Background: Surfactant protein A stimulates macrophage chemotaxis.
Results: SPA interaction with TLR2 stimulates JNK- and ERK-dependent TGFβ production, which in turn stimulates RHAMM- and hyaluronan-dependent macrophage chemotaxis.
Conclusion: SPA activates a novel and specific pathway to stimulate macrophage chemotaxis.
Significance: Uncovering the mechanisms that regulate innate immunity in the lung is crucial for understanding the responses to infection and injury.
Keywords: Chemotaxis, Extracellular Matrix, Macrophages, Pulmonary Surfactant, Toll-like Receptors (TLR), Hyaluronan, RHAMM
Abstract
The innate immune system protects the host from bacterial and viral invasion. Surfactant protein A (SPA), a lung-specific collectin, stimulates macrophage chemotaxis. However, the mechanisms regulating this function are unknown. Hyaluronan (HA) and its receptors RHAMM (receptor for HA- mediated motility, CD168) and CD44 also regulate cell migration and inflammation. We therefore examined the role of HA, RHAMM, and CD44 in SPA-stimulated macrophage chemotaxis. Using antibody blockade and murine macrophages, SPA-stimulated macrophage chemotaxis was dependent on TLR2 but not the other SPA receptors examined. Anti-TLR2 blocked SPA-induced production of TGFβ. In turn, TGFβ1-stimulated chemotaxis was inhibited by HA-binding peptide and anti-RHAMM antibody but not anti-TLR2 antibody. Macrophages from TLR2−/− mice failed to migrate in response to SPA but responded normally to TGFβ1 and HA, effects that were blocked by anti-RHAMM antibody. Macrophages from WT and CD44−/− mice had similar responses to SPA, whereas those from RHAMM−/− mice had decreased chemotaxis to SPA, TGFβ1, and HA. In primary macrophages, SPA-stimulated TGFβ production was dependent on TLR2, JNK, and ERK but not p38. Pam3Cys, a specific TLR2 agonist, stimulated phosphorylation of JNK, ERK, and p38, but only JNK and ERK inhibition blocked Pam3Cys-stimulated chemotaxis. We have uncovered a novel pathway for SPA-stimulated macrophage chemotaxis where SPA stimulation via TLR2 drives JNK- and ERK-dependent TGFβ production. TGFβ1, in turn, stimulates macrophage chemotaxis in a RHAMM and HA-dependent manner. These findings are highly relevant to the regulation of innate immune responses by SPA with key roles for specific components of the extracellular matrix.
Introduction
Lung infections and many noninfectious lung disorders, such as bronchopulmonary dysplasia, acute respiratory distress syndrome, cystic fibrosis, and occupational lung diseases, involve an inflammatory response with elaboration of growth factors and cytokines and an abnormal remodeling of the extracellular matrix. Toll-like receptors (TLRs)3 and a family of proteins called collectins play central roles in regulation of host defense via recognition of specific pathogen-associated molecular patterns (PAMPs) on various microorganisms (1). Although these innate immune receptors are widely expressed, they are of particular importance to dendritic cells and macrophages.
The pulmonary collectins, surfactant proteins A (SPA) and D, are critical regulators of macrophage activity (2). They have important roles in aggregation, opsonization, and phagocytosis of pulmonary pathogens, as well as production of reactive oxygen species and cytokines (3, 4). In particular, SPA stimulates macrophage chemotaxis (5) and phagocytosis of apoptotic neutrophils, mechanisms that result in production of TGFβ (6). A number of cell surface proteins bind to SPA. Key among these are TLR2, TLR4, SIRPα, and calreticulin/CD91, proteins that intimately regulate the innate immune response (7–9).
Transforming growth factor-β (TGFβ) is a multifunctional regulator of cell growth and differentiation that induces the expression of various extracellular matrix components, including hyaluronan, collagen, and fibronectin (10). Additionally, TGFβ is also a strong chemoattractant for macrophages, lymphocytes, mast cells, and fibroblasts (11). Interestingly, higher concentrations of TGFβ are anti-inflammatory and contribute to the abrogation of the inflammatory response after injury (6).
Hyaluronan (HA), a nonsulfated glycosaminoglycan polymer of repeating disaccharide units of N-acetylglucosamine and glucuronic acid, is regulated by TGFβ and also modifies inflammatory cell behavior, in particular, chemotaxis (reviewed in Ref. 12). An increased recovery of HA in bronchoalveolar lavage has been found in a variety of human lung disorders, such as acute respiratory distress syndrome and occupational lung disorders, and in animal models, such as bleomycin-induced lung injury (13, 14). In this rodent model, elevated low molecular weight HA content in bronchoalveolar lavage temporally correlates with an influx of macrophages into the lung. Furthermore, administration of an HA-binding peptide (HABPep) inhibits macrophage accumulation after bleomycin injury, suggesting that HA is critical in macrophage recruitment to the lung (14).
HA exerts its biologic effects via specific cell surface receptors. Although a number of HA-binding proteins have been isolated, two distinct cell surface receptors, CD44 and RHAMM (Hmmr, CD168), have been studied in some detail. Binding of HA to CD44 has been implicated in lymphocyte homing, tumorigenesis, and activation of monocytes (15). Binding of HA to RHAMM promotes cell migration in association with tyrosine phosphorylation and focal adhesion turnover (16). Furthermore, blockade of either HA or RHAMM inhibits TGFβ1-stimulated fibroblast motility (17), as well as macrophage motility after bleomycin lung injury (18). Interestingly, TLR2/4 double knock-out macrophages do not respond to HA stimulation (19), and CD44 associates with TLR4, suggesting that TLRs and HA receptors likely cooperate to mediate intracellular signaling by HA (20).
Because HA has been implicated in macrophage migration through interactions with RHAMM and CD44, and both SPA and HA effects are influenced by TLR2/4, we examined whether SPA stimulation of macrophage chemotaxis is dependent upon HA/receptor interactions. We here report that SPA-stimulated chemotaxis results from TLR2- and JNK/ERK-dependent TGFβ production, which in turn requires RHAMM and HA, but not CD44, to promote macrophage migration. These studies are highly relevant to inflammatory responses in the lung, suggest that interactions of the extracellular matrix with inflammatory cells are key in this novel pathway, and reveal a new role for SPA in innate immune responses.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
SPA was purified from bronchoalveolar lavage of human alveolar proteinosis patients as described previously (21). Purified SPA contained no detectable endotoxin as determined by the Limulus assay (data not shown) and no TGFβ as determined by the mink lung epithelial cell assay (supplemental Fig. 1). Highly purified and defined HA oligosaccharides, including HA, a six sugar oligosaccharide of HA, 8-, 14-, and 34-mer and a 900-kDa HA (HA900, HMW HA) that were free of endotoxin, protein, or nucleic acid, were the kind gifts of Seikagaku Corp. (Tokyo, Japan). Anti-RHAMM antibody (R36), generated in rabbits against amino acids 585–605 encoded in the full-length RHAMM cDNA (22, 23), has been described previously (24). CD44 antibodies included KM81 (generously provided by Ellen Puré, Wistar Institute, University of Pennsylvania), CD44v3 (Calbiochem), and IM-7 (BD Biosciences). Other antibodies used in the study included anti-SIRPα (Upstate, Charlottesville, VA), anti-calreticulin (Affinity Bioreagents, Golden, CO), anti-TLR2 (Zymed Laboratories Inc.), and anti-TLR4 (e-Bioscience, San Diego). All signaling antibodies were obtained from Cell Signaling Technology (Danvers, MA) and included rabbit monoclonal antibodies to total ERK1/2 (p42 MAPK, catalog no. 4695) and phospho-p38 (pMAPKAPK-2-T222, catalog no. 3316), and rabbit polyclonal antibodies to phospho-ERK1/2 (p-p44/42 MAPK-T202/Y204, catalog no. 9101), total p38 (p38 MAPK, catalog no. 9212), total JNK (SAPK/JNK, catalog no. 9252), phospho-JNK (pSAPK/JNK, catalog no. 9251), and β-actin (catalog no. 4967). Pan-specific TGFβ antibody was purchased from R&D (Minneapolis, MN), and TGFβ1 was purchased from Sigma (catalog no. T 7039). The synthetic TLR2 ligands, tripalmitoyl-S-glycero-Cys-(Lys)4 (Pam3Cys), and FSL1, are endotoxin-free and were obtained from Invivogen. Pharmacologic blockers of the MAPKs ERK (PD98059, 10 μm), JNK (SP600125, 10 μm), and p38 (SB202190, 10 μm) were obtained from Calbiochem.
Peptides
HA-binding peptide (HABPep), mimicking a charged sequence previously shown to bind HA (RGGGRGRRR) (25) and scrambled peptide containing the same amino acids but in a random, non-HA binding order (RGRRGRGRG), have been described previously (14). Peptides were commercially produced to >95% purity (Invitrogen).
Cell Culture
RAW264.7 murine macrophages were obtained from ATCC (catalog no. TIB-71, Manassas, VA) and were maintained in DMEM supplemented with 10% heat-inactivated FBS, 2 mm l-glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin at 37 °C under 5% CO2. Macrophages older than 10 passages were not used. The optimal dose of TGFβ1 to stimulate HA production in RAW264.7 cells was first determined as a dose response, and 3–10 ng/ml TGFβ1 stimulated maximal HA production (supplemental Fig. 2).
Mouse Lines and Bone Marrow-derived Macrophages (BMDM)
All mice were housed in the Animal Care Facilities of either The Children's Hospital of Philadelphia or University of Texas Southwestern Medical Center, Dallas. All animal experiments were reviewed and authorized by the Institutional Animal Care and Utilization Committees of both institutions, and experiments were conducted in accordance with National Institutes of Health guidelines. All mice were on the C57/Bl6 background, and wild type littermates were used as controls. TLR2−/− mice were the kind gift of Dr. S. Akira (Osaka University, Osaka, Japan); mice expressing a TGFβ-responsive construct (12 repeats of the CAGA sequence of the plasminogen activator inhibitor 1 (PAI1) promoter) driving GFP (CAGA-GFP mice) were the kind gift of Dr. Hal Dietz (Johns Hopkins University, Baltimore, MD); CD44−/− mice were the kind gift of Dr. Tak Mak (Amgen, Toronto, Canada), and RHAMM−/− mice were the kind gift of Dr. Eva A. Turley (London Regional Cancer Centre, London, Ontario, Canada).
Bone marrow-derived macrophages were obtained by sacrificing mice and flushing the bone marrow from the tibia and femur of each mouse with macrophage medium (RPMI 1640 with 10% FCS, 20% L929 conditioned medium, 1% penicillin/streptomycin/glutamine) under sterile conditions. Marrow cells were made into a single cell suspension by gentle pipetting and strained. Cells were centrifuged and resuspended; red blood cells were lysed by flash exposure to hypotonic solution and then cultured in macrophage medium. Nonadherent cells were replated and provided fresh medium. Cells were allowed to further differentiate for 3 days and were then used for assays.
Chemotaxis Assay
Macrophage chemotaxis was determined using a modified Boyden chemotaxis chamber containing a 96-well microchemotaxis plate (MBA-96, Neuro Probe, Cabin John, MD) as described previously (26). Briefly, the bottom wells of the chamber contained 40 μl of various concentrations of chemoattractants (SPA, TGFβ, HA, and Pam3Cys) in defined medium (DMEM or DM), without fetal calf serum (FCS). Positive and negative controls included 10% FCS or DMEM, respectively. The upper wells were filled with 5 × 105 cells/ml suspended in 100 μl of DMEM in the presence or absence of blocking reagents (antibodies to RHAMM, CD44, SIRPα, calreticulin, TLR2, TLR4, HABPep, and JNK/ERK/p38 and inhibitors) with, as appropriate, nonimmune rabbit, mouse, chicken IgG, HABP preincubated with an excess of HA, and DMSO serving as controls. A 5-μm pore polycarbonate membrane filter was placed between the bottom and top chamber. The chamber was incubated for 6 h at 37 °C. Adherent cells on the upper surface of the membrane were treated with 200 μl of 1 mm EDTA for 15–20 min and wiped off. Cells that had migrated into the membrane were stained with Diff-QuickTM and counted in five randomly selected high power fields in each well. Each chemoattractant solution was tested in 6 wells, and each experiment was repeated at least three times. Data were expressed as the number of macrophages that migrated into the membrane for each test solution, converted to a percentage of control (DM), and combined for at least three separate experiments.
To confirm that the migration of macrophages was indeed chemotaxis, a checkerboard analysis was performed according to the method established by Zigmond and Hirsch (27). Chemotaxis of macrophages to the chemoattractant was confirmed if directional migration toward the chemoattractant in the lower chamber was negated by inclusion of the same concentration of the chemoattractant in the top chamber.
Actin-based Cytoskeletal Rearrangements
Macrophages were plated at a concentration of 1 × 106 cells/ml in DMEM with 10% FCS in chamber slides (catalog no. 154526, Lab-Trek ΙΙ Chamber Slide System, Fisher). After cells were allowed to adhere for 24 h, the medium was changed to DMEM with 1% FCS overnight. The next day, cells were exposed to SPA (100 μg/ml), TGFβ1 (10 ng/ml), HA6 oligosaccharide (4 mm), or Pam3Cys (5 μm) in defined medium for 1 h. Cells were then washed twice with PBS, fixed with 3% paraformaldehyde in PBS for 10 min, and washed twice again with PBS. Cytoskeletal staining was achieved by using FITC-phalloidin (Molecular Probes, Eugene, OR) treatment for 10 min at RT in a dark environment. After washing in double distilled H2O, chambers were removed, and slides were mounted with Fluoromount (28). The cells were examined under oil-immersion at ×600 magnification.
TGFβ Mink Lung Bioassay and TGFβ ELISA
SPA preparations and conditioned media were assayed for active TGFβ content using the mink lung epithelial cell line according to the protocol described by Abe et al. (29). This TGF-β-responsive cell line was stably transfected with the human plasminogen activator inhibitor (PAI-1) promoter linked to a luciferase reporter gene. Briefly, 1.8 × 105 mink lung epithelial cell line/ml were allowed to attach for 3 h and then cultured overnight with 30 μl of SPA, medium, or 40–1200 pg/ml TGFβ standard (Sigma). Mink lung epithelial cell line extracts were collected the next day and assayed for luciferase activity using the luciferase assay system per the manufacturer's instructions (Promega). Data were expressed as picograms/ml of TGFβ presented as a percent of control (PBS).
For the active TGFβ ELISA, 5 × 106 macrophages were plated onto 6-well dishes in DMEM supplemented with 10% FBS and maintained at 37 °C. The medium was replaced with DMEM without FBS overnight to make cells quiescent. Macrophages were then exposed to SPA (100 μg/ml) or Pam3Cys at differing concentrations from 0.5 to 10 μm for 24 h. Active and total TGFβ was measured using an ELISA kit from R&D Systems (catalog no. DY1679; Minneapolis, MN) as per the manufacturer's instructions. TGFβ was measured in both the cell pellets and supernatants. Activation of TGFβ to obtain total TGFβ content was achieved by acidification as per the manufacturer's instructions. To determine their contribution to SPA-stimulated TGFβ production, macrophages exposed to Pam3Cys (5 μm) were also incubated with JNK, ERK, or p38 inhibitors (each 10 μm) for 24 h, and TGFβ content was again determined in the supernatant.
ELISA-like Assay for HA
Supernatants collected from 1 × 106 cells/ml were assayed for HA content by an ELISA-like assay as described previously (30) with several modifications. This ELISA measures the competition of HA present in the sample versus HA coated on a 96-well plate for binding to a biotinylated HA-binding protein (Seikagaku, Japan). Briefly, 60 μl of cellular supernatant or Healon standard (GE Healthcare) were loaded onto nonfat dry milk-blocked Covalink-NH 96-Microwell plates (Nunc, Fisher Corp.) after overnight protease digestion. After addition of 60 μl of biotinylated HA-binding protein to each well and incubation at 37 °C for 1 h, 100 μl of the sample biotinylated HA-binding protein incubation solution were transferred to a HA-coated Covalink plate and incubated for 1 h at 37 °C to allow to competitive binding (0.2 mg/ml HA, ICN Inc.). HA binding was detected by an avidin-biotin complex reagent (Vectastain) and o-phenylenediamine (Sigma). The change in absorbance at 450 nm after a 15-min incubation was measured. HA concentrations of the sample were normalized to cellular protein and expressed as nanograms of HA/mg of protein.
Western Blot Analysis
Western blot analysis was performed using the NOVEX NuPAGE system from Invitrogen with 1-mm of 4–15% BisTris gels according to standard protocol as directed by the manufacturer and as described previously (18). Briefly, 10 μg of cell lysate was loaded to each well; gels were run at 100 V at 4 °C for 1 h in NuPAGE MOPS/SDS running buffer under reducing conditions. Protein was transferred to nitrocellulose membrane and run at 30 V for 60 min at room temperature. The membrane was blocked using 5% nonfat dry milk in Tween/Tris-buffered saline (TTBS) (100 mm Tris base, 1.5 m NaCl adjusted to a pH of 7.4 with 0.1% Tween 20). Primary antibodies were applied overnight at 4 °C. The next day, the membrane was washed using TTBS four times for 10 min each time. A horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was applied for 1 h at room temperature. Following this, the membrane was washed with TTBS followed by two 15-min washes with TBS. The blots were developed using a chemiluminescence system from Amersham Biosciences.
Statistical Analysis
All experiments were repeated at least three times, and chemotaxis was assessed with 5–6 wells per condition in each experiment. Data from triplicate experiments were combined using percent of control for each experiment. All data are presented as means ± S.E. Analysis of variance with Neuman-Keuls post hoc testing was used to assess differences between three or more groups. Significance was accepted at the 0.05 level of probability. Comparisons between two groups of observations were determined by Student's t test assuming unequal variances. A p value of less than 0.05 was considered significant.
RESULTS
SPA-stimulated Chemotaxis Is Dependent on TLR2 but Independent of TLR4, SIRPα, and Calreticulin
Among a number of its innate immune functions, the pulmonary collectin SPA stimulates macrophage migration. To determine the mechanisms regulating this function, we first determined the optimal SPA dose for macrophage chemotaxis. SPA stimulated macrophage chemotaxis in a dose-dependent manner, with maximum 5–7-fold stimulation at a dose of 100 μg/ml (Fig. 1A). To confirm the chemotactic nature of this response, a checkerboard analysis was performed with SPA either in the bottom, top, or in both chambers of a modified Boyden chamber (Table 1). As expected, 10% FCS or SPA in the bottom chamber significantly stimulated directed migration from the top to the bottom chamber (Table 1). However, when the concentration gradient was abolished by adding SPA or 10% FCS to the top and bottom chambers, no chemotaxis was observed, and migration was similar to the negative control (Table 1). These results confirm that SPA-stimulated migration is a chemotactic response, and we chose to use 100 μg/ml as the dose of SPA for all subsequent experiments.
FIGURE 1.

SPA-stimulated macrophage chemotaxis requires TLR-2, RHAMM, and HA. A, as assessed in a modified Boyden chemotaxis chamber assay, SPA stimulated macrophage chemotaxis in a dose-dependent manner with maximum stimulation at 100 μg/ml (*, p < 0.05 versus DM). Defined medium (DMEM) was used as a negative control. B, SPA (100 μg/ml)-stimulated chemotaxis was tested in the presence of blocking antibodies against SIRPα, calreticulin/CD91, TLR4, and TLR2 with nonimmune rabbit, rat, and chicken IgGs as species-specific controls. SPA alone significantly stimulated macrophage chemotaxis (*, p < 0.05 versus DMEM). SPA-stimulated chemotaxis was only inhibited by anti-TLR2 antibody (#, p < 0.05 versus SPA alone, SPA + IgG). C, SPA-stimulated chemotaxis (100 μg/ml) in the presence of HABPep, anti-RHAMM, and three CD44 antibodies, IM-7, KM81, or CD44v3, with nonimmune IgG and HABPep incubated with an excess of HA used as controls. SPA alone significantly stimulated macrophage chemotaxis (*, p < 0.05 versus DMEM). SPA-stimulated chemotaxis was significantly inhibited to base line in the presence of HABPep or anti-RHAMM antibody (#, p < 0.05 versus SPA alone/IgG/HA + HABPep), but not with any CD44 antibody tested. All data are normalized as percent of control (DM) presented as mean ± S.E. for three independent separate experiments, each with samples run at least in triplicate.
TABLE 1.
Checkerboard analysis of SPA-stimulated chemotaxis
Chemotaxis was assessed in a modified Boyden chamber assay as described under “Experimental Procedures.” Both 10% FCS or SPA (100 μg/ml), when added in the bottom chamber, produced a significant increase in migration (*, p < 0.05 versus DM/DM). Abrogation of the gradient by adding SPA to both the bottom and top chambers completely inhibited the chemotactic response (#, p < 0.05 versus DM/SPA). Data are presented as means ± S.E. normalized to percent of control (DM) for three separate experiments. These results confirm that SPA-stimulated migration is a chemotactic response. ND means not determined.
| Bottom chamber | Top chamber |
||
|---|---|---|---|
| DM | 10% FCS | SPA 100 μg/ml | |
| DM | 100 ± 34 | 113 ± 14 | 91 ± 22 |
| 10% FCS | 394 ± 63* | 127 ± 15# | ND |
| SPA 100 μg/ml | 594 ± 168* | ND | 94 ± 44# |
SPA binds a number of cell surface proteins, including TLR2, where it competitively inhibits the binding of peptidoglycan (31), zymosan (32), and TLR4 (33). In addition, Gardai et al. (7) developed a model to explain the pro- and anti-inflammatory actions of SPA. During quiescent conditions, in the absence of bacterial invasion, the globular head of SPA binds to SIRPα (signal inhibitory regulator protein α) on resident macrophages, an anti-inflammatory signal, whereas in the presence of pathogens and/or their products, the globular head of SPA is occupied, thereby allowing the binding of the collagenous tails of SPA to calreticulin/CD91, a pro-inflammatory signal. To determine the specific SPA/receptor interaction mediating macrophage chemotaxis, we examined whether this response could be inhibited by using the blocking antibodies to SIRPα, calreticulin, TLR2, or TLR4 as used in the study of Gardai et al. (7). Anti-TLR2 antibody significantly inhibited SPA-stimulated chemotaxis, whereas anti-SIRPα, anti-calreticulin/CD91, and anti-TLR4 had no effect (Fig. 1B). Interestingly, blocking SIRPα, normally an anti-inflammatory signal, did not result in increased chemotaxis, suggesting that SPA-stimulated chemotaxis is an active signal and not just removal of repression due to SIRPα. Additionally, nonimmune rabbit, rat, and chicken IgG used as controls also had no effect. These data suggested that TLR2 is necessary for SPA-stimulated chemotaxis, whereas SIRPα, calreticulin, and TLR4 are not.
SPA-stimulated chemotaxis Is Mediated by RHAMM and HA but Not CD44
Because cell motility is regulated by HA and its receptors RHAMM and CD44, we next examined whether SPA-mediated chemotaxis was dependent on HA and its receptors by using either HA-binding peptide (HABPep) or anti-HA receptor antibodies in the top chamber. HABPep preincubated with an excess of HA and rabbit IgG served as controls. SPA-stimulated macrophage chemotaxis was inhibited to base line in the presence of either HABPep or anti-RHAMM antibody (Fig. 1C), but three CD44 antibodies, IM-7, KM81, and CD44v3, previously shown to block murine cell migration (34–36), failed to block SPA-stimulated chemotaxis (Fig. 1C). These results indicated that HA interaction with RHAMM, and not CD44, mediates SPA-stimulated macrophage chemotaxis.
SPA Causes a Time-dependent Increase in HA Production That Is Dependent on TGFβ
Because our data suggested that HA acting through RHAMM was necessary for SPA-stimulated macrophage chemotaxis, we next determined whether SPA could stimulate HA synthesis. TGFβ1, which is known to stimulate HA synthesis (37), was used as a positive control. Macrophages were treated with SPA, TGFβ1, or DMEM for 1, 6, 12, and 24 h. HA content of the media was determined using an ELISA-like assay for HA, and data were normalized to total protein of the cell pellet. Untreated macrophages did not accumulate HA in the medium (Fig. 2A). Interestingly, treatment with SPA or TGFβ1 produced similar 3–4-fold time-dependent increases in HA production that reached statistical significance at 12 and 24 h (Fig. 2A). Because the SPA that we were using was not contaminated with TGFβ (supplemental Fig. 1), we next examined whether SPA-stimulated HA production could be blocked by a pan-specific TGFβ antibody using rabbit IgG as a control. SPA alone or with IgG produced a significant 3-fold increase in HA production as compared with DMEM (Fig. 2B). This response was completely inhibited by anti-TGFβ antibody (Fig. 2B), suggesting that SPA-stimulated HA synthesis was an indirect process and as result of TGFβ production.
FIGURE 2.
SPA-stimulated increase in HA and chemotaxis is dependent on TGFβ, and SPA-stimulated TGFβ production is dependent on TLR2. A, media were assayed for HA content by an ELISA-like assay after incubation with SPA (100 μg/ml) or TGFβ1 (10 ng/ml) for 1, 6, 12, and 24 h and normalized to the protein content of the cells in each culture. Data are presented as nanograms of HA per mg of protein ± S.E. for three independent experiments with samples run in triplicate within each experiment. Macrophages cultured in DMEM had no increase in HA content of the medium over time. SPA or TGFβ1 treatment produced similar time-dependent increases in HA production that were significant at 12 and 24 h (*, p < 0.05 versus DMEM). B, SPA stimulated HA production in the presence of TGFβ antibody or rabbit IgG (each 50 μg/ml). SPA alone or with IgG resulted in a significant increase in media HA concentration (*, p < 0.05 versus DMEM). SPA stimulation of HA was significantly inhibited to base line in the presence of a pan-specific TGFβ antibody (#, p < 0.05 versus SPA/SPA+IgG). C, SPA stimulated chemotaxis in the presence of anti-TGFβ, anti-RHAMM, and anti-TLR2 antibodies. SPA alone significantly stimulated macrophage chemotaxis (*, p < 0.05 versus DMEM). Anti-TGFβ antibody inhibited SPA-stimulated chemotaxis to levels similar to that achieved by anti-RHAMM and anti-TLR-2 antibodies (#, p < 0.05 versus SPA/SPA + IgG). Data are representative of three independent experiments with at least four replicates within each experiment. D, media were assayed for TGFβ content after incubation with SPA in the presence of either anti-TLR2 or anti-RHAMM antibodies. Data are presented as mean ± S.E. of percent of DM control. SPA + IgG produced significant induction of TGFβ (*, p < 0.05 versus DMEM). SPA induction of TGFβ was significantly inhibited to base line in the presence of anti-TLR2 antibody (#, p < 0.05 versus SPA/SPA + IgG). However, anti-RHAMM antibody failed to block SPA stimulation of TGFβ secretion. Data are representative of three experiments with at least three replicates within each experiment. Collectively, these data show that SPA stimulates TGFβ production via interaction with TLR2, and TGFβ stimulates macrophage chemotaxis in a RHAMM-dependent manner. E, genetic confirmation of TLR2 requirement for SPA-stimulated TGFβ production. BMDM from WT, RHAMM−/−, and TLR2−/− mice were exposed to SPA, and active TGFβ content of the media was measured by ELISA. SPA stimulated TGFβ production in both WT and RHAMM−/− mice, a response that was significantly blunted in TLR2−/− macrophages. Data are presented as mean ± S.E. and are representative of two independent experiments with samples run in triplicate within each experiment.
Because SPA-stimulated HA production was dependent on TGFβ, SPA-stimulated chemotaxis was examined in the presence of anti-TGFβ and anti-RHAMM antibodies, with anti-TLR2 antibody used as a positive control for inhibition. Consistent with results for HA synthesis, TGFβ antibody significantly inhibited SPA-stimulated chemotaxis to levels similar to those achieved by anti-RHAMM and anti-TLR2 antibodies (Fig. 2C). These data indicated that TLR2, TGFβ, and RHAMM are all necessary for SPA induction of macrophage chemotaxis.
SPA Stimulation of TGFβ Synthesis Is Dependent on TLR2 but Not RHAMM
Because SPA stimulates TGFβ secretion in macrophages (38), we next attempted to block this effect using anti-TLR2 or anti-RHAMM antibody. RAW264.7 macrophages were treated with SPA in the presence of either anti-TLR2 or anti-RHAMM antibody or rabbit IgG used as a control. Although anti-TLR2 antibody inhibited SPA induction of TGFβ to base line, anti-RHAMM antibody had no effect on this response (Fig. 2D). To confirm these effects using a genetic approach, we obtained primary BMDM from wild type, TLR2−/−, and RHAMM−/− mice, stimulated them with SPA, and measured TGFβ accumulation in the medium. There was little to no TGFβ secretion in the absence of SPA stimulation (Fig. 2E, RPMI). SPA significantly stimulated TGFβ production in WT and RHAMM−/− macrophages, but this response was significantly inhibited in TLR2−/− macrophages (Fig. 2E).
To further confirm the role of TLR2 in TGFβ production with SPA treatment, we obtained bone marrow-derived macrophages from mice harboring a transgene in which the TGFβ-responsive PAI1 promoter was fused to a GFP reporter (39). Quiescent cells were treated with either TGFβ as a positive control or SPA. In the absence of stimulation, there was little to no GFP staining. SPA treatment caused a robust expression of GFP, equivalent to that stimulated by TGFβ. Both anti-TLR2 and anti-TGFβ antibodies blocked the SPA-stimulated effect on the expression of GFP (Fig. 3). Collectively, these data suggested that SPA-stimulated TGFβ production requires TLR2 but not RHAMM. Furthermore, data showing that anti-RHAMM antibody blocked SPA-stimulated chemotaxis (Fig. 2C) suggested that RHAMM is a more downstream player in the pathway.
FIGURE 3.
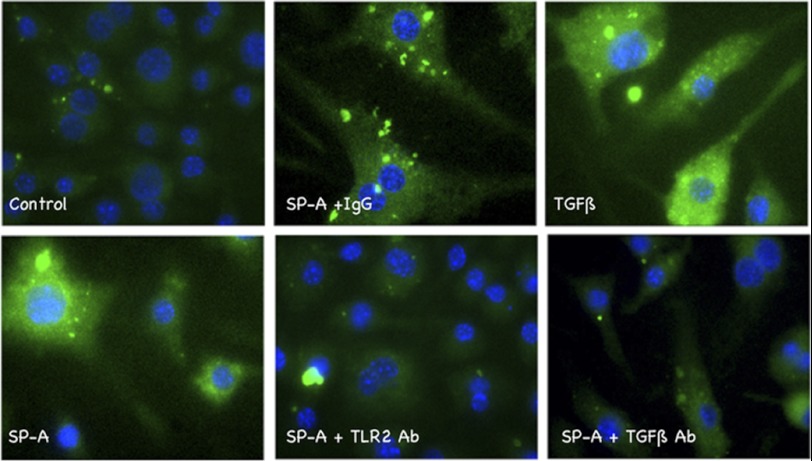
Effect of SPA on macrophages obtained from CAGA-GFP mice. BMDM were obtained from CAGA-GFP mice expressing a transgene consisting of 12 repeats of the PAI1 promoter linked to GFP as a reporter (39). The PAI1 promoter is highly TGFβ-sensitive, and GFP expression correlates with active TGFβ expression and signaling. Cells were plated and made quiescent overnight in 1% FCS. Three hours after stimulation, they were observed under direct fluorescence microscopy and imaged at ×400 magnification. Quiescent cells (Control) showed little to no GFP. Stimulation with active TGFβ1, a positive control, showed robust GFP staining. Similar increases in GFP staining were observed with SPA, and both anti-TLR2 and anti-TGFβ antibodies blocked this response. Nonimmune IgG had little to no effect on the GFP signal stimulated by SPA. Data shown are representative of two independent experiments with samples examined in triplicate within each experiment.
TGFβ1 Stimulation of Chemotaxis Is Dependent on RHAMM
Because TGFβ was the likely mediator of SPA-stimulated chemotaxis, and RHAMM and TLR2 were critical for this response, we examined whether TGFβ1-stimulated chemotaxis was also dependent upon these receptors. TGFβ1 stimulated a 3–4-fold increase in macrophage chemotaxis (Fig. 4A), an effect that was blocked by anti-RHAMM but not by anti-TLR2 antibody (Fig. 4A). As expected, TGFβ antibody, used as a positive inhibitory control, blocked TGFβ-stimulated chemotaxis. These results suggested that RHAMM/HA interactions were downstream of TGFβ, whereas TLR2 was upstream of TGFβ.
FIGURE 4.

TGFβ1 stimulation of chemotaxis is dependent on RHAMM and HA. A, TGFβ1-stimulated macrophage chemotaxis was examined in the presence of anti-TGFβ, anti-TLR2, and anti-RHAMM antibodies. TGFβ1 alone significantly stimulated macrophage chemotaxis (*, p < 0.05 versus DMEM). This effect was significantly inhibited to base line in the presence of anti-TGFβ and anti-RHAMM antibodies (#, p < 0.05 versus TGFβ1 or TGFβ1 + ΙgG) but not by anti-TLR2 antibody. B, we next determined the chemotactic response to HA6 and the receptors involved in this response. HA6 (4 mm) stimulated a 4–5-fold response in macrophage chemotaxis (*, p < 0.05 versus DMEM). Only anti-RHAMM antibody and HABPep blocked this response to base line (#, p < 0.05 versus HA6 alone). Anti-TLR2, -TLR4, and -TGFβ and three antibodies to CD44 (IM7, KM81, and CD44v3) had no effect. C, to compare the effects of HMW and low molecular weight HA, we used HA6 (6-mer HA, 100 μg/ml) and HA900 (900-kDa HA, 100 μg/ml) as chemoattractants. HA6 stimulated macrophage chemotaxis 6-fold (*, p < 0.05 versus DMEM and HA900), whereas HA900 had no effect. Interestingly, HA900 inhibited HA6-stimulated chemotaxis when the two HA products were combined (#, p < 0.05 versus HA6). D, HA of various molecular sizes, each at 4 mm except HA900 (100 μg/ml), were tested in the modified Boyden chamber assay. A molecular size-dependent increase in HA-stimulated chemotaxis was observed with maximal chemotaxis observed with 34-mer HA (*, p < 0.05 versus DMEM; #, p < 0.01 versus DMEM; n.s. means not significant versus DMEM). HA900 had no effect on chemotaxis. All chemotaxis data are presented as mean ± S.E. normalized as percent of control (DM) for three separate experiments, with at least four replicates within each experiment.
HA Stimulation of Chemotaxis Is Dependent on RHAMM and Not CD44
The data thus far suggested that RHAMM/HA interactions were necessary and were downstream of TGFβ-stimulated chemotaxis. To examine this further, we obtained endotoxin-, protein-, and nucleic acid-free HA oligosaccharide (HA6) and used it as a chemoattractant. Preliminary dose-response experiments defined 4 mm as the optimal concentration for this response (data not shown). HA6 stimulated a 4–5-fold increase in chemotaxis (Fig. 4B). This effect was blocked to base line by anti-RHAMM antibody and HABPep but not by antibodies to TLR2, TLR4, TGFβ, or three separate antibodies to CD44 (IM7, KM81, and CD44v3) (Fig. 4B). Preincubation of HABPep with HMW HA (900 kDa, HA900) abrogated the inhibitory effect of HABPep, thereby confirming the specificity of the inhibition. The results of the various blockers used are summarized in Table 2.
TABLE 2.
Summary of antibody blockade results
Because there is a distinct specificity of antibody blockade of chemotaxis to SPA, TGFβ, and HA, a hierarchy is established where SPA-stimulated chemotaxis requires TLR2, TGFβ, HA, and RHAMM and RHAMM/HA interactions universally required for all chemoattractants tested.
| Blockers | Chemoattractants |
||
|---|---|---|---|
| SPA | TGFβ | HA | |
| αTLR2 | + | − | − |
| αTGFβ | + | + | − |
| HABPep + | + | + | |
| αRHAMM | + | + | + |
| αCD44 | − | − | − |
To further define the effects of molecular size on HA-stimulated macrophage motility, we used HA6 versus HA900 in the chemotaxis assay. HABPep alone had no effect on macrophage chemotaxis (Fig. 4C). A 6-fold increase in chemotaxis was seen with HA6 stimulation, whereas HA900 did not stimulate chemotaxis (Fig. 4C). Interestingly, when both HA6 and HMW HA were added as chemoattractants together, no chemotaxis was observed suggesting that HMW HA acts in a dominant negative manner. Finally, we used HA oligosaccharides of varying sizes to determine the effect of HA size on macrophage chemotaxis. All HA oligosaccharides (6-, 8-, 14-, and 34-mer) were used at 4 mm, whereas HA900 was used at the highest dose possible without prohibitive viscosity of the material (0.01 mm). Increasing HA oligosaccharide size was associated with increased stimulation of chemotaxis, whereas HA900 had no effect (Fig. 4D). These data demonstrated that HA-stimulated macrophage chemotaxis is both dose- and molecular size-dependent, requires RHAMM, and HMW HA acts in a dominant negative manner to inhibit HA6-stimulated chemotaxis.
Chemotactic Responses to SPA, TGFβ, and HA in TLR2−/−, CD44−/−, and RHAMM−/− Macrophages
To genetically confirm the antibody blocking experiments, we obtained BMDM from WT and TLR2−/− mice and examined their responses to SPA, TGFβ, and HA as chemoattractants. As expected, WT macrophages had a 5–6-fold response to each of the three chemoattractants (Fig. 5A, top panel), and each of the responses was blocked to base line by anti-RHAMM antibody. TLR2−/− macrophages did not respond to SPA (Fig. 5A, bottom panel). Interestingly, however, macrophages from TLR2−/− mice had robust chemotactic responses to both TGFβ and HA6 (Fig. 5A, bottom panel), and each of these responses was blocked to base line by anti-RHAMM antibody (Fig. 5A). These data confirm that SPA interaction with TLR2 mediates the pathway upstream from TGFβ.
FIGURE 5.

Chemotactic responses in TLR2−/−, CD44−/−, and RHAMM−/− macrophages. A, BMDM were obtained from WT and TLR2−/− mice, and chemotaxis to SPA, TGFβ, and HA6 was determined. Chemotaxis is represented by cells per high power field in three independent experiments with four replicates per group in each experiment. Data are presented as mean ± S.E. In concordance with the data obtained with the RAW264.7 murine macrophage cell line, WT macrophages had 5–6-fold chemotactic responses to SPA (100 μg/ml), TGFβ (10 ng/ml), and HA6 (4 mm) (*, p < 0.05 versus DM). Each of these responses was blocked to base line by anti-RHAMM antibody (#, p < 0.05 versus SPA, TGFβ, or HA6) but not by normal IgG. Complete medium (CM, DMEM + 10% FCS) was used as a positive control. TLR2−/− macrophages did not respond to SPA (^, p < 0.05 versus CM, TGFβ, and HA) but had robust 4-fold responses to TGFβ and HA6, both of which were inhibited to base line by anti-RHAMM antibody (#, p < 0.05 versus TGFβ or HA alone or with normal IgG). B, BMDM were obtained from WT and CD44−/− mice, and chemotactic responses to SPA and TGFβ were examined. Both sets of macrophages showed equivalent chemotactic responses to SPA and TGFβ (*, p < 0.05 versus DM), and these responses were inhibited to base line by anti-RHAMM antibody (#, p < 0.05 versus SPA or TGFβ alone or with IgG). C, WT and CD44−/− macrophages also had similar 4–6-fold chemotactic responses to HA6 (*, p < 0.05 versus RPMI). D, BMDM were obtained from WT and RHAMM−/− mice, and chemotaxis to SPA, TGFβ and HA6 was determined. RHAMM WT macrophages demonstrated significant chemotactic responses to all three stimulants. RHAMM−/− macrophages showed significantly lower chemotactic responses to SPA and no response to TGFβ and HA6 (#, p < 0.05 versus WT macrophages). Collectively, these data confirm that CD44 is dispensable, and RHAMM is a key mediator in the chemotactic responses to SPA, TGFβ, and HA.
Next, we examined BMDM from littermate WT and CD44−/− mice. Both WT and CD44−/− macrophages had similar 4-fold responses to SPA and TGFβ (Fig. 5B). In both genotypes, SPA responses were inhibited by anti-TLR2 antibody (Fig. 5B), and TGFβ responses were inhibited to base line by anti-RHAMM antibody (Fig. 5B). These data confirmed that CD44−/− is completely dispensable in the chemotactic responses to SPA and TGFβ. We next determined whether CD44 was involved with HA6-stimulated chemotaxis. BMDM from WT and CD44−/− mice had similar 5–6-fold chemotactic responses to HA6 (Fig. 5C), suggesting that CD44 was not required for HA6-stimulated chemotaxis.
To definitively confirm the role of RHAMM in these chemotactic responses, we obtained BMDM from littermate RHAMM WT and KO mice. SPA, TGFβ, and HA6 all stimulated macrophage chemotaxis in WT mice (Fig. 5D). RHAMM KO macrophages had a significantly lower chemotactic response with SPA, TGFβ, and HA6 (Fig. 5D). Collectively, these data genetically confirm that SPA stimulates chemotaxis via TLR2 and that RHAMM, but not CD44, is necessary for chemotaxis stimulated by SPA, TGFβ, and HA.
SPA Stimulation of Cytoskeletal Rearrangement Is Dependent upon TLR2 and RHAMM
The assembly of actin-based extensions, namely filopodia and lamellipodia, are integral components of cell migration. In previous studies, Tino and Wright (28) demonstrated filopodial extensions with SPA stimulation of macrophages. Because anti-RHAMM antibody blocked SPA-stimulated migration, we examined the effect of this blockade on actin organization. RAW264.7 cells were stimulated with SPA, and cytoskeletal rearrangements were determined using FITC-phalloidin (green) and nuclei stained using DAPI (blue) (supplemental Fig. 3). Quiescent cells in DM had few filopodia or cytoplasmic extensions. SPA treatment of macrophages, with or without normal IgG, showed increased spike-like filopodia. However, these cytoskeletal rearrangements were completely blocked by R36, and cell morphology was similar to quiescent cells.
BMDM from WT and TLR2−/− mice were made quiescent overnight and examined after SPA, TGFβ, or HA6 stimulation. WT macrophages responded to all three conditions, with or without normal IgG, and showed extensive filopodia and lamellipodia formation (Fig. 6, A and B). TLR2−/− macrophages failed to respond to SPA but had robust filopodial and lamellipodial formation with TGFβ and HA6 treatment, and both of these responses were blocked by anti-RHAMM antibody (Fig. 6, A and B). Collectively, these data confirm the critical roles of TLR2 and RHAMM in SPA-mediated cytoskeletal rearrangements relevant to cell motility.
FIGURE 6.
Cytoskeletal changes in response to SPA, TGFβ, and HA6 in WT and TLR2−/− mice. BMDM from WT and TLR2−/− mice were plated and made quiescent overnight. Three hours after stimulation with SPA, TGFβ, or HA6, the cytoskeleton was stained using FITC-phalloidin (green) and nuclei with DAPI (blue). A, WT macrophages that were mostly round in shape at base line showed increased formation of filopodia and lamellipodia when treated with SPA with or without normal IgG. Treatment with anti-RHAMM antibody (R36) inhibited SPA-stimulated cytoskeletal changes. B, both WT and TLR2−/− macrophages exposed to TGFβ or HA6 showed similar robust cytoskeletal changes with formation of lamellipodia and filopodia as seen in WT macrophages exposed to SPA, and these responses were completely inhibited when cells were also exposed to R36.
TLR2-mediated TGFβ Production and Chemotaxis Require the MAPK JNK and ERK
Because we had demonstrated TLR2 as the principal receptor that mediated SPA-stimulated TGFβ production, we tested whether Pam3Cys, a TLR2-specific ligand, could stimulate macrophage chemotaxis and whether blockade of TGFβ, RHAMM, and HA could inhibit this response. First, we conducted a dose response study that showed that 5 μm Pam3Cys stimulated a 5–6-fold increase in macrophage chemotaxis (Fig. 7A). Using this concentration of Pam3Cys, we next tested whether HABPep and antibodies to TGFβ and RHAMM could block the chemotaxis stimulated by TLR2 ligation. Pam3Cys stimulated chemotaxis to the same degree as 10 ng/ml TGFβ used as positive control (Fig. 7B). All three blockers, HABPep, anti-TGFβ, and anti-RHAMM antibodies blocked Pam3Cys-stimulated chemotaxis to base line, whereas scrambled peptide and nonimmune rabbit IgG had no effect (Fig. 7B). We next determined if Pam3Cys stimulated latent and active TGFβ production in macrophages. Concentrations of Pam3Cys as low as 0.1 μm stimulated a 5-fold increase in production of both latent and active TGFβ compared with control cells maintained in DMEM alone (Fig. 7C).
FIGURE 7.

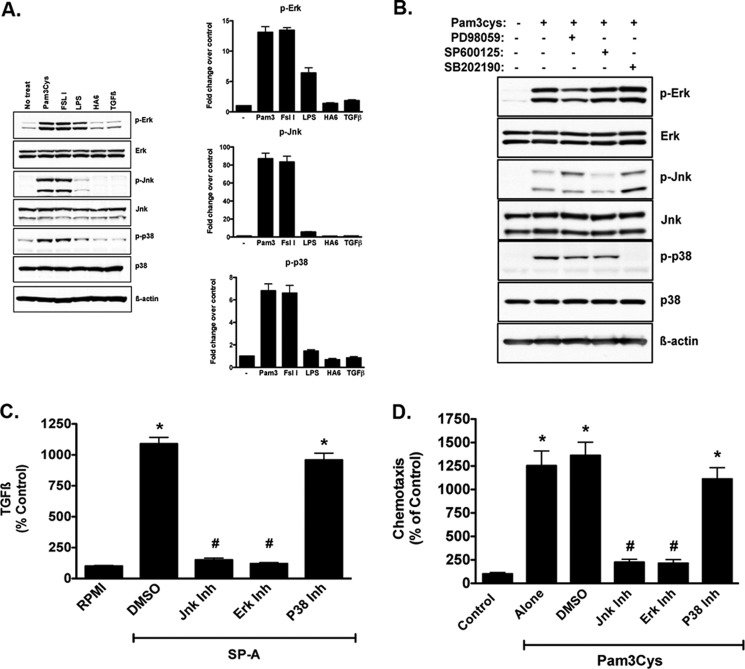
Pam3Cys, a TLR2 ligand, stimulates TGFβ production and chemotaxis that requires RHAMM. A, dose response to Pam3Cys-stimulated chemotaxis. RAW264.7 cells, examined in the chemotaxis assay, showed a dose-dependent increase in chemotaxis to Pam3Cys with the optimal dose being 5 μm. This dose of Pam3Cys was used for all subsequent experiments. B, Pam3Cys stimulated chemotaxis to the same degree as 10 ng/ml TGFβ1. Chemotaxis to Pam3Cys was inhibited by antibodies to TGFβ and RHAMM (R36), as well as HABPep, but not by scrambled peptide or normal rabbit IgG used as controls. (*, p < 0.05 versus Pam3Cys + TGFβ and Pam3Cys + R36; #, p < 0.05 versus Pam3Cys alone.) C, Pam3Cys stimulated the production of both latent and active TGFβ with maximal effect at even the lowest concentrations studied (*, p < 0.05 versus DMEM without Pam3Cys).
MAPKs play an important role for TLR2-induced inflammation (40, 41). We therefore examined if MAPK signaling was also important in TLR2-stimulated macrophage chemotaxis. We first determined the phosphorylation of MAPKs after Pam3Cys treatment in both RAW264.7 cells (Fig. 8A) and primary BMDM obtained from WT mice (Fig. 8B). RAW264.7 macrophages were exposed to Pam3Cys (a TLR2/1 agonist), FSL1 (a TLR2/6 agonist), LPS (a TLR4 agonist), HA6, or TGFβ1, and cells were harvested in orthovanadate-containing lysis buffer at 10 min. Immunoblot analyses for phospho-ERK, phospho-JNK, and phospho-p38, as well as total ERK, JNK, p38, and β-actin as loading controls, were performed. Macrophages exposed to Pam3Cys showed robust phosphorylation of JNK, ERK, and p38, and LPS treatment was associated with ERK phosphorylation, but none of the other treatments were associated with significant effects on phosphorylation (Fig. 8A). In primary BMDM from WT mice, we also examined the effects of the signaling inhibitors on the phosphorylation pattern with Pam3Cys treatment (Fig. 8B). Similar to the findings in RAW264.7 cells (Fig. 8A), WT BMDM had robust phosphorylation of ERK, JNK, and p38 with Pam3Cys treatment, and each pharmacologic inhibitor blocked the phosphorylation of the relevant signaling component (Fig. 8B), thereby confirming the specificity of the inhibitors in these cells.
FIGURE 8.
Pam3Cys-stimulated TGFβ production and chemotaxis requires ERK and JNK but not p38. We examined the intracellular signaling events, specifically the MAPKs ERK, JNK, and p38, stimulated by TLR2 ligation, TLR4 ligation, HA6, and TGFβ1, and we used pharmacologic inhibitors to test the contribution of each pathway to Pam3Cys-stimulated TGFβ production and chemotaxis. A, in RAW264.7 cells, Pam3Cys, a TLR2/1 ligand (2nd lane), and FSL1, a TLR2/6 ligand (3rd lane), stimulated the phosphorylation of ERK, JNK, and p38. LPS (25 μg/ml, 4th lane), a TLR4 ligand, only stimulated ERK phosphorylation. HA6 (4 mm, 5th lane) and TGFβ1 (10 ng/ml, 6th lane) had no significant effects on any MAPK and remained similar to that of DMEM control cells (No treat, 1st lane). Data are presented as mean ± S.E. of densitometry from three independent experiments. B, using BMDM from WT mice, we examined the effects of Pam3Cys and blockers of ERK, JNK, and p38 on the phosphorylation of these proteins. Pam3Cys treatment resulted in a robust phosphorylation of ERK, JNK, and p38 and all three blockers (ERK, PD98059; JNK, SP600125; and p38, SB202190; each at 10 μm concentration) blocked the relevant pathway confirming the specificity of each blocker in these primary cells. C, in WT BMDM, SPA (100 μg/ml) stimulated a 10-fold increase in TGFβ production (*, p < 0.05 DMSO versus RPMI). Pharmacologic inhibition of JNK and ERK, but not p38, inhibited Pam3Cys-stimulated TGFβ production to base line (#, p < 0.05 versus SPA + DMSO). D, also in WT BMDM, pharmacologic inhibition of JNK and ERK, but not p38, inhibited Pam3Cys-stimulated chemotaxis (*, p < 0.05 versus control; #, p < 0.05 versus Pam3Cys + DMSO). Data are presented as mean ± S.E. from three experiments with samples run in triplicate within each experiment. Collectively, these data confirm that TLR2 stimulation by SPA or Pam3Cys results in JNK- and ERK-dependent TGFβ production and subsequent chemotaxis and confirm that universal ligation of TLR2 has the same effects as SPA.
We then tested the effects of pharmacologic inhibition of JNK, ERK, and p38 on SPA-stimulated TGFβ production in primary WT BMDM (Fig. 8C). SPA stimulated a 10-fold increase in TGFβ as compared with unstimulated controls, and this response was completely inhibited using JNK and ERK, but not p38 inhibitor (Fig. 8C). Because inhibition of JNK and ERK decreased Pam3Cys-induced TGFβ expression, we sought to determine whether JNK and ERK blockade would also inhibit Pam3Cys-stimulated macrophage chemotaxis. Chemotaxis to Pam3Cys was determined in WT BMDM in the presence or absence of JNK, ERK, or p38 inhibitors. Pam3Cys-stimulated a 10–12-fold increase in macrophage chemotaxis, with no effect noted with DMSO, the vehicle for the inhibitors (Fig. 8D). Interestingly, and in line with the effects on TGFβ production, JNK and ERK, but not p38 blocker, inhibited Pam3Cys-stimulated chemotaxis to base line (Fig. 8D), suggesting that Pam3Cys stimulation of TLR2 results in the activation of JNK and ERK, which are critical steps in TGFβ expression and subsequent macrophage chemotaxis.
DISCUSSION
We describe a novel pathway regulating SPA-stimulated macrophage chemotaxis. Interaction of SPA with TLR2 results in JNK- and ERK-dependent TGFβ production. This growth factor then stimulates macrophage chemotaxis, a process that requires HA and its receptor RHAMM. Ligation of RHAMM by HA promotes actin cytoskeletal rearrangements that are required for cell motility. These data suggest the pulmonary collectin SPA regulates macrophage recruitment via TLR2 and that the extracellular matrix components HA and RHAMM regulate the inflammatory response in concert with the innate host defense system of the lung.
SPA, the most abundant surfactant protein in the lung, is an integral part of the innate immune system. It binds to and enhances the uptake of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and various other bacteria and viruses, as well as stimulates macrophage chemotaxis (42). SPA can modulate the inflammatory response to a variety of stimuli (43). For instance, SPA abrogates inflammation caused by LPS exposure. Intratracheal instillation of LPS increases lung SPA protein and mRNA content, changes that correlate with diminished inflammation and secretion of cytokines. In addition, administration of LPS to SPA−/− mice results in an increased production of inflammatory cytokines and nitric oxide as compared with wild type mice. It is likely that SPA competes with LPS for TLR4 binding. However, SPA directly enhances nitric oxide and TNFα production by macrophages in response to various stimuli (44), with this response likely being mediated by interaction with calreticulin/CD91.
A number of cell-associated binding proteins and receptors have been identified for SPA. A 210-kDa SPA receptor exists on BMDM, alveolar macrophages, and the U937 macrophage cell line (45), and increased SPA binding to this receptor occurs with LPS and IFNγ treatment. Additionally, expression of this receptor is dependent upon the activation state of macrophages with highest affinity binding occurring on monocytes and reduced binding on GM-CSF-activated macrophages (46). Another potential receptor for SPA is the receptor for the complement protein C1q implicated in SPA-stimulated phagocytosis in monocytes (47). Additionally, the scavenger protein glycoprotein-340 binds SPA in a carbohydrate recognition domain-independent manner, but it does not affect binding of SPA to alveolar macrophages or SPA stimulation of macrophage chemotaxis. However, glycoprotein-340 directly stimulates random migration (chemokinesis) of alveolar macrophages (48). SPA can also bind to CD14, a known pattern recognition co-receptor for LPS and PGN, that mediates release of pro-inflammatory cytokines (49).
Gardai et al. (7) showed that SPA acts as a dual function surveillance molecule to enhance or suppress cytokine production depending on the binding orientation of the carbohydrate recognition domain of SPA. SPA maintains normal lung homeostasis by binding to SIRPα that blocks pro-inflammatory signaling by activating SHP-1, a phosphatase that in turn dephosphorylates p38. However, upon recognition of PAMPs on foreign organisms, the collagenous tail of SPA binds to calreticulin/CD91 and elicits the phosphorylation of p38 and the downstream activation of the NFκB pro-inflammatory signal pathway (7). Thus, intraperitoneal injection of mice with SPA half an hour before LPS exposure suppresses pro-inflammatory cytokine production. However, simultaneous injection of the two results in enhanced inflammation.
Our studies define a novel pathway where SPA binds TLR2 and activates JNK and ERK to increase TGFβ production. In the context of the whole animal model, we predict different phases of the response, with early lower concentrations of TGFβ stimulating macrophage chemotaxis to recruit these cells, and later higher concentrations of TGFβ being inhibitory and anti-inflammatory to stop macrophage motility and limit the inflammatory response. In support of the pathway proposed in the report, Kramer et al. (50) instilled SPA into the lungs of ventilated preterm lambs and observed increased accumulation of inflammatory cells confirming that SPA promotes the recruitment of inflammatory cells to the lung.
As noted above, TLRs, primary receptors of the innate immune system, provide immediate recognition of PAMPs on foreign pathogens to allow for clearance and phagocytosis minutes to hours after infectious challenge (51). TLR4 is responsible for LPS recognition and signaling (52), whereas TLR2 is responsible for recognition and binding to Gram-positive PGN, which also elicits proinflammatory cytokine release (31). Indeed, macrophages from TLR2−/− mice are unable to produce pro-inflammatory cytokines in response to PGN (53). SPA inhibits PGN-induced NFκB activation and TNFα secretion through a specific competitive binding of SPA to TLR2 on U937 cells and alveolar macrophages. In addition, SPA can also alter the interaction of TLR2 with zymosan, a yeast cell wall component, also causing down-regulation of NFκB activation and TNFα secretion (53). Collectively, these data strongly support an anti-inflammatory role for SPA in controlling host defense in response to foreign challenge through TLR2 interactions. However, our data show that blockade of TLR-2 abolishes SPA stimulation of chemotaxis and that SPA/TLR2 interactions result in the production and release of TGFβ, an anti-inflammatory growth factor. These effects likely promote the recruitment of activated macrophages to affected areas of the lung and, when the concentration of TGFβ is high enough, inhibits further macrophage migration.
TGFβ1 is a key regulator of inflammatory cell motility and is a strong chemoattractant for a variety of cell types, including macrophages, neutrophils, T-cells, and fibroblasts (17, 54). TGFβ1 increases HA synthesis (37) and the expression of RHAMM in a dose- and time-dependent manner (55). Furthermore, TGFβ1-stimulated fibroblast migration can be blocked by RHAMM antibody and by HABPep, suggesting that RHAMM and HA regulation of TGFβ1-stimulated cell motility is likely a universal mechanism (17). Our data show that SPA/TLR2 interaction stimulates TGFβ release and that TLR2 blockade does not inhibit TGFβ-mediated chemotaxis; TGFβ is therefore a downstream component of the chemotactic response to SPA.
CD44 is a type 1 transmembrane HA receptor that was initially implicated in lymphocyte homing and tumorigenesis. More recently, CD44 is required for the clearance of low molecular weight HA from injured sites and is crucial to resolving lung inflammation (56). Indeed, Gallo and co-workers (57) have recently reported mechanisms by which CD44 mediates internalization of HA. Furthermore, co-immunoprecipitation of CD44 and TLR4 suggests a close relationship between HA receptors and TLR signaling (20). However, in our studies of SP-A-stimulated chemotaxis, CD44−/− cells responded to SP-A in a manner similar to that of WT macrophages, indicating that CD44 is not involved in TLR2-stimulated TGFβ production and cell motility.
The cell-associated receptor RHAMM (Hmmr, CD168) regulates the motility of various cell types such as fibroblasts, lymphocytes, smooth muscle cells, endothelial cells, and macrophages (58). Binding of HA to RHAMM results in tyrosine phosphorylation, focal adhesion turnover processes, and activation of various signaling molecules, including Src, and Ras, through the ERK kinase cascade (16, 59, 60). Several injury models have demonstrated an increased expression of RHAMM and HA in macrophages, fibroblasts, and smooth muscle cells migrating in response to tissue injury (24, 60, 61). Furthermore, we have previously shown that blocking RHAMM inhibits immune cell chemotaxis and random migration in vitro (58) and inflammation in vivo (18). The roles of CD44 and RHAMM in inflammatory cell responses have been studied using knock-out mice and blocking antibody experiments. CD44, acting through HA, has been implicated in inflammatory cell activation as well as rolling and adhesion to the endothelium (62). However, bleomycin lung injury in CD44 knock-out animals results in an unresolved exaggerated inflammation primarily consisting of macrophages in association with elevated HA concentrations (56). Furthermore, blockade of RHAMM inhibits macrophage motility in vitro and recruitment to the lung after bleomycin injury (18). These previous reports suggest differential roles for these two receptors with respect to inflammation after lung injury, with RHAMM mediating recruitment and CD44 required for resolution of inflammation. The data in this report clearly demonstrate that SPA, TGFβ, and HA-stimulated migration are dependent on RHAMM and not CD44. Additionally, our data demonstrate that HA-stimulated macrophage chemotaxis is dose- and molecular size-dependent, with low molecular weight HA maximally stimulating and HMW HA inhibiting cell motility.
A number of previous reports have implicated intracellular signaling pathways in TLR-stimulated inflammatory functions. These include activation of NFκB, JNK, ERK, and p38 (63). Adhikary et al. (40) studied TLR2-specific signaling pathways and showed that Pam3Cys stimulation of corneal epithelial cells resulted in phosphorylation of JNK, ERK, and p38 but that only blockade of JNK had an inhibitory effect on activation of NFκB. Indeed, JNK−/− mice had decreased responses to S. aureus-stimulated inflammation. Our results differ somewhat from those of Adhikary et al. (40) in that both JNK and ERK were required for the expression of TGFβ. These differences are possibly due to cell type specificity in responses.
In summary, our data confirm an important role for TLR2-stimulated and JNK/ERK-mediated TGFβ production and macrophage recruitment, as shown in the schematic model in Fig. 9. Our data are consistent with the model that TLR2 recognition of PAMPs differs from that of collectins such as SPA, in that SPA competitively inhibits the binding of peptidoglycan to TLR2, thereby decreasing NFκB activation (31), but it leads to activation of a signaling pathway that results in TGFβ production. TGFβ-stimulated macrophage motility, in turn, is dependent on HA and RHAMM, indicating that these components of the extracellular matrix perform vital tasks in the recruitment of macrophages to the site of infection, but they also serve to dampen the inflammatory process so as to protect the host from unrelenting inflammation and tissue damage. Further understanding of the mechanisms involved in TLR2-stimulated chemotaxis may allow the development of novel agents to attenuate unmitigated inflammation after lung injury.
FIGURE 9.
Schematic describing the pathway for TLR2-mediated TGFβ production and chemotaxis. The studies presented here show that SPA or Pam3Cys ligation of TLR2 increases TGFβ production in a JNK/ERK-dependent manner. In turn, TGFβ acts to recruit macrophages to the site of infection/injury, an event that is mediated by TGFβ receptors, but requires RHAMM and HA. When TGFβ concentrations are high enough at the site of infection/injury, there is a general suppression of inflammation. Modulation of RHAMM and HA may provide novel therapeutic targets to modulate innate immune responses.
Acknowledgments
We thank the following for their kind gifts of reagents: Dr. Hal Dietz (The Johns Hopkins University, Baltimore, MD) for the CAGA-GFP mice; Dr. Tak Mak (Amgen, Toronto, Ontario, Canada) for the CD44−/− mice; and Dr. Eva A. Turley (London Regional Cancer Centre, London, Ontario, Canada) for the RHAMM−/− mice. The assistance of Masaki Kosemura and Takahiro Masa (Seikagaku Corp.) in preparation of hyaluronan oligosaccharides is greatly appreciated. We thank Drs. Ralph DeBerardinis, Felix Yarovinsky, and Carole Mendelson (University of Texas Southwestern) for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL62472 and HL073896 (to R. C. S.) and HL068072 (to J. R. W.). This work was also supported by a Sponsored Research Agreement from Seikagaku Corp., Japan (to R. C. S.).
We dedicate this manuscript to Jo Rae Wright, who passed away on January 11, 2012. Her inspiration, encouragement, and enthusiasm, together with scientific rigor and insight, helped guide and shape this project to its fruition.

This article contains supplemental Figs. 1–3.
- TLR
- Toll-like receptor
- BMDM
- bone marrow-derived macrophage
- Pam3Cys
- tripalmitoyl-S-glycero-Cys-(Lys)4
- HA
- hyaluronan (hyaluronic acid)
- HABP
- hyaluronan-binding protein
- HABPep
- synthetic HA-binding peptide
- HA6
- 6-mer HA oligosaccharide
- HA900
- 900-kDa HA
- RHAMM
- receptor for HA-mediated motility (CD168)
- SPA
- surfactant protein A
- PAMP
- pathogen-associated molecular pattern
- HMW
- high molecular weight
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- SIRPα
- signal inhibitory regulatory protein α
- PGN
- peptidoglycan.
REFERENCES
- 1. Waters P., Vaid M., Kishore U., Madan T. (2009) Lung surfactant proteins A and D as pattern recognition proteins. Adv. Exp. Med. Biol. 653, 74–97 [DOI] [PubMed] [Google Scholar]
- 2. Wright J. R. (2005) Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 5, 58–68 [DOI] [PubMed] [Google Scholar]
- 3. Chroneos Z. C., Sever-Chroneos Z., Shepherd V. L. (2010) Pulmonary surfactant. An immunological perspective. Cell Physiol. Biochem. 25, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCormack F. X., Whitsett J. A. (2002) The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Invest. 109, 707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright J. R., Youmans D. C. (1993) Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophage. Am. J. Physiol. 264, L338–L344 [DOI] [PubMed] [Google Scholar]
- 6. Huynh M. L., Fadok V. A., Henson P. M. (2002) Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gardai S. J., Xiao Y. Q., Dickinson M., Nick J. A., Voelker D. R., Greene K. E., Henson P. M. (2003) By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115, 13–23 [DOI] [PubMed] [Google Scholar]
- 8. Kishore U., Greenhough T. J., Waters P., Shrive A. K., Ghai R., Kamran M. F., Bernal A. L., Reid K. B., Madan T., Chakraborty T. (2006) Surfactant proteins SP-A and SP-D. Structure, function, and receptors. Mol. Immunol. 43, 1293–1315 [DOI] [PubMed] [Google Scholar]
- 9. Kuroki Y., Takahashi M., Nishitani C. (2007) Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 9, 1871–1879 [DOI] [PubMed] [Google Scholar]
- 10. Roberts A. B., Heine U. I., Flanders K. C., Sporn M. B. (1990) Transforming growth factor-β. Major role in regulation of extracellular matrix. Ann. N. Y. Acad. Sci. 580, 225–232 [DOI] [PubMed] [Google Scholar]
- 11. Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. (1987) Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. U.S.A. 84, 5788–5792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savani R. C., DeLisser H. M. (2003) in Proteoglycans and Lung Disease (Garg H. G., Roughley P. J., Hales C. A., eds) pp 73–106, Marcel Dekker, New York [Google Scholar]
- 13. Teder P., Nettelbladt O., Heldin P. (1995) Characterization of the mechanism involved in bleomycin-induced increased hyaluronan production in rat lung. Am. J. Respir. Cell Mol. Biol. 12, 181–189 [DOI] [PubMed] [Google Scholar]
- 14. Savani R. C., Hou G., Liu P., Wang C., Simons E., Grimm P. C., Stern R., Greenberg A. H., DeLisser H. M., Khalil N. (2000) A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 23, 475–484 [DOI] [PubMed] [Google Scholar]
- 15. Turley E. A., Noble P. W., Bourguignon L. Y. (2002) Signaling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 [DOI] [PubMed] [Google Scholar]
- 16. Hall C. L., Wang C., Lange L. A., Turley E. A. (1994) Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J. Cell Biol. 126, 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samuel S. K., Hurta R. A., Spearman M. A., Wright J. A., Turley E. A., Greenberg A. H. (1993) TGF-β1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM and hyaluronan. J. Cell Biol. 123, 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaman A., Cui Z., Foley J. P., Zhao H., Grimm P. C., Delisser H. M., Savani R. C. (2005) Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am. J. Respir. Cell Mol. Biol. 33, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 20. Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 21. McIntosh J. C., Swyers A. H., Fisher J. H., Wright J. R. (1996) Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 15, 509–519 [DOI] [PubMed] [Google Scholar]
- 22. Entwistle J., Zhang S., Yang B., Wong C., Li Q., Hall C. L., Jingbo A., Mowat M., Greenberg A. H., Turley E. A. (1995) Characterization of the murine gene encoding the hyaluronan receptor RHAMM. Gene 163, 233–238 [DOI] [PubMed] [Google Scholar]
- 23. Fieber C., Plug R., Sleeman J., Dall P., Ponta H., Hofmann M. (1999) Characterisation of the murine gene encoding the intracellular hyaluronan receptor IHABP (RHAMM). Gene 226, 41–50 [DOI] [PubMed] [Google Scholar]
- 24. Savani R. C., Wang C., Yang B., Zhang S., Kinsella M. G., Wight T. N., Stern R., Nance D. M., Turley E. A. (1995) Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J. Clin. Invest. 95, 1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang B., Yang B. L., Savani R. C., Turley E. A. (1994) Identification of a common hyaluronan-binding motif in the hyaluronan-binding proteins RHAMM, CD44, and link protein. EMBO J. 13, 286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi Y., Kornovski B. S., Savani R., Turley E. A. (1993) A rapid, multiwell colorimetric assay for chemotaxis. J. Immunol. Methods 164, 149–154 [DOI] [PubMed] [Google Scholar]
- 27. Zigmond S. H., Hirsch J. G. (1973) Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J. Exp. Med. 137, 387–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tino M. J., Wright J. R. (1999) Surfactant proteins A and D specifically stimulate directed actin-based responses in alveolar macrophages. Am. J. Physiol. 276, L164–L174 [DOI] [PubMed] [Google Scholar]
- 29. Abe M., Harpel J. G., Metz C. N., Nunes I., Loskutoff D. J., Rifkin D. B. (1994) An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 [DOI] [PubMed] [Google Scholar]
- 30. Delpech B., Bertrand P., Maingonnat C. (1985) Immunoenzymoassay of the hyaluronic acid-hyaluronectin interaction. Application to the detection of hyaluronic acid in serum of normal subjects and cancer patients. Anal. Biochem. 149, 555–565 [DOI] [PubMed] [Google Scholar]
- 31. Murakami S., Iwaki D., Mitsuzawa H., Sano H., Takahashi H., Voelker D. R., Akino T., Kuroki Y. (2002) Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-α secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J. Biol. Chem. 277, 6830–6837 [DOI] [PubMed] [Google Scholar]
- 32. Sato M., Sano H., Iwaki D., Kudo K., Konishi M., Takahashi H., Takahashi T., Imaizumi H., Asai Y., Kuroki Y. (2003) Direct binding of Toll-like receptor 2 to zymosan and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 171, 417–425 [DOI] [PubMed] [Google Scholar]
- 33. Yamada C., Sano H., Shimizu T., Mitsuzawa H., Nishitani C., Himi T., Kuroki Y. (2006) Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J. Biol. Chem. 281, 21771–21780 [DOI] [PubMed] [Google Scholar]
- 34. Nedvetzki S., Walmsley M., Alpert E., Williams R. O., Feldmann M., Naor D. (1999) CD44 involvement in experimental collagen-induced arthritis (CIA). J. Autoimmun. 13, 39–47 [DOI] [PubMed] [Google Scholar]
- 35. Katoh S., Matsumoto N., Kawakita K., Tominaga A., Kincade P. W., Matsukura S. (2003) A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J. Clin. Invest. 111, 1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puré E., Camp R. L., Peritt D., Panettieri R. A., Jr., Lazaar A. L., Nayak S. (1995) Defective phosphorylation and hyaluronate binding of CD44 with point mutations in the cytoplasmic domain. J. Exp. Med. 181, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heldin P., Laurent T. C., Heldin C. H. (1989) Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem. J. 258, 919–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reidy M. F., Wright J. R. (2003) Surfactant protein A enhances apoptotic cell uptake and TGF-β1 release by inflammatory alveolar macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L854–L861 [DOI] [PubMed] [Google Scholar]
- 39. Neptune E. R., Frischmeyer P. A., Arking D. E., Myers L., Bunton T. E., Gayraud B., Ramirez F., Sakai L. Y., Dietz H. C. (2003) Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 33, 407–411 [DOI] [PubMed] [Google Scholar]
- 40. Adhikary G., Sun Y., Pearlman E. (2008) c-Jun NH2-terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J. Leukocyte Biol. 83, 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang S., Qi D., Liang J., Miao R., Minagawa K., Quinn T., Matsui T., Fan D., Liu J., Fu M. (2012) The putative tumor suppressor Zc3h12d modulates toll-like receptor signaling in macrophages. Cell. Signal. 24, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crouch E., Wright J. R. (2001) Surfactant proteins a and d and pulmonary host defense. Annu. Rev. Physiol. 63, 521–554 [DOI] [PubMed] [Google Scholar]
- 43. Crouch E., Hartshorn K., Ofek I. (2000) Collectins and pulmonary innate immunity. Immunol. Rev. 173, 52–65 [DOI] [PubMed] [Google Scholar]
- 44. Borron P., McIntosh J. C., Korfhagen T. R., Whitsett J. A., Taylor J., Wright J. R. (2000) Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L840–L847 [DOI] [PubMed] [Google Scholar]
- 45. Chroneos Z. C., Abdolrasulnia R., Whitsett J. A., Rice W. R., Shepherd V. L. (1996) Purification of a cell-surface receptor for surfactant protein A. J. Biol. Chem. 271, 16375–16383 [DOI] [PubMed] [Google Scholar]
- 46. Chroneos Z., Shepherd V. L. (1995) Differential regulation of the mannose and SP-A receptors on macrophages. Am. J. Physiol. 269, L721–L726 [DOI] [PubMed] [Google Scholar]
- 47. Geertsma M. F., Nibbering P. H., Haagsman H. P., Daha M. R., van Furth R. (1994) Binding of surfactant protein A to C1q receptors mediates phagocytosis of Staphylococcus aureus by monocytes. Am. J. Physiol. 267, L578–L584 [DOI] [PubMed] [Google Scholar]
- 48. Tino M. J., Wright J. R. (1999) Glycoprotein-340 binds surfactant protein A (SP-A) and stimulates alveolar macrophage migration in an SP-A-independent manner. Am. J. Respir. Cell Mol. Biol. 20, 759–768 [DOI] [PubMed] [Google Scholar]
- 49. Sano H., Sohma H., Muta T., Nomura S., Voelker D. R., Kuroki Y. (1999) Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J. Immunol. 163, 387–395 [PubMed] [Google Scholar]
- 50. Kramer B. W., Jobe A. H., Bachurski C. J., Ikegami M. (2001) Surfactant protein A recruits neutrophils into the lungs of ventilated preterm lambs. Am. J. Respir. Crit. Care Med. 163, 158–165 [DOI] [PubMed] [Google Scholar]
- 51. Akira S. (2000) Toll-like receptors, Lessons from knockout mice. Biochem. Soc. Trans. 28, 551–556 [DOI] [PubMed] [Google Scholar]
- 52. Abreu M. T., Arditi M. (2004) Innate immunity and toll-like receptors. Clinical implications of basic science research. J. Pediatr. 144, 421–429 [DOI] [PubMed] [Google Scholar]
- 53. Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. (1999) Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11, 443–451 [DOI] [PubMed] [Google Scholar]
- 54. Adams D. H., Hathaway M., Shaw J., Burnett D., Elias E., Strain A. J. (1991) Transforming growth factor-β induces human T lymphocyte migration in vitro. J. Immunol. 147, 609–612 [PubMed] [Google Scholar]
- 55. Amara F. M., Entwistle J., Kuschak T. I., Turley E. A., Wright J. A. (1996) Transforming growth factor-β1 stimulates multiple protein interactions at a unique cis-element in the 3′-untranslated region of the hyaluronan receptor RHAMM mRNA. J. Biol. Chem. 271, 15279–15284 [DOI] [PubMed] [Google Scholar]
- 56. Teder P., Vandivier R. W., Jiang D., Liang J., Cohn L., Puré E., Henson P. M., Noble P. W. (2002) Resolution of lung inflammation by CD44. Science 296, 155–158 [DOI] [PubMed] [Google Scholar]
- 57. Yamasaki K., Muto J., Taylor K. R., Cogen A. L., Audish D., Bertin J., Grant E. P., Coyle A. J., Misaghi A., Hoffman H. M., Gallo R. L. (2009) NLRP3/cryopyrin is necessary for interleukin-1β (IL-1β) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 284, 12762–12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Savani R. C., Bagli D. J., Harrison R. E., Turley E. A. (2000) in Scarless Wound Healing (Garg H. G., Longaker M. T. eds.) 19 Ed., pp. 115–142, Marcel Dekker, New York [Google Scholar]
- 59. Hall C. L., Lange L. A., Prober D. A., Zhang S., Turley E. A. (1996) pp60(c-src) is required for cell locomotion regulated by the hyaluronan receptor RHAMM. Oncogene 13, 2213–2224 [PubMed] [Google Scholar]
- 60. Zhang S., Chang M. C., Zylka D., Turley S., Harrison R., Turley E. A. (1998) The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J. Biol. Chem. 273, 11342–11348 [DOI] [PubMed] [Google Scholar]
- 61. Savani R. C., Khalil N., Turley E. A. (1995) Hyaluronan receptor antagonists alter skin inflammation and fibrosis following injury. Proc. W. Pharmacol. Soc. 38, 131–136 [PubMed] [Google Scholar]
- 62. DeGrendele H. C., Estess P., Picker L. J., Siegelman M. H. (1996) CD44 and its ligand hyaluronate mediate rolling under physiologic flow. A novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 183, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kawai T., Akira S. (2006) TLR signaling. Cell Death Differ. 13, 816–825 [DOI] [PubMed] [Google Scholar]