-CTTGTTCAGAGCGGAGAAAGC-3′ |
| Vegfa R | 5′-ACATCTGCAAGTACGTTCGTT-3′ |
| Glut1 F | 5′-CAGTTCGGCTATAACACTGGTG-3′ |
| Glut1 R | 5′-GCCCCCGACAGAGAAGATG-3′ |
| P4ha1 F | 5′-AAGGCTGAGCCGAGCTACA-3′ |
| P4ha1 R | 5′-GCCAAGCACTCTTAGATACTCTG-3′ |
| P4ha2 F | 5′-AGACAGGTGTCCTCACTGTTG-3′ |
| P4ha2 R | 5′-GCATCTTCGTCATCGCTCCT-3′ |
| Aggrecan F | 5′-CCTGCTACTTCATCGACCCC-3′ |
| Aggrecan R | 5′-AGATGCTGTTGACTCGAACCT-3′ |
| Col2a1 F | 5′-ACTTGCCAAGACCTGAAACTCTG-3′ |
| Col2a1 R | 5′-AAACTTTCATGGCGTCCAAGG-3′ |
| EPO F | 5′-CATCTGCGACAGTCGAGTTCTG-3′ |
| EPO R | 5′-CACAACCCATCGTGACATTTTC-3′ |
a F, forward; R, reverse.
Western Blotting, C-P4H Activity Assays, and Analysis of 4-Hydroxyproline Formation
Chondrocytes isolated from the growth plates of newborn mice were lysed on ice in a buffer containing 137 mm NaCl, 20 mm Tris-HCl, pH 8, 10% glycerol, 1% Nonidet P-40, 2 mm EDTA, and protease inhibitors (Roche Applied Science). EDTA was excluded from the preparation of the samples used in the C-P4H activity measurements. Protein concentrations were determined by the Bradford method (Bio-Rad Protein Assay). 40-μg aliquots of the lysates were analyzed by 8% SDS-PAGE under reducing conditions followed by Western blotting with a HIF-1α antibody (NB100-479; Novus Biologicals). To be able to detect HIF-2α in Western blots, the remaining lysates after taking aliquots for the C-P4H analyses were pooled, and 70 μg of the pool was analyzed by 8% SDS-PAGE followed by Western blotting with a HIF-2α antibody (AF2997; R&D Systems). Antibodies against α-tubulin (Sigma-Aldrich) or β-actin (AC-15; Novus Biologicals) were used as loading controls. Alternatively, samples were analyzed by 8% nondenaturing PAGE followed by Western blotting with polyclonal antibodies generated by Innovagen against purified recombinant peptide-substrate-binding domains of the human C-P4H α(I) and α(II) subunits (25), respectively. Signals were detected with the addition of ECL (Amersham Biosciences). To control for the isoenzyme specificity of the C-P4H antibodies, recombinant human and mouse C-P4H-I and C-P4H-II tetramers were expressed in insect cells by coexpressing the different α subunits with the human PDI/β subunit, and Triton X-100-soluble fractions of the cell lysates were analyzed by nondenaturing PAGE followed by Western blotting, as described previously (20).
The C-P4H activity/μg of protein of the chondrocyte lysates was analyzed by a method based on the formation of 4-hydroxy[14C]proline in a [14C]proline-labeled substrate consisting of nonhydroxylated procollagen polypeptide chains (26). The C-P4H activity of the insect lysates expressing recombinant human and mouse C-P4H-I and C-P4H-II tetramers was analyzed by a method based on the hydroxylation-coupled decarboxylation of 2-oxo[1-14C]glutarate (26). The same method was used to study the activity of purified recombinant human C-P4H-I (27) under hypoxic conditions. The enzyme preparation and reaction components were stabilized, and the C-P4H reactions were carried out in 1% O2, 5% CO2, and 94% N2 in an In vivo2 400 hypoxic work station (Ruskin Technologies).
To study formation of 4-hydroxyproline Hif-1af/f chondrocytes isolated from the growth plates of newborn mice were cultured in the presence of 0.1 mm ascorbate and infected with an adenovirus encoding either β-gal or Cre recombinase as described above. On day 10 the cells were exposed to hypoxia (1% O2) for 24 h, or the culture was continued in normoxia in the presence of 30 μCi of l-[2,3,4,5-3H]proline (PerkinElmer Life Sciences). Cell lysates were prepared as described above, and medium samples were collected from two independent experiments. The amount of 4-hydroxy[3H]proline formed in nondialyzable protein was determined with a radiochemical method after acid hydrolysis of the samples (28) relative to total protein content of the cell lysates determined by the Bradford method.
ELISA Analysis of VEGF Protein
The amount of VEGF in the culture medium was determined using the mouse VEGF ELISA kit (R&D Systems) as described previously (8). Briefly, cell culture medium from Hif-1af/f newborn growth plate chondrocytes transduced with β-gal or Cre recombinase as described above and exposed to 21% O2 or 1% O2 for 24 h was harvested and stored at −20 °C. VEGF ELISA was conducted according to the manufacturer's instructions. The data were normalized to total protein content measured by a BCA protein assay (Pierce Biotechnology). Experiments were performed in biological duplicates.
Statistical Analyses
The statistical analyses were performed using Student's two-tailed t test. Data are shown as means ± S.D.
RESULTS
Expression of P4ha1 and P4ha2 mRNAs Is Increased in Hypoxic Newborn Mouse Epiphyseal Growth Plate Chondrocytes
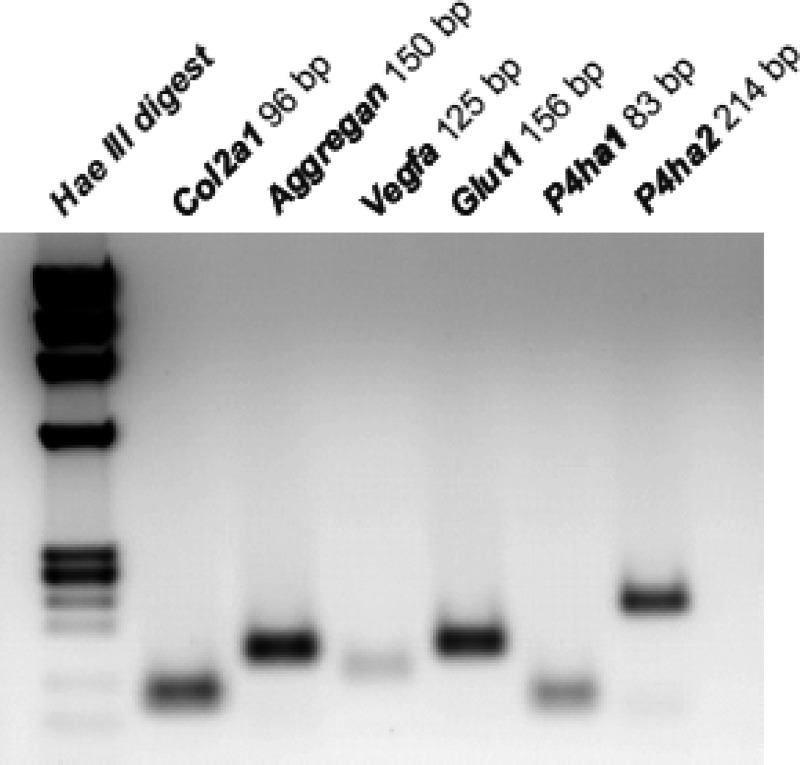
To study the effect of hypoxia and the roles of the key regulators of the hypoxia response pathway, VHL, HIF-1, and HIF-2, on the expression of C-P4Hs in chondrocytes, primary chondrocytes were isolated from the epiphyseal growth plates of newborn Vhlf/f, Hif-1af/f, and Hif-2af/f mice and cultured in a monolayer. At day 10 they were exposed to 1% O2 (hypoxia) or cultured further in 21% O2 (normoxia) for 8 h. RT-PCR analysis of the chondrocytes cultured in normoxia showed that they expressed mRNAs encoding the proα1 chain of type II collagen (Col2a1) and aggrecan, proteins characteristic of proliferating chondrocytes, thus confirming their phenotypic identity (Fig. 1). Moreover, as reported previously (7), these cells also had detectable levels of mRNAs for vascular endothelial growth factor-A (Vegfa) and glucose transporter type I (Glut1), two well characterized HIF target genes (Fig. 1). Finally, RT-PCR analysis provided evidence that the primary chondrocytes expressed mRNAs for the catalytic α(I) and α(II) subunits of the C-P4H isoenzymes I and II (P4ha1 and P4ha2), respectively (Fig. 1). This finding is in agreement with our previously published microarray data set generated using fetal metatarsal cultures (9).
FIGURE 1.
RT-PCR of total RNA extracted from primary mouse epiphyseal growth plate chondrocytes cultured for 10 days under normoxic conditions. The products of amplification of mRNAs coding for the proα1 chain of type II collagen (Col2a1), aggrecan, vascular endothelial growth factor A (Vegfa), glucose transporter 1 (Glut1), and the C-P4H α(I) and α(II) subunits (P4ha1 and P4ha2, respectively) are shown.
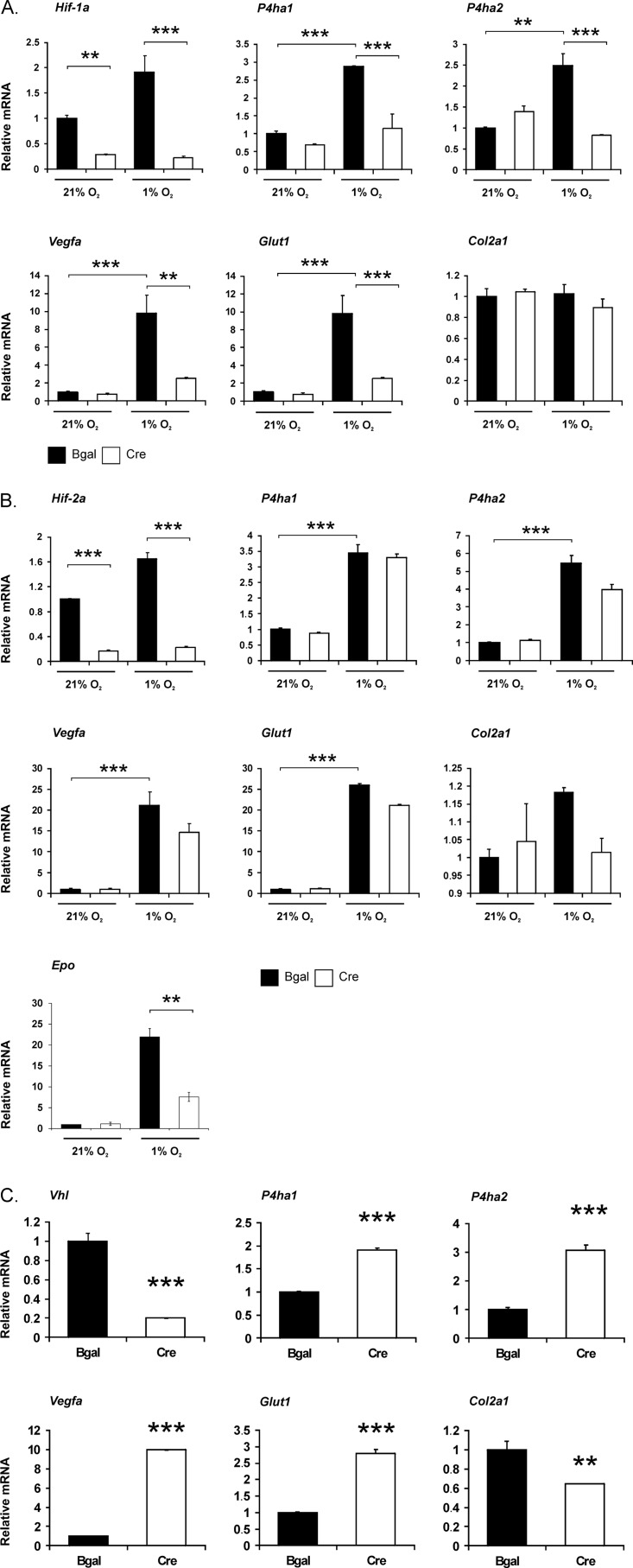
Exposure to hypoxia for 8 h led to an approximately 3–3.5-fold increase in the expression of the P4ha1 mRNA in the Hif-1af/f and Hif-2af/f growth plate chondrocytes infected with the control adenovirus encoding β-gal, a 2.5–5.5-fold increase being seen in the case of the P4ha2 mRNA (Fig. 2, A and B). As reported previously (7, 9), acute hypoxia did not increase the expression of Col2a1 mRNA (Fig. 2, A and B). Conversely, it significantly increased the expression of the Vegfa and Glut1 mRNAs by approximately 10–25-fold (Fig. 2, A and B).
FIGURE 2.
Quantification of Hif-1a, Hif-2a, Vhl, P4ha1, P4ha2, Vegfa, Glut1, Epo, and Col2a1 mRNAs in control and HIF-1α (A), HIF-2α (B), and VHL (C) knock-down chondrocytes. Primary chondrocytes were isolated from growth plates of newborn Hif-1af/f, Hif-2af/f, and Vhlf/f mice, cultured in a monolayer and transduced on day 1 after plating with adenoviruses producing either β-galactosidase (black columns) or Cre recombinase (gray columns) to generate control and HIF-1α, HIF-2α, and VHL knock-down chondrocytes, respectively. On day 10 the cells were exposed to hypoxia for 8 h. Data are given as means ± S.D. (error bars; triplicates of one representative experiment are shown). Statistical differences were calculated as ***, p < 0.01; **, p < 0.05.
Inactivation of VHL Increases the Expression of P4ha1 and P4ha2 mRNAs
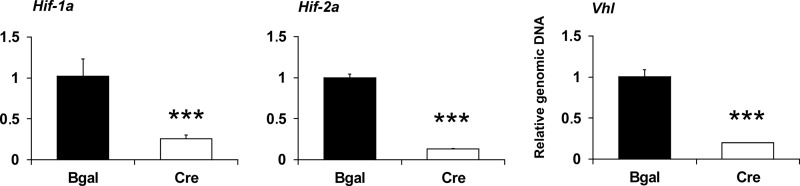
We then asked whether a lack of VHL with consequent stabilization and activation of HIF-1α and HIF-2α mimics the effects of hypoxia in chondrocytes. For this purpose, primary chondrocytes were isolated from newborn epiphyseal growth plates of Vhlf/f mice, cultured in a monolayer in normoxia, and transduced at day 1 after plating with adenoviruses producing either β-gal or Cre recombinase to generate control and VHL knock-down chondrocytes, respectively. The excellent efficiency of Vhl deletion (>80%) was confirmed by analyses of both total RNA (Fig. 2C) and genomic DNA (Fig. 3) isolated at day 10.
FIGURE 3.
Quantification of the efficiency of deletion of the Hif-1a, Hif-2a, and Vhl genes by analysis of genomic DNA. Primary chondrocytes were isolated from growth plates of newborn Hif-1af/f, Hif-2af/f, and Vhlf/f mice, cultured in a monolayer and transduced on day 1 after plating with adenoviruses producing either β-galactosidase (black columns) or Cre recombinase (gray columns). Genomic DNA was isolated on day 10. Error bars, S.D. ***, p < 0.01.
Like hypoxia, a lack of VHL up-regulated the P4ha1 and P4ha2 mRNA levels by approximately 2–3-fold and those of the Vegfa and Glut1 mRNAs by approximately 10- and 3-fold, respectively (Fig. 2C). This indicates that the hypoxia-induced increase in P4ha1 and P4ha2 mRNA levels is likely to be dependent on HIF-1α or HIF-2α, or both. Interestingly, expression of Col2a1 mRNA was modestly, though significantly, reduced in the cells in which VHL had been knocked down (Fig. 2C). This finding differs from what we have observed previously in VHL knock-down newborn mouse growth plate chondrocytes that had been cultured for a shorter period of time (9), but it is consistent with a detectable change in the shape of these cells, which had a fibroblastoid appearance at day 10 rather than the classical cobblestone morphology of the control chondrocytes (data not shown).
HIF-1 but Not HIF-2 Is Responsible for the Hypoxia Inducibility of the P4ha1 and P4ha2 mRNAs
We next studied whether the hypoxia-induced increase in P4ha1 and P4ha2 mRNAs is dependent on HIF-1α or HIF-2α. For this purpose, primary chondrocytes were isolated from growth plates of newborn Hif-1af/f and Hif-2af/f mice, cultured in a monolayer, and transduced with adenoviruses producing either β-gal or Cre recombinase, as above, to generate control, HIF-1α and HIF-2α knock-down chondrocytes. At day 10 the cells were exposed to hypoxia for 8 h or maintained under normoxic culture conditions. The excellent efficiency of deletion of the Hif-1a and Hif-2a genes (at least 70%) was confirmed by analyses of total RNA (Fig. 2, A and B) and genomic DNA (Fig. 3).
A lack of HIF-1α completely abolished the hypoxia-induced up-regulation of both P4ha1 and P4ha2 mRNAs (Fig. 2A), whereas a lack of HIF-2α had no significant effect on the up-regulation of these two transcripts under hypoxic conditions (Fig. 2B). These findings indicate that the increase in P4ha1 and P4ha2 mRNAs observed in hypoxia is exclusively dependent on HIF-1α in the newborn mouse growth plate chondrocytes. To confirm the downstream efficacy of HIF-1α and HIF-2α inactivation, the expression of known target genes was analyzed. The hypoxia-induced expression of Vegfa and Glut1 mRNAs was significantly down-regulated in hypoxic HIF-1α knock-down chondrocytes relative to controls (Fig. 2A), in agreement with previous observations (7). This was further verified by analyzing the VEGF protein level in the cell culture medium. Exposure to 1% O2 for 24 h led to a 2.6 ± 0.1-fold increase in VEGF accumulation in the cell culture medium isolated from Hif-1af/f newborn growth plate chondrocytes transduced with Bgal compared with normoxic cells, whereas the increase was only 1.87 ± 0.09-fold in cells lacking HIF-1α. Inactivation of HIF-2α in chondrocytes had no significant effect on the hypoxia-induced increase in Glut1 mRNA, which is known to be regulated exclusively by HIF-1α, at least in Hep3B and Kelly cells (29), or on Vegfa mRNA, the hypoxia inducibility of which has been reported to be dependent on both HIF-1α and HIF-2α in other cell types (30) (Fig. 2B). By contrast, inactivation of HIF-2α markedly inhibited the hypoxic induction of erythropoietin mRNA (Epo) (Fig. 2B). Epo has recently been shown to be expressed in the chondrocytes of developing porcine cartilage (31), and HIF-2α is known to be the critical isoform in the regulation of its expression (32, 33). The lack of HIF-1α or HIF-2α had no effect on Col2a1 mRNA levels in normoxia or hypoxia, which is in accordance with the lack of Col2a1 mRNA induction by hypoxia (Fig. 2, A and B).
The Hypoxia-induced Increases in the Expression of P4ha1 and P4ha2 mRNAs Are Associated with Similar Increases in the Accumulation of C-P4H-I and C-P4H-II Tetramers
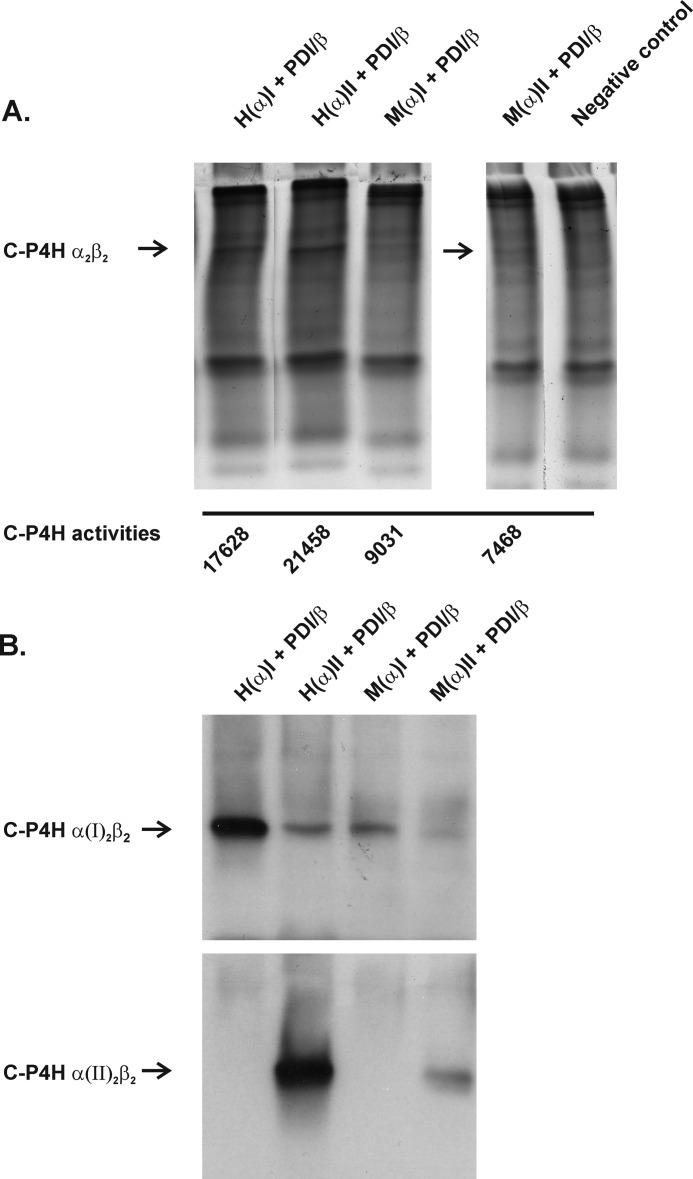
To study whether the above hypoxia-related changes in the expression of P4ha1 and P4ha2 mRNAs are concomitant with similar changes at the protein level, we analyzed C-P4H tetramer formation and C-P4H activity in the above growth plate chondrocytes isolated from newborn mice. To study the assembly of active C-P4H tetramers, we used novel polyclonal antibodies generated against recombinant peptide-substrate-binding domains of the human C-P4H α(I) and α(II) subunits (25). We first analyzed the isoenzyme specificity and sensitivity of the C-P4H antibodies by expressing recombinant human and mouse C-P4H-I and C-P4H-II tetramers in insect cells (20). The amounts of the two isoenzymes in the insect cell samples were estimated based on an analysis using 8% nondenaturing PAGE followed by Coomassie Blue staining and measurement of C-P4H activity (Fig. 4A). Approximately equal amounts of the recombinant human C-P4H-I and C-P4H-II (C-P4H activities 17,630 and 21,460 dpm, respectively) and mouse C-P4H-I and C-P4H-II (C-P4H activities 9,030 and 7,470 dpm, respectively) (Fig. 4A) were then run on 8% denaturing PAGE followed by ECL Western blotting with the α(I) and α(II) subunit antibodies (Fig. 4B). Because similar staining intensities were obtained for the two isoenzymes with 1:100 and 1:500–1,000 dilutions of antibodies to the α(I) and α(II) subunits, respectively (Fig. 4B), these dilutions were used in the subsequent analyses of the chondrocyte samples. The α(II) antibody is specific for the C-P4H-II isoenzyme, whereas the α(I) antibody shows some cross-reactivity with the C-P4H-II isoenzyme (Fig. 4B). It must also be noted that the antibodies demonstrate similar affinity for the recombinant human and mouse C-P4H isoenzymes, the weaker staining of the mouse isoenzymes (Fig. 4B) being due to the fact that their expression levels in insect cells were only approximately 30–50% of those of the corresponding human ones (Fig. 4A).
FIGURE 4.
Staining of recombinant human and mouse C-P4H-I and C-P4H-II tetramers with anti-human C-P4H α(I) and α(II) antibodies. A, protein lysates from insect cells expressing human (H) or mouse (M) C-P4H α(I) or α(II) subunits together with PDI/β were run on 8% PAGE under nondenaturing conditions followed by Coomassie Blue staining and subjected to C-P4H activity measurements. B, equal amounts of the human and mouse C-P4H-I and C-P4H-II were run on 8% PAGE under nondenaturing conditions and analyzed by Western blotting using an anti-human C-P4H α(I) or α(II) antibody. The arrows indicate C-P4H α2β2 tetramers (A) and individual isoenzymes (B). The lanes in A were regrouped from different parts of the same gel.
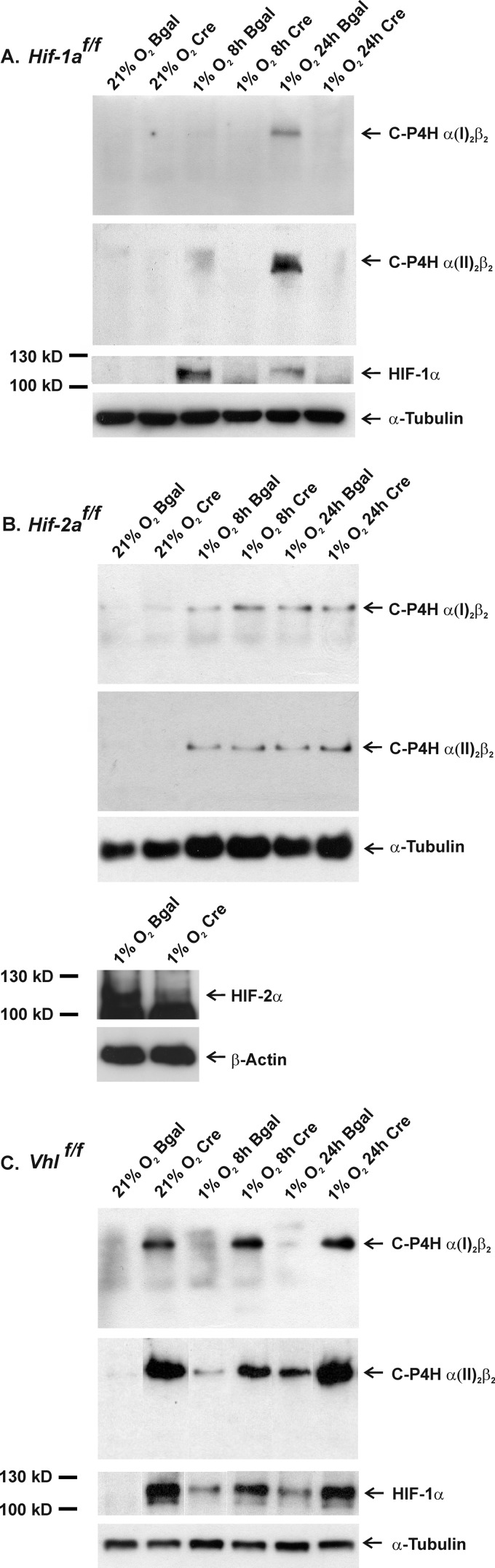
The Hif-1af/f, Hif-2af/f, and Vhlf/f growth plate chondrocytes were cultured and transduced with the adenoviruses as above and then exposed to hypoxia for 8 or 24 h at day 10. Protein lysates of the chondrocytes were then analyzed by nondenaturing PAGE followed by Western blotting with the optimized C-P4H-I and C-P4H-II antibodies. In the control cells infected with the β-gal-producing adenovirus, the amounts of the C-P4H-I and C-P4H-II tetramers were found to be increased relative to normoxia in all three cell types after 8 or 24 h of exposure to hypoxia (Fig. 5, A–C). Inactivation of HIF-1α completely abolished the hypoxia-induced accumulation of the C-P4H-I and C-P4H-II tetramers (Fig. 5A), whereas inactivation of HIF-2α had no effect (Fig. 5B). Inactivation of VHL led to robust accumulation of C-P4H-I and C-P4H-II already in normoxia, and exposure to hypoxia did not lead to any further increases in the amounts of the C-P4Hs in the VHL knock-down chondrocytes (Fig. 5C). The efficacies of the Cre recombinase-mediated HIF-1α, HIF-2α, and VHL deletions were verified by SDS-PAGE analysis followed by Western blotting with an antibody recognizing HIF-1α or HIF-2α. The Cre recombinase-expressing Hif-1af/f cells showed no accumulation of HIF-1α in hypoxia (Fig. 5A), whereas in Cre recombinase-expressing Vhlf/f cells HIF-1α was no longer targeted for proteasomal degradation but was stabilized already in normoxia (Fig. 5C). Similarly, no accumulation of HIF-2α was detected in the Cre recombinase-expressing Hif-2af/f cells in hypoxia (Fig. 5B). Unfortunately, the quality of HIF-2α antibodies available was not sufficient for the detection of HIF-2α from individual chondrocyte lysates after taking aliquots for the C-P4H assays, and therefore analysis is shown from a pool of lysates. It must also be noted that the Western blots shown in Fig. 5, A–C, are representatives of three independent experiments, and quantitation of the data is given below via determination of the amount of C-P4H activity formed (representing the amount of C-P4H tetramers) in the different conditions.
FIGURE 5.
Analysis of C-P4H-I and C-P4H-II protein expression in control and Hif-1a (A), Hif-2a (B), and Vhl (C) floxed chondrocytes. The Hif-1af/f, Hif-2af/f, and Vhlf/f chondrocytes were cultured in a monolayer and transduced on day 1 after plating with adenoviruses producing either β-galactosidase or Cre recombinase. On day 10 the cells were exposed to 1% O2 for 8 or 24 h, or the culture was continued in normoxia. Total cell lysates were analyzed by 8% nondenaturing PAGE followed by Western blotting with antibodies against the C-P4H α(I) and α(II) subunits. The accumulation of HIF-1α (A and C) and HIF-2α (B) was studied by 8% SDS-PAGE followed by Western blotting with an antibody recognizing HIF-1α or HIF-2α, respectively. In the latter case the lysates from cells cultured in 1% O2 for 24–72 h were pooled for analysis. α-Tubulin or β-actin was used as a loading control. The lanes in the C-P4H-II and HIF-1α panel in C were regrouped from different parts of the same Western blot.
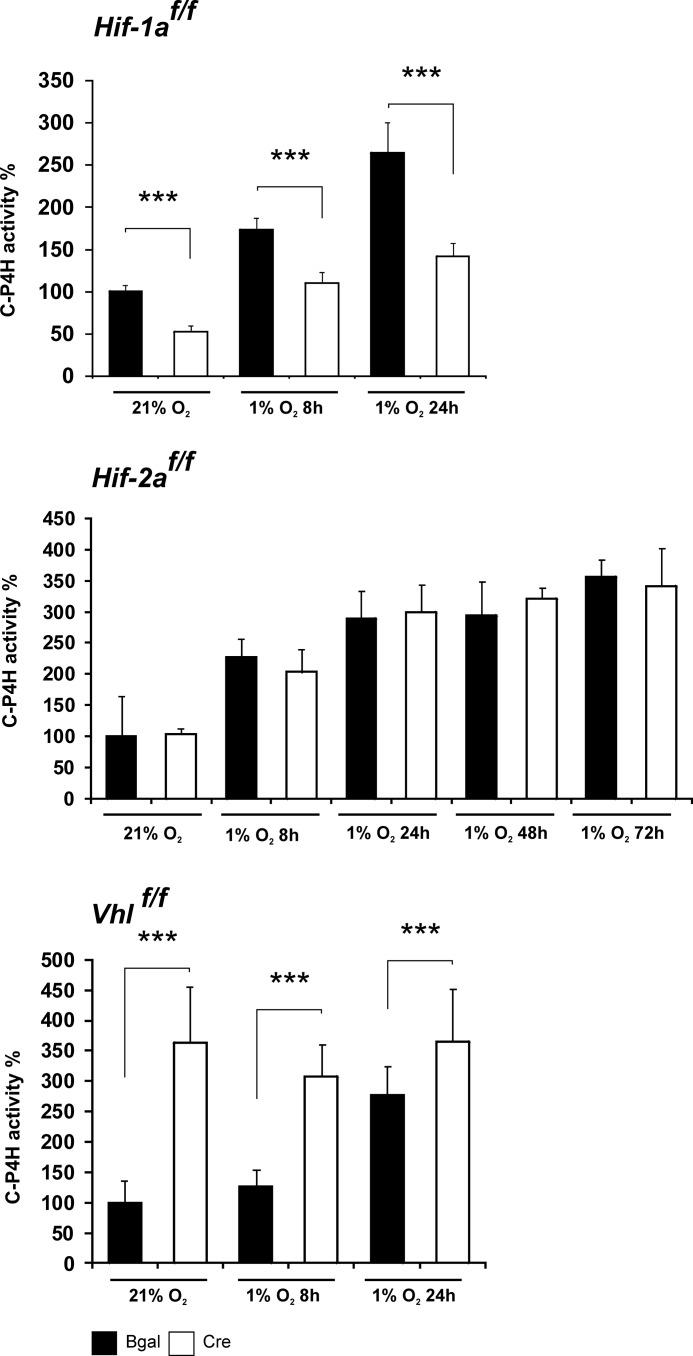
The quantity of active C-P4H tetramers assembled in the Hif-1af/f, Hif-2af/f, and Vhlf/f growth plate chondrocytes was further studied by analyzing the amount of C-P4H activity (Fig. 6). Chondrocytes were cultured and infected with the adenoviruses as above and subjected to 24–72 h of hypoxia on day 10. The C-P4H activity of the cell lysates was then analyzed by a method based on the formation of 4-hydroxy[14C]proline in a [14C]proline-labeled substrate consisting of nonhydroxylated procollagen polypeptide chains (26). As the enzyme reaction is carried out under normoxic conditions, the activity generated exclusively reflects the quantity of active C-P4H tetramers assembled in the live cells, which thus serves as a surrogate for the quantity of C-P4H tetramers in these cells (see below). The amount of activity was increased ∼3-fold in the hypoxia-treated Hif-1af/f, Hif-2af/f, and Vhlf/f chondrocytes that had been transduced with β-gal-producing adenovirus (Fig. 6). Inactivation of HIF-1α markedly reduced the hypoxia-induced increase in the amount of C-P4H activity, whereas inactivation of HIF-2α had no effect (Fig. 6). Inactivation of VHL led to an increased amount of C-P4H activity in normoxia (Fig. 6).
FIGURE 6.
Analysis of C-P4H activity in Hif-1af/f, Hif-2af/f, and Vhlf/f chondrocytes transduced with adenoviruses producing either β-galactosidase (black columns) or Cre recombinase (gray columns). On day 10 after transduction the cells were exposed to 1% O2 for 8, 24, 48, and 72 h, or the culture was continued in normoxia. This C-P4H activity assay measures the formation of 4-hydroxy[14C]proline in a [14C]proline-labeled substrate consisting of nonhydroxylated procollagen polypeptide chains. Data are given as means ± S.D. (error bars; triplicates of at least three independent experiments). Statistical differences were calculated as ***, p < 0.01.
The Decreased Specific Activity of C-P4H in Hypoxia Is Partially Compensated for by the Hypoxia-induced Increase in the Quantities of C-P4H Tetramers
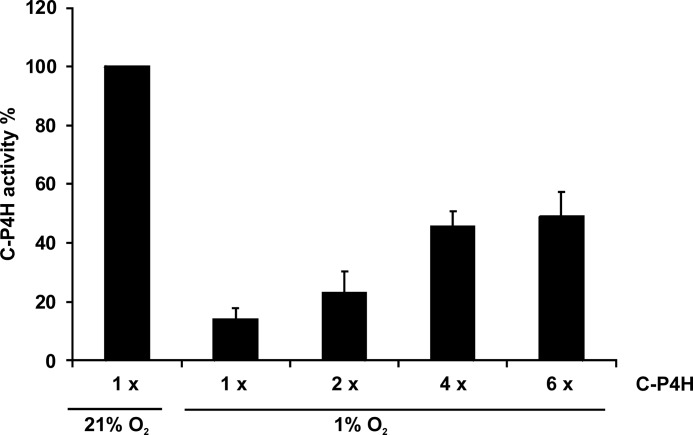
The C-P4Hs require oxygen for their reaction, the reported Km value of C-P4H-I for O2 being 20 μm (34, 35), which is probably higher than the O2 concentration in the hypoxic environment of growth plate chondrocytes. To study whether the observed hypoxia-induced increase in the C-P4H tetramers is of biological relevance under hypoxic conditions, we assessed the activity of a recombinant human C-P4H-I in 1% O2. The amount of C-P4H activity generated in 1% O2 was only approximately 15% of that generated by the same quantity of enzyme in normoxia (Fig. 7), whereas a 2–6-fold increase in the amount of C-P4H tetramer increased the C-P4H activity generated in 1% O2 to approximately 20–50% of that in normoxia (Fig. 7). Thus, the hypoxia-induced reduction in C-P4H activity is partially compensated for by a hypoxia-induced increase in C-P4H tetramers. This illustrates the delicate balance maintained by chondrocytes in their hypoxic environment and the compensation that must occur to allow for critical enzyme activity, effective production of ECM, and subsequent cellular stability.
FIGURE 7.
Analysis of the activity of purified recombinant human C-P4H-I in 21% O2 and 1% O2. The amount of enzyme increased 6-fold (6×) in the assays carried out in 1% O2 relative to that present in the reactions carried out in 21% O2. This C-P4H activity assay measures the hydroxylation-coupled decarboxylation of 2-oxo-[1-14C]glutarate.
This aspect was studied further by analyzing the effect of hypoxia in the presence or absence of HIF-1α on the synthesis of 4-hydroxyproline in the primary newborn mouse growth plate chondrocytes. The cells were cultured in the presence of [3H]proline, and collagen synthesis was analyzed by radiochemical determination of the amount of 4-hydroxy[3H]proline formed in nondialyzable proteins, i.e. mainly collagens, of the cell lysates and culture media (28). Hypoxia caused a 63 and 47% reduction in the amount of 4-hydroxyproline in the cell lysates and a 26 and 43% reduction in the culture media in two independent experiments (Table 2). The reduction was markedly aggravated upon inactivation of HIF-1α, being 94 and 67% in the lysates and 72 and 71% in the culture media (Table 2). Thus, HIF-1 activity plays an important role in the regulation collagen prolyl 4-hydroxylation capacity in hypoxia.
TABLE 2.
Effect of hypoxia in the presence (β-gal) or absence (Cre) of HIF-1α on 4-hydroxyproline formation in cultured newborn mouse growth plate chondrocytes
The cells were cultured in the presence of [3H]proline in normoxia or hypoxia for 24 h, and collagen synthesis was analyzed by radiochemical determination of the amount of 4-hydroxy[3H]proline formed in nondialysable proteins, i.e. mainly collagens, of the cell lysates and culture media.
| Cell culture conditions | Relative hydroxyproline amount |
|
|---|---|---|
| Cell lysates | Culture medium | |
| % | ||
| Experiment 1 | ||
| β-Gal HIF-1af/f, normoxia | 100 | 100 |
| β-Gal HIF-1af/f, hypoxia | 37 | 74 |
| Cre HIF-1af/f , hypoxia | 6 | 28 |
| Experiment 2 | ||
| β-Gal HIF-1af/f, normoxia | 100 | 100 |
| β-Gal HIF-1af/f, hypoxia | 53 | 57 |
| Cre HIF-1af/f , hypoxia | 33 | 29 |
DISCUSSION
The expression of several genes involved in the synthesis, maintenance, and degradation of ECM has been shown to be up-regulated by hypoxia, including those encoding the C-P4Hs, lysyl hydroxylases, and lysyl oxidases, enzymes that are required in collagen synthesis and thus for the proper formation of the ECM (3, 17, 18). Specifically, the mRNAs encoding the C-P4H α(I) and α(II) subunits are up-regulated by hypoxia in a variety of cell types, including primary human articular chondrocytes. Typical increases have been reported to be 2–8-fold, and figures as high as approximately 30-fold have been quoted (3, 9, 24, 36–39). Hypoxia also leads to increased accumulation of C-P4H α(I) protein (36). However, accumulation of the α(I) protein does not necessarily indicate that the amount of active C-P4H tetramers is increased. Because O2 is an essential substrate for the C-P4Hs, a hypoxia-induced increase in their amount is likely to be of special importance in hypoxic tissues that are active in collagen synthesis. The avascular developing cartilage is an example of such a tissue. We show here that the expression of P4ha1 and P4ha2 mRNAs is increased approximately 2–6-fold in primary newborn mouse growth plate chondrocytes. More importantly, we demonstrate for the first time that these hypoxia-induced increases are associated with similar increases in C-P4H-I and C-P4H-II tetramers and in C-P4H activity and that they are completely dependent on HIF-1 and not on HIF-2. Furthermore, we show that inactivation of HIF-1 leads to a marked reduction in the collagen prolyl 4-hydroxylation capacity in hypoxic chondrocyte cultures.
A functional hypoxia-responsive element has been identified in the gene encoding C-P4H α(I), approximately 120 bp upstream of the transcription start site, and HIF-1α has been shown to bind to this site (36). Consistent with these findings, no hypoxic induction of P4ha1 and P4ha2 mRNAs was detected in a mutant mouse hepatoma cell line lacking HIF-1 (37). Moreover, chromatin immunoprecipitation analysis using a human breast cancer cell line has identified high stringency HIF-1 binding sites in the genes encoding the C-P4H α(I) and α(II) subunits, whereas a HIF-2 binding site was identified only in the gene encoding the α(I) subunit (40). Altogether, these data indicate that it is HIF-1 that is mainly responsible for the hypoxic up-regulation of C-P4Hs.
An analysis of genetically modified mice has demonstrated that a lack of HIF-1α in growth plate chondrocytes leads to a dramatic shortening of the limbs (6). HIF-1α-null chondrocytes undergo massive cell death, particularly in the center of the developing growth plate, and timely differentiation of chondrocytes is impaired (9). Altogether, these findings indicate that HIF-1α is essential for the survival and differentiation of hypoxic chondrocytes in vivo (6, 9). HIF-1α can elicit its role as a survival and differentiation factor in hypoxic chondrocytes by several mechanisms, e.g. via the regulation of VEGF-A expression and metabolic pathways (41, 42). Our data show that HIF-1α up-regulates the amounts of the C-P4Hs I and II in hypoxic newborn mouse growth plate chondrocytes and is thus likely to improve the efficiency of the prolyl 4-hydroxylation of collagen polypeptides in the developing growth plate. Regulation of collagen prolyl 4-hydroxylation may therefore be an additional mechanism by which HIF-1α operates as a survival and differentiation factor in growth plate chondrocytes, as proper ECM accumulation is not only essential for organ development as such but also promotes cell differentiation and survival through specific cell-matrix interactions (43, 44).
Our data show that HIF-2 has no role in the hypoxic up-regulation of C-P4Hs in newborn mouse growth plate chondrocytes. This is an interesting finding, as a complete lack of HIF-2 in the mouse limb bud mesenchyme was recently shown to cause only a moderate and transient delay in endochondral bone development, most probably due to modest impairment of the differentiation of hypertrophic growth plate chondrocytes into late hypertrophic cells (12). Therefore, in contrast to HIF-1, the role of HIF-2 is not crucial for growth plate development. Instead, HIF-2 has been shown to be vital for homeostasis of the mouse articular cartilage surface, at least partially by regulating the genes for collagen X, matrix metalloproteinase 13, and VEGF-A (10, 11). Furthermore, HIF-2 induces a number of catabolic factors involved in cartilage destruction and is thus likely to be involved in the development of osteoarthritis (10, 11).
Altogether, our current data indicate that the C-P4Hs are likely to be key HIF-1 targets required for proper cartilage and bone development. It will thus be important to study the individual roles of the C-P4H I and II isoenzymes in endochondral bone development in vivo.
Acknowledgments
We thank Dr. M. Celeste Simon (University of Pennsylvania) for the Hif-2a floxed mouse line and for helpful discussions, Dr. Amato J. Giaccia (Stanford University) for helpful discussions, and M. Siurua for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AR04819 (to E. S.). This work was also supported by Health Science Council Grants 200471 and 202469 and the Center of Excellence 2012–2017 programme from the Academy of Finland, and the S. Juselius Foundation (to J. M.).
- HIF
- hypoxia-inducible factor
- C-P4H
- collagen prolyl 4-hydroxylase
- Ct
- cycle threshold
- ECM
- extracellular matrix
- Epo
- erythropoietin
- Glut
- glucose transporter
- HIF-P4H
- HIF prolyl 4-hydroxylase
- PDI
- protein disulfide isomerase
- qPCR
- quantitative real-time PCR
- VHL
- von Hippel-Lindau.
REFERENCES
- 1. Chandel N. S., Simon M. C. (2008) Hypoxia-inducible factor: roles in development, physiology, and disease. Cell Death Differ. 15, 619–620 [DOI] [PubMed] [Google Scholar]
- 2. Kaelin W. G., Jr., Ratcliffe P. J. (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 [DOI] [PubMed] [Google Scholar]
- 3. Myllyharju J., Schipani E. (2010) Extracellular matrix genes as hypoxia-inducible targets. Cell Tissue Res. 339, 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Myllyharju J. (2009) HIF prolyl 4-hydroxylases and their potential as drug targets. Curr. Pharm. Des. 15, 3878–3885 [DOI] [PubMed] [Google Scholar]
- 5. Ratcliffe P. J. (2007) HIF-1 and HIF-2: working alone or together in hypoxia? J. Clin. Invest. 117, 862–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schipani E., Ryan H. E., Didrickson S., Kobayashi T., Knight M., Johnson R. S. (2001) Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 15, 2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pfander D., Cramer T., Schipani E., Johnson R. S. (2003) HIF-1α controls extracellular matrix synthesis by epiphyseal chondrocytes. J. Cell Sci. 116, 1819–1826 [DOI] [PubMed] [Google Scholar]
- 8. Pfander D., Kobayashi T., Knight M. C., Zelzer E., Chan D. A., Olsen B. R., Giaccia A. J., Johnson R. S., Haase V. H., Schipani E. (2004) Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development 131, 2497–2508 [DOI] [PubMed] [Google Scholar]
- 9. Provot S., Zinyk D., Gunes Y., Kathri R., Le Q., Kronenberg H. M., Johnson R. S., Longaker M. T., Giaccia A. J., Schipani E. (2007) Hif-1α regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 177, 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saito T., Fukai A., Mabuchi A., Ikeda T., Yano F., Ohba S., Nishida N., Akune T., Yoshimura N., Nakagawa T., Nakamura K., Tokunaga K., Chung U. I., Kawaguchi H. (2010) Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 [DOI] [PubMed] [Google Scholar]
- 11. Yang S., Kim J., Ryu J. H., Oh H., Chun C. H., Kim B. J., Min B. H., Chun J. S. (2010) Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 16, 687–693 [DOI] [PubMed] [Google Scholar]
- 12. Araldi E., Khatri R., Giaccia A. J., Simon M. C., Schipani E. (2011) Lack of HIF-2α in limb bud mesenchyme causes a modest and transient delay of endochondral bone development. Nat. Med. 17, 25–26; author reply 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lafont J. E., Talma S., Murphy C. L. (2007) Hypoxia-inducible factor 2α is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 56, 3297–3306 [DOI] [PubMed] [Google Scholar]
- 14. Lafont J. E., Talma S., Hopfgarten C., Murphy C. L. (2008) Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 283, 4778–4786 [DOI] [PubMed] [Google Scholar]
- 15. Duval E., Leclercq S., Elissalde J. M., Demoor M., Galéra P., Boumédiene K. (2009) Hypoxia-inducible factor 1α inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1α-dependent redifferentiation of chondrocytes. Arthritis Rheum. 60, 3038–3048 [DOI] [PubMed] [Google Scholar]
- 16. Thoms B. L., Murphy C. L. (2010) Inhibition of hypoxia-inducible factor-targeting prolyl hydroxylase domain-containing protein 2 (PHD2) enhances matrix synthesis by human chondrocytes. J. Biol. Chem. 285, 20472–20480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Myllyharju J. (2008) Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann. Med. 40, 402–417 [DOI] [PubMed] [Google Scholar]
- 18. Myllyharju J., Kivirikko K. I. (2004) Collagens, modifying enzymes and their mutations in humans, flies, and worms. Trends Genet. 20, 33–43 [DOI] [PubMed] [Google Scholar]
- 19. Helaakoski T., Annunen P., Vuori K., MacNeil I. A., Pihlajaniemi T., Kivirikko K. I. (1995) Cloning, baculovirus expression, and characterization of a second mouse prolyl 4-hydroxylase α-subunit isoform: formation of an α2β2 tetramer with the protein disulfide-isomerase/β subunit. Proc. Natl. Acad. Sci. U.S.A. 92, 4427–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Annunen P., Helaakoski T., Myllyharju J., Veijola J., Pihlajaniemi T., Kivirikko K. I. (1997) Cloning of the human prolyl 4-hydroxylase α subunit isoform α(II) and characterization of the type II enzyme tetramer: the α(I) and α(II) subunits do not form a mixed α(I)α(II)β2 tetramer. J. Biol. Chem. 272, 17342–17348 [DOI] [PubMed] [Google Scholar]
- 21. Kukkola L., Hieta R., Kivirikko K. I., Myllyharju J. (2003) Identification and characterization of a third human, rat, and mouse collagen prolyl 4-hydroxylase isoenzyme. J. Biol. Chem. 278, 47685–47693 [DOI] [PubMed] [Google Scholar]
- 22. Van Den Diepstraten C., Papay K., Bolender Z., Brown A., Pickering J. G. (2003) Cloning of a novel prolyl 4-hydroxylase subunit expressed in the fibrous cap of human atherosclerotic plaque. Circulation 108, 508–511 [DOI] [PubMed] [Google Scholar]
- 23. Annunen P., Autio-Harmainen H., Kivirikko K. I. (1998) The novel type II prolyl 4-hydroxylase is the main enzyme form in chondrocytes and capillary endothelial cells, whereas the type I enzyme predominates in most cells. J. Biol. Chem. 273, 5989–5992 [DOI] [PubMed] [Google Scholar]
- 24. Grimmer C., Balbus N., Lang U., Aigner T., Cramer T., Müller L., Swoboda B., Pfander D. (2006) Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen levels. Am. J. Pathol. 169, 491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myllyharju J., Kivirikko K. I. (1999) Identification of a novel proline-rich peptide-binding domain in prolyl 4-hydroxylase. EMBO J. 18, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kivirikko K. I., Myllylä R. (1982) Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 82, 245–304 [DOI] [PubMed] [Google Scholar]
- 27. Vuori K., Pihlajaniemi T., Marttila M., Kivirikko K. I. (1992) Characterization of the human prolyl 4-hydroxylase tetramer and its multifunctional protein disulfide-isomerase subunit synthesized in a baculovirus expression system. Proc. Natl. Acad. Sci. U.S.A. 89, 7467–7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Juva K., Prockop D. J. (1966) Modified procedure for the assay of H-3- or C-14-labeled hydroxyproline. Anal. Biochem. 15, 77–83 [DOI] [PubMed] [Google Scholar]
- 29. Warnecke C., Zaborowska Z., Kurreck J., Erdmann V. A., Frei U., Wiesener M., Eckardt K. U. (2004) Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2α target gene in Hep3B and Kelly cells. FASEB J. 18, 1462–1464 [DOI] [PubMed] [Google Scholar]
- 30. Gordan J. D., Simon M. C. (2007) Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr. Opin. Genet. Dev. 17, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Spiegelaere W., Cornillie P., Van den Broeck W. (2010) Localization of erythropoietin in and around growing cartilage. Mol. Cell. Biochem. 337, 287–291 [DOI] [PubMed] [Google Scholar]
- 32. Gruber M., Hu C. J., Johnson R. S., Brown E. J., Keith B., Simon M. C. (2007) Acute postnatal ablation of Hif-2α results in anemia. Proc. Natl. Acad. Sci. U.S.A. 104, 2301–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rankin E. B., Biju M. P., Liu Q., Unger T. L., Rha J., Johnson R. S., Simon M. C., Keith B., Haase V. H. (2007) Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest. 117, 1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kivirikko K. I., Myllylä R., Pihlajaniemi T. (1992) in Post-Translational Modifications of Proteins (Harding J. J., Crabbe J. C., eds) pp. 1–51, CRC Press, Boca Raton, FL [Google Scholar]
- 35. Hirsilä M., Koivunen P., Günzler V., Kivirikko K. I., Myllyharju J. (2003) Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 278, 30772–30780 [DOI] [PubMed] [Google Scholar]
- 36. Takahashi Y., Takahashi S., Shiga Y., Yoshimi T., Miura T. (2000) Hypoxic induction of prolyl 4-hydroxylase α(I) in cultured cells. J. Biol. Chem. 275, 14139–14146 [DOI] [PubMed] [Google Scholar]
- 37. Hofbauer K. H., Gess B., Lohaus C., Meyer H. E., Katschinski D., Kurtz A. (2003) Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur. J. Biochem. 270, 4515–4522 [DOI] [PubMed] [Google Scholar]
- 38. Fähling M., Mrowka R., Steege A., Nebrich G., Perlewitz A., Persson P. B., Thiele B. J. (2006) Translational control of collagen prolyl 4-hydroxylase-α(I) gene expression under hypoxia. J. Biol. Chem. 281, 26089–26101 [DOI] [PubMed] [Google Scholar]
- 39. Elvidge G. P., Glenny L., Appelhoff R. J., Ratcliffe P. J., Ragoussis J., Gleadle J. M. (2006) Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J. Biol. Chem. 281, 15215–15226 [DOI] [PubMed] [Google Scholar]
- 40. Schödel J., Oikonomopoulos S., Ragoussis J., Pugh C. W., Ratcliffe P. J., Mole D. R. (2011) High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117, e207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Araldi E., Schipani E. (2010) Hypoxia, HIFs and bone development. Bone 47, 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maes C., Araldi E., Haigh K., Khatri R., Van Looveren R., Giaccia A. J., Haigh J. J., Carmeliet G., Schipani E. (2012) VEGF-independent cell-autonomous functions of HIF-1α regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J. Bone Miner. Res. 27, 596–609 [DOI] [PubMed] [Google Scholar]
- 43. Svoboda K. K. (1998) Chondrocyte-matrix attachment complexes mediate survival and differentiation. Microsc. Res. Tech. 43, 111–122 [DOI] [PubMed] [Google Scholar]
- 44. Egerbacher M., Haeusler G. (2003) Integrins in growth plate cartilage. Pediatr. Endocrinol. Rev. 1, 2–8 [PubMed] [Google Scholar]