Background: Two-pore domain K+ channels mediate background K+ conductance and regulate cellular function.
Results: Low extracellular pH (pHo) significantly increases the Na+ to K+ relative permeability of TWIK-1, TASK-1, and TASK-3 K+ channels.
Conclusion: TWIK-1, TASK-1, and TASK-3 channels change ion selectivity in lowered pHo.
Significance: The findings provide insights on the mechanism of regulation of acid-sensitive K2P channels by low pHo.
Keywords: Biophysics, Electrophysiology, Ion Channels, Membrane Biophysics, Physiology, Potassium Channels, Reconstitution of Membrane Transporters, Ion Selectivity, K2P Channel, Two-pore Domain Potassium Channels
Abstract
Two-pore domain K+ channels (K2P) mediate background K+ conductance and play a key role in a variety of cellular functions. Among the 15 mammalian K2P isoforms, TWIK-1, TASK-1, and TASK-3 K+ channels are sensitive to extracellular acidification. Lowered or acidic extracellular pH (pHo) strongly inhibits outward currents through these K2P channels. However, the mechanism of how low pHo affects these acid-sensitive K2P channels is not well understood. Here we show that in Na+-based bath solutions with physiological K+ gradients, lowered pHo largely shifts the reversal potential of TWIK-1, TASK-1, and TASK-3 K+ channels, which are heterologously expressed in Chinese hamster ovary cells, into the depolarizing direction and significantly increases their Na+ to K+ relative permeability. Low pHo-induced inhibitions in these acid-sensitive K2P channels are more profound in Na+-based bath solutions than in channel-impermeable N-methyl-d-glucamine-based bath solutions, consistent with increases in the Na+ to K+ relative permeability and decreases in electrochemical driving forces of outward K+ currents of the channels. These findings indicate that TWIK-1, TASK-1, and TASK-3 K+ channels change ion selectivity in response to lowered pHo, provide insights on the understanding of how extracellular acidification modulates acid-sensitive K2P channels, and imply that these acid-sensitive K2P channels may regulate cellular function with dynamic changes in their ion selectivity.
Introduction
Two-pore domain K+ channels (K2P)2 comprise the newest subfamily of K+ channels. Unlike other K+ channels that are tetramers, K2P channels are dimers with each subunit containing four transmembrane segments and two pore-forming loops (P-loop) (see Fig. 1A) (1). They mediate background K+ conductance and play a key role in regulation of cellular excitability as well as other cellular functions. Mammalian K2P channels are encoded by 15 KCNK genes and subdivided into six subfamilies, based on their sequence similarities and functional resemblance: TWIK, TREK, TASK, TALK, THIK, and TRESK. They respond to various physical and chemical stimuli such as temperature, mechanical stretch, arachidonic acid, lysophospholipids, pHo, and volatile anesthetics (2, 3).
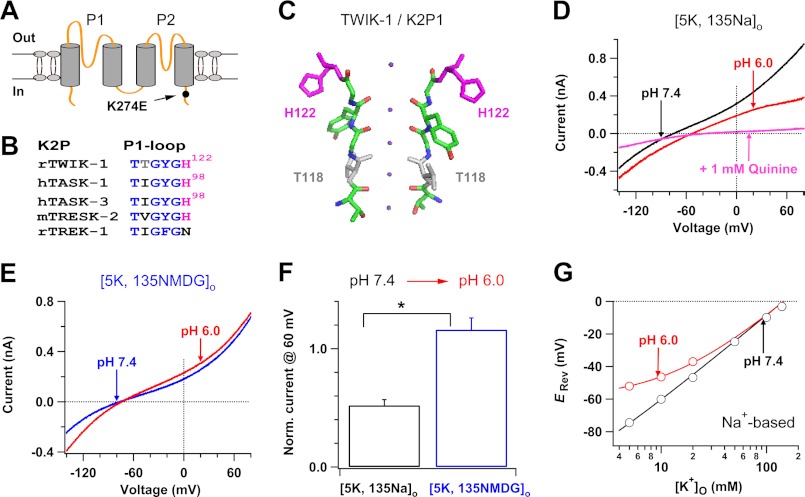
FIGURE 1.
Lowered pHo shifted the reversal potential and inhibited outward currents in TWIK-1 K+ channels. A, topology of a TWIK-1·K274E subunit. B, alignment of the selectivity sequences of P1-loops in five rat, human, and mouse K2P channels. C, the crystal structure of the selectivity filter (P1-loop) of TWIK-1 K+ channels (24). D and E, whole-cell currents of TWIK-1·K274E K+ channels are shown before (black or blue line) and after (red lines) change of pH from 7.4 to 6.0 in Na+-based (D) or NMDG+-based (E) bath solutions with 5 mm K+. Quinine blockade confirmed TWIK-1 currents in pHo 6.0 in D (pink line). F, comparison of low pHo-induced inhibitions of outward TWIK-1 K+ currents in Na+-based and NMDG+-based bath solutions. Currents at 60 mV obtained in Na+-based or NMDG+-based bath solutions with pH 6.0 were normalized (Norm.) by those recorded in pH 7.4 from a group of experiments in D or E. *, p < 0.001. G, reversal potentials (Erev) of the channels were plotted as a function of [K+]o in Na+-based solutions with pH 7.4 (black circles) or 6.0 (red circles) (n = 4–11). The continuous curves are fits with the Goldman-Hodgkin-Katz equation: Erev = RT/zF × ln((ρ[Na+]o + [K+]o)/(ρ[Na+]i + [K+]i)), yielding a Na+ to K+ relative permeability ρ of 0.005 at pH 7.4 and 0.09 at pH 6.0, respectively. Whole-cell currents in all figures were obtained in transfected CHO cells. The currents measured in lowered pHo represent those at equilibrium in all figures, unless indicated specifically.
Several subtypes of K2P channels are sensitive to external acidification. Decreases of pHo in physiological or subphysiological ranges strongly inhibit outward currents of TWIK-1 (K2P1), TREK-1 (K2P2), TASK-1 (K2P3), TASK-3 (K2P9), and TRESK-2 (K2P18) K+ channels (4–11). The low pHo-induced inhibition is dependent on extracellular K+ concentration ([K+]o), and increases in [K+]o reduce the inhibition (7, 12). Although histidine protonation has been identified as the pHo sensor, the mechanism of how low pHo affects acid-sensitive K2P channels is not well understood (2). The pHo-sensing histidine in TREK-1 K+ channels is located in the first turret loop of the outer pore. Such a histidine is also conserved in voltage-gated K+ channels (Kv1.1, Kv1.4, and Kv1.5) and inward rectifying K+ channels (Kir2.1) (13, 14). External protons inhibit TREK-1 K+ channels by inducing closure of the outer pore gate (5), similar to the C-type inactivation of Kv1 channels that has been well studied under external acidification (15, 16). In contrast, the pHo-sensing histidine in conventional acid-sensitive K2P channels, TASK-1 and TASK-3, along with TWIK-1 and TRESK-2 K+ channels, immediately follow the K+ channel selectivity sequence TXGYG of the P1-loop, whereas other K2P channels have an asparagine or methionine or tyrosine in the corresponding residue (see Fig. 1, B and C). Site-directed mutagenesis studies have identified the conserved histidine that is primarily responsible for the pHo sensitivity in TWIK-1, TASK-1, and TASK-3 K+ channels (4, 7, 8). Two related hypotheses have been proposed to interpret the mechanism of how lowered pHo results in inhibition of TASK-1 and TASK-3 K+ channels: a pore-blocking mechanism by protons or proton-induced inhibition of the channel gating (2), based on the effects of lowered pHo on open probability and/or unitary current (6, 8, 9, 17). These hypotheses face a challenge in interpreting the [K+]o dependence of low pHo-induced inhibition of the channels (2). It was previously hypothesized that increases in [K+]o decrease low pHo-induced effects on open probability, but it does not rule out the possibility that it is due to the reduction of [Na+]o rather than the increase in [K+]o (18).
We hypothesize that extracellular acidification modulates the selectivity filter and influences ion selectivity of acid-sensitive TWIK and TASK K+ channels, based on previously reported observations and the implications that are logically derived from these observations. First, we recently demonstrated that TWIK-1 K+ channels change ion selectivity, become permeable to extracellular Na+, and conduct inward leak Na+ currents in response to decreases of [K+]o in physiological and subphysiological ranges (19), suggesting that the ion selectivity of TWIK-1, and perhaps other K2P channels, can be regulated by physiological stimuli (20), although it was generally thought that ion selectivity of highly selective K+ channels does not change in response to external stimuli (21). Second, acid-sensitive TWIK and TASK K+ channels conserve a unique K+ channel selectivity sequence TXGYGH in the P1-loop (see Fig. 1B). The conserved histidine confers the pHo sensitivity in these TWIK and TASK K+ channels, and substitution of this histidine possibly influences ion selectivity of TASK-1 K+ channels (22, 23). Recently published crystal structures of TWIK-1 K+ channels confirm the location of the pHo-sensing histidine in the extracellular mouth of the selectivity filter (24) (PDB: 3UKM) (see Fig. 1C). Protonation of the pHo-sensing histidine is likely to have an impact on the conformation of the selectivity filter (25). Our previous work suggests that the reversal potential of TWIK-1 K+ channels may move in the depolarizing direction in lowered pHo (26). Third, previously published data indicate that lowered pHo potentially shifted the reversal potential of TASK-1 and TASK-3 K+ channels heterologously expressed in Xenopus oocytes or mammalian cell lines (7, 27). Such an effect was either noticed without further interpretation or neglected possibly due to small inward currents in outward rectifying TASK K+ channels. It was previously thought that TASK-3 K+ channels do not change ion selectivity in lowered pHo because replacement of extracellular Na+ with channel-impermeable choline or N-methyl-d-glucamine (NMDG) in bath solutions does not alter the sensitivity of TASK-3 K+ channels to changes in pHo (18). However, definitive evidence that could lead to this conclusion is not available.
TWIK-1 K+ channels are highly expressed in the brain, kidney, and heart (28–31). They contribute to a large passive K+ conductance in rat hippocampal astrocytes (26), conduct inward leak Na+ currents in human cardiac myocytes under pathological hypokalemia (32), and regulate phosphate and water transport in mouse proximal tubule and medullary collecting duct, respectively (33). Low pHo-induced changes in TWIK-1 function may influence cardiac excitability and K+ homeostasis in astrocytes and renal cells. TASK-1 and TASK-3 K+ channels are mainly expressed in the brain but also in other tissues, including heart and adrenal gland (1, 3). Homomeric and/or heteromeric TASK K+ channels regulate membrane potentials of several types of neurons that are involved in sensing O2 and CO2 (34). Low pHo-induced functional alteration of TASK K+ channels may affect the activity of these neurons.
Here we report that TWIK-1, TASK-1, and TASK-3 K+ channels, which are heterologously expressed in Chinese hamster ovary (CHO) cells, change ion selectivity and become permeable to extracellular Na+ ions in response to lowered pHo in subphysiological ranges. These findings improve the understanding of the mechanism of how external acidification regulates acid-sensitive K2P channels, provide another evidence of dynamic ion selectivity of highly selective K+ channels under physiological conditions, and imply a potential mechanism through which K2P channels influence cellular function by changing ion selectivity.
EXPERIMENTAL PROCEDURES
Molecular Biology and Cell Culture
K2P cDNA (rat TWIK-1, rat TREK-1, human TASK-1, human TASK-3, and mouse TRESK-2) was subcloned into pRAT or pMAX, a dual purpose vector for Xenopus oocyte or mammalian cell expression (4). K2P mutations were created by Pfu-based mutagenesis kits (Stratagene) and confirmed by automated DNA sequencing.
CHO cells were maintained in DMEM supplemented with 10% FBS in a 5% CO2 incubator and seeded in a 35-mm dish 24 h prior to transfection. Cells with at least 80% confluence were transfected by Lipofectamine 2000 (Invitrogen) with 3 μg of K2P plasmids and 1 μg of pEGFP plasmids and studied 24 h later. GFP expression was used to identify effectively transfected CHO cells.
Electrophysiology
Whole-cell patch clamp recordings were performed, and data were analyzed as described previously (19). Briefly, whole-cell voltage clamp recordings were performed with the EPC-10 USB amplifier and a Dell 745 computer using PatchMaster software (HEKA Elektronik). Patch pipettes with resistances of 2.0–3.5 megaohms were used. The resistance was compensated at least 80% to minimize voltage errors. The currents were recorded with a 2.2-s voltage ramp from −140 mV to +80 mV each 15 s. Data analysis was performed using Fitmaster (HEKA Elektronik), IGOR Pro (WaveMetrics), and Excel (Microsoft). All data are presented as means ± S.E. Two-tailed Student's t tests were used to check for significant differences between two groups of data.
The pipette solution contained (in mm): 140 KCl, 1 MgCl2, 10 EGTA, 1 K2-ATP, and 10 HEPES (pH 7.4 adjusted with KOH). The Na+-based bath solution contained (in mm): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 15 glucose, 10 Hepes (pH 7.4, 6.8, 6.0 adjusted with NaOH). The total concentration of Na+ and K+ in bath solutions is 140 mm. Bath solutions with various [K+]o were obtained by exchanges of equimolar K+ and Na+. The NMDG+-based bath solutions were obtained by replacing extracellular Na+ by equimolar NMDG+, and the pH of bath solutions was adjusted with HCl.
RESULTS
TWIK-1 K+ Channels Change Ion Selectivity and Become Permeable to Na+ in Lowered pHo
We first examined the influence of lowering pHo on the reversal potential and ion selectivity of TWIK-1 K+ channels heterologously expressed in CHO cells under physiological K+ gradients. TWIK-1 wild type K+ channels produced small detectable macroscopic currents in only about 1 of 18 transfected CHO cells because the TWIK-1 K+ channels in the cell surface are silenced by sumoylation, a posttranslational modification of lysine residues by conjugation of a small ubiquitin modifier protein. Desumoylation in TWIK-1 Lys-274 residue leads to the opening of TWIK-1 K+ channels (4). Thus, we employed TWIK-1·K274E mutant K+ channels, which contain an intracellular point mutation that disrupts the sumoylation motif and enables the detection of TWIK-1 currents (Fig. 1A), as a tool for this study; these channels had been used in our previous study (19). TWIK-1·K274E mutant K+ channels maintain the K+ selectivity in physiological pHo and K+ gradients, and the K274E mutation does not change ion selectivity of TWIK-1 K+ channels (19, 32). We monitored the reversal potential of the channels before and after the change of pH from 7.4 to 6.0 in Na+-based bath solutions with 5 mm K+ because such a change of pHo caused an ∼50% inhibition of outward TWIK-1 K+ currents in transfected CHO cells (26). Lowering pHo from 7.4 to 6.0 shifted the reversal potential of the channels from −74.5 ± 0.8 mV to −51.6 ± 1.0 mV (n = 10) (Fig. 1D), suggesting that the Na+ to K+ relative permeability of the channels increased from ∼0.005 into ∼0.10. In contrast, the reversal potential of the channels did not significantly move after the same change of pH in NMDG+-based bath solutions (−74.2 ± 1.1 mV versus −72.5 ± 1.2 mV, n = 6) (Fig. 1E). Thus, TWIK-1 K+ channels become permeable to Na+ in Na+-based bath solutions with pH 6.0. This result is reminiscent of altered ion selectivity of TWIK-1 K+ channels in lowered [K+]o (19). We also investigated ion selectivity of TWIK-1 K+ channels in lowered pHo by directly measuring reversal potentials in Na+-based bath solutions with various [K+]o. The reversal potentials recorded in pH 6.0 bath solutions with 10 and 20 mm K+ were also much more depolarized than those obtained in pHo 7.4, implying that the Na+ to K+ relative permeability of the channels significantly increases (Fig. 1G). These results support the hypothesis that TWIK-1 K+ channels change ion selectivity and become permeable to external Na+ in response to lowered pHo.
The altered ion selectivity and increase in the Na+ to K+ relative permeability of TWIK-1 K+ channels in lowered pHo were also reflected by different inhibitions of outward TWIK-1 K+ currents in Na+-based and NMDG+-based bath solutions with 5 mm K+. Lowering pHo from 7.4 into 6.0 in Na+-based bath solutions induced an ∼55% decrease of the TWIK-1 current at 60 mV, whereas this reduction did not occur in NMDG+-based bath solutions (Fig. 1, D–F). Such a difference in low pHo-induced inhibitions is consistent with a significant increase in the Na+ to K+ relative permeability and decrease in the electrochemical driving forces for outward TWIK-1 K+ currents in Na+-based, but not NMDG+-based, bath solutions with pH 6.0 because low pHo presumably has the same effects on open probability and/or unitary currents of the channels in Na+-based and NMDG+-based bath solutions.
We next attempted to examine whether substitution of the pHo-sensing histidine (His-122, Fig. 1, B and C) could eliminate the observed effect of lowered pHo on the reversal potential and ion selectivity of TWIK-1 K+ channels as the mutations in the His-122 position abolish the pHo sensitivity of TWIK-1 K+ channels (4). However, substitutions of the histidine with asparagine (H122N) or aspartic acid (H122D) did not produce functional channels in transfected CHO cells, although TWIK-1 K+ channels with these mutations give measurable currents in Xenopus oocytes (4, 35). In addition, direct measurement of inward Na+ currents through TWIK-1 K+ channels in Na+-based bath solutions with pH 6.0 and 0 mm K+ is not feasible because lowering normal [K+]o alone can induce large changes in ion selectivity of TWIK-1 K+ channels (19). We examined the permeability of divalent cation Mg2+ in TWIK-1 K+ channels in lowered pHo in the absence of extracellular K+. In pHo 6.0, TWIK-1 K+ channels did not conduct inward Mg2+ currents in Mg2+-based bath solutions with 0 mm K+ (see Fig. 4F). Therefore, TWIK-1 K+ channels change ion selectivity and increase the Na+ to K+ relativity permeability in pHo 6.0, but they do not simply lose the ion selectivity.
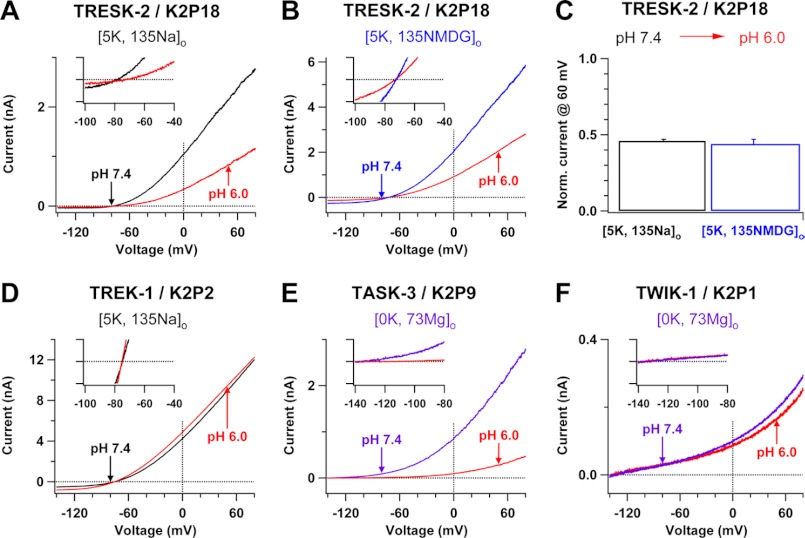
FIGURE 4.
Negative controls for low pHo-induced changes in ion selectivity of TWIK-1, TASK-1, and TASK-3 K+ channels. A and B, whole-cell currents of TRESK-2 K+ channels are shown before (black or blue line) and after (red lines) change of pH from 7.4 to 6.0 in Na+-based (A) or NMDG+-based (B) bath solutions with 5 mm K+. C, comparison of low pHo-induced inhibitions of outward TRESK-2 K+ currents in Na+-based and NMDG+-based bath solutions. Currents at 60 mV obtained in Na+-based or NMDG+-based bath solutions with pH 6.0 were normalized (Norm.) by those recorded in pH 7.4 from a group of experiments in A or B (n = 4–5). D–F, whole-cell currents of TREK-1, TASK-3, and TWIK-1 K+ channels are shown before (black or purple lines) and after (red lines) change of pH from 7.4 to 6.0 in Na+-based bath solutions with 5 mm K+ (D) or Mg2+-based bath solutions with 0 mm K+ (E and F) (n = 5–8). Insets in A and B and D–F: current traces are shown in shorter voltage ranges and narrow current amplitudes (−100 to +100 pA) so that the reversal potential or no inward Mg2+ current is clearly visible.
Kinetics of Changes in the Reversal Potential and Current Amplitude Reveal Dynamic Ion Selectivity of TWIK-1 K+ Channels in Extracellular Acidification
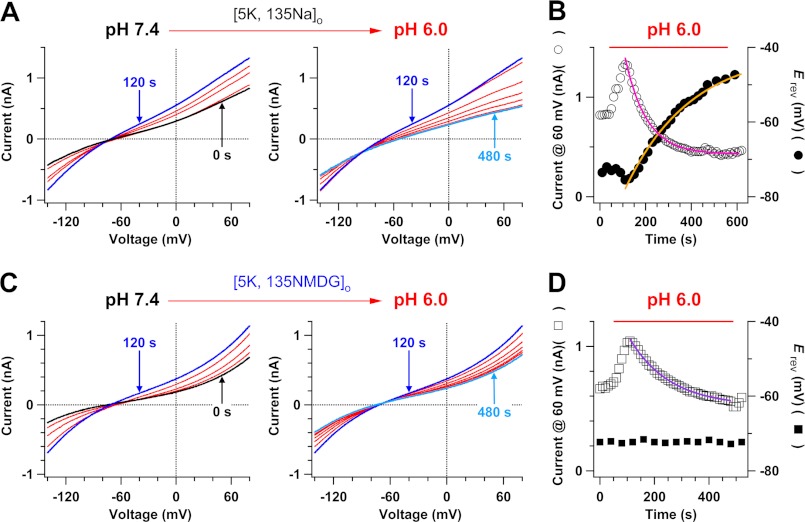
We monitored the kinetics of low pHo-induced effects on TWIK-1 K+ channels in Na+-based bath solutions with 5 mm K+. The effects of lowered pHo on TWIK-1 K+ channels were slow, and they took ∼8 min to reach the equilibrium. Lowered pHo induced two-phase effects on the current amplitude and reversal potential of TWIK-1 K+ channels. In the first 2 min after the change of pHo from 7.4 into 6.0, whole-cell TWIK-1 currents increased transiently, the current at 60 mV went up to the peak by 65 ± 3% (n = 10), and the reversal potential moved toward the hyperpolarizing direction by ∼4 mV, indicating that the channels are highly K+-selective (Fig. 2A, left panel). In the second phase, whole-cell TWIK-1 currents decreased progressively, and the current at 60 mV went down to a steady-state level at 45 ± 4% of the current obtained in pHo 7.4 (Fig. 1F and Fig. 2A, right panel), with a time constant of ∼94 s (Fig. 2B, open circles and pink line). The reversal potential began to shift in the depolarizing direction until it reached the equilibrium at approximately −52 mV, indicating that the channels altered their Na+ to K+ relative permeability during this period. The time constant of the change in the Na+ to K+ relative permeability in the second phase was ∼284 s (Fig. 2B, filled circles and orange line), reflecting the process whereby the selectivity filter changes from a K+-selective to a Na+-permeable conformation. We attempted to restore the K+ selectivity of the channels by switching pHo back to 7.4 from 6.0. The recovery of K+ selectivity was slow and took ∼12 min. This hysteresis is not surprising as the recovery of the K+ selectivity is extremely slow from lowered [K+]o to normal [K+]o (19).
FIGURE 2.
Kinetics of low pHo-induced changes in the reversal potential and current amplitude of TWIK-1 K+ channels. A and C, two-phase effects of lowered pH of Na+-based (A) or NMDG+-based (C) bath solutions on whole-cell currents of TWIK-1·K274E K+ channels. Whole-cell currents are each shown in 30 and 60 s in the left and right panels, respectively. B and D, kinetics of the changes in the reversal potential (filled circles and squares) and TWIK-1 currents at 60 mV (open circles and squares) after pHo was lowered in A or C. The superimposed single-exponential fit yields a time constant of 284 ± 25 s (orange line, n = 9), 94 ± 8 s (pink line, n = 9), and 148 ± 11 s (purple line, n = 6), respectively.
We studied kinetics of effects of lowered pHo on TWIK-1 K+ channels in NMDG+-based bath solutions with 5 mm K+ to differentiate the effects of low pHo on the reversal potential and the current amplitude of the channels. As expected, lowering pHo from 7.4 to 6.0 did not change the reversal potential of TWIK-1 K+ channels (Figs. 1E and 2, C and D). However, it still induced two-phase changes in TWIK-1 K+ currents, similar to those observed in Na+-based bath solutions (Fig. 2, A and B). In the first phase, the current at 60 mV increased by 73 ± 6% (n = 6), and in the second phase, the current at 60 mV reduced with a time constant of ∼148 s until it reached the levels similar to that in pH 7.4 (Figs. 1F and 2, C and D). Dynamic changes in the current amplitude reflect a process in which lowered pHo modulates the pore and alters the single channel properties of the channels. In addition, the time constant of the current decay in the second phase in NMDG+-based bath solutions is significantly slower than that in Na+-based bath solutions, consistent with the increase in the Na+ to K+ relative permeability and decrease in the electrochemical driving force of outward TWIK-1 K+ current in Na+-based bath solutions with pH 6.0.
TASK-3 K+ Channels Exhibit Dynamic Ion Selectivity in Lowered pHo
We further examined the effects of lowered pHo on the reversal potential and ion selectivity of TASK-3 K+ channels in Na+-based bath solutions under physiological K+ gradients in transfected CHO cells. Unlike those observed in TWIK-1 K+ channels, low pHo-induced effects on TASK-3 K+ channels were rapid, and they took just around 1 min to reach the equilibrium. Lowering pHo from 7.4 to 6.0 shifted the reversal potential of TASK-3 K+ channels from −77.7 ± 0.4 mV to −51.9 ± 2.5 mV (n = 10), consistent with a previous study (27), and such a shift was reversible (Fig. 3A). In contrast, it did not significantly change the reversal potential of TASK-3 K+ channels in NMDG+-based bath solutions (−77.9 ± 0.7 mV versus −74.4 ± 1.0 mV, n = 9; Fig. 3B). Surprisingly, lowering pHo from 7.4 to 6.0 in Na+-based bath solutions had no significant effect on the reversal potential of TRESK-2 K+ channels (−78.8 ± 0.6 mV versus −75.3 ± 1.2 mV, n = 5; Fig. 4A), which also conserve the pHo-sensing histidine following the selectivity sequence TXGYG of the P1-loop (Fig. 1B), although the currents at 60 mV of TRESK-2 K+ channels were reduced by ∼54%, consistent with a previous study (10) (Fig. 4, A–C). Thus, TRESK-2 K+ channels maintain the K+ selectivity in low pHo. In another negative control, lowered pHo did not change ion selectivity of rat TREK-1 K+ channels, which are insensitive to pHo and do not conserve the pHo-sensing histidine in the selectivity filter (Fig. 1B), because changes of pH from 7.4 to 6.0 in Na+-based bath solutions did not influence the reversal potential (−76.2 ± 1.0 mV versus −75.8 ± 0.6 mV, n = 6; Fig. 4D). TASK-3 K+ channels are outward rectifying and conduct small inward K+ currents under physiological K+ gradients, so it was not always easy to precisely measure their reversal potentials in 5 mm [K+]o. However, the trend of reversal potential movements in negative controls was different from that in TASK-3 K+ channels, indicating that these measured reversal potentials represent ion selectivity of TASK-3 K+ channels. We also directly measured the reversal potentials of TASK-3 K+ channels in Na+-based bath solutions with various [K+]o. The reversal potentials recorded in pH 6.0 bath solutions with 2.5 mm K+ were much more depolarized than those obtained in pHo 7.4, implying that the Na+ to K+ relative permeability of the channels significantly increases (Fig. 3F).
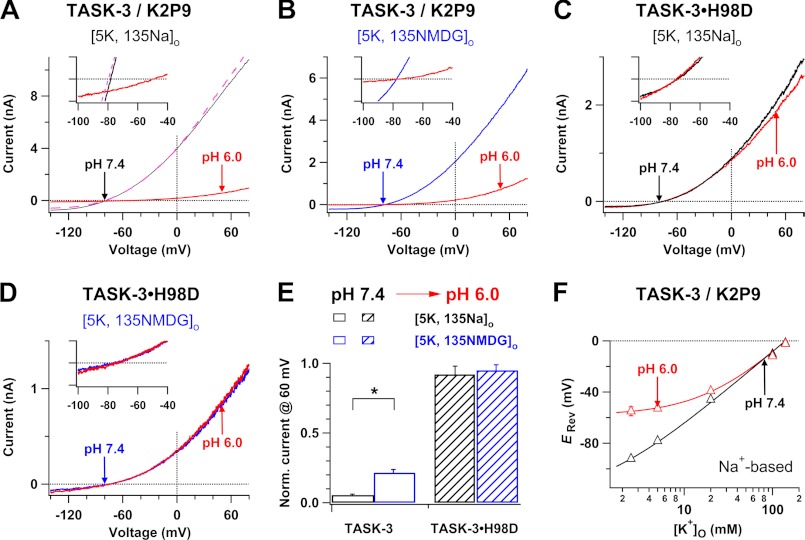
FIGURE 3.
Effects of lowered pHo on the reversal potential and outward currents of TASK-3 wild type and TASK-3·H98D mutant K+ channels. A–D, whole-cell currents are shown for TASK-3 (A and B) and TASK-3·H98D (C and D) K+ channels before (black or blue line) and after (red lines) change of pH from 7.4 to 6.0 in Na+-based (A and C) or NMDG+-based (B and D) bath solutions. The dashed pink line in A represents the current after pHo was switched backed to 7.4 from 6.0. Insets: current traces are shown in shorter voltage ranges and narrow current amplitudes (−100 to +100 pA) so that the reversal potentials are clearly visible. E, comparison of low pHo-induced inhibitions of outward K+ currents in Na+-based and NMDG+-based bath solutions for the channels. Whole-cell currents at 60 mV obtained in Na+-based or NMDG+-based bath solutions with pH 6.0 were normalized by those recorded in pH 7.4. *, p < 0.001. F, reversal potentials of TASK-3 K+ channels were plotted as a function of [K+]o in Na+-based solutions with pH 7.4 (black triangles) or 6.0 (red triangles) (n = 11–29). The continuous curves are fits with the Goldman-Hodgkin-Katz equation, yielding a Na+ to K+ relative permeability of 0.006 at pH 7.4 and 0.13 at pH 6.0, respectively.
We compared low pHo-induced inhibitions of TASK-3 K+ channels in Na+-based and NMDG+-based bath solutions with 5 mm K+ because increases in the Na+ to K+ relative permeability in TASK-3 K+ channels could cause more significant inhibitions in Na+-based bath solutions than in NMDG+-based bath solutions, as we observed for TWIK-1 K+ channels (Fig. 1F). Lowering pH from 7.4 into 6.0 in Na+-based bath solutions decreased the TASK-3 current at 60 mV by 95 ± 1% (n = 10), whereas only 79 ± 2% (n = 9) reduction was seen in NMDG+-based bath solutions (Fig. 3E). In addition, we examined whether the mutation in the pHo-sensing histidine (His-98) eliminated the effects of low pHo on the reversal potentials of TASK-3 K+ channels. Lowering pH from 7.4 into 6.0 in Na+-based bath solutions neither influenced the reversal potential of TASK-3·H98D mutant K+ channels (−74.3 ± 2.2 mV versus −73.7 ± 1.4 mV, n = 4) nor induced significant inhibition of outward TASK-3 K+ currents (Fig. 3, C–E). Similar to TWIK-1 K+ channels, TASK-3 K+ channels did not conduct inward Mg2+ currents in Mg2+-based bath solutions with pH 6.0 in the absence of extracellular K+ (Fig. 4E), supporting that TASK-3 K+ channels do not simply lose the ion selectivity. These results support the hypothesis that TASK-3 K+ channels change ion selectivity and become permeable to external Na+ in response to lowered pHo.
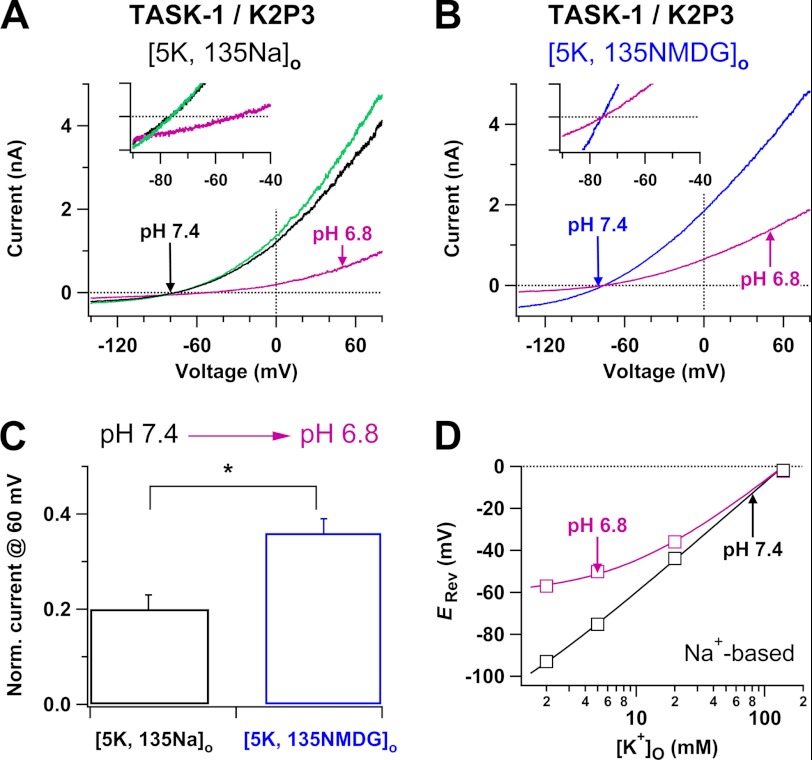
TASK-1 K+ Channels Show Dynamic Ion Selectivity in Subphysiological pHo
We examined the effects of lowered pHo on the reversal potential and ion selectivity of TASK-1 K+ channels in transfected CHO cells by using the same strategies with which we have studied TWIK-1 and TASK-3 K+ channels. TASK-1 K+ channels are more sensitive to changes of pHo than TWIK-1 and TASK-3 K+ channels (2), so we studied the effects of low pHo in subphysiological ranges on the reversal potential of TASK-1 K+ channels in Na+-based bath solutions with 5 mm K+. TASK-1 K+ channels did not yield detectable whole-cell currents in most transfected CHO cells, so we used a TASK-1·Δi20 mutant with a 20-amino acid deletion within the C-terminal, which increases abundance at the plasma membrane relative to TASK-1 wild type channels (36), as a tool. Compatible with those seen in TASK-3 K+ channels, low pHo-induced effects on TASK-1 K+ channels were fast, and they took ∼1 min to reach equilibrium. The reversal potential of TASK-1 K+ channels shifted from −75.2 ± 1.2 mV to −50.1 ± 2.9 mV (n = 10) (Fig. 5A) after changes of pH from 7.4 to 6.8 in Na+-based bath solutions, implying that the Na+ to K+ relative permeability of the channels is significantly increased. In contrast, the movement of the reversal potential was not seen in NMDG+-based bath solutions before and after changes of pHo (−73.6 ± 1.2 mV versus −74.5 ± 1.6 mV, n = 6) (Fig. 5B). We directly measured the reversal potential of the channels in Na+-based bath solutions with various [K+]o. At 2 mm [K+]o, the reversal potential measured in pH 6.8 was much more depolarized than that obtained in pH 7.4. The fits of measured reversal potentials in various [K+]o with the Goldman-Hodgkin-Katz equation indicate that the Na+ to K+ relative permeability of the channels significantly increases in pH 6.8 (Fig. 5D). Moreover, we compared low pHo-induced inhibitions of TASK-1 K+ channels in Na+-based and NMDG+-based bath solutions with 5 mm K+. Lowering pH from 7.4 into 6.8 decreased the TASK-1 current at 60 mV by 80 ± 1% (n = 10) in Na+-based bath solutions, whereas it induced a reduction of only 64 ± 3% (n = 6) in NMDG+-based bath solutions (Fig. 5C), consistent with increases in the Na+ to K+ relative permeability and decreases in electrochemical driving forces of outward TASK-1 K+ currents in pH 6.8. These results suggest that TASK-1 K+ channels change ion selectivity and become permeable to external Na+ in response to lowering pHo.
FIGURE 5.
Effects of lowered pHo on the reversal potential and outward currents of TASK-1 K+ channels. A and B, whole-cell currents of TASK-1 K+ channels are shown before (black or blue line) and after (pink lines) change of pH from 7.4 to 6.8 in Na+-based (A) or NMDG+-based (B) bath solutions. Insets: current traces were shown in shorter voltage ranges and narrow current amplitudes (−100 to +100 pA) so that the reversal potentials are clearly visible. The green line in A represents the current after pHo was switched back to 7.4 from 6.8. C, comparison of low pHo-induced inhibitions of outward TASK-1 currents in Na+-based and NMDG+-based bath solutions. Whole-cell currents at 60 mV obtained in Na+-based or NMDG+-based bath solutions with pH of 6.8 were normalized (Norm.) by those recorded in pH of 7.4. *, p < 0.001. D, reversal potentials of TASK-1 K+ channels were plotted as a function of [K+]o in Na+-based solutions with pH 7.4 (black squares) or 6.8 (pink squares) (n = 3–11). The continuous curves are fits with the Goldman-Hodgkin-Katz equation, yielding a Na+ to K+ relative permeability of 0.005 at pH 7.4 and 0.06 at pH 6.8, respectively.
DISCUSSION
Dynamic Ion Selectivity of Acid-sensitive TWIK and TASK K+ Channels in Extracellular Acidification
We provided three lines of evidence supporting that TWIK-1, TASK-1, and TASK-3 K+ channels change ion selectivity and become permeable to extracellular Na+ in lowered pHo. First, we investigated ion selectivity of TWIK-1, TASK-1, and TASK-3 K+ channels in both normal and lowered pHo by using the reversal potential analysis and whole-cell current recordings in transfected CHO cells. Our results indicate that the reversal potentials of TWIK-1, TASK-1, and TASK-3 K+ channels are significantly shifted in the depolarizing direction in Na+-based, but not in NMDG+-based, bath solutions with lowered pH. TWIK-1 K+ channels have a nearly linear current-voltage curve and conduct large inward K+ currents in physiological K+ gradients, so their reversal potential can be precisely measured during lowering pHo (19). Outward rectifying TASK-1 and TASK-3 K+ channels conduct very small inward K+ currents in physiological K+ gradients, so precisely monitoring their reversal potential is challenging. This may be the reason why the effects of lowered pHo on their reversal potentials were neglected. However, low pH-induced, reversible shifts of the reversal potentials in TASK-1 and TASK-3 K+ channels should reflect changes of their ion selectivity. Because the reversal potential analysis on outward rectifying TASK-1 and TASK-3 K+ channels may be challenged, we secondly compared low pHo-induced inhibitions of outward currents of TWIK-1, TASK-1, and TASK-3 K+ channels in Na+-based and NMDG+-based bath solutions with 5 mm K+. If the K2P channels become significantly permeable to external Na+ in lowered pHo such as pH 6.0, the electrochemical driving force for outward K+ currents should become smaller in Na+-based, but not in NMDG+-based, bath solutions. Such a difference could result in more profound low pHo-induced inhibitions in Na+-based bath solutions than in NMDG+-based bath solutions. This is exactly what we observed for TWIK-1, TASK-1, and TASK-3 K+ channels (Figs. 1F, 3E, and 5C). Third, we examined the effects of lowered pHo on TASK-3·H98D mutant K+ channels, in which the pHo-sensing histidine is substituted by aspartic acid. Lowered pHo neither changes the reversal potential and ion selectivity of TASK-3·H98D mutant K+ channels in Na+-based bath solutions with 5 mm K+ nor induces different inhibitions in Na+-based and NMDG+-based bath solutions with 5 mm K+ (Fig. 3, C–E).
A most recent publication reported that the TWIK-1·K274E·I293A·I294A mutant (TWIK-1surf) channels heterologously expressed in Xenopus oocytes, which largely increase detectable TWIK-1 currents, exhibit altered ion selectivity in lowered pHo (35). However, whether TWIK-1surf channels represent TWIK-1 wild type K+ channels is questionable in terms of ion selectivity because the data presented in the study showed that TWIK-1surf channels are K+-selective in pH 7.4 Na+-based bath solutions with 0 and 2 mm K+, not consistent with previous observations that TWIK-1 wild type channels are nonselective channels significantly permeable to extracellular Na+ in 0 and 2 mm [K+]o (19, 32). The TWIK-1·K274E mutant K+ channels, which we used in this study, share the same behaviors as TWIK-1 wild type K+ channels in ion selectivity (19, 32). Therefore, our study suggests that TWIK-1 wild type K+ channels exhibit dynamic ion selectivity in lowered pHo.
How does protonation in the pHo-sensing histidine change ion selectivity of acid-sensitive TWIK and TASK K+ channels? A previous study with molecular dynamics simulations predicts that protonation of the pHo-sensing His-98 residue of TASK-1 K+ channels induces rotation of the Tyr-96 and Gly-97 peptide, and then the conformation of the selectivity filter changes (25). Voltage-gated Kv2.1 channels change ion selectivity during C-type inactivation (37). Extracellular acidification enhances C-type inactivation in Kv1 channels (16, 38, 39). K2P channels are thought to have a C-type inactivation gate at the selectivity filter close to the extracellular side of the channels (40–42). Extracellular acidification facilitates C-type inactivation in human TREK-1 K+ channels (5). Protonation in the pHo-sensing histidine of TASK-1 and TASK-3 K+ channels is likely to involve enhancement of C-type inactivation.
Although TWIK-1, TASK-1, TASK-3, and TRESK-2 K+ channels contain the same selectivity sequence TXGYGH with the pHo-sensing histidine in the P1-loop in the selectivity filter, they show different responses to lowered pHo. TRESK-2 K+ channels maintain the K+ selectivity in lowered pHo, whereas TWIK-1, TASK-1, and TASK-3 K+ channels become permeable to external Na+. Extracellular acidification has rapid effects on TASK-1 and TASK-3 K+ channels, but it causes much slower functional changes in TWIK-1 K+ channels. These four acid-sensitive K2P channels belong to three different subfamilies of K2P channels, and their sequence similarities are very low. Therefore, the other part of the channels contributes to their different responses to lowering pHo.
Although it is generally thought that ion selectivity of highly selective K+ channels does not change in response to external stimuli, recently we demonstrated that TWIK-1 K+ channels change ion selectivity and become permeable to extracellular Na+ in response to physiological decreases of extracellular K+ levels (19). In this study, we showed another example of dynamic ion selectivity of K2P channels under physiological conditions; TWIK-1, TASK-1, and TASK-3 K+ channels change ion selectivity and become permeable to extracellular Na+ under extracellular acidification. It will not be surprising to find that other physiological stimuli regulate ion selectivity of K2P channels.
In physiological or pathophysiological processes (e.g. hypoxia), in which pHo is lowered to a range of 6.0–6.8, acid-sensitive TWIK and TASK channels may behave with dynamic ion selectivity. TASK-1 and TASK-3 K+ channels conduct much smaller background K+ currents in lowered pHo than in normal pHo, whereas TWIK-1 K+ channels conduct significant inward Na+ currents and contribute to the transportation of both K+ and Na+ across the membrane, which are involved in cellular excitability and/or electrolyte homeostasis.
Does pHo regulation of TWIK-1, TASK-1, and TASK-3 K+ channels in heterologous expression systems apply to these channels in native tissues? These native channels should exhibit low pHo-induced phenotypes that were observed in cloned channels, as long as they reach the plasma membrane. However, many K2P channels including TWIK-1 and TASK-1 are poorly expressed in the plasma membrane. One possible mechanism is that the channels conserve endoplasmic reticulum retention motifs that hold the channels in the endoplasmic reticulum (36, 43). Another possibility is that the channels may lack accessory subunits and/or interacting proteins in the heterologous expression systems. In fact, interacting proteins such as 14-3-3 determine the trafficking and expression levels of TASK-1 K+ channels (44).
The Mechanism of Regulation of Acid-sensitive K2P Channels by Extracellular Acidification
Although the effects of extracellular acidification on TASK-1 and TASK-3 K+ channels have been extensively studied, the mechanism of how low pHo affects these channels is not well understood (2). Previous studies showed that lowered pHo changes single channel properties such as open probability and/or unitary current of TASK-1 and TASK-3 K+ channels (7, 8, 17). In this study, we show that lowered pHo also modulates the selectivity filter and then influences ion selectivity of TWIK-1, TASK-1, and TASK-3 K+ channels. Therefore, low pHo affects both the ion selectivity and the single channel property of these acid-sensitive K2P channels. The effects of lowered pHo on the single channel property of these channels alone could be studied in NMDG+-based bath solutions, as we did in whole-cell current levels in Figs. 1E, 2C, 3B, and 5B. Lowered pHo in NMDG+-based bath solutions induced large reduction of TRESK-2, TASK-1, and TASK-3 outward currents, whereas it did not significantly change TWIK-1 outward currents. A possible mechanism of such a difference is that lowered pHo inhibits unitary currents, induces C-type inactivation, and dramatically decreases open probability in TRESK-2, TASK-1, and TASK-3 K+ channels, whereas it may not cause C-type inactivation and may have minor effects on unitary current and open probability in TWIK-1 K+ channels.
Previous findings that lowered pHo affects single channel properties could not fully interpret a well known observation that low pHo-induced inhibitions of acid-sensitive TASK K+ channels are dependent on [K+]o. Our findings provide a supplemental interpretation on the observation. Low pHo increases the Na+ to K+ relative permeability and then decreases the driving forces of outward K+ currents, which are determined by the electrochemical gradients of both K+ and Na+. Assuming that the effects of low pHo on single channel property are constant, the driving forces of outward K+ current became smaller with increases of [K+]o or decreases of [Na+]o in Na+-based bath solutions. This will result in the observation that low pHo-induced inhibitions depend on both [K+]o and [Na+]o.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R01GM102943-01A1 (to H. C.) through the NIGMS. This work was also supported by American Heart Association (11GRNT7270014) (to H. C.).
- K2P channel
- two-pore domain potassium channel
- pHo
- extracellular pH
- [K+]o
- extracellular potassium concentration
- [Na+]o
- extracellular sodium concentration
- NMDG
- N-methyl-d-glucamine.
REFERENCES
- 1. Goldstein S. A., Bayliss D. A., Kim D., Lesage F., Plant L. D., Rajan S. (2005) International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 57, 527–540 [DOI] [PubMed] [Google Scholar]
- 2. Lotshaw D. P. (2007) Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem. Biophys. 47, 209–256 [DOI] [PubMed] [Google Scholar]
- 3. Enyedi P., Czirják G. (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605 [DOI] [PubMed] [Google Scholar]
- 4. Rajan S., Plant L. D., Rabin M. L., Butler M. H., Goldstein S. A. (2005) Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 121, 37–47 [DOI] [PubMed] [Google Scholar]
- 5. Cohen A., Ben-Abu Y., Hen S., Zilberberg N. (2008) A novel mechanism for human K2P2.1 channel gating: facilitation of C-type gating by protonation of extracellular histidine residues. J. Biol. Chem. 283, 19448–19455 [DOI] [PubMed] [Google Scholar]
- 6. Duprat F., Lesage F., Fink M., Reyes R., Heurteaux C., Lazdunski M. (1997) TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 16, 5464–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopes C. M., Gallagher P. G., Buck M. E., Butler M. H., Goldstein S. A. (2000) Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. J. Biol. Chem. 275, 16969–16978 [DOI] [PubMed] [Google Scholar]
- 8. Kim Y., Bang H., Kim D. (2000) TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem. 275, 9340–9347 [DOI] [PubMed] [Google Scholar]
- 9. Rajan S., Wischmeyer E., Xin Liu G., Preisig-Müller R., Daut J., Karschin A., Derst C. (2000) TASK-3, a novel tandem pore domain acid-sensitive K+ channel: an extracellular histidine as pH sensor. J. Biol. Chem. 275, 16650–16657 [DOI] [PubMed] [Google Scholar]
- 10. Kang D., Mariash E., Kim D. (2004) Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J. Biol. Chem. 279, 28063–28070 [DOI] [PubMed] [Google Scholar]
- 11. Keshavaprasad B., Liu C., Au J. D., Kindler C. H., Cotten J. F., Yost C. S. (2005) Species-specific differences in response to anesthetics and other modulators by the K2P channel TRESK. Anesth. Analg. 101, 1042–1049 [DOI] [PubMed] [Google Scholar]
- 12. Lopes C. M., Zilberberg N., Goldstein S. A. (2001) Block of Kcnk3 by protons: evidence that 2-P-domain potassium channel subunits function as homodimers. J. Biol. Chem. 276, 24449–24452 [DOI] [PubMed] [Google Scholar]
- 13. Steidl J. V., Yool A. J. (1999) Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Mol. Pharmacol. 55, 812–820 [PubMed] [Google Scholar]
- 14. Coulter K. L., Périer F., Radeke C. M., Vandenberg C. A. (1995) Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron 15, 1157–1168 [DOI] [PubMed] [Google Scholar]
- 15. Claydon T. W., Boyett M. R., Sivaprasadarao A., Orchard C. H. (2002) Two-pore residues mediate acidosis-induced enhancement of C-type inactivation of the Kv1.4 K+ channel. Am. J. Physiol. Cell Physiol. 283, C1114–C1121 [DOI] [PubMed] [Google Scholar]
- 16. Li X., Bett G. C., Jiang X., Bondarenko V. E., Morales M. J., Rasmusson R. L. (2003) Regulation of N- and C-type inactivation of Kv1.4 by pHo and K+: evidence for transmembrane communication. Am. J. Physiol. Heart Circ. Physiol. 284, H71–H80 [DOI] [PubMed] [Google Scholar]
- 17. Kim Y., Bang H., Kim D. (1999) TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am. J. Physiol. 277, H1669–H1678 [DOI] [PubMed] [Google Scholar]
- 18. Clarke C. E., Veale E. L., Wyse K., Vandenberg J. I., Mathie A. (2008) The M1P1-loop of TASK3 K2P channels apposes the selectivity filter and influences channel function. J. Biol. Chem. 283, 16985–16992 [DOI] [PubMed] [Google Scholar]
- 19. Ma L., Zhang X., Chen H. (2011) TWIK-1 two-pore domain potassium channels change ion selectivity and conduct inward leak sodium currents in hypokalemia. Sci. Signal. 4, ra37. [DOI] [PubMed] [Google Scholar]
- 20. Goldstein S. A. (2011) K2P potassium channels, mysterious and paradoxically exciting. Sci. Signal. 4, pe35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hille B. (2001) Ion Channels of Excitable Membranes, Third Ed., p. 469 Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 22. O'Connell A. D., Morton M. J., Sivaprasadarao A., Hunter M. (2005) Selectivity and interactions of Ba2+ and Cs+ with wild type and mutant TASK1 K+ channels expressed in Xenopus oocytes. J. Physiol. 562, 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuill K., Ashmole I., Stanfield P. R. (2004) The selectivity filter of the tandem pore potassium channel TASK-1 and its pH sensitivity and ionic selectivity. Pflugers Arch. 448, 63–69 [DOI] [PubMed] [Google Scholar]
- 24. Miller A. N., Long S. B. (2012) Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 [DOI] [PubMed] [Google Scholar]
- 25. Stansfeld P. J., Grottesi A., Sands Z. A., Sansom M. S., Gedeck P., Gosling M., Cox B., Stanfield P. R., Mitcheson J. S., Sutcliffe M. J. (2008) Insight into the mechanism of inactivation and pH sensitivity in potassium channels from molecular dynamics simulations. Biochemistry 47, 7414–7422 [DOI] [PubMed] [Google Scholar]
- 26. Zhou M., Xu G., Xie M., Zhang X., Schools G. P., Ma L., Kimelberg H. K., Chen H. (2009) TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 29, 8551–8564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meadows H. J., Randall A. D. (2001) Functional characterization of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology 40, 551–559 [DOI] [PubMed] [Google Scholar]
- 28. Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 15, 1004–1011 [PMC free article] [PubMed] [Google Scholar]
- 29. Lesage F., Lauritzen I., Duprat F., Reyes R., Fink M., Heurteaux C., Lazdunski M. (1997) The structure, function, and distribution of the mouse TWIK-1 K+ channel. FEBS Lett. 402, 28–32 [DOI] [PubMed] [Google Scholar]
- 30. Ordög B., Brutyó E., Puskás L. G., Papp J. G., Varró A., Szabad J., Boldogkoi Z. (2006) Gene expression profiling of human cardiac potassium and sodium channels. Int. J. Cardiol. 111, 386–393 [DOI] [PubMed] [Google Scholar]
- 31. Gaborit N., Le Bouter S., Szuts V., Varro A., Escande D., Nattel S., Demolombe S. (2007) Regional and tissue-specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 582, 675–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma L., Xie Y. P., Zhou M., Chen H. (2012) Silent TWIK-1 potassium channels conduct monovalent cation currents. Biophys. J. 102, L34–L36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nie X., Arrighi I., Kaissling B., Pfaff I., Mann J., Barhanin J., Vallon V. (2005) Expression and insights on function of potassium channel TWIK-1 in mouse kidney. Pflugers Arch. 451, 479–488 [DOI] [PubMed] [Google Scholar]
- 34. Lesage F., Barhanin J. (2011) Molecular physiology of pH-sensitive background K2P channels. Physiology (Bethesda) 26, 424–437 [DOI] [PubMed] [Google Scholar]
- 35. Chatelain F. C., Bichet D., Douguet D., Feliciangeli S., Bendahhou S., Reichold M., Warth R., Barhanin J., Lesage F. (2012) TWIK1, a unique background channel with variable ion selectivity. Proc. Natl. Acad. Sci. U.S.A. 109, 5499–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Renigunta V., Yuan H., Zuzarte M., Rinné S., Koch A., Wischmeyer E., Schlichthörl G., Gao Y., Karschin A., Jacob R., Schwappach B., Daut J., Preisig-Müller R. (2006) The retention factor p11 confers an endoplasmic reticulum-localization signal to the potassium channel TASK-1. Traffic 7, 168–181 [DOI] [PubMed] [Google Scholar]
- 37. Kiss L., LoTurco J., Korn S. J. (1999) Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 76, 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kehl S. J., Eduljee C., Kwan D. C., Zhang S., Fedida D. (2002) Molecular determinants of the inhibition of human Kv1.5 potassium currents by external protons and Zn2+. J. Physiol. 541, 9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Claydon T. W., Boyett M. R., Sivaprasadarao A., Ishii K., Owen J. M., O'Beirne H. A., Leach R., Komukai K., Orchard C. H. (2000) Inhibition of the K+ channel kv1.4 by acidosis: protonation of an extracellular histidine slows the recovery from N-type inactivation. J. Physiol. 526, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mathie A., Al-Moubarak E., Veale E. L. (2010) Gating of two-pore domain potassium channels. J. Physiol. 588, 3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen A., Ben-Abu Y., Zilberberg N. (2009) Gating the pore of potassium leak channels. Eur. Biophys. J. 39, 61–73 [DOI] [PubMed] [Google Scholar]
- 42. Zilberberg N., Ilan N., Goldstein S. A. (2001) KCNKØ: opening and closing the 2-P-domain potassium leak channel entails “C-type” gating of the outer pore. Neuron 32, 635–648 [DOI] [PubMed] [Google Scholar]
- 43. Feliciangeli S., Tardy M. P., Sandoz G., Chatelain F. C., Warth R., Barhanin J., Bendahhou S., Lesage F. (2010) Potassium channel silencing by constitutive endocytosis and intracellular sequestration. J. Biol. Chem. 285, 4798–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Kelly I., Butler M. H., Zilberberg N., Goldstein S. A. (2002) Forward transport: 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell 111, 577–588 [DOI] [PubMed] [Google Scholar]