Background: Hepcidin, the systemic iron regulator, is induced during inflammation and leads to low circulating and increased intracellular iron levels.
Results: (Patho)physiologically relevant H2O2 levels up-regulate hepcidin via STAT3 in cultured liver cells.
Conclusion: Intracellular and extracellular H2O2 acts similarly to IL-6 on hepcidin up-regulation and requires a functional STAT3-binding site.
Significance: H2O2 is an important link between inflammation and iron metabolism.
Keywords: Hepatocyte, Iron Metabolism, Liver Metabolism, Oxidative Stress, Signaling, Anemia of Chronic Diseases, Hepcidin, Hydrogen Peroxide
Abstract
The peptide hormone hepcidin regulates mammalian iron homeostasis by blocking ferroportin-mediated iron export from macrophages and the duodenum. During inflammation, hepcidin is strongly induced by interleukin 6, eventually leading to the anemia of chronic disease. Here we show that hepatoma cells and primary hepatocytes strongly up-regulate hepcidin when exposed to low concentrations of H2O2 (0.3–6 μm), concentrations that are comparable with levels of H2O2 released by inflammatory cells. In contrast, bolus treatment of H2O2 has no effect at low concentrations and even suppresses hepcidin at concentrations of >50 μm. H2O2 treatment synergistically stimulates hepcidin promoter activity in combination with recombinant interleukin-6 or bone morphogenetic protein-6 and in a manner that requires a functional STAT3-responsive element. The H2O2-mediated hepcidin induction requires STAT3 phosphorylation and is effectively blocked by siRNA-mediated STAT3 silencing, overexpression of SOCS3 (suppressor of cytokine signaling 3), and antioxidants such as N-acetylcysteine. Glycoprotein 130 (gp130) is required for H2O2 responsiveness, and Janus kinase 1 (JAK1) is required for adequate basal signaling, whereas Janus kinase 2 (JAK2) is dispensable upstream of STAT3. Importantly, hepcidin levels are also increased by intracellular H2O2 released from the respiratory chain in the presence of rotenone or antimycin A. Our results suggest a novel mechanism of hepcidin regulation by nanomolar levels of sustained H2O2. Thus, similar to cytokines, H2O2 provides an important regulatory link between inflammation and iron metabolism.
Introduction
During infection and inflammation, mammalian iron metabolism undergoes typical changes. Plasma iron is rapidly withdrawn from the circulation and is safely stored intracellularly in the form of ferritin. This redistribution limits iron availability for erythropoiesis and eventually leads to the so-called anemia of chronic disease (1). Hepcidin, a 25-amino acid peptide has been identified as the central systemic iron-regulating hormone, and its discovery has provided new insights into the molecular mechanisms underlying hypoferremia and iron retention in the reticuloendothelial system during inflammation (2–4). Under physiological conditions, replenished iron stores and inflammatory signals lead to hepcidin secretion by hepatocytes. Hepcidin then blocks duodenal uptake and macrophage release of iron by binding to ferroportin, finally causing its internalization and proteasomal degradation (5). The hepcidin-mediated blockage of ferroportin efficiently prevents iron export to the extracellular space and is a major mechanistic pathway of the anemia of chronic disease (6).
Among other cytokines, interleukin-6 (IL-6) is the most potent inducer of hepcidin during inflammation (6, 7), a response controlled by STAT3 signaling (8). In addition, multiple intra- and extracellular signals cross-talk to hepcidin, and the hepcidin promoter contains binding sites for AP-1 (9), GATA-4 (10, 11), C/EBPα5 (8), and SMADs (mothers against decapentaplegic homologs; binding to the BMP-responsive elements) (12) besides STAT3. Some of the promoter elements are essential for hepcidin basal expression, such as BMP-responsive elements (12) or C/EBPα (8), whereas others (e.g. the STAT3-binding site) are predominantly involved in hepcidin up-regulation upon inflammatory stimuli (8).
Inflammatory conditions not only change the cytokine milieu; they also increase the concentration of reactive oxygen species (ROS) locally and systemically. Upon activation, inflammatory cells, such as neutrophils and macrophages, undergo an “oxidative burst” that results in the release of large amounts of reactive oxygen species to kill invading bacteria (13). The membrane-associated NADPH oxidase (NOX2) first generates superoxide that is rapidly dismutated to the more stable H2O2 by superoxide dismutases (14). During inflammation, tissue H2O2 concentrations are persistently elevated and can reach H2O2 concentrations in the low micromolar range, mostly <10 μm (15).
During inflammation cells and tissues are exposed to persistently elevated concentrations of H2O2 demanding a tight regulation of iron homeostasis to prevent tissue damage via Fenton and Fenton-like reactions. Previous studies showed direct regulatory control of iron homeostasis by H2O2; the iron regulatory protein 1 (IRP1), which usually stabilizes the mRNA of TfR1 by binding to iron-responsive elements in the 3′-untranslated region (16), shows increased binding activity under persistently elevated H2O2 concentrations (17, 18). In addition, TfR1 is up-regulated independent of IRP-1 at the translational level under sustained levels of H2O2 (19). Both pathways finally lead to an iron shift into cells and removal of circulating free iron.
Little information is available how ROS regulate hepcidin expression. Previous in vitro and in vivo studies on common oxidative stress-associated liver diseases, such as alcoholic liver disease (20) and chronic hepatitis C (21), demonstrated hepcidin down-regulation. However, a detailed mechanistic participation of individual ROS on hepcidin down-regulation was not demonstrated, and some reported observations were performed under artificially high ROS conditions. Miura et al. (21) exposed cultured cells to a single bolus of 100 μm H2O2, which is commonly employed to dissect redox regulatory pathways. Such conditions, however, hardly mimic the continuous release of H2O2 from inflammatory cells, where H2O2 concentrations do not exceed 10 μm (15). Under normal culture conditions, H2O2 is rapidly degraded within 30 min (22), and artificially high H2O2 concentrations have to be used that may cause nonspecific oxidative damage. We have previously established and optimized an enzymatic system that allows the continuous generation of H2O2 at steady state levels over 24 h (19, 22–25). This system allows mimicking the continuous H2O2 flux from inflammatory cells.
Using this system, we show that in contrast to bolus application of high doses of H2O2, sustained levels of H2O2 as low as 0.3 μm rapidly and potently induce hepcidin independent of the cytokine network. Thus, H2O2 acts synergistically to inflammatory cytokines contributing to the anemia of chronic disease.
EXPERIMENTAL PROCEDURES
Cell Culture
Huh7 cells (from the Japanese Cancer Research Resources Bank, Tokyo, Japan) were maintained in DMEM with 4.5 g/liter glucose (PAA Laboratories, Pasching, Austria) and 10% fetal calf serum (PAA Laboratories) under 5% CO2. Cells were screened routinely for mycoplasma contamination by PCR, and no infection could be detected. For experiments, cells were seeded at a density of 6–7 × 104/well in 12-well plates with a working volume of 1 ml/well. For immunoblotting experiments, we used 6-well plates with 1.8 × 105 cells/well with a working volume of 2.5 ml/well, and for luciferase assays, cells were kept in 96-well plates (5000 cells/well and a working volume of 100 μl).
Cytokines and Reagents
IL-6 was purchased from Sigma-Aldrich and used diluted in culture medium at concentrations between 0.15 and 10 ng/ml. Bone morphogenetic protein 6 (BMP-6) was purchased from R&D Systems Inc. (Minneapolis, MN) and used at concentrations between 10 and 50 ng/ml. N-Acetylcysteine was purchased from Sigma-Aldrich and used in a final concentration of 2 mm. Rotenone and antimycin A were also purchased from Sigma-Aldrich and used at final concentrations of 10 and 3 μm, respectively.
Exposure of Cell Culture Cells to H2O2
H2O2 Bolus
Concentration of H2O2 (Merck) was determined spectrophotometrically (ϵ230 = 74 liters mol−1 cm−1). H2O2 was diluted into cell culture medium at concentrations between 25 and 2000 μm.
Steady State H2O2 (H2O2ss) Treatment Using Glucose Oxidase and Catalase
Glucose oxidase (GOX) and catalase (CAT) were purchased from Sigma-Aldrich. Actual activities of GOX and CAT were determined at very low H2O2 concentrations prior to the experiment using a sensitive chemiluminescence technique (19, 22, 25, 26). During all experiments, kGOX was kept at 4 × 10−8 m/s, whereas kCAT was adjusted to reach defined H2O2ss concentrations between 0.15 and 6 μm. Because GOX metabolizes glucose and oxygen stochiometrically to H2O2 and δ-gluconolactone, kGOX was selected to not decrease glucose levels during 24 h by more than 3 mm, which does not influence glucose metabolism. In addition, no hypoxia was observed under these conditions (27).
Measurement of H2O2 Concentrations in Cell Culture Medium
Huh7 cells were seeded at a density of 6–7 × 104 cells/well in 12-well plates. After 24 h, cell culture medium was changed to phenol red-free DMEM (4.5 g of glucose/liter) with 10% FCS. H2O2 was added either as a bolus or generated by GOX/CAT as described above. H2O2 determination was done as described by Mueller et al. (15, 25). Briefly, a white 96-well plate (Greiner Bio-One, Frickenhausen, Germany) was prefilled with 100 μl/well of a 100 μm luminol, PBS solution (Sigma-Aldrich). Then 100 μl of cell culture supernatant from various time points of incubation with H2O2 and GOX/CAT, respectively, were added. Luminescence was then determined by adding 50 μl of a 0.4 mm NaOCl solution by the injection device of the luminometer (Fluostar, BMG Labtech, Ortenberg, Germany). Luminescence was measured immediately after NaOCl injection over 2 s.
H2O2 Intracellular Imaging by Peroxy Green-1 (PG-1)
For imaging studies, Huh7 cells were seeded onto chamber slides (Nunc LabTek, Langenselbold, Germany) and grown overnight. The next day, cells were loaded with PG-1 (28) (a kind gift of Christopher Chang (University of California, Berkeley)) at a concentration of 5 μm in Hanks' balanced salt solution, 25 mm glucose for 20 min. Then the cells were exposed to H2O2ss ranging from 0.15 to 3 μm generated by the GOX/CAT system in Hanks' balanced salt solution, 25 mm glucose, 1% FCS for 6 h. Confocal fluorescence imaging was performed with a Zeiss LSM-710 laser-scanning microscope and a ×40 (0.8 numerical aperture) oil immersion objective lens. Excitation of PG-1-loaded cells at 488 nm was carried out with an argon laser, and emission was collected at 520 nm.
RNA Isolation, Reverse Transcription, and Real-time Quantitative PCR
Cells were lysed with TriReagent (Molecular Research Center Inc. Cincinnati, OH), and RNA was isolated with a standard chloroform/isopropyl alcohol extraction. RNA concentration was adjusted after photometric measurement, and 500 ng of RNA were transcribed using Moloney murine leukemia virus reverse transcriptase, 50 pmol of random hexamer (both from Promega, Mannheim, Germany), and 100 pmol of oligo(dT) primers (Carl Roth, Karlsruhe, Germany). Relative mRNA transcript levels were quantified using the LightCycler FastStart DNA Master Hybridization Probes kit on a LightCycler (Roche Applied Science) and applying the TaqMan methodology. The housekeeping genes β2-microglobulin and hypoxanthine-guanine-phosphoribosyltransferase were amplified in a parallel reaction for normalization. The TaqMan probes were positioned on exon-exon boundaries of corresponding genes to exclude co-amplification of genomic DNA. Primers and probes were designed using the Primer Express software (PerkinElmer Life Sciences) or the online Universal Probe Library Assay Design Center (Roche Applied Science) and synthesized at Eurofins MWG Operon (Ebersbach, Germany). Sense and antisense primer (each at 0.5 μm) and 0.125 μm 5′-phosphorylated probe were labeled at their 5′-end with the reporter dye 6-carboxyfluorescein and at the 3′-end with the quencher dye 6-carboxytetramethylrhodamine.
Transfection Experiments
Huh7 cells were seeded in 96-well plates or 12-well plates depending on the experiment. Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For promoter studies, hepcidin promoter constructs containing firefly luciferase and a control plasmid containing Renilla luciferase were cotransfected. Details of the constructs have been published previously (8, 12, 29). The catalase-containing plasmid was a kind gift of Thomas Kietzmann (University of Kaiserslautern, Germany) (30, 31). The SOCS3 plasmid and the empty vector control were a kind gift from Julia Strebovsky (University of Heidelberg, Germany).
Analysis of Hepcidin Promoter Activity
Huh7 cells were transfected as described above. 24 h after transfection, cells were treated with cytokines, steady state H2O2, or a combination of both. After another 24 h, luciferase activity was assessed by the Dual Luciferase assay (Promega, Mannheim, Germany). Firefly luciferase expression was normalized to CMV promoter-controlled Renilla luciferase. Simultaneous overexpression of SOCS3 (suppressor of cytokine signaling 3) was performed by co-transfection of the SOCS3 expression vector or its corresponding empty control vector.
RNA Silencing
For RNA silencing experiments, Huh7 cells were transfected using Lipofectamine 2000 and 10 nm siRNA. Validated siRNA for STAT3, gp130, JAK1, and JAK2 were purchased by Applied Biosystems Inc. (Foster City, CA). Equimolar concentrations of universal negative siRNA (Sigma-Aldrich) were used as negative control.
Immunoblotting
Cells were rinsed in ice-cold PBS and harvested in radioimmune precipitation assay buffer, 1× Complete® protease inhibitor with EDTA (Roche Applied Science) on ice. Protein concentration was determined by a BCA assay (Pierce) and adjusted to 1 mg/ml in Laemmli buffer containing 0.2% 2-mercaptoethanol. Equal amounts of protein (20–40 μg/lane) were separated on 10% SDS-polyacrylamide gels and blotted on nitrocellulose membranes. Equal protein loading was confirmed by protein staining with Ponceau-S solution. Membranes were blocked in 5% skim milk, PBS-Tween 0.05% (T-PBS) for 1 h at room temperature and then incubated in primary antibody (see Table 1) diluted in skim milk/T-PBS overnight at 4 °C. 5% BSA, T-PBS was used for blocking of nonspecific binding and for dilution of the anti-pSTAT3 antibody. After a wash in T-PBS for 20 min, the secondary antibody (see Table 2) diluted in 5% skim milk was incubated at room temperature for 2 h followed by another washing step in T-PBS. The blot was developed using chemiluminescence (Rotilumin, Carl Roth, Karlsruhe, Germany) and exposed to autoradiography film for 1–3 min.
TABLE 1.
Primer list
| Primer | Sequence |
|---|---|
| Human β2-microglobulin | |
| Forward | 5′-tga ctt tgt cac agc cca aga ta-3′ |
| Reverse | 5′-aat cca aat gcg gca tct tc-3′ |
| Probe | FAM-tga tgc tgc tta cat gtc tcg atc cca-TAMa |
| Human hepcidin | |
| Forward | 5′-cag gac aga gct gga gcc a -3′ |
| Reverse | 5′-gca gca cat ccc aca ctt tg-3′ |
| Probe | FAM-ctg ctg cgg ctg ctg tca tcg a-TAM |
| Human IL-6 | |
| Forward | 5′-gcc cag cta tga act cct tct-3′ |
| Reverse | 5′-ctt ctc ctg ggg gta ctg g-3′ |
| Probe | UPL 68 (Roche Applied Science) |
a FAM, 6-carboxyfluorescein; TAM, 6-carboxytetramethylrhodamine.
TABLE 2.
Antibodies and dilutions
| Antigen | Host species | Dilution | Company/source | Catalog number |
|---|---|---|---|---|
| pSTAT3 (Tyr-705) | Rabbit | 1:1000 | Cell Signaling | 9145 |
| STAT3 | Mouse | 1:1000 | Cell Signaling | 9139 |
| Catalase | Rabbit | 1:1000 | Dr. A. Völkl, University of Heidelberg (48) | |
| gp130 | Rabbit | 1:1000 | Cell Signaling | 3732 |
| JAK1 | Rabbit | 1:1000 | Cell Signaling | 3344 |
| JAK2 | Rabbit | 1:1000 | Cell Signaling | 3230 |
| Rabbit Ig | Goat HRP conjugate | 1:3000 | Rockland | 611–1322 |
| Mouse Ig | Goat HRP conjugate | 1:2000 | Abcam | ab6789-1 |
RESULTS
Sustained Non-toxic Levels of H2O2 Strongly Up-regulate Hepcidin
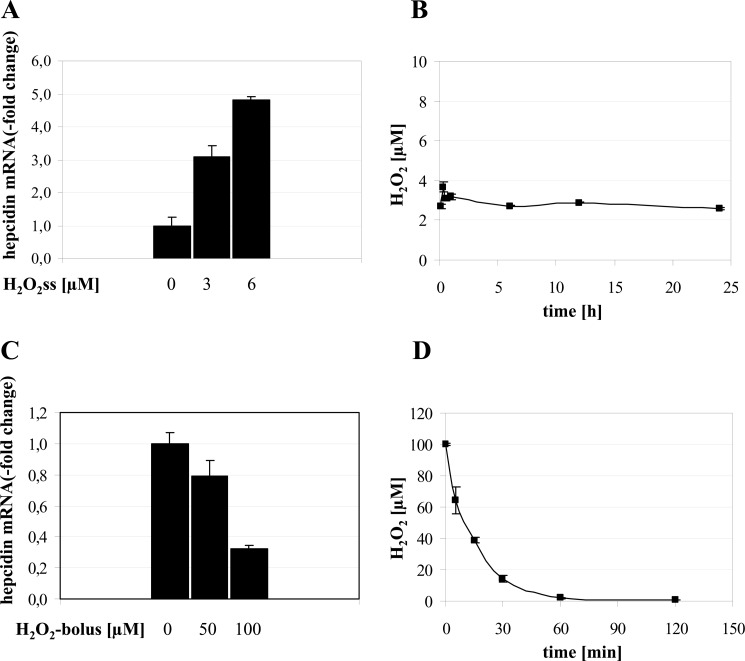
In this study, we investigated whether hepatic cells exposed to low non-toxic steady state levels of H2O2 as produced by the glucose oxidase and catalase system (GOX/CAT) alter hepcidin mRNA levels. We show that sustained H2O2 treatment (6 μm) of HuH7 cells for a period of 24 h up-regulates hepcidin mRNA expression by ∼5-fold in a dose-dependent manner (Fig. 1A). Because similar results could be observed in other hepcidin-secreting hepatoma cells (Hep3B and HepG2) and primary mouse hepatocytes (data not shown), H2O2-mediated up-regulation of hepcidin seems to be a general mechanism. Application of the luminol-hypochlorite assay confirms the stable maintenance of H2O2 levels at 3 μm over 24 h as generated by the GOX/CAT system (Fig. 1B). By contrast, a H2O2 bolus of 100 μm drastically suppressed mRNA expression of hepcidin (Fig. 1C) as reported previously (21). No changes in hepcidin expression were observed using H2O2 boli of 50 μm H2O2 or below. As shown in Fig. 1D, an H2O2 bolus of 100 μm was completely removed from the cell culture medium within 60 min. The exponential first order kinetic is highly suggestive of a catalase-mediated decomposition of H2O2 and, in confirmation, could be blocked by sodium azide (not shown). Thus, in the bolus experiment, cultured cells are exposed for a limited time of less than 1 h to artificially high and toxic concentrations of H2O2. Taken together, these studies indicate that hepcidin mRNA expression responds in an opposite fashion to sustained low levels of H2O2 in the range of those generated by inflammatory cells and a strong H2O2 bolus.
FIGURE 1.
A, low dose steady state H2O2 treatment induces hepcidin mRNA in hepatocytes. Huh7 cells were treated with 3 or 6 μm H2O2ss over 24 h. RNA was isolated, and hepcidin mRNA was quantified by quantitative real-time PCR. Results are normalized to β2-microglobulin and represented as -fold increase ± S.D. (error bars) compared with untreated control. Hepcidin is significantly up-regulated by H2O2ss. **, p < 0.01. B, the glucose oxidase/catalase system allows the stable maintenance of low dose H2O2 (H2O2ss) over 24 h. Glucose oxidase and catalase were diluted into cell culture medium according to enzymatic activity to result in an H2O2ss concentration of 3 μm over 24 h. H2O2 concentration was followed using a luminol-NaOCl-based chemiluminescence assay. Note the different time and H2O2 scale used to depict the H2O2-kinetics between B and D. C, H2O2 bolus treatment down-regulates hepcidin mRNA in hepatocytes. Huh7 cells were treated with H2O2 boli of 50 and 100 μm. RNA was isolated 24 h after treatment, and hepcidin mRNA was quantified by real-time PCR. Results are normalized to β2-microglobulin and represented as -fold decrease ± S.D. compared with untreated control. **, p < 0.01. D, bolus H2O2 is rapidly removed by cultured hepatoma cells due to intrinsic catalase activity. H2O2 was diluted into cell culture medium at an initial concentration of 100 μm. Changes in H2O2 concentration were followed using a luminol-NaOCl-based chemiluminescence assay. After 1 h, there is virtually no H2O2 left in the cell culture medium. Note the different time and H2O2 scale used to depict the H2O2 kinetics between B and D.
Ultralow Levels of H2O2 Are Sufficient to Induce Hepcidin Promoter Activity
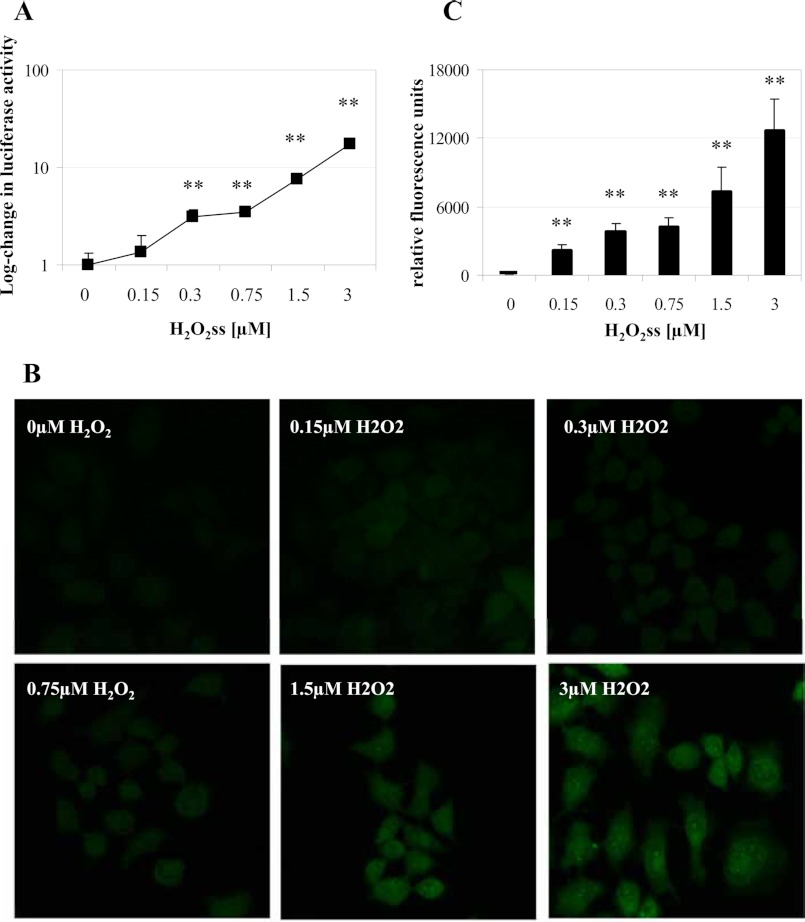
By titrating H2O2 levels using various GOX/CAT enzyme ratios, we next demonstrate that steady state levels of H2O2 as low as 0.3 μm are sufficient to induce hepcidin promoter activity (Fig. 2A). For these experiments, a firefly luciferase reporter construct containing the full-length hepcidin promoter was transfected together with a control plasmid containing the Renilla luciferase. Hepcidin promoter activity was determined by the ratio of firefly/Renilla luciferase activity. In order to demonstrate that the application of such low extracellular H2O2ss levels affects the intracellular H2O2 status, fluorescence studies were performed using the intracellular H2O2 dye PG-1 (Fig. 2, B and C). In contrast to conventional dyes like dichlorofluorescein diacetate, which are not specific to H2O2 and are prone to various artifacts, PG-1 is based on a boronate-caged fluorophore that is specifically deprotected by H2O2 (28). In parallel to the hepcidin induction, a homogenous increase of PG-1 fluorescence was observed when exposing cells to 0.3 μm sustained H2O2 or higher concentrations over 6 h (Fig. 2, B and C). To our knowledge, hepcidin is one of the most H2O2-sensitive molecules, responding already to submicromolar changes of the extracellular H2O2 environment.
FIGURE 2.
A, submicromolar concentrations of H2O2 activate hepcidin promoter activity. Huh7 cells were transfected with reporter constructs in which the wild type hepcidin promoter was fused to firefly luciferase and a control plasmid expressing Renilla luciferase under the control of the CMV promoter. 24 h later, cells were incubated for 24 h with increasing concentrations of H2O2ss ranging from 0.15 to 3 μm. A significant increase in hepcidin promoter activity was seen starting at 0.3 μm H2O2ss concentration. Transfection was performed in triplicates, and results are presented as -fold change ± S.D. (error bars) of firefly/Renilla luciferase activity on a semilogarithmic scale. **, p < 0.01. B and C, exposure of cultured cells to low dose steady state H2O2 increases intracellular H2O2. Low dose steady state H2O2 application leads to an increase of intracellular H2O2 levels as shown by an increase in PG-1 fluorescence in Huh7 cells. Cells were loaded with PG-1 and then exposed to H2O2ss ranging from 0.15 to 3 μm for 6 h. Fluorescence was determined in a laser-scanning microscope using the same settings for all conditions. Magnification was ×200. Quantification of the increase in fluorescence is depicted in C and shows a dose-dependent increase during stimulation with H2O2ss starting from 0.15 μm. **, p < 0.01.
H2O2-mediated Up-regulation of Hepcidin Requires the STAT3-binding Site in the Hepcidin Promoter
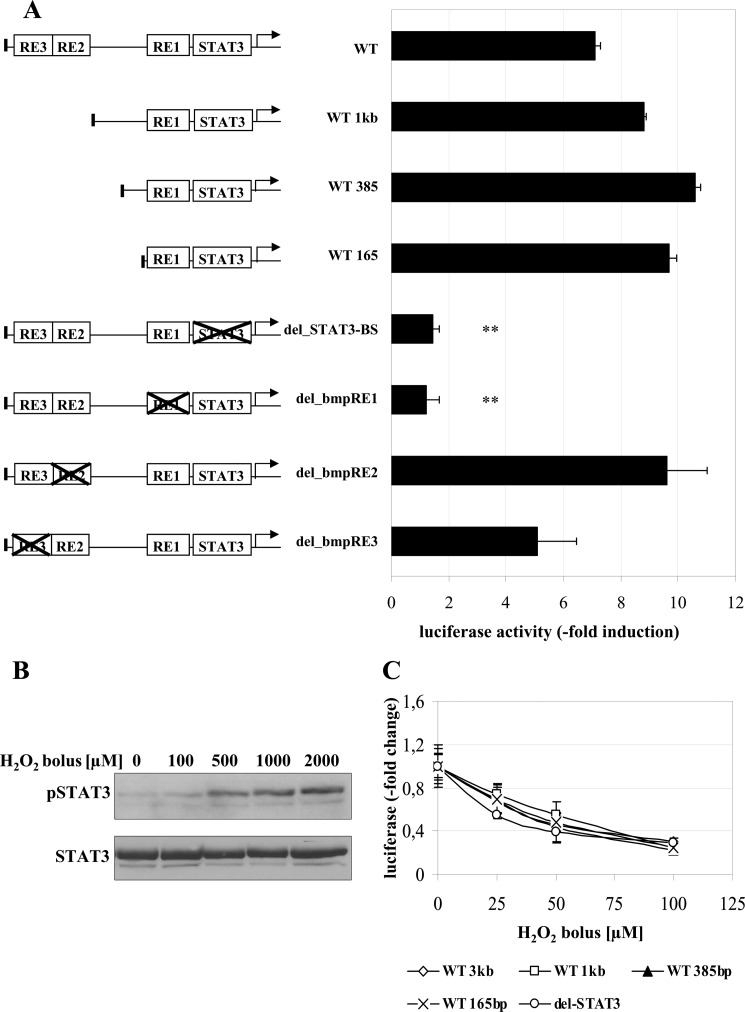
We next identified the promoter elements involved in H2O2-dependent hepcidin induction. For this purpose, we transfected luciferase reporter constructs containing either the full-length hepcidin wild type promoter (WT 3 kb), three increasingly truncated wild type promoter derivatives (WT 1 kb, WT 385 bp, and WT 165 bp), or four full-length hepcidin promoters with selective deletions of specific binding sites, namely the STAT3-binding site (del-STAT3) and the BMP-responsive elements 1, 2, and 3 (BMP-RE1, -2, and -3), respectively. As shown in Fig. 3A, no changes of promoter activity in response to H2O2ss were observed when truncating the hepcidin promoter stepwise from the 5′-end. However, a selective deletion of the STAT3-binding site almost completely prevented H2O2-mediated up-regulation of promoter activity, as did deletion of BMP-RE1. Selective deletions of BMP-RE2 and -3 still allowed for H2O2ss-dependent promoter activation. This represents the typical pattern of STAT3-dependent hepcidin promoter activation, which requires both the STAT3-binding site and the BMP-RE1 (12, 29). Because it is established that deletions of the STAT3-binding site or the BMP-RE1 decrease basal expression hepcidin levels (8, 29), the results are shown as -fold induction compared with the untreated control.
FIGURE 3.
A, STAT3 is required for the H2O2-dependent increase of hepcidin promoter activity. Huh7 cells were transfected with hepcidin promoter constructs containing the full-length wild type promoter (WT); promoter regions with decreasing length of the 5′-flanking region (WT 1kb, WT 385bp, and WT 165bp); or the WT full-length promoter with specific deletions of transcription factor binding sites (i.e. the STAT3-binding site (delSTAT3)) and the BMP-responsive elements 1–3 (del_bmp-RE1 to -3). Transfection and expression control was performed by a Renilla control plasmid. 24 h after transfection, cells were incubated with 3 μm H2O2ss for 24 h. Although increasing truncation of the WT promoter did not change responsiveness to H2O2ss, the deletion of the STAT3-binding site completely abrogated H2O2ss-dependent promoter activation as did deletion of BMP-RE1. Because full STAT3 activation needs the presence of BMP-RE1, H2O2ss-induced hepcidin induction represents the typical profile of a STAT3-dependent activation. Transfections were performed in triplicates, and results are presented as -fold change ± S.D. (error bars) of firefly/Renilla luciferase activity compared with the untreated control of each construct. **, p < 0.01. B, H2O2 bolus application induces STAT3 phosphorylation at supraphysiological doses. Huh7 cells were treated with H2O2 boli between 100 μm and 2 mm for 1 h. Only H2O2 bolus concentrations of 500 μm or above induced STAT3 phosphorylation after 1 h but led to cell death later on. Normalization was performed using a STAT3 antibody. C, H2O2 bolus-induced down-regulation of hepcidin is STAT3-independent. Huh7 cells were transfected as described in A. 24 h after transfection, cells were incubated with H2O2 boli of 25, 50, or 100 μm for 24 h. Luciferase activity significantly decreased with increasing concentrations of bolus H2O2 application in all promoter constructs. Transfections were performed in triplicates, and results are presented as -fold change ± S.D. of firefly/Renilla luciferase activity compared with the untreated control of each construct.
Importantly, although H2O2 is able to induce STAT3 phosphorylation (Fig. 3B) as a bolus at toxic concentrations, promoter activity of the hepcidin promoter constructs was down-regulated. Thus, application of H2O2 boli between 25 and 100 μm decreased hepcidin in a dose-dependent manner and independent of the STAT3-binding site (Fig. 3C). Taken together, only low and sustained and not high dose bolus H2O2 up-regulates hepcidin expression and requires the STAT3-responsive element, whereas down-regulation of hepcidin expression by high dose bolus H2O2 occurs independent of STAT3 binding to the promoter.
Low Levels of Sustained H2O2 Are Sufficient to Activate the STAT3 Signaling Cascade
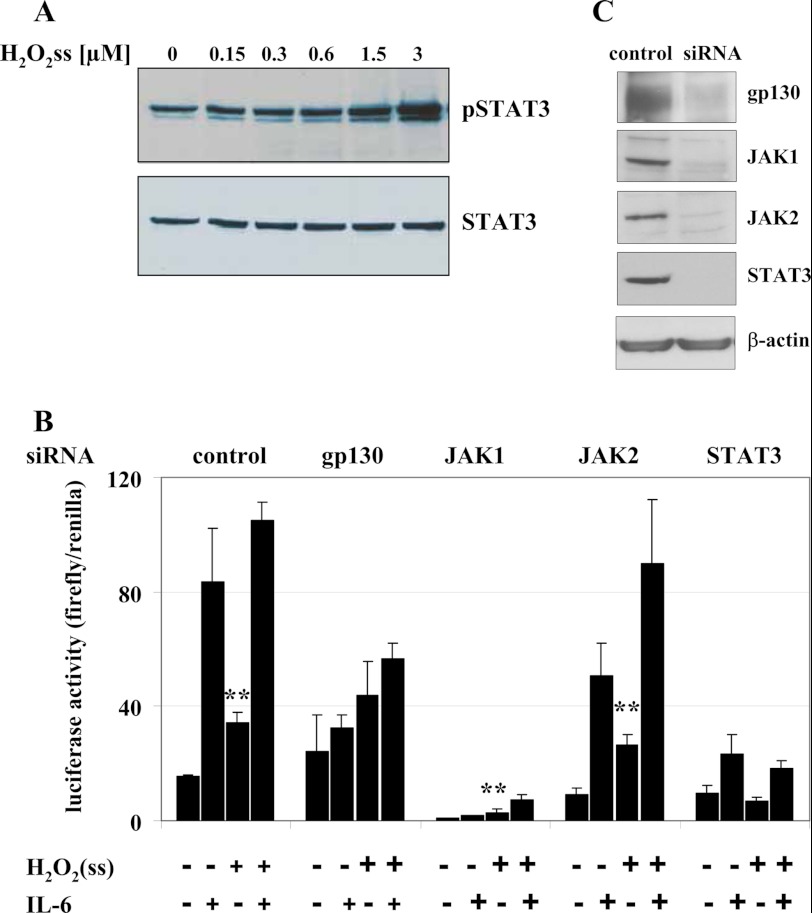
H2O2 boli higher than 500 μm are known to induce STAT3 phosphorylation (Fig. 3B). We now tested whether our low sustained H2O2 levels provided by the GOX/CAT system are likewise able to activate the STAT3 signaling cascade. Using a phospho-specific STAT3 antibody, we were able to show that STAT3 is phosphorylated dose-dependently at the tyrosine residue at position 705 during a 6-h treatment of Huh7 cells with low and sustained levels of H2O2 (Fig. 4A). We next tested whether the H2O2-mediated STAT3 phosphorylation affects hepcidin regulation. Luciferase reporter constructs with the wild type hepcidin promoter were transfected into HUH7 cells, and the STAT3 signaling cascade was then disrupted by siRNA-mediated silencing of each molecule involved in the gp130-JAK-STAT3 signaling cascade. These experiments identified three types of involvement in H2O2ss signaling (Fig. 4B and supplemental Fig. S1); gp130 and STAT3 confer H2O2ss responsiveness, which is abrogated after silencing of these two molecules. STAT3 silencing even shows a complete suppression of H2O2ss-mediated hepcidin induction. JAK1 is responsible for basal hepcidin expression but not H2O2ss responsiveness. JAK2, finally, was shown to be completely dispensable for H2O2ss as well as for IL-6 responsiveness, as has been shown previously (32, 33). The importance of STAT3 for H2O2ss-mediated hepcidin expression was additionally confirmed by overexpression of SOCS3 (suppressor of cytokine signaling 3), the endogenous antagonist of STAT3 (see supplemental Fig. S2). These experiments confirmed the important role of STAT3 phosphorylation, gp130, and JAK1 for the induction of hepcidin by H2O2.
FIGURE 4.
A, STAT3 Tyr-705 is phosphorylated in response to low levels of H2O2. Increasing H2O2ss concentrations cause increasing STAT3 phosphorylation. Huh7 cells were treated with increasing concentrations of H2O2ss (range 0.15–3 μm) for 6 h. B and C, siRNA-mediated silencing of STAT3 and gp130 prevents H2O2ss-dependent activation of hepcidin. The H2O2-mediated effect on hepcidin induction is abolished completely by silencing of STAT3. Silencing of gp130 also diminished the H2O2 effect significantly. JAK1 silencing decreases basal hepcidin promoter activity but does not influence H2O2 responsiveness, whereas JAK2 is dispensable for H2O2-mediated as well as IL-6-mediated hepcidin induction. Efficient silencing is shown by Western blotting. Huh7 cells were transfected with a full-length wild type hepcidin promoter fused to a firefly luciferase construct plus a Renilla control plasmid. Co-transfection with universal negative siRNA or siRNA directed specifically against individual molecules of the JAK-STAT3 signaling cascade was performed at the same time. Cells were treated 24 h after transfection with H2O2ss (3 μm), IL-6 (10 ng/ml), or a combination of both. Hepcidin promoter activity was assayed 12 h after treatment. Transfections were performed in triplicates, and results are presented as the ratio of firefly/Renilla luciferase activity ± S.D. (error bars) Significant differences in H2O2-mediated promoter activity compared with medium control are marked by asterisks (**, p < 0.01).
H2O2ss Induces Hepcidin Synergistically to IL-6 and BMP6
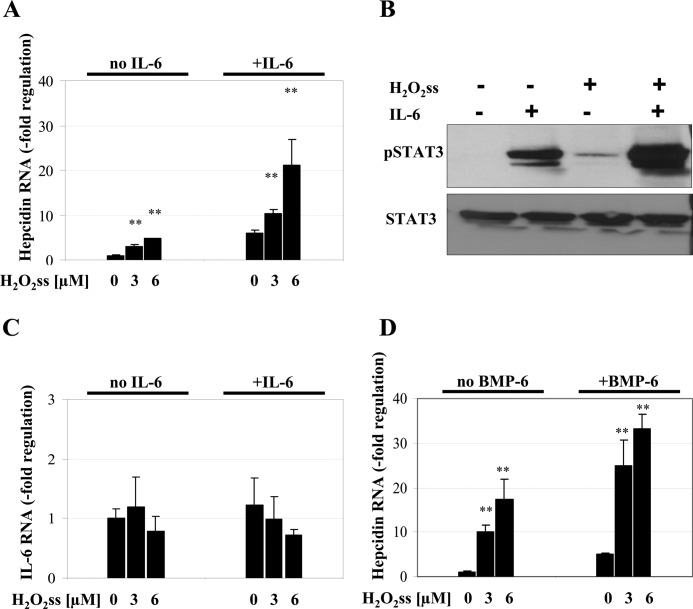
Cytokines, such as IL-6, and bone morphogenetic proteins, such as BMP6, are powerful transcriptional activators of hepcidin promoter activity, with BMPs being required for basal expression. As shown in Fig. 5A, H2O2ss-mediated up-regulation of hepcidin was drastically enhanced in the presence of 10 ng/ml IL-6. Using immunoblotting for pSTAT3, we establish that the combined application of low H2O2ss and IL-6 further increases phosphorylation of STAT3 as compared with either of the stimuli alone (Fig. 5B). This effect is not dependent on endogenous IL-6 production in hepatocytes after treatment with H2O2ss (Fig. 5C). In addition, H2O2ss further increases the BMP6-induced hepcidin response (Fig. 5D). We thus conclude that H2O2ss up-regulates hepcidin additively with IL-6 and BMP6 via increased STAT3 phosphorylation.
FIGURE 5.
H2O2ss acts synergistically with the hepcidin activator IL-6 or BMP-6. A, H2O2ss increases hepcidin mRNA levels in addition to IL-6. Combining increasing concentrations of H2O2ss (3 and 6 μm) with 10 ng/ml IL-6 leads to a further increase in hepcidin mRNA. Cells were incubated for 24 h with either H2O2ss or the cytokines alone or in combination. Hepcidin was determined by quantitative real-time PCR and normalized to β2-microglobuline. Results are presented as -fold change ± S.D. (error bars). **, p < 0.01. B, combined treatment with IL-6 and H2O2ss leads to additive STAT3 Tyr-705 phosphorylation. Huh7 cells were treated with 3 μm H2O2ss, IL-6 (10 ng/ml), or a combination of both for 1 h. The exposure was set to no background on purpose to allow detection of the additive effect of H2O2 and IL-6. C, the effect of H2O2ss on hepcidin mRNA is not mediated by an increased production of IL-6. Huh7 cells were incubated with H2O2ss with or without IL-6 10 ng/ml. Endogenous IL-6 production was assessed by quantitative real-time PCR and normalized to β2-microglobulin. Endogenous production did not change significantly after incubation with H2O2ss, IL-6, or a combination of both. Results are presented as -fold change ± S.D. **, p < 0.01. D, H2O2ss can increase BMP6-induced hepcidin mRNA up-regulation. Cells were incubated for 24 h with either H2O2ss or BMP6 (50 ng/ml) alone or in combination. Hepcidin was determined by quantitative PCR and normalized to β2-microglobulin. Results are presented as -fold change ± S.D. **, p < 0.01.
Modulation of Hepcidin Expression by Intracellular H2O2
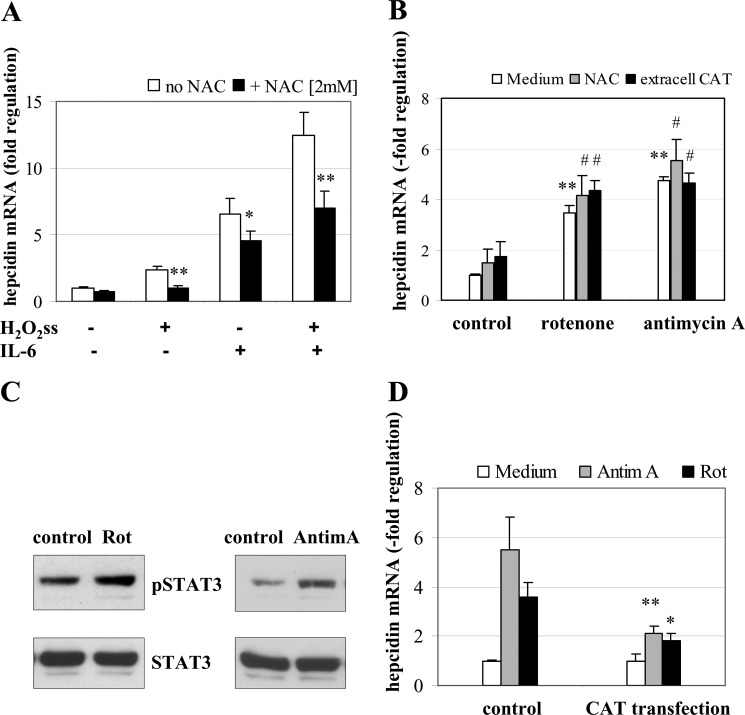
Thus far, we could show that minuscule amounts of extracellular H2O2ss suffice to induce hepcidin. We next analyzed whether H2O2ss also affects hepcidin expression when modulated intracellularly (e.g. when produced from intracellular sources or when scavenged in the presence of an antioxidant, such as N-acetylcysteine (NAC)). 2 mm NAC completely blocked up-regulation of hepcidin either by H2O2ss alone or in combination with IL-6 (Fig. 6A). This was not due to direct interaction of NAC with the GOX/CAT system because this effect was likewise observed when cells were pretreated with NAC for 24 h and, after washing, exposed to H2O2ss (data not shown). Interestingly, NAC alone also partly blocked hepcidin up-regulation by IL-6 alone by approximately 25%, suggesting that H2O2 could be involved in the genuine mechanism of IL-6 mediated induction of hepcidin.
FIGURE 6.
A, N-acetylcysteine efficiently blocks H2O2ss-induced hepcidin up-regulation. Huh7 cells were incubated with medium alone, 3 μm H2O2ss, or 3 μm H2O2ss and 2 mm N-acetylcysteine (NAC) with or without IL-6 (10 ng/ml). Total RNA was isolated 6 h after treatment, and hepcidin mRNA was quantified by real-time PCR. Results are normalized to β2-microglobulin and represented as -fold decrease ± S.D. (error bars) compared with untreated control. The hepcidin-inducing effect of H2O2ss was completely blocked by NAC. The additive effect of IL-6 and 3 μm H2O2ss was reduced to the effect of IL-6 alone when NAC was added. *, p < 0.05; **, p < 0.01. B, intracellular H2O2 induces hepcidin mRNA. Huh7 cells treated with rotenone or antimycin A show a significant up-regulation of hepcidin mRNA. This increase in hepcidin cannot be reversed by adding either NAC or extracellular catalase to the cell culture medium. Results are normalized to β2-microglobulin and represented as -fold change ± S.D. compared with untreated control. **, p < 0.01 compared with the medium control; #, no statistically significant change compared with rotenone or antimycin A treatment without NAC or extracellular catalase. C, treatment with rotenone or antimycin A increase STAT3 phosphorylation. Huh7 cells were treated for 6 h with either rotenone (10 μm) or antimycin A (3 μm). Cell lysates were then blotted for pSTAT3 and STAT3. Both reagents led to increased STAT3 phosphorylation. D, overexpressed intracellular catalase blocks hepcidin up-regulation due to intracellular H2O2. Huh7 cells were transiently transfected with catalase. 24 h after transfection, cells were treated for 6 h with either rotenone (10 μm) or antimycin A (3 μm). Results are normalized to β2-microglobulin and represented as -fold change ± S.D. compared with untransfected control. *, p < 0.05; **, p < 0.01.
To increase the intracellular generation of H2O2, Huh7 cells were incubated with 10 μm rotenone or 3 μm antimycin A for 6 h. Both agents are known to induce mitochondrial release of H2O2 by blocking the mitochondrial respiratory chain (complex I and III, respectively) (34, 35). As shown in Fig. 6B, rotenone and antimycin A both increase the expression of hepcidin mRNA. Notably, this intracellular effect cannot be modulated by co-incubation with 2 mm NAC or purified extracellular bovine catalase in the medium (kCAT = 0.05 s−1) (Fig. 6B). Both rotenone and antimycin A are also able to induce increased STAT3 phosphorylation (Fig. 6C). Transient overexpression of catalase significantly suppressed hepcidin induction by rotenone and antimycin A (Fig. 6D) and partially blunted phosphorylation of STAT3 (supplemental Fig. S3).
DISCUSSION
Hepcidin plays a key role in systemic iron regulation, but so far little is known about its regulation by ROS. We here show that even submicromolar concentrations of sustained H2O2 are sufficient for a potent up-regulation of hepcidin in hepatocytes. The levels of sustained H2O2 utilized in our experiments are nontoxic and mimic inflammatory conditions. We further demonstrate that H2O2 acts synergistically to other classical inducers of hepcidin, such as IL-6 or BMP-6. Our studies further show that the H2O2 effect on hepcidin is mainly mediated via STAT3, which represents the classical inflammatory pathway for the regulation of hepcidin. Importantly, specific STAT3 knockdown as well as overexpression of the STAT3 inhibitor SOCS3 blocked the hepcidin response to H2O2. Further analysis of the JAK-STAT signaling cascade revealed that in order to get a full H2O2-dependent response, several elements are needed: gp130 and STAT3 for the H2O2 responsiveness as well as JAK1 for a normal basic hepcidin promoter activity.
Our results also show that JAK2 is completely dispensable for hepcidin signaling. This was already known for IL-6 (32, 33) and has now also been confirmed for H2O2, which obviously signals along the same STAT3 pathway as IL-6. The exact mechanism of gp130 activation by H2O2ss will most likely involve thiol groups and sulfenic acid signaling. Human gp130 contains a total of 18 cysteine residues, 13 of them in the extracellular domain and 10 of these known to form disulfide bonds (36), which would offer ample possibilities for thiol-based signaling.
Low dose H2O2 is a considerable inducer of hepcidin mRNA (2.5–10-fold induction depending on experimental setting and time course). Because hepcidin is primarily regulated at the transcriptional level, this is supposed to translate into a significant amount of secreted protein and influence iron fluxes in ferroportin-expressing cells. In conclusion, our studies establish H2O2 as an important inducer of hepcidin during acute or chronic inflammation, further potentiating hypoferremia caused by cytokines.
At first sight, our results seem to challenge a previous publication by Miura et al. (21), who showed hepcidin down-regulation by H2O2 due to increased histone deacetylase activity. However, these authors applied bolus H2O2 at unphysiologically high concentrations. In our study, we could confirm that such supraphysiological H2O2 levels suppress hepcidin. Although we did not measure histone deacetylase activity, the decrease in hepcidin transcription after H2O2 bolus is independent of any specific promoter element and, therefore, compatible with a nonspecific, most likely toxic suppression of hepcidin. Indeed, H2O2 levels are typically around 0.1 μm in non-inflamed tissue and can maximally reach concentrations of ∼10 μm during acute inflammation (15, 37). Experiments with 100 μm H2O2 or even higher concentrations may therefore result in nonspecific oxidative damage of the cell and disrupt cellular redox homeostasis and most likely do not reflect upon endogenously occurring metabolic processes. The observation that different doses and application modes of H2O2 affect the same signaling pathway in an opposite manner is not unique for hepcidin. Similar findings were reported by De Oliveira-Marques et al. (38) for NFκB, whereby a low dose of H2O2ss stimulated NFκB activity, whereas an H2O2 bolus inhibited NFκB activation. Our data resolve the obvious contradiction previously established by the H2O2 bolus experiments; because it has been known for more than 2 decades that H2O2 is a potent activator of the STAT3 pathway, the suppression of STAT-controlled hepcidin by H2O2 was difficult to comprehend.
Although STAT3 seems to be responsible almost exclusively for the H2O2-mediated up-regulation of hepcidin in our experiments, the hepcidin promoter contains other redox-sensitive elements. For example, a C/EBPα binding site has been previously suggested to down-regulate hepcidin in response to oxidative stress in mouse models for HCV and alcoholic liver disease (39). However, elimination of the C/EBPα binding site at positions −231 to −222 (40) in the truncated promoter construct WT 165 kb neither prevented hepcidin down-regulation by H2O2 bolus application nor influenced hepcidin up-regulation by H2O2ss. Because in previous publications the type of ROS that activated C/EBPα has not been identified, the repressive effect might be exerted by a type of ROS other than H2O2. The hepcidin promoter also contains a putative AP-1 binding site, which is known to be sensitive to H2O2 (41). However, similar to C/EBPα, loss of the only AP-1 binding site in the promoter constructs at positions −242 to −232 (8) had no effect on the responsiveness to H2O2. Taken together, up-regulation of hepcidin by H2O2 seems to be exclusively mediated via STAT3.
The finding that hepcidin can be induced by very low levels of H2O2 starting from 0.3 μm has wide reaching implications. To the best of our knowledge, hepcidin is one of the most H2O2-sensitive molecules described to date. Even the redox-sensitive IRP1 required ∼10 times higher levels of H2O2 at ∼5 μm (42). Besides the amount of H2O2, the localization of the H2O2 source is of critical importance for hepcidin regulation. It is especially relevant for the question of whether H2O2 could serve as an important if not mandatory intracellular signaling molecule within the hepcidin pathway. By applying the intracellular H2O2 dye PG-1, we show that exposure of cells to submicromolar H2O2 concentrations affects the intracellular H2O2 milieu. Furthermore, we demonstrate that N-acetylcysteine blocks up-regulation of hepcidin not only by H2O2 but also by IL-6 alone. This suggests that H2O2 could be involved in IL-6 signaling to hepcidin (e.g. by activation of an NADPH oxidase). The requirement of the NADPH oxidase in JAK/STAT-dependent signaling has been previously shown for angiotensin II (43). In addition, we demonstrate that also intracellularly released H2O2 is able to induce hepcidin. H2O2 release by inhibition of the mitochondrial respiratory chain by rotenone (complex I) and antimycin A (complex III) (35) significantly induced hepcidin promoter activity, an effect that could be abrogated by overexpressed catalase.
We further propose that apart from its role in inflammation, H2O2 could also contribute to the regulation of hepcidin under non-inflammatory conditions, including metabolic diseases. Indeed, a new iron overload entity termed dysmetabolic iron overload syndrome has been introduced recently (44), which is often associated with the metabolic syndrome and non-alcoholic fatty liver disease (45). In these patients, circulating hepcidin levels are elevated for so far unknown reasons. They interrupt iron recycling and subsequently lead to iron accumulation in the reticuloendothelial system. Because insulin signaling (46), especially during conditions of insulin resistance (47), has been associated with increased intracellular release of H2O2, it would be very interesting to further investigate the role of H2O2 in dysmetabolic iron overload syndrome in future studies. Finally, although highly speculative, up-regulation of hepcidin by intracellular H2O2 could be an attractive mechanism of iron-mediated control of hepcidin. In this context, it should be noted that it still remains unresolved why iron-mediated induction of hepcidin in vivo cannot be reproduced under in vitro conditions.
In summary, our results establish H2O2 as an important upstream regulator and potent inducer of hepcidin that acts independent of but synergistically with the cytokine network, thus contributing to the anemia of chronic disease. On a final note, our results also demonstrate the importance of choosing an appropriate H2O2 model for studies of cellular signaling that closely mimics H2O2 conditions in vivo.
This work was supported in part by the Dietmar Hopp-Stiftung and the Manfred Lautenschläger-Stiftung.

This article contains supplemental Figs. S1–S3.
- C/EBPα
- CCAAT/enhancer-binding protein α
- BMP
- bone morphogenetic protein
- BMP-RE
- BMP-responsive element
- ROS
- reactive oxygen species
- H2O2ss
- steady state H2O2
- GOX
- glucose oxidase
- CAT
- catalase
- PG-1
- Peroxy Green 1
- NAC
- N-acetylcysteine.
REFERENCES
- 1. Cartwright G. E., Lauretsen M. A. (1946) The anemia associated with chronic infection. Science 103, 72. [PubMed] [Google Scholar]
- 2. Ganz T. (2002) The role of hepcidin in iron sequestration during infections and in the pathogenesis of anemia of chronic disease. Isr. Med. Assoc. J. 4, 1043–1045 [PubMed] [Google Scholar]
- 3. Nemeth E., Valore E. V., Territo M., Schiller G., Lichtenstein A., Ganz T. (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101, 2461–2463 [DOI] [PubMed] [Google Scholar]
- 4. Nicolas G., Bennoun M., Porteu A., Mativet S., Beaumont C., Grandchamp B., Sirito M., Sawadogo M., Kahn A., Vaulont S. (2002) Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. U.S.A. 99, 4596–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 6. Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B. K., Ganz T. (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 113, 1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roy C. N., Custodio A. O., de Graaf J., Schneider S., Akpan I., Montross L. K., Sanchez M., Gaudino A., Hentze M. W., Andrews N. C., Muckenthaler M. U. (2004) An Hfe-dependent pathway mediates hyposideremia in response to lipopolysaccharide-induced inflammation in mice. Nat. Genet. 36, 481–485 [DOI] [PubMed] [Google Scholar]
- 8. Verga Falzacappa M. V., Vujic Spasic M., Kessler R., Stolte J., Hentze M. W., Muckenthaler M. U. (2007) STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109, 353–358 [DOI] [PubMed] [Google Scholar]
- 9. Truksa J., Lee P., Beutler E. (2007) The role of STAT, AP-1, E-box and TIEG motifs in the regulation of hepcidin by IL-6 and BMP-9. Lessons from human HAMP and murine Hamp1 and Hamp2 gene promoters. Blood Cells Mol. Dis. 39, 255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Island M. L., Fatih N., Leroyer P., Brissot P., Loreal O. GATA-4 transcription factor regulates hepatic hepcidin expression. Biochem. J. 437, 477–482 [DOI] [PubMed] [Google Scholar]
- 11. Bagu E. T., Santos M. M. Friend of GATA suppresses the GATA-induced transcription of hepcidin in hepatocytes through a GATA-regulatory element in the HAMP promoter. J. Mol. Endocrinol. 47, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Casanovas G., Mleczko-Sanecka K., Altamura S., Hentze M. W., Muckenthaler M. U. (2009) Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J. Mol. Med. 87, 471–480 [DOI] [PubMed] [Google Scholar]
- 13. Klebanoff S. J. (1988) Phagocytic cells. Products of oxygen metabolism. in Inflammation: Basic Principles and Clinical Correlates (Snyderman R., ed) pp. 391–444, Raven Press, New York [Google Scholar]
- 14. Geiszt M., Leto T. L. (2004) The Nox family of NAD(P)H oxidases. Host defense and beyond. J. Biol. Chem. 279, 51715–51718 [DOI] [PubMed] [Google Scholar]
- 15. Mueller S., Arnhold J. (1995) Fast and sensitive chemiluminescence determination of H2O2 concentration in stimulated human neutrophils. J. Biolumin. Chemilumin. 10, 229–237 [DOI] [PubMed] [Google Scholar]
- 16. Hentze M. W., Muckenthaler M. U., Andrews N. C. (2004) Balancing acts. Molecular control of mammalian iron metabolism. Cell 117, 285–297 [DOI] [PubMed] [Google Scholar]
- 17. Martins E. A., Robalinho R. L., Meneghini R. (1995) Oxidative stress induces activation of a cytosolic protein responsible for control of iron uptake. Arch. Biochem. Biophys. 316, 128–134 [DOI] [PubMed] [Google Scholar]
- 18. Pantopoulos K., Hentze M. W. (1995) Rapid responses to oxidative stress mediated by iron regulatory protein. EMBO J. 14, 2917–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andriopoulos B., Hegedüsch S., Mangin J., Riedel H. D., Hebling U., Wang J., Pantopoulos K., Mueller S. (2007) Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the iron regulatory protein/iron-responsive element network. J. Biol. Chem. 282, 20301–20308 [DOI] [PubMed] [Google Scholar]
- 20. Harrison-Findik D. D., Schafer D., Klein E., Timchenko N. A., Kulaksiz H., Clemens D., Fein E., Andriopoulos B., Pantopoulos K., Gollan J. (2006) Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 281, 22974–22982 [DOI] [PubMed] [Google Scholar]
- 21. Miura K., Taura K., Kodama Y., Schnabl B., Brenner D. A. (2008) Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology 48, 1420–1429 [DOI] [PubMed] [Google Scholar]
- 22. Mueller S., Millonig G., Waite G. N. (2009) The GOX/CAT system. A novel enzymatic method to independently control hydrogen peroxide and hypoxia in cell culture. Adv. Med. Sci. 54, 121–135 [DOI] [PubMed] [Google Scholar]
- 23. Mütze S., Hebling U., Stremmel W., Wang J., Arnhold J., Pantopoulos K., Mueller S. (2003) Myeloperoxidase-derived hypochlorous acid antagonizes the oxidative stress-mediated activation of iron regulatory protein 1. J. Biol. Chem. 278, 40542–40549 [DOI] [PubMed] [Google Scholar]
- 24. Mueller S., Pantopoulos K., Hübner C. A., Stremmel W., Hentze M. W. (2001) IRP1 activation by extracellular oxidative stress in the perfused rat liver. J. Biol. Chem. 276, 23192–23196 [DOI] [PubMed] [Google Scholar]
- 25. Mueller S. (2000) Sensitive and nonenzymatic measurement of hydrogen peroxide in biological systems. Free Radic. Biol. Med. 29, 410–415 [DOI] [PubMed] [Google Scholar]
- 26. Mueller S., Riedel H. D., Stremmel W. (1997) Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal. Biochem. 245, 55–60 [DOI] [PubMed] [Google Scholar]
- 27. Millonig G., Hegedüsch S., Becker L., Seitz H. K., Schuppan D., Mueller S. (2009) Hypoxia-inducible factor 1 α under rapid enzymatic hypoxia. Cells sense decrements of oxygen but not hypoxia per se. Free Radic. Biol. Med. 46, 182–191 [DOI] [PubMed] [Google Scholar]
- 28. Miller E. W., Tulyathan O., Isacoff E. Y., Chang C. J. (2007) Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 3, 263–267 [DOI] [PubMed] [Google Scholar]
- 29. Verga Falzacappa M. V., Casanovas G., Hentze M. W., Muckenthaler M. U. (2008) A bone morphogenetic protein (BMP)-responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J. Mol. Med. 86, 531–540 [DOI] [PubMed] [Google Scholar]
- 30. Franke K., Curth K., Lenart J., Knochenhauer D., Kietzmann T. (2004) Enhanced plasminogen activator inhibitor-1 expression in transgenic mice with hepatocyte-specific overexpression of superoxide dismutase or glutathione peroxidase. Antioxid. Redox Signal. 6, 721–728 [DOI] [PubMed] [Google Scholar]
- 31. BelAiba R. S., Djordjevic T., Bonello S., Flügel D., Hess J., Kietzmann T., Görlach A. (2004) Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol. Chem. 385, 249–257 [DOI] [PubMed] [Google Scholar]
- 32. Murray P. J. (2007) The JAK-STAT signaling pathway. Input and output integration. J. Immunol. 178, 2623–2629 [DOI] [PubMed] [Google Scholar]
- 33. Guschin D., Rogers N., Briscoe J., Witthuhn B., Watling D., Horn F., Pellegrini S., Yasukawa K., Heinrich P., Stark G. R. (1995) A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 14, 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loschen G., Flohé L., Chance B. (1971) Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett. 18, 261–264 [DOI] [PubMed] [Google Scholar]
- 35. Boveris A., Oshino R., Erecińska M., Chance B. (1971) Reduction of mitochondrial components by durohydroquinone. Biochim. Biophys. Acta 245, 1–16 [DOI] [PubMed] [Google Scholar]
- 36. Moritz R. L., Hall N. E., Connolly L. M., Simpson R. J. (2001) Determination of the disulfide structure and N-glycosylation sites of the extracellular domain of the human signal transducer gp130. J. Biol. Chem. 276, 8244–8253 [DOI] [PubMed] [Google Scholar]
- 37. Test S. T., Weiss S. J. (1984) Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J. Biol. Chem. 259, 399–405 [PubMed] [Google Scholar]
- 38. de Oliveira-Marques V., Cyrne L., Marinho H. S., Antunes F. (2007) A quantitative study of NF-κB activation by H2O2. Relevance in inflammation and synergy with TNF-α. J. Immunol. 178, 3893–3902 [DOI] [PubMed] [Google Scholar]
- 39. Nishina S., Hino K., Korenaga M., Vecchi C., Pietrangelo A., Mizukami Y., Furutani T., Sakai A., Okuda M., Hidaka I., Okita K., Sakaida I. (2008) Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology 134, 226–238 [DOI] [PubMed] [Google Scholar]
- 40. Courselaud B., Pigeon C., Inoue Y., Inoue J., Gonzalez F. J., Leroyer P., Gilot D., Boudjema K., Guguen-Guillouzo C., Brissot P., Loréal O., Ilyin G. (2002) C/EBPα regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J. Biol. Chem. 277, 41163–41170 [DOI] [PubMed] [Google Scholar]
- 41. Devary Y., Gottlieb R. A., Lau L. F., Karin M. (1991) Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol. Cell. Biol. 11, 2804–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mueller S., Pantopoulos K. (2002) Activation of iron regulatory protein-1 (IRP1) by oxidative stress. Methods Enzymol. 348, 324–337 [DOI] [PubMed] [Google Scholar]
- 43. Schieffer B., Luchtefeld M., Braun S., Hilfiker A., Hilfiker-Kleiner D., Drexler H. (2000) Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ. Res. 87, 1195–1201 [DOI] [PubMed] [Google Scholar]
- 44. Moirand R., Mortaji A. M., Loréal O., Paillard F., Brissot P., Deugnier Y. (1997) A new syndrome of liver iron overload with normal transferrin saturation. Lancet 349, 95–97 [DOI] [PubMed] [Google Scholar]
- 45. Riva A., Trombini P., Mariani R., Salvioni A., Coletti S., Bonfadini S., Paolini V., Pozzi M., Facchetti R., Bovo G., Piperno A. (2008) Revaluation of clinical and histological criteria for diagnosis of dysmetabolic iron overload syndrome. World J. Gastroenterol. 14, 4745–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Livingston J. N., Gurny P. A., Lockwood D. H. (1977) Insulin-like effects of polyamines in fat cells. Mediation by H2O2 formation. J. Biol. Chem. 252, 560–562 [PubMed] [Google Scholar]
- 47. Ikemura M., Nishikawa M., Hyoudou K., Kobayashi Y., Yamashita F., Hashida M. (2010) Improvement of insulin resistance by removal of systemic hydrogen peroxide by PEGylated catalase in obese mice. Mol. Pharm. 7, 2069–2076 [DOI] [PubMed] [Google Scholar]
- 48. Litwin J. A., Völkl A., Müller-Höcker J., Hashimoto T., Fahimi H. D. (1987) Immunocytochemical localization of peroxisomal enzymes in human liver biopsies. Am J Pathol 128, 141–150 [PMC free article] [PubMed] [Google Scholar]