Background: PKCϵ, a potential oncogene, is up-regulated in prostate cancer.
Results: PKCϵ facilitates the formation of TNFR-I complex to regulate the NF-κB pathway via a C1 domain/diacylglycerol-dependent mechanism.
Conclusion: PKCϵ is an upstream regulator of NF-κB signaling in prostate cancer.
Significance: Mechanisms identified here may reveal novel PKCϵ effectors that contribute to prostate cancer progression and highlight the potential relevance of this pathway for therapeutic purposes.
Keywords: Diacylglycerol, NF-κB, Prostate Cancer, Protein Kinase C (PKC), Transgenic Mice, Tumor Necrosis Factor (TNF)
Abstract
Protein kinase C ϵ (PKCϵ) has emerged as an oncogenic kinase and plays important roles in cell survival, mitogenesis and invasion. PKCϵ is up-regulated in most epithelial cancers, including prostate, breast, and lung cancer. Here we report that PKCϵ is an essential mediator of NF-κB activation in prostate cancer cells. A strong correlation exists between PKCϵ overexpression and NF-κB activation status in prostate cancer cells. Moreover, transgenic overexpression of PKCϵ in the mouse prostate causes preneoplastic lesions that display significant NF-κB hyperactivation. PKCϵ RNAi depletion or inhibition in prostate cancer cells diminishes NF-κB translocation to the nucleus with subsequent impairment of both activation of NF-κB transcription and induction of NF-κB responsive genes in response to the proinflammatory cytokine tumor necrosis factor α (TNFα). On the other hand, PKCϵ overexpression in normal prostate cells enhances activation of the NF-κB pathway. A mechanistic analysis revealed that TNFα activates PKCϵ via a C1 domain/diacylglycerol-dependent mechanism that involves phosphatidylcholine-phospholipase C. Moreover, PKCϵ facilitates the assembly of the TNF receptor-I signaling complex to trigger NF-κB activation. Our studies identified a molecular link between PKCϵ and NF-κB that controls key responses implicated in prostate cancer progression.
Introduction
The NF-κB family of transcription factors plays a crucial role in inflammation as well as in the development and progression of cancer. Extensive evidence indicates that the NF-κB pathway is implicated in controlling the expression of genes involved in cell survival, proliferation, angiogenesis, and invasion (1, 2). NF-κB is a dimer formed by proteins of the Rel family (RelA/p65, RelB, c-Rel, NF-κB1/p50, and NF-κB2/p52) that is retained in the cytoplasm as a complex with inhibitory IκB proteins. In the canonical pathway, external stimuli such as proinflammatory cytokines promote the dissociation of the ternary complex (mainly that composed of IκBα-p50-p65), an event triggered by phosphorylation of IκBα at Ser-32 and Ser-36 by IκBα kinase (IKK),2 followed by proteasomal degradation of IκBα. The released NF-κB dimer is subsequently translocated into the nucleus where it binds specific elements in the promoters of NF-κB-responsive genes (3). Abnormally high NF-κB activity and aberrant expression of NF-κB-regulated gene products are clinical hallmarks of chronic inflammation and have been widely linked to the cancer phenotype. Inflammation has indeed been shown to contribute to prostate cancer development via multiple mechanisms such as oxidative stress, genomic instability, and DNA damage or indirectly by increasing levels of proinflammatory factors such as tumor necrosis factor α (TNFα), which themselves affect cancer risk (4). NF-κB hyperactivation may result from enhanced production of tumor-promoting cytokines, enhanced stimulation of growth factor receptors, and/or aberrant expression/activation of upstream NF-κB kinases such as IKK and NF-κB-inducing kinase (NIK) (5). The functional association of NF-κB with oncogenic and tumor suppressor signaling networks, including the Ras/ERK, PI3K/Akt, and p53 pathways, argues for a high level of complexity in the mechanisms leading to NF-κB hyperactivation (6, 7).
Several lines of evidence strongly suggest that the NF-κB pathway is dysregulated in prostate cancer and has been implicated in the progression to the androgen-independent state that ultimately leads to patient death (8). Constitutive NF-κB activation has been reported in prostate tumors and has prognostic importance as it correlates with poor outcome in a subset of primary tumors (5). Nuclear p65 NF-κB can be observed in organ-confined prostate tumors, suggesting that constitutive NF-κB activation may be an early event in prostate cancer development (5, 9). NF-κB hyperactivation is a feature of prostate cancer cell lines lacking androgen receptor expression (such as PC3 and DU145 cells), whereas androgen-dependent cell lines (such as LNCaP and CWR22Rv1 cells) generally display low levels of basal NF-κB activity (10). NF-κB has been shown to mediate the effect of proinflammatory cytokines in prostate cancer cells, including TNFα, IFNγ, and IL-1β (11). Binding of TNFα to its receptor TNFR-I in cancer cells, including prostate cancer cells, leads to the recruitment of adaptor proteins (TRADD, TRAF2, RIP) and the formation of a signaling complex that regulates NF-κB activation and transcriptional activation of inflammatory, survival, and anti-apoptotic genes such as BCL2, BCL2L1, PTGS2 (COX2), and XIAP (3, 11–13).
There is ample evidence that PKC serine-threonine kinases are involved in the activation of NF-κB. Although many studies highlighted the relevance of the atypical PKCs ζ and ι as NF-κB modulators (14, 15), Diacylglycerol (DAG)/phorbol ester responsive PKCs also emerged as potential modifiers of NF-κB signaling (16–18). Both classical/conventional cPKCs (α, β, and γ) and novel nPKCs (δ, ϵ, η, and θ) have been implicated as regulators of apoptosis, survival, differentiation, mitogenesis, and transformation in a strict cell-type dependent manner. Studies from several laboratories, including ours, revealed that PKCδ generally acts as a negative regulator of proliferation and/or mediates apoptotic responses, whereas PKCϵ is a prosurvival and mitogenic kinase (19–22). In prostate cancer cells, activation of PKCϵ accelerates G1/S transition, mediates survival through Bad-dependent and Bad-independent mechanisms, and confers androgen independence (23–25). Most interestingly, PKCϵ emerged as a potential oncogene and cancer biomarker and it is up-regulated not only in prostate cancer but also in several other epithelial cancers including lung, breast, and thyroid cancer (19, 26, 27). PKCϵ up-regulation can be observed in > 95% of human prostate tumors and is common in advanced stages of the disease (19, 28, 29). Notably, overexpression of PKCϵ in normal immortalized RWPE-1 prostate cells to levels observed in prostate cancer cells confers growth advantage and causes ERK and Akt activation (30). Our laboratory recently demonstrated that transgenic overexpression of PKCϵ but not PKCα or PKCδ in the mouse prostate induces prostatic intraepithelial neoplasia (PIN) (30). These findings thereby suggest a crucial role of PKCϵ in prostate cancer development. However, little is known regarding the potential mechanisms underlying the effects of PKCϵ in prostate tumorigenesis.
By means of cellular and animal models, in this study we identified a key role for PKCϵ as a mediator of NF-κB signaling in prostate cancer. PKCϵ turned out to be an essential effector of TNFα and mediates constitutive activation of NF-κB in androgen-independent prostate cancer cells. PKCϵ regulates the expression of NF-κB-responsive gene products implicated in prostate cancer development and progression. Interestingly, transgenic overexpression of PKCϵ in mice conferred NF-κB hyperactivation in preneoplastic lesions, arguing for a critical role for this nPKC in NF-κB signaling.
EXPERIMENTAL PROCEDURES
Materials
TNFα was purchased from Pepro Tech (Rocky Hill, NJ). PMA was procured from LC Laboratories (Woburn, MA). The pan-PKC inhibitor GF 109302X (bisindolylmaleimide I) was obtained from BioMol (Plymouth Meeting, PA). The PKCϵ inhibitor peptide ϵV1–2 (Tat-fused) and the carrier Tat peptide were kindly provided by Dr. Daria Mochly-Rosen (Stanford University, CA). [32P]α-deoxy adenosine triphosphate (dATP) was from PerkinElmer Life Sciences (Santa Clara, CA). Fetal bovine serum was purchased from Hyclone (Logan, UT). Keratinocyte serum-free medium was purchased from Invitrogen. Other cell culture reagents and media were from the ATCC.
Cell Culture
Human prostate cancer cells (LNCaP, PC3, and DU145) cells were obtained from the ATCC and cultured in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C in a humidified 5% CO2 atmosphere. Human normal immortalized prostate epithelial RWPE-1 cells were cultured as described previously (30).
Western Blots
Western blot analysis was carried out essentially as described previously (31). Bands were visualized by the ECL Western blotting detection system. Images were captured using a Fujifilm LAS-3000 system and the LAS-2000 software. The following antibodies were used: anti-PKCϵ, anti-IκBα, anti-NF-κB p65, anti-RIP (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-phospho-IκBα (1:1000, Cell Signaling Technology Inc., Danvers, MA), anti-TRAF2 (1:1000, BD Biosciences, San Jose, CA), anti-TRADD (1:1000, EMD Millipore Corp., Billerica, MA), anti-vinculin, and anti-β-actin (1:50,000, Sigma-Aldrich, St. Louis, MO). Anti-mouse or anti-rabbit secondary antibodies conjugated to horseradish peroxidase (1:5000, Bio-Rad) were used.
Generation of PKCϵ Expression Constructs
PKCϵ was amplified by PCR from pMyr-PKCϵ-FLAG (generous gift from Dr. Alex Toker, Harvard Medical School, Boston, MA) and flanked with the 5′-XhoI and 3′-NotI restriction sites. The PCR product was cloned into XhoI and NotI sites in the pCMV/myc/cyto, pCMV/myc/nuc, pCMV/myc/mito, and pCMV/myc/ER Shooter vectors (Invitrogen).
Transfection of Mammalian Expression Vectors
RWPE-1 and LNCaP cells were transfected with various PKCϵ expression vectors or empty vector using Lipofectamine Plus (Invitrogen). Experiments were carried out 24 h after transfection.
Adenoviral Infections
Generation of the PKCϵ adenovirus (AdV) has been described previously (32). Subconfluent LNCaP or RWPE-1 cells in 6-well plates were infected with the PKCϵ AdV (multiplicity of infection = 1 pfu/cell) in RPMI 1640 supplemented with 2% FBS or in keratinocyte serum-free medium without supplements, respectively. A LacZ AdV was used as control. After 4 h, viral particles were removed, and cells were incubated for 24 h in complete medium. Expression of PKCϵ was readily detected 24 h after infection and remained stable for several days (Ref. 24) and data not shown).
Analysis of PKCϵ Translocation by Ultracentrifugation
PKCϵ translocation was determined by Western blot analysis of soluble and particulate fractions obtained by ultracentrifugation, as described previously (33). Briefly, LNCaP cells were harvested into a lysis buffer containing 20 mm Tris-HCl (pH 7.4), 5 mm EGTA, 5 μg/ml 4-(2-aminoethyl)-benzenesulfonylfluoride, 1 μg/ml pepstatin A, 5 μg/ml aprotinin, and 5 μg/ml leupeptin, and sonicated. The cytosolic (soluble) fraction was obtained by collection of the supernatant after centrifugation of the cell lysate (1 h at 100,000 × g at 4 °C), and the remaining pellet represented the particulate fraction.
RNAi
We used two different RNAi sequences in each case. For transient depletion of PKCϵ we used ON-TARGET Plus RNAi duplexes purchased from Dharmacon (Lafayette, CO). The following target sequences were used: PKCϵ RNAi #1, CAGAGGAGAUUAAGACUAU (catalog no. J-004653-06); PKCϵ RNAi #2, GUAAUGAGUCGUCUUUCUA (catalog no. J-004653-08). Control Negative Silencer® siRNA was from Ambion (Austin, TX). Cells were transfected with different siRNAs (120 pmol) using the Amaxa nucleofector (Amaxa Biosystems, Gaithersburg, MD) and 24–48 h later were used for the indicated experiments.
Luciferase Reporter Assays
Cells in 12-well plates (7 × 104 cells/well) were transfected with 0.5 μg of a NF-κB firefly luciferase reporter (kind gift from Dr. Dave Manning, University of Pennsylvania) vector using Lipofectamine Plus. The Renilla luciferase expression vector pRL-TK (50 ng, Promega, Madison, WI) was cotransfected for normalization of transfection efficiency. After 48 h, cells were stimulated with TNFα or vehicle and lysed 6 h later with passive lysis buffer (Promega). Luciferase activity was determined in cell extracts using the dual-luciferase reporter assay system (Promega). Results were expressed as the ratio between firefly and Renilla luciferase activities.
Preparation of Nuclear Extracts and EMSA
Nuclear and cytosolic fractions were obtained after cell lysis using the NE-PER nuclear protein extraction kit (Pierce Biotechnology Inc., Rockford, IL). NF-κB DNA-binding in nuclear extracts was determined by EMSA as described previously (34). Briefly, the NF-κB oligonucleotide probe (5′-agcttGAGGGGATTCCCTTA-3′) was labeled with [α-32P]deoxyadenosine triphosphate using Klenow enzyme and purified on a Sephadex G-25 column. The binding reaction was carried out at 25 °C for 10 min with or without 5 μg of nuclear proteins, 1 μg of poly(dI-dC), and 106 cpm of labeled probe in a final volume of 20 μl of binding buffer (10× buffer) (100 mm Tris-HCl (pH 7.5), 500 mm NaCl, 50 mm MgCl2, 100 mm EDTA, 10 mm DTT, 1% Triton X-100, and 50% glycerol). Binding specificity was confirmed by cold competition. Decreased signal intensity was observed with 25- and 50-fold molar excess of cold NF-κB probe, whereas the signal did not diminish with a cold AP-1 probe (Ref. 35 and data not shown). DNA-protein complexes were separated on a 6% non-denaturing polyacrylamide gel at 200 V. The gel was fixed and dried, and DNA-protein complexes were visualized by autoradiography.
RNA Isolation and cDNA Synthesis
Subconfluent LNCaP cells were treated with either TNFα (10 ng/ml) or vehicle, and 6 h later RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). 2 μg of RNA/sample were reverse-transcribed using random hexamers as primers and the Taqman reverse transcription reagent kit (Applied Biosystems, Branchburg, NJ).
Real-time PCR
PCR primers and fluorogenic probes for COX2, VEGF, MMP9, and IL6 were purchased from Applied Biosystems. The probes were 5′ end-labeled with 6-carboxyfluorescein. PCR amplifications were performed using an ABI PRISM 7700 detection system in a total volume of 12.5 μl containing Taqman Universal PCR MasterMix (Applied Biosystems), commercial target primers (300 nm), the fluorescent probe (200 nm), and 1 μl of cDNA. PCR product formation was continuously monitored using the Sequence detection system software version 1.7 (Applied Biosystems). The 6-carboxyfluorescein signal was normalized to endogenous 18 S.
Site-directed Mutagenesis
Mutagenesis was carried out using the Quick Change II site-directed mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA). For introducing a Cys-to-Ala mutation in position 204 in the C1a region of pEGFP-PKCϵ (31), we used the following primers (mismatch mutations are underlined): 5′-CAGGGATATCAATGTCAAGTTGCCACTTGCGTTGTCCACAAGCG-3′ (forward) and 5′-CGCTTGTGGACAACGCAAGTGGCAACTTGACATTGATATCCCTG-3′ (reverse). The resulting mutant in pEGFP was then used as a template to mutate Cys to Ala in position 276 in the PKCϵ C1b domain using the following primers: 5′-CAGGGCTTGCAGTGTAAAGTCGCCAAAATGAATGTTCACCGGCG-3′ (forward) and 5′-CGCCGGTGAACATTCATTTTGGCGACTTTACACTGCAAGCCCTG-3′ (reverse).
Immunofluorescence and Confocal Microscopy
For NF-κB nuclear translocation studies, LNCaP cells were plated on coverslides in 12-well plates and 48 h later stimulated with either TNFα (10 ng/ml) or vehicle. At the indicated times, cells were washed twice with PBS, fixed for 10 min with precooled methanol, washed three times for 5 min with PBS, and permeabilized for 15 min with 0.5% Triton X-100 in PBS followed by a 10-min incubation in 100 mm glycine in PBS. After a blocking incubation with goat serum in PBS for 30 min, sections were incubated with a rabbit anti-NF-κB p65 antibody (1:250) overnight. The following day, sections were washed twice for 5 min with PBS and incubated with a CY3-conjugated anti-rabbit antibody (1:2000, Jackson ImmunoResearch Laboratories, Inc.) for 1 h. After additional washings, DNA was stained using DAPI (0.1 μg/ml, 10 min). Coverslides were washed three times with PBS, mounted with Vectashield, and visualized by confocal microscopy.
For live cell imaging, cells were transfected with pEGFP plasmids using the Amaxa nucleofector, grown in glass-bottomed culture dishes (MatTek, Ashland, MA), and cultured for 24 h before being subjected to various treatments. Living cells were visualized with a confocal laser scanning fluorescence microscope (LSM 410 or 510, Carl Zeiss).
Coimmunoprecipitation Assays
LNCaP cells (4 × 106) were stimulated for 10 min with either TNFα (10 ng/ml) or vehicle and lysed in ice-cold immunoprecipitation (IP) buffer (10 mm HEPES (pH 7.4), 150 mm NaCl, 5 mm EDTA, 1 mm sodium orthovanadate, 2 mm phenylmethylsulfonyl fluoride, 0.2% Nonidet P-40, 5 mg/ml BSA, and protease inhibitor mixture). Lysates were vortexed in IP buffer for 20 min at 4 °C to solubilize the membrane fraction and centrifuged, and the cleared extracts were incubated with 1 μg of mouse monoclonal antibody to p55 TNFR-I (Upstate Biotechnology, Billerica, MA) overnight at 4 °C with gentle rocking. After addition of anti-mouse IgG-agarose beads (Sigma), the complexes were incubated for 1 h at 4 °C. Immunoprecipitated proteins were washed three times with cold IP buffer. The samples were eluted by incubation at 65 °C for 15 min in 2× SDS-PAGE sample buffer, resolved through SDS-PAGE, and subsequently immunoblotted with PKCϵ, RIP, TRAF2, or TRADD antibodies. For reverse coimmunoprecipitation, cell lysates were immunoprecipitated with 1 μg of anti-rabbit PKCϵ (Santa Cruz Biotechnology, Inc.). The samples were then analyzed by immunoblotting with an anti-TNFR-I antibody.
Generation of Prostate PKCϵ Transgenic Mice and Animal Care
The generation and phenotypic characterization of probasin (PB)-driven PKCϵ transgenic mice are described elsewhere (30). The standard nomenclature for this line is FVB/N-Tg (Pbsn-Prkce) and is referred to as PB-PKCϵ. FVB/N inbred mice (used in maintenance of transgenic lines) were acquired from Charles River Laboratories, Inc. (strain code 207). Mice were housed in individually ventilated cages on autoclaved hardwood bedding in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility at the MD Anderson Cancer Center, Science Park, Smithville, TX. All procedures were performed in compliance with the Public Health Service Guide for the Care and Use of Laboratory Animals, 8th edition, 2010.
Immunohistochemistry
Immunohistochemical analysis was carried on 12-month-old homozygous Tg/Tg males on a pure FVB/N background and WT FVB/N males. Complete necropsy, macroscopic examination, tissue collection, and processing were carried out as described previously (30). Briefly, mice were sacrificed with CO2, and their entire genitourinary tract was removed. Prostates were submitted “en bloc” and processed in standard formalin-fixed, paraffin-embedded sections. For staining we used anti-phospho NF-κB p65 antibody (1:100, Cell Signaling Technology, Inc.) and Envision plus labeled polymer anti-rabbit-HRP (Dako, Carpinteria, CA), followed by incubation with Dako 3,3′-diaminobenzidine (DAB) substrate.
Statistical Analysis
Results were compared by analysis of variance using GraphPad Prism 5.0. In all cases, p < 0.05 was considered statistically significant.
RESULTS
Prostate-specific Expression of PKCϵ Correlates with NF-κB Hyperactivation
Extensive evidence suggests a key role for PKCϵ in mitogenic, survival, and oncogenic signaling (27). PKCϵ is distinctively up-regulated in human prostate cancer and mediates anti-apoptotic responses in prostate cancer cell lines in culture (28, 29). As the NF-κB pathway also plays a prominent role in human prostate cancer development, we decided to investigate a potential association between these two pathways. The PC3 and DU145 androgen-independent prostate cancer cell lines display elevated PKCϵ levels relative to a normal immortalized prostate epithelial cell line (RWPE-1) or androgen-dependent LNCaP prostate cancer cells (Fig. 1A), as reported previously (30). A clear translocation of NF-κB from the cytosol to the nuclear fraction could be seen in PC3 and DU145 prostate cancer cell lines, indicating the constitutive activation of NF-κB in these cells, as reported (10). Notably, there is a strong correlation between PKCϵ levels and nuclear NF-κB expression (Fig. 1A) as well as with NF-κB DNA-binding activity (as determined by EMSA) (Fig. 1B) across different prostate cells. These results argue for a potential relationship between PKCϵ and NF-κB activation status in prostate cancer cells.
FIGURE 1.
Correlation between PKCϵ expression and NF-κB activity in prostate cell lines. A, PKCϵ and NF-κB p65 expression in total cell lysates and cytosolic and nuclear extracts were determined by Western blot analysis. Vinculin and ATF2 were used as loading controls for each fraction. B, NF-κB DNA-binding activity, as determined by EMSA, across different prostate cells. F, free probe. Similar results were observed in three independent experiments. Relative optical density is indicated underneath each lane. C, phospho-NF-κB p65 immunohistochemical staining in prostates from 12 month-old PB-PKCϵ or wild-type (FVB/N) mice. Representative figures are shown.
To determine whether an association between PKCϵ and NF-κB occurs in an in vivo setting, we took advantage of a mouse transgenic model that we recently developed in which prostate-specific expression of PKCϵ is driven by the PB promoter. PB-PKCϵ mice develop a preneoplastic phenotype characterized by hyperplasia and PIN, with a penetrance of 100% at 12 months (30). Interestingly, preneoplastic lesions in PB-PKCϵ mice display a characteristic nuclear NF-κB staining. This effect was particularly prominent in PIN lesions relative to normal areas or regions with mild hyperplasia and could not be detected in prostates from age-matched WT control FVB/N male mice (Fig. 1C).
PKCϵ Mediates NF-κB Activation in Prostate Cancer Cells
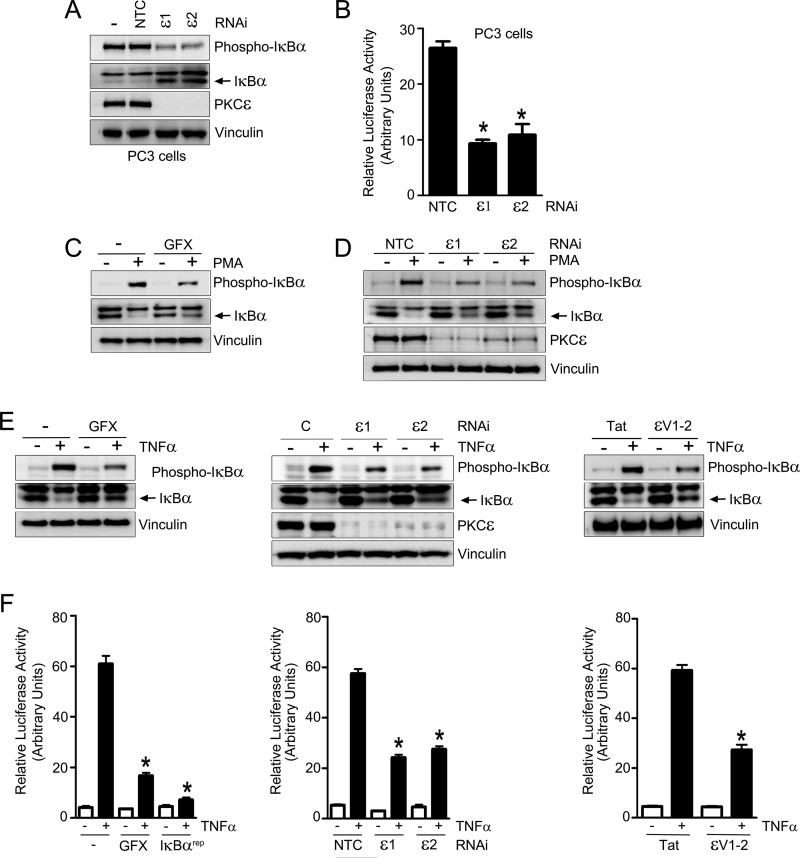
First, we examined whether NF-κB hyperactivation in androgen-independent PC3 cells is mediated by PKCϵ. When we silenced PKCϵ in PC3 cells using two different RNAi duplexes, we noticed a significant reduction in phospho-IκBα levels. Moreover, total IκBα levels in PKCϵ-depleted cells were higher than in parental PC3 cells or cells subjected to control RNAi (Fig. 2A). To further assess the role of PKCϵ in the maintenance of NF-κB hyperactivation, we took advantage of a NF-κB luciferase reporter. As shown in Fig. 2B, silencing of PKCϵ from PC3 cells led to a pronounced inhibition of NF-κB luciferase activity. Similar results were observed in androgen-independent DU145 cells (supplemental Fig. S1). Therefore, PKCϵ mediates NF-κB constitutive activation in androgen-independent prostate cancer cells.
FIGURE 2.
PKCϵ mediates constitutive and PMA- or TNFα-induced NF-κB activation in prostate cancer cells. A, PC3 cells were transfected with ϵ1, ϵ2, or NTC. PKCϵ, total IκBα, and phospho-IκBα levels were determined by Western blot analysis 48 h later. B, PC3 cells were transfected with ϵ1, ϵ2, or NTC. After 24 h, cells were cotransfected with NF-κB firefly luciferase reporter and pTK-Renilla plasmids. Luciferase activity was determined 24 h later. The firefly/Renilla ratio was calculated, and results were expressed as mean ± S.E. (n = 3). *, p < 0.05 versus control. C, effect of the pan-PKC inhibitor GF1090203X (GFX, 5 μm, 1 h) on IκBα phosphorylation after treatment with either PMA (100 nm, 30 min) or vehicle. D, LNCaP cells were transfected with two different PKCϵ RNAi duplexes (ϵ1 or ϵ2) or a non-target control RNAi duplex (NTC) and, 48 h later, treated with vehicle or PMA (100 nm, 30 min). Total and phosphorylated IκBα were determined by Western blot analysis. E, phosphorylated and total IκBα levels were determined by Western blot analysis in LNCaP cells after TNFα (10 ng/ml, 30 min) or vehicle treatment. Left panel, effect of GF 1090203X (GFX, 5 μm, 1 h). Center panel, effect of RNAi duplexes ϵ1, ϵ2,or NTC transfected 48 h before TNFα treatment. Right panel, effect of the PKCϵ inhibitor ϵV1–2 or Tat carrier (1 μm, 1 h). F, LNCaP cells were cotransfected with NF-κB firefly luciferase reporter and pTK-Renilla plasmids and, 24 h later, stimulated with TNFα (10 ng/ml) or vehicle. Luciferase activity was determined 6 h after TNFα stimulation. The firefly/Renilla ratio was calculated, and results were expressed as mean ± S.E. (n = 3). *, p < 0.05 versus control. Left panel, effect of GF 1090203X (GFX, 5 μm, 1 h) or the IκBα super-repressor (IκBαrep) that was transfected 24 h before. Center panel, effect of RNAi duplexes ϵ1, ϵ2, or NTC, transfected 48 h before TNFα or vehicle treatment. Right panel, effect of the PKCϵ inhibitor ϵV1–2 or Tat carrier (1 μm).
We then examined whether PKCϵ plays a role in the activation of the NF-κB pathway in prostate cancer cells. Phorbol 12-myristate 13-myristate (PMA), a prototypical PKC activator, is a known activator of NF-κB responses (11). As expected, treatment of LNCaP cells with PMA (100 nm) induced a time-dependent phosphorylation of IκBα, a well established readout of NF-κB activation, with concomitant degradation of IκBα (supplemental Fig. S2). Both PMA-induced IκBα phosphorylation and degradation were sensitive to the “pan” PKC inhibitor GF 1090203X (Fig. 2C), suggesting that it is mediated by PKC and not by other phorbol ester receptors unrelated to PKC that have been described in prostate cancer cells (36–38). To determine whether PKCϵ mediates PMA-induced NF-κB activation in prostate cancer cells, LNCaP cells were subjected to PKCϵ RNAi using two different duplexes. A scrambled RNAi duplex was used as control. PKCϵ depletion of > 80% was achieved with either PKCϵ duplex (Fig. 2D) and did not affect the expression of other PKCs (see Refs. 24, 39). IκBα phosphorylation by PMA was markedly reduced in PKCϵ-depleted cells. Moreover, PKCϵ RNAi rescued the degradation of IκBα in response to PMA (Fig. 2D).
PKCϵ Is Required for TNFα-induced Activation of NF-κB in LNCaP Cells
Inflammation is an important factor in the development of prostate cancer. NF-κB is an established mediator of responses by inflammatory cytokines such as TNFα, IFNγ, and IL-1β, including in prostate cancer cells (11). Stimulation of LNCaP cells with TNFα (10 ng/ml) led to a time-dependent elevation in phospho-IκBα levels with a concomitant decrease in total IκBα levels (supplemental Fig. S3). TNFα-induced phosphorylation of IκBα as well as the subsequent degradation of total IκBα were inhibited by GF 1090203X (Fig. 2E, left panel), indicating that it is PKC-mediated. Furthermore, silencing PKCϵ with two different RNAi duplexes significantly attenuated TNFα-induced IκBα phosphorylation and IκBα degradation (Fig. 2E, center panel). To further establish the requirement of PKCϵ in this response, we used a pharmacological approach. The cell-permeable, Tat-fused peptide ϵV1–2 selectively blocks the translocation and activation of PKCϵ but not of other PKCs (40). As shown in Fig. 2E (right panel), ϵV1–2 significantly inhibited TNFα-induced IκBα phosphorylation compared with the Tat control peptide.
As a complementary approach to determine the involvement of PKCϵ in TNFα-induced activation of NF-κB, we used a luciferase reporter assay. Upon transfection of a NF-κB luciferase reporter plasmid into LNCaP cells, a significant activation of luciferase activity was observed in response to TNFα, which was maximal at 6 h (data not shown) and was essentially blunted by expression of the IκBα super-repressor (IκBαrep). Pretreatment with the PKC inhibitor GF 1090203X impaired TNFα-induced activation of NF-κB luciferase reporter activity (Fig. 2F, left panel). Moreover, RNAi silencing of PKCϵ (Fig. 2F, center panel) or pretreatment with the PKCϵ inhibitor ϵV1–2 (right panel) caused a marked reduction in NF-κB luciferase reporter activity induced by TNFα. Neither PKCα nor PKCδ RNAi depletion reduced NF-κB luciferase activity in response to TNFα (supplemental Fig. S4). From these results we concluded that PKCϵ is required for the activation of NF-κB by both PMA and TNFα in prostate cancer cells.
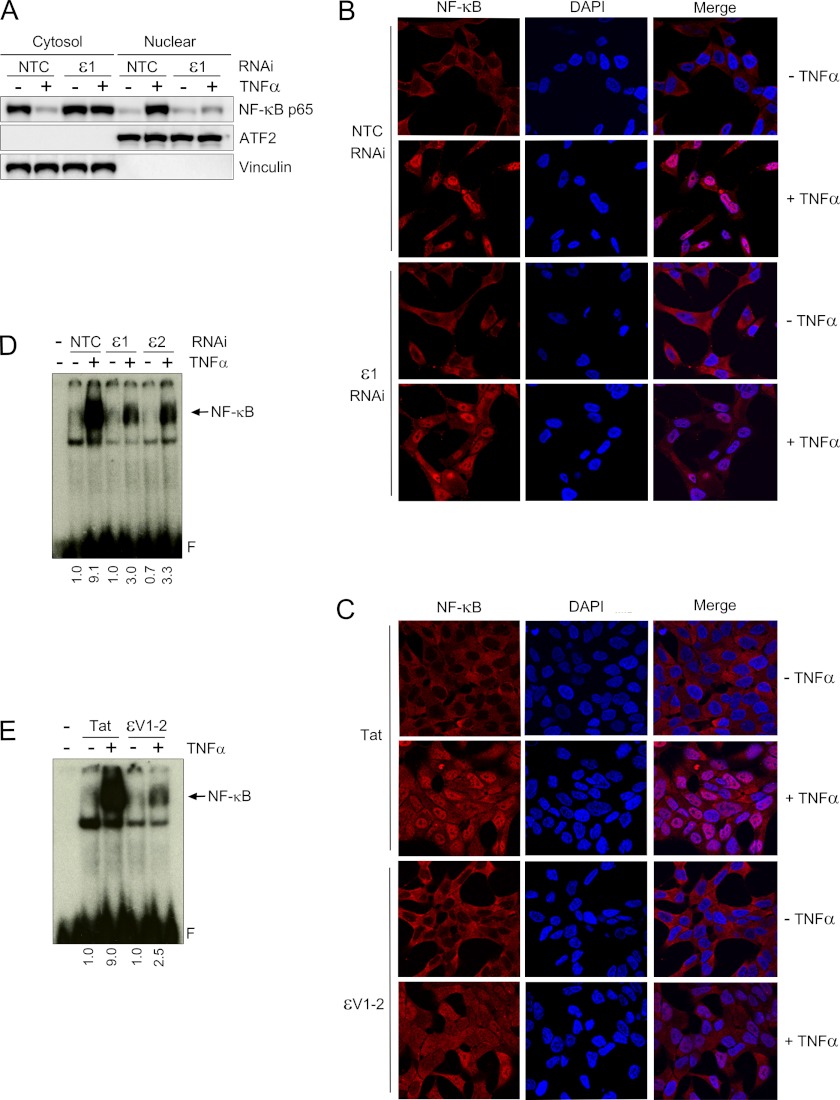
PKCϵ Is Required for Nuclear Translocation of NF-κB and Activation of NF-κB DNA-binding Activity
Upon activation, NF-κB p65 dissociates from phosphorylated IκB and translocates to the nucleus where it binds to specific elements in NF-κB-responsive gene promoters (2, 3). The effect of PKCϵ depletion on NF-κB nuclear translocation was examined using a fractionation assay. In LNCaP cells, TNFα promotes the translocation of NF-κB to the nucleus, as judged by the disappearance of NF-κB from the cytosolic fraction and the concomitant elevation of NF-κB in the nuclear fraction. This effect was reduced in PKCϵ depleted cells (Fig. 3A). Similar conclusions were obtained using immunocytochemistry. Indeed, as shown in Fig. 3B, nuclear relocalization of NF-κB can be readily observed in response to TNFα treatment. However, this effect was barely detected in PKCϵ-depleted LNCaP cells. Similar results were observed with PMA (data not shown). Moreover, NF-κB nuclear translocation was significantly lower in LNCaP cells treated with the ϵV1–2 peptide inhibitor compared with the control Tat carrier peptide (Fig. 3C).
FIGURE 3.
PKCϵ mediates TNFα-induced NF-κB activation in LNCaP cells. A, LNCaP cells were transfected with either PKCϵ (ϵ1) or non-target control (NTC) RNAi duplexes and, 48 h later, stimulated with either TNFα (10 ng/ml) or vehicle for 30 min. Western blot analysis for p65 NF-κB was carried out in cytosolic and nuclear fractions using vinculin and ATF2 as loading controls for each fraction. B, nuclear translocation of NF-κB was assessed by immunocytochemistry in LNCaP cells treated with either TNFα (10 ng/ml) or vehicle for 30 min. Experiments were carried out 48 h after transfection with either ϵ1 or NTC RNAi duplexes. Nuclei were stained with DAPI. Cells were visualized by confocal microscopy. Similar results were obtained in at least three independent experiments. C, similar experiments as those in B were carried out in LNCaP cells treated with either ϵV1–2 or Tat peptides (1 μm). D, NF-κB-DNA binding was assessed by EMSA in nuclear extracts prepared 30 min after TNFα or vehicle treatment. Experiments were carried out 48 h after transfection with either ϵ1 or NTC RNAi duplexes. Relative optical density is indicated underneath each lane. E, similar experiments as those in D were carried out in LNCaP cells treated with either ϵV1–2 or Tat peptides (1 μm, 1 h). In all cases, similar results were obtained in at least three independent experiments. Relative optical density is indicated underneath each lane.
To determine whether PKCϵ was implicated in the activation of NF-κB DNA-binding activity by TNFα, we used an EMSA approach. TNFα caused a pronounced activation of NF-κB DNA-binding activity in nuclear extracts of LNCaP cells. NF-κB DNA-binding activity was reduced upon treatment with GF 109203X (data not shown). Most notably, PKCϵ-depleted LNCaP cells show defective activation of NF-κB DNA binding activity in nuclear extracts (Fig. 3D). A similar effect was observed by treatment with ϵV1–2 (Fig. 3E). Overall, these results strongly advocate a role of PKCϵ as a mediator of TNFα-induced NF-κB activation in prostate cancer cells.
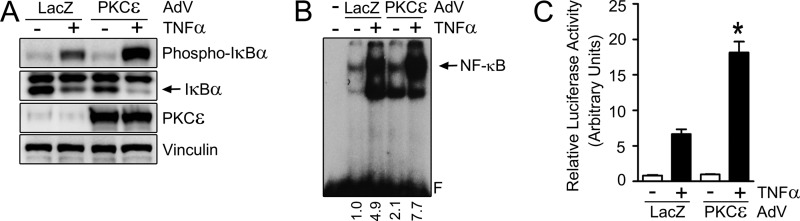
PKCϵ Overexpression in Normal Prostate Epithelial Cells Promotes NF-κB Activation
“Normal” immortalized prostate RWPE-1 epithelial cells express very low PKCϵ levels relative to prostate cancer cells (30) and have low basal NF-κB activity (see Fig. 1). PKCϵ was overexpressed in RWPE-1 cells using an adenoviral approach. A LacZ AdV was used as a control. Notably, ectopic overexpression of PKCϵ potentiates TNFα-induced phosphorylation of IκBα and enhances IκBα degradation in RWPE-1 cells (Fig. 4A). PKCϵ overexpression in RWPE-1 cells also augmented TNFα-induced NF-κB DNA-binding activity (Fig. 4B), NF-κB luciferase reporter activity (C), and NF-κB nuclear translocation (supplemental Fig. S5). Similar results were observed upon PKCϵ overexpression in LNCaP cells (supplemental Fig. S6).
FIGURE 4.
Overexpression of PKCϵ in RWPE-1 prostate epithelial cells potentiates NF-κB activation. RWPE-1 cells were infected with either PKCϵ or control (LacZ) AdVs (multiplicity of infection = 1 pfu/cell) and 24 h later treated with either TNFα (10 ng/ml) or vehicle. A, phosphorylated and total IκBα levels, as determined by Western blot analysis 30 min after TNFα stimulation. B, nuclear extracts of RWPE-1 cells were prepared 30 min after TNFα stimulation, and NF-κB binding activity was assayed by EMSA. Relative optical density is indicated underneath each lane. C, RWPE-1 cells were cotransfected with NF-κB firefly luciferase reporter and pTK-Renilla plasmids and, 24 h later, stimulated with TNFα (10 ng/ml) or vehicle. Luciferase activity was determined 6 h after TNFα stimulation. The firefly/Renilla ratio was calculated, and results were expressed as mean ± S.E. (n = 3). *, p < 0.05 versus control.
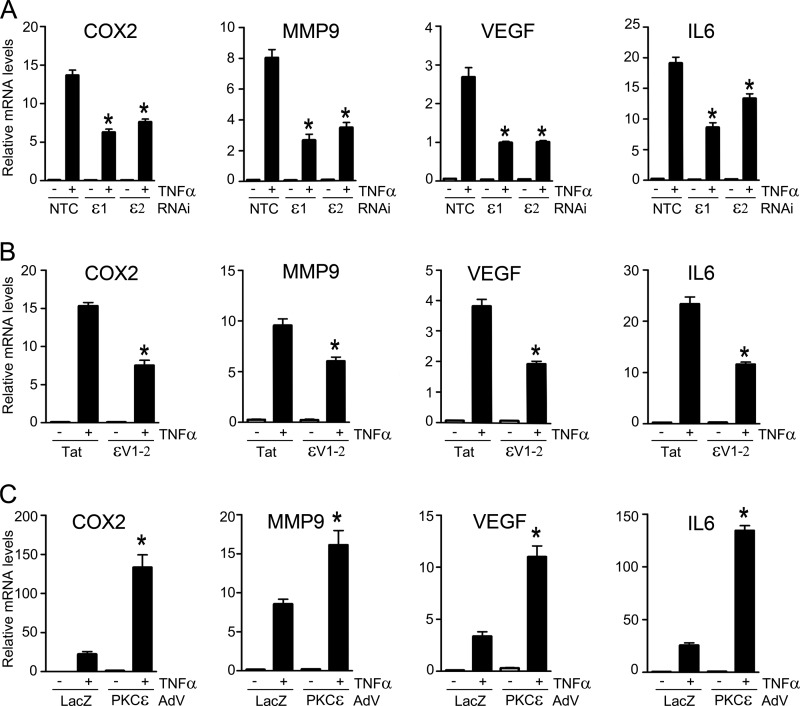
PKCϵ Controls the Expression of NF-κB-regulated Genes
NF-κB regulates the expression of genes implicated in survival, proliferation, metastasis, and invasion (3, 11). To establish the biological significance of the regulation of NF-κB transcriptional activity by PKCϵ, we examined the induction of NF-κB-responsive genes using quantitative PCR. Treatment of LNCaP cells with TNFα caused a marked elevation in COX2, VEGF, MMP9, and IL6 mRNA levels. When we carried out similar experiments in PKCϵ-depleted LNCaP cells, the induction of all these NF-κB-responsive genes was significantly diminished (Fig. 5A). A similar reduction was observed upon treatment with the ϵV1–2 peptide inhibitor (Fig. 5B). Conversely to PKCϵ silencing/inhibition, adenoviral overexpression of PKCϵ in LNCaP cells potentiated the induction of COX2, VEGF, MMP9, and IL6 mRNA levels by TNFα (Fig. 5C). Therefore, PKCϵ mediates the induction of NF-κB-responsive genes by this inflammatory cytokine.
FIGURE 5.
PKCϵ mediates the induction of NF-κB-responsive genes. LNCaP cells were treated with either TNFα (10 ng/ml) or vehicle, and RNA was isolated 6 h later for qPCR analysis of COX2, VEGF, MMP9, and IL6. A, effect of RNAi duplexes ϵ1, ϵ2, or NTC transfected 48 h before TNFα treatment. B, effect of the PKCϵ inhibitor ϵV1–2 or Tat carrier (1 μm). C, effect of infection with either PKCϵ or control (LacZ) AdVs (multiplicity of infection = 1 pfu/cell) carried out 24 h before treatment. Results are shown as fold induction over vehicle-treated cells and expressed as mean ± S.E. (n = 3). *, p < 0.05 versus control.
PKCϵ Translocation to the Plasma Membrane Is a Requirement for the Activation of NF-κB by TNFα
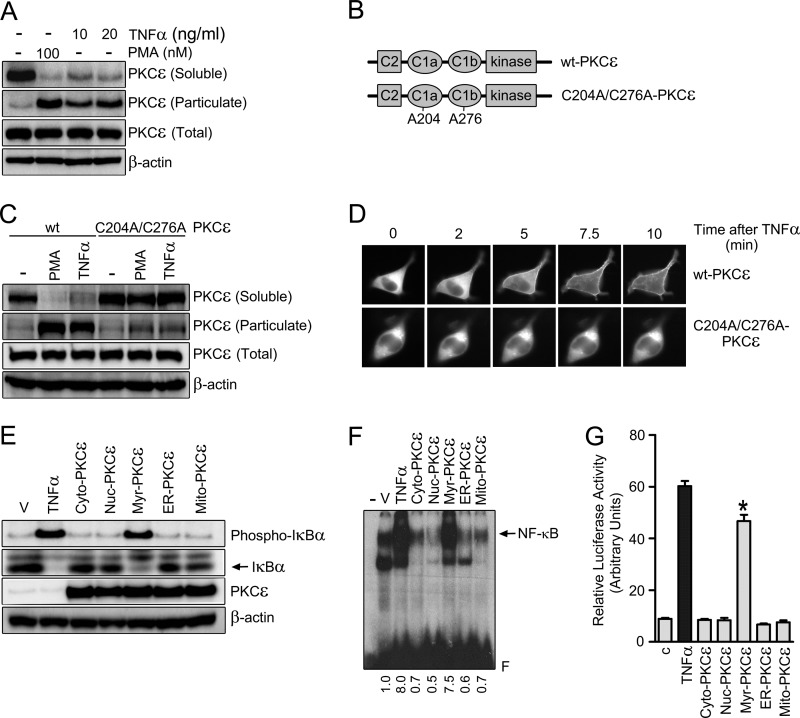
PKC translocation to membranes is a hallmark of enzyme activation. PKC isozymes can distinctly relocalize to multiple intracellular compartments, ultimately leading to a differential access to substrates (41). We raised the question whether TNFα could induce changes in the subcellular localization of PKCϵ in LNCaP cells. In the first set of experiments, we examined the redistribution of PKCϵ using an ultracentrifugation approach (42). Fig. 6A shows that TNFα treatment caused the disappearance of PKCϵ immunoreactivity from the cytosolic (soluble) fraction with a concomitant increase in the particulate fraction, as well established for PMA (31, 34).
FIGURE 6.
PKCϵ translocation to the plasma membrane is required for TNFα-induced activation of NF-κB. A, LNCaP cells were treated for 30 min with either TNFα or PMA at the indicated concentrations and subject to fractionation into soluble and particulate fractions. Endogenous PKCϵ levels in each fraction were determined by Western blot analysis. B, schematic representation of WT and the C1a/C1b Cys-to-Ala PKCϵ mutant. C, LNCaP cells were transfected with pEGFP vectors encoding either the WT or C1 domain PKCϵ mutant. After 24 h, cells were treated with TNFα (20 ng/ml), PMA (100 nm), or vehicle for 30 min and subject to fractionation. GFP-PKCϵ levels in each fraction were determined by Western blot analysis using an anti-PKCϵ antibody. D, GFP-PKCϵ (WT or C1 domain mutant) localization in LNCaP cells was determined in response to TNFα (50 ng/ml) by real-time microscopy. E, LNCaP cells were transfected with different PKCϵ targeting expression vectors. After 24 h, cell extracts were prepared and subject to Western blot analysis for phosphorylated and total IκBα. F, NF-κB-DNA binding by EMSA was measured in nuclear cell extracts 24 h after transfection with the different constructs. V, vehicle. G, NF-κB luciferase reporter activity was measured 24 h after transfection of the different PKCϵ constructs. In E, F, and G, TNFα (10 ng/ml) was used as a positive control. Results are expressed as mean ± S.E. (n = 3). *, p < 0.05 versus control. In all cases, three independent experiments gave the similar results.
Translocation of PKCϵ is mediated by binding of the ligand (either phorbol ester or DAG generated by stimulation of membrane receptors) to their C1a and C1b domains located in the regulatory N-terminal region. Cys residues in C1 domains of PKCs are essential for folding and thus required for proper ligand binding and enzyme translocation (41, 43). A PKCϵ C1a-C1b domain double mutant was generated (C204A/C276A-PKCϵ) (Fig. 6B) and expressed in LNCaP cells. Unlike WT-GFP-PKCϵ, the C1 domain PKCϵ mutant failed to translocate from the cytosolic to the particulate fraction in response to PMA or TNFα (Fig. 6C). These findings were further corroborated by real-time microscopy using GFP-fused constructs. As shown in Fig. 6D, a time-dependent translocation of WT-PKCϵ to the cell periphery was readily detected 5 min after TNFα treatment. On the other hand, the C1 domain PKCϵ mutant failed to translocate in response to TNFα (Fig. 6D), even at longer times (data not shown). The C1 domain requirement for translocation by TNFα argues for the involvement of DAG in the activation of PKCϵ in response to the cytokine.
To authenticate the requirement of PKCϵ peripheral translocation in NF-κB activation by TNFα, a number of constructs to target PKCϵ to specific intracellular compartments were generated. PKCϵ was fused at the N terminus to different tags that direct the kinase to plasma membrane, nucleus, cytoplasm, endoplasmic reticulum or mitochondria (31). Remarkably, at similar levels of expression, only myr-PKCϵ, which is targeted to the plasma membrane (31), was capable of inducing IκBα phosphorylation and degradation, similar to TNFα in non-transfected cells (Fig. 6E). Likewise, only myr-PKCϵ activated NF-κB binding activity (Fig. 6F) and induced NF-κB luciferase reporter activity when expressed in LNCaP cells (G), an indication that targeting PKCϵ to the plasma membrane, is sufficient to promote NF-κB activation. Together with the translocation experiments, our data strongly argue that translocation of PKCϵ to the plasma membrane is required for the activation of NF-κB by TNFα.
PKCϵ Activation by TNFα Is Mediated by PC-PLC
G-protein-coupled receptors activate PKCs through the production of DAG via phosphatidylinositol (PI)- or phosphatidylcholine (PC)-specific PLCs (44, 45). To determine which mechanism(s) mediate(s) TNFα-induced activation of PKCϵ in prostate cancer cells, we used the PI-specific PLC inhibitor U73122 and the PC-specific PLC inhibitor D609. TNFα-mediated PKCϵ membrane translocation in LNCaP cells was significantly attenuated by D609 but not by U73122 (Fig. 7A), suggesting the involvement of PC-PLC. This conclusion was confirmed using real-time microscopy, which revealed that translocation of GFP-PKCϵ to the plasma membrane is sensitive to D609 but not affected by U73122 (Fig. 7B). These results also authenticate the involvement of DAG in the activation of PKCϵ by TNFα.
FIGURE 7.
TNFα activates PKCϵ and NF-κB via PC-PLC. LNCaP cells were incubated for 30 min with either the PI-PLC inhibitor U73122 (30 μm) or the PC-PLC inhibitor D609 (50 μm) and then stimulated with TNFα (10 ng/ml) or vehicle. A, cytosolic and particulate fractions were prepared by ultracentrifugation 30 min after TNFα treatment. Endogenous PKCϵ levels were determined in each fraction by Western blot analysis. B, effect of PI-PLC and PC-PLC inhibitors on GFP-PKCϵ membrane translocation by TNFα (50 ng/ml), as determined by real-time microscopy. C, NF-κB DNA binding activity was evaluated in nuclear extracts by EMSA 30 min after stimulation with TNFα. Relative optical density is indicated underneath each lane. NC, negative control, no protein added. D, LNCaP cells were infected with either PKCϵ or control (LacZ) AdVs (multiplicity of infection = 1 pfu/cell) and, 24 h later, treated with either TNFα (10 ng/ml) or vehicle for 30 min. Phosphorylated and total IκBα levels were determined by Western blot analysis. In all cases, similar results were observed at least in three independent experiments.
Next, we wished to determine whether PC-PLC was required for NF-κB activation by TNFα. As shown in Fig. 7C, activation of NF-κB binding activity by TNFα can be essentially blunted by pretreatment with D609 but not by U73122. Moreover, both the induction of IκB phosphorylation by TNFα and its potentiation by PKCϵ overexpression (using a PKCϵ AdV) were impaired by D609 but not by U73122 (Fig. 7D). Therefore, PC-PLC mediates the activation of the PKCϵ-NF-κB axis by TNFα in prostate cancer cells.
PKCϵ Is Implicated in the Formation of the TNFR-I Complex
The mobilization of PKCϵ to the plasma membrane upon TNFα stimulation led us to speculate on its potential association with TNFR-I, the receptor for TNFα. Interestingly, when we immunoprecipitated TNFR-I from LNCaP cells, we observed that endogenous PKCϵ coimmunoprecipitates with this receptor (Fig. 8A). The association was enhanced by treatment with TNFα. A reverse coimmunoprecipitation assay using an anti-PKCϵ antibody led to a similar conclusion (Fig. 8B).
FIGURE 8.
PKCϵ is required for the formation of the TNFR-I receptor complex. LNCaP cells were treated with TNFα (10 ng/ml) and subject to IP with either an anti-TNFR-I antibody (A) or an anti-PKCϵ antibody (B). Total and immunoprecipitated TNFR-I and PKCϵ levels were determined by Western blot analysis. IgG was used as a control for the immunoprecipitation. Similar results were observed in at least three independent experiments. C, levels of adaptor proteins RIP, TRAF2, and TRADD were determined in TNFR-I immunoprecipitates from LNCaP cells transfected 48 h earlier with either PKCϵ (ϵ1) or non-target control (NTC) RNAi duplexes. Densitometric analysis of three independent experiments is also shown. p < 0.05 versus NTC.
TNFR-I recruits adaptor proteins TRADD, TRAF2 and RIP upon stimulation to form a signaling complex that promotes the activation of NF-κB (12, 46). To determine whether PKCϵ modulates the formation of the complex we examined the association of adaptor proteins to TNFR-I by coimmunoprecipitation. These experiments revealed that in PKCϵ-depleted LNCaP cells the association of the adaptors with the receptor is markedly diminished. Specifically, the association of RIP, TRAF2, and TRADD with TNFR-I was reduced by 41%, 56%, and 39%, respectively, in PKCϵ-depleted cells compared with control cells (Fig. 8C). These results thus suggest a role for PKCϵ in the formation of the TNFR-I complex.
DISCUSSION
NF-κB is an inducible transcription factor activated by a wide array of stimuli, including cytokines, bacterial endotoxins, and cytotoxic stimuli such as chemotherapeutic agents, oxidative stress, and ionizing radiation (1, 11). There is mounting evidence that NF-κB is constitutively activated in prostate cancer and several other cancer types and that this pathway is a key mediator of inflammatory responses associated with disease initiation and progression (5, 8, 9). Inhibition of NF-κB activity in prostate cancer cell lines drastically reduces their ability to form colonies in soft agar and suppresses both growth and development of metastatic lesions in vivo (47). Moreover, expression of oncogenes or loss of tumor suppressor genes in the mouse prostate leads to the development of invasive prostate carcinoma through NF-κB (6, 48, 49). Despite the growing evidence for a role of NF-κB in prostate tumorigenesis and resistance to therapy, the mechanisms underlying the activation of NF-κB in prostate cancer remain only partially understood.
The involvement of PKC in regulating NFκB has been known for several years (14, 16). However, the implication of individual PKC isozymes in the control of this pathway, and in particular in prostate cancer, remained to be elucidated. Studies highlighted the involvement of phorbol ester/DAG unresponsive (“atypical”) PKCs in prostate cancer progression (14). For example, atypical PKCι promotes prostate cancer growth and transcription of the IL6 gene through an NF-κB-dependent pathway (15). PKCι also promotes the phosphorylation of IKK in response to TNFα and is required for prostate cancer cell survival (50). PKCϵ, a member of the “novel” DAG-responsive PKC isozymes, has emerged as an important player in prostate cancer progression (27). Genetic deletion of the PKCϵ gene inhibits the formation of prostate tumors in transgenic adenocarcinoma of mouse prostate (TRAMP) mice (51). Most importantly, PKCϵ is in the vast majority of human prostate cancer specimens (19, 28, 29). PKCϵ emerged as an oncogenic kinase and, when ectopically expressed in fibroblasts, it promotes growth advantage and transformation (52). Similarly, when we overexpressed PKCϵ in non-transformed RWPE-1 epithelial prostate cells to levels similar to those in prostate cancer cells, we observed a manifest growth advantage with concomitant elevations in phospho-ERK and phospho-Akt (30). Notably, in this study we found that ectopic overexpression of PKCϵ in RWPE-1 cells potentiates the activation of NF-κB and the induction of NF-κB-responsive genes by TNFα. We recently reported that prostate-specific transgenic overexpression of PKCϵ in mice induces preneoplastic lesions characterized by hyperplasia and PIN. These lesions display elevated phospho-Akt, phospho-S6, and phospho-Stat3 levels and resistance to apoptotic stimuli (30). As shown here, PINs from PB-PKCϵ transgenic mice also display high nuclear NF-κB staining, a hallmark of NF-κB pathway activation. We have found recently that PKCϵ cooperates with Pten deficiency to promote prostate cancer. The resulting adenocarcinomas in prostates from mice overexpressing PKCϵ and haplodeficient in Pten display remarkable NF-κB hyperactivation, even stronger than in PINs3. Thus, PKCϵ drives the activation of signaling pathways implicated in the development and progression of prostate cancer in conjunction with other oncogenic alterations.
This study provides evidence that in prostate cancer cells, PKCϵ depletion or inhibition diminishes TNFα-induced IκBα phosphorylation and degradation, NF-κB nuclear translocation, and transactivation potential. Moreover, PKCϵ is required for the maintenance of constitutive NF-κB activation in androgen-independent cell lines such as PC3 and DU145 cells. It is interesting that depletion of other DAG-responsive PKCs, namely PKCα or PKCδ, did not significantly affect NF-κB transcriptional activity, arguing for a remarkable PKC isozyme-specificity for the activation of the pathway in prostate cancer cells. In support of this conclusion, a study in 293T cells revealed that NF-κB activating kinase (NAK), an IKK kinase, mediates IKK and NF-κB activation in response to growth factors in a manner that is dependent on PKCϵ but not PKCα or PKCθ (53). Notably, unlike PB-PKCϵ, PB-PKCα and PB-PKCδ mice do not develop preneoplastic lesions (30). Our study also provides evidence that TNFα is a bona fide stimulus for PKCϵ activation. TNFα promotes the translocation of PKCϵ from the cytosol to the plasma membrane in LNCaP cells to activate the NF-κB pathway, an effect mediated by DAG generated by PC- but not PI-PLC. Although death receptors do not generally couple directly to DAG generation, PKC activation in response to TNFα has been shown in various cellular models (18, 54, 55). It should be noted that PKCϵ redistributes to different intracellular compartments in response to distinct stimuli. For example, translocation of PKCϵ to mitochondria and the endoplasmic reticulum/Golgi has been reported in prostate cancer cells (56, 57). The characteristic redistribution of PKCϵ to the plasma membrane that we observed in prostate cancer cells in response to TNFα results in its association with the TNFR-I complex and is consistent with the idea that allosteric activation of PKCϵ by DAG facilitates its access to substrates and binding partners at the plasma membrane (41, 57). Binding of TNFα to its receptor in cancer cells triggers the rapid assembly of a TNFR-I-TRADD-TRAF2-RIP complex at the plasma membrane (12, 13). This complex triggers a NF-κB response but no apoptosis (46). Subsequently, a second cytosolic complex is formed that lacks TNFR-I but includes FADD and procaspases 8 and 10. Apoptosis would be activated by complex II, provided the signal from complex I fails to activate NF-κB. Our results show that in the absence of PKCϵ there is an evident reduction in the association of TNFR-I with the adaptor proteins required for NF-κB signaling. PKCϵ may phosphorylate the TNFα receptor in prostate cancer cells to differentially modulate the association of adaptor proteins, as shown previously for PKCδ in neutrophils (55). Another possibility is that PKCϵ phosphorylates adaptor proteins to promote the association with the TNFα receptor. Alternatively, PKCϵ may serve as a docking protein for different components of the TNFR-I complex. These mechanisms are currently under investigation in our laboratory.
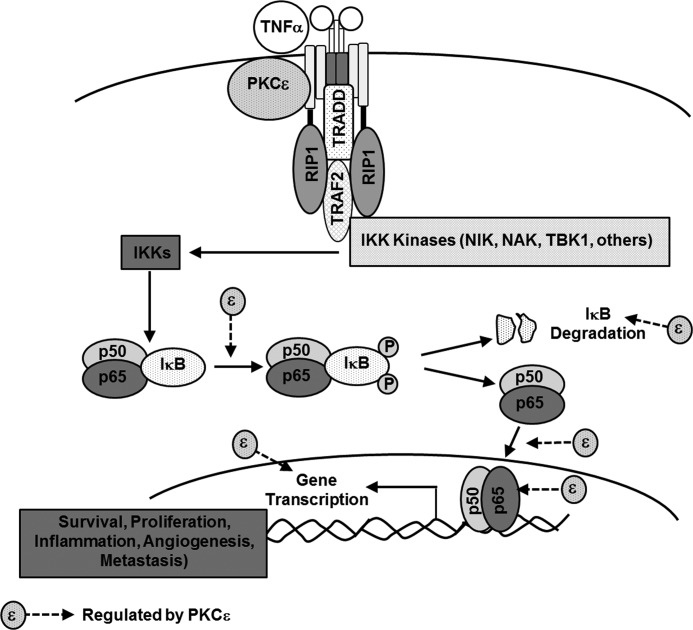
In summary, our study provides evidence that PKCϵ plays a key role in constitutive and cytokine-mediated NF-κB signaling activation in prostate cancer. PKCϵ modulates the expression of NF-κB-regulated genes relevant for cell survival, angiogenesis, and invasiveness (illustrated in Fig. 9). The identification of PKCϵ as a crucial upstream regulator of NF-κB signaling in prostate cancer argues for a central role of this kinase in the control of pathways involved in prostate cancer development and progression. It is important to highlight that there has been significant interest in the development of PKCϵ inhibitors as anti-inflammatory and anti-cancer agents (58, 59). Selective PKCϵ inhibitors can be effective candidates for sensitization of cancer cells to chemotherapeutic agents and radiotherapy, as shown previously for NF-κB inhibitors. Proof of principle has been recently provided in lung cancer models, where inhibition or depletion of PKCϵ impairs the tumorigenic and invasive capacity of these cells and “normalizes” the expression of apoptotic and survival genes implicated in disease progression, including well established NF-κB-regulated genes (60, 61). Thus, targeting the PKCϵ-NF-κB pathway may provide novel means for the treatment of prostate cancer or other cancers where this pathway may prove to be relevant.
FIGURE 9.
Diagram illustrating the regulation of TNFα-induced activation of NF-κB by PKCϵ.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-CA89202 (to M. G. K.).

This article contains supplemental Figs. S1–S6.
R. Garg, J. Blando, C. J. Perez, H. Wang, F. J. Benavides, and M. G. Kazanietz, unpublished data.
- IKK
- IκBα kinase
- TNFα
- tumor necrosis factor α
- PIN
- prostatic intraepithelial neoplasia
- AdV
- adenovirus
- IP
- immunoprecipitation
- PB
- probasin
- PMA
- phorbol 12-myristate 13-myristate
- PI
- phosphatidylinositol
- PC-PLC
- phosphatidylcholine-phospholipase C
- TRADD
- tumor necrosis factor receptor type-I associated death domain protein
- RIP
- receptor-interacting protein
- DAG
- diacylglycerol
- PMA
- phorbol myristate acetate
- NTC
- non-target control
- TNFR-I
- tumor necrosis factor receptor-I.
REFERENCES
- 1. Karin M., Lin A. (2002) NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221–227 [DOI] [PubMed] [Google Scholar]
- 2. May M. J., Ghosh S. (1997) Rel/NF-κB and IκB proteins. An overview. Semin. Cancer Biol. 8, 63–73 [DOI] [PubMed] [Google Scholar]
- 3. Hayden M. S., Ghosh S. (2004) Signaling to NF-κB. Genes Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 4. Stock D., Groome P. A., Siemens D. R. (2008) Inflammation and prostate cancer. A future target for prevention and therapy? Urol. Clin. North Am. 35, 117–130 [DOI] [PubMed] [Google Scholar]
- 5. Suh J., Payvandi F., Edelstein L. C., Amenta P. S., Zong W. X., Gélinas C., Rabson A. B. (2002) Mechanisms of constitutive NF-κB activation in human prostate cancer cells. Prostate 52, 183–200 [DOI] [PubMed] [Google Scholar]
- 6. Madrid L. V., Baldwin A. S., Jr. (2003) Regulation of NF-κB by oncoproteins and tumor suppressor proteins. Methods Mol. Biol. 223, 523–532 [DOI] [PubMed] [Google Scholar]
- 7. Romashkova J. A., Makarov S. S. (1999) NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401, 86–90 [DOI] [PubMed] [Google Scholar]
- 8. Jin R. J., Lho Y., Connelly L., Wang Y., Yu X., Saint Jean L., Case T. C., Ellwood-Yen K., Sawyers C. L., Bhowmick N. A., Blackwell T. S., Yull F. E., Matusik R. J. (2008) The nuclear factor κB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 68, 6762–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shukla S., MacLennan G. T., Fu P., Patel J., Marengo S. R., Resnick M. I., Gupta S. (2004) Nuclear factor κB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia 6, 390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zerbini L. F., Wang Y., Cho J. Y., Libermann T. A. (2003) Constitutive activation of nuclear factor κB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 63, 2206–2215 [PubMed] [Google Scholar]
- 11. Pahl H. L. (1999) Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18, 6853–6866 [DOI] [PubMed] [Google Scholar]
- 12. Chen G., Goeddel D. V. (2002) TNF-R1 signaling. A beautiful pathway. Science 296, 1634–1635 [DOI] [PubMed] [Google Scholar]
- 13. MacEwan D. J. (2002) TNF ligands and receptors. A matter of life and death. Br. J. Pharmacol. 135, 855–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diaz-Meco M. T., Moscat J. (2012) The atypical PKCs in inflammation. NF-κB and beyond. Immunol. Rev. 246, 154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishiguro H., Akimoto K., Nagashima Y., Kojima Y., Sasaki T., Ishiguro-Imagawa Y., Nakaigawa N., Ohno S., Kubota Y., Uemura H. (2009) aPKCλ/ι promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc. Natl. Acad. Sci. U.S.A. 106, 16369–16374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holden N. S., Squires P. E., Kaur M., Bland R., Jones C. E., Newton R. (2008) Phorbol ester-stimulated NF-κB-dependent transcription. Roles for isoforms of novel protein kinase C. Cell. Signal. 20, 1338–1348 [DOI] [PubMed] [Google Scholar]
- 17. Lu Z. G., Liu H., Yamaguchi T., Miki Y., Yoshida K. (2009) Protein kinase CΔ activates RelA/p65 and nuclear factor κB signaling in response to tumor necrosis factor α. Cancer Res. 69, 5927–5935 [DOI] [PubMed] [Google Scholar]
- 18. Satoh A., Gukovskaya A. S., Nieto J. M., Cheng J. H., Gukovsky I., Reeve J. R., Jr., Shimosegawa T., Pandol S. J. (2004) PKC-Δ and -ϵ regulate NF-κB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G582–591 [DOI] [PubMed] [Google Scholar]
- 19. Aziz M. H., Manoharan H. T., Church D. R., Dreckschmidt N. E., Zhong W., Oberley T. D., Wilding G., Verma A. K. (2007) Protein kinase Cϵ interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 67, 8828–8838 [DOI] [PubMed] [Google Scholar]
- 20. Basu A., Sivaprasad U. (2007) Protein kinase Cϵ makes the life and death decision. Cell. Signal. 19, 1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griner E. M., Caino M. C., Sosa M. S., Colón-González F., Chalmers M. J., Mischak H., Kazanietz M. G. (2010) A novel cross-talk in diacylglycerol signaling. The Rac-GAP β2-chimaerin is negatively regulated by protein kinase CΔ-mediated phosphorylation. J. Biol. Chem. 285, 16931–16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez-Guerrico A. M., Kazanietz M. G. (2005) Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade. A key role for protein kinase C Δ. J. Biol. Chem. 280, 38982–38991 [DOI] [PubMed] [Google Scholar]
- 23. Gavrielides M. V., Frijhoff A. F., Conti C. J., Kazanietz M. G. (2004) Protein kinase C and prostate carcinogenesis. Targeting the cell cycle and apoptotic mechanisms. Curr. Drug Targets 5, 431–443 [DOI] [PubMed] [Google Scholar]
- 24. Meshki J., Caino M. C., von Burstin V. A., Griner E., Kazanietz M. G. (2010) Regulation of prostate cancer cell survival by protein kinase Cϵ involves bad phosphorylation and modulation of the TNFα/JNK pathway. J. Biol. Chem. 285, 26033–26040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu D., Foreman T. L., Gregory C. W., McJilton M. A., Wescott G. G., Ford O. H., Alvey R. F., Mohler J. L., Terrian D. M. (2002) Protein kinase Cϵ has the potential to advance the recurrence of human prostate cancer. Cancer Res. 62, 2423–2429 [PubMed] [Google Scholar]
- 26. Bae K. M., Wang H., Jiang G., Chen M. G., Lu L., Xiao L. (2007) Protein kinase C ϵ is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner. Cancer Res. 67, 6053–6063 [DOI] [PubMed] [Google Scholar]
- 27. Gorin M. A., Pan Q. (2009) Protein kinase C ϵ. An oncogene and emerging tumor biomarker. Mol. Cancer 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cornford P., Evans J., Dodson A., Parsons K., Woolfenden A., Neoptolemos J., Foster C. S. (1999) Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am. J. Pathol. 154, 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koren R., Ben Meir D., Langzam L., Dekel Y., Konichezky M., Baniel J., Livne P. M., Gal R., Sampson S. R. (2004) Expression of protein kinase C isoenzymes in benign hyperplasia and carcinoma of prostate. Oncol. Rep. 11, 321–326 [PubMed] [Google Scholar]
- 30. Benavides F., Blando J., Perez C. J., Garg R., Conti C. J., DiGiovanni J., Kazanietz M. G. (2011) Transgenic overexpression of PKCϵ in the mouse prostate induces preneoplastic lesions. Cell Cycle 10, 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Burstin V. A., Xiao L., Kazanietz M. G. (2010) Bryostatin 1 inhibits phorbol ester-induced apoptosis in prostate cancer cells by differentially modulating protein kinase C (PKC) Δ translocation and preventing PKCΔ-mediated release of tumor necrosis factor α. Mol. Pharmacol. 78, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shinohara H., Kayagaki N., Yagita H., Oyaizu N., Ohba M., Kuroki T., Ikawa Y. (2001) A protective role of PKCϵ against TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 284, 1162–1167 [DOI] [PubMed] [Google Scholar]
- 33. Caloca M. J., Fernandez N., Lewin N. E., Ching D., Modali R., Blumberg P. M., Kazanietz M. G. (1997) β2-Chimaerin is a high-affinity receptor for the phorbol ester tumor promoters. J. Biol. Chem. 272, 26488–26496 [DOI] [PubMed] [Google Scholar]
- 34. Garg R., Ramchandani A. G., Maru G. B. (2008) Curcumin decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation to modulate downstream targets in mouse skin. Carcinogenesis 29, 1249–1257 [DOI] [PubMed] [Google Scholar]
- 35. Garg R., Ingle A., Maru G. (2008) Dietary turmeric modulates DMBA-induced p21ras, MAP kinases and AP-1/NF-κB pathway to alter cellular responses during hamster buccal pouch carcinogenesis. Toxicol. Appl. Pharmacol. 232, 428–439 [DOI] [PubMed] [Google Scholar]
- 36. Yang D., Kedei N., Li L., Tao J., Velasquez J. F., Michalowski A. M., Tóth B. I., Marincsák R., Varga A., Bíró T., Yuspa S. H., Blumberg P. M. (2010) RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res. 70, 7905–7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gandellini P., Folini M., Longoni N., Pennati M., Binda M., Colecchia M., Salvioni R., Supino R., Moretti R., Limonta P., Valdagni R., Daidone M. G., Zaffaroni N. (2009) miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cϵ. Cancer Res. 69, 2287–2295 [DOI] [PubMed] [Google Scholar]
- 38. Lavalle C. R., Bravo-Altamirano K., Giridhar K. V., Chen J., Sharlow E., Lazo J. S., Wipf P., Wang Q. J. (2010) Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem. Biol. 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caino M. C., von Burstin V. A., Lopez-Haber C., Kazanietz M. G. (2011) Differential regulation of gene expression by protein kinase C isozymes as determined by genome-wide expression analysis. J. Biol. Chem. 286, 11254–11264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Begley R., Liron T., Baryza J., Mochly-Rosen D. (2004) Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem. Biophys. Res. Commun. 318, 949–954 [DOI] [PubMed] [Google Scholar]
- 41. Griner E. M., Kazanietz M. G. (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 42. Fujii T., García-Bermejo M. L., Bernabó J. L., Caamaño J., Ohba M., Kuroki T., Li L., Yuspa S. H., Kazanietz M. G. (2000) Involvement of protein kinase C Δ (PKCΔ) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCΔ. J. Biol. Chem. 275, 7574–7582 [DOI] [PubMed] [Google Scholar]
- 43. Wang S., Kazanietz M. G., Blumberg P. M., Marquez V. E., Milne G. W. (1996) Molecular modeling and site-directed mutagenesis studies of a phorbol ester-binding site in protein kinase C. J. Med. Chem. 39, 2541–2553 [DOI] [PubMed] [Google Scholar]
- 44. Hammond G., Thomas C., Schiavo G. (2004) in Phosphoinositides in Subcellular Targeting and Enzyme Activation (Stenmark H. ed) Vol. 282, pp. 177–206, Springer Publishing, Berlin [Google Scholar]
- 45. Schütze S., Berkovic D., Tomsing O., Unger C., Krönke M. (1991) Tumor necrosis factor induces rapid production of 1′2′diacylglycerol by a phosphatidylcholine-specific phospholipase C. J. Exp. Med. 174, 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Micheau O., Tschopp J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 [DOI] [PubMed] [Google Scholar]
- 47. Yemelyanov A., Gasparian A., Lindholm P., Dang L., Pierce J. W., Kisseljov F., Karseladze A., Budunova I. (2006) Effects of IKK inhibitor PS1145 on NF-κB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene 25, 387–398 [DOI] [PubMed] [Google Scholar]
- 48. Fernandez-Marcos P. J., Abu-Baker S., Joshi J., Galvez A., Castilla E. A., Cañamero M., Collado M., Saez C., Moreno-Bueno G., Palacios J., Leitges M., Serrano M., Moscat J., Diaz-Meco M. T. (2009) Simultaneous inactivation of Par-4 and PTEN in vivo leads to synergistic NF-κB activation and invasive prostate carcinoma. Proc. Natl. Acad. Sci. U.S.A. 106, 12962–12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Min J., Zaslavsky A., Fedele G., McLaughlin S. K., Reczek E. E., De Raedt T., Guney I., Strochlic D. E., Macconaill L. E., Beroukhim R., Bronson R. T., Ryeom S., Hahn W. C., Loda M., Cichowski K. (2010) An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-κB. Nat. Med. 16, 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Win H. Y., Acevedo-Duncan M. (2008) Atypical protein kinase C phosphorylates IKKαβ in transformed non-malignant and malignant prostate cell survival. Cancer Lett. 270, 302–311 [DOI] [PubMed] [Google Scholar]
- 51. Hafeez B. B., Zhong W., Weichert J., Dreckschmidt N. E., Jamal M. S., Verma A. K. (2011) Genetic ablation of PKC ϵ inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res. 71, 2318–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mischak H., Goodnight J. A., Kolch W., Martiny-Baron G., Schaechtle C., Kazanietz M. G., Blumberg P. M., Pierce J. H., Mushinski J. F. (1993) Overexpression of protein kinase C-Δ and -ϵ in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J. Biol. Chem. 268, 6090–6096 [PubMed] [Google Scholar]
- 53. Tojima Y., Fujimoto A., Delhase M., Chen Y., Hatakeyama S., Nakayama K., Kaneko Y., Nimura Y., Motoyama N., Ikeda K., Karin M., Nakanishi M. (2000) NAK is an IκB kinase-activating kinase. Nature 404, 778–782 [DOI] [PubMed] [Google Scholar]
- 54. Chang Q., Tepperman B. L. (2003) Effect of selective PKC isoform activation and inhibition on TNF-α-induced injury and apoptosis in human intestinal epithelial cells. Br. J. Pharmacol. 140, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kilpatrick L. E., Sun S., Korchak H. M. (2004) Selective regulation by Δ-PKC and PI 3-kinase in the assembly of the antiapoptotic TNFR-1 signaling complex in neutrophils. Am. J. Physiol. Cell Physiol. 287, C633–642 [DOI] [PubMed] [Google Scholar]
- 56. Wang H., Kazanietz M. G. (2010) p23/Tmp21 differentially targets the Rac-GAP β2-chimaerin and protein kinase C via their C1 domains. Mol. Biol. Cell 21, 1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jaken S., Parker P. J. (2000) Protein kinase C binding partners. BioEssays 22, 245–254 [DOI] [PubMed] [Google Scholar]
- 58. Bao L., Gorin M. A., Zhang M., Ventura A. C., Pomerantz W. C., Merajver S. D., Teknos T. N., Mapp A. K., Pan Q. (2009) Preclinical development of a bifunctional cancer cell homing, PKCϵ inhibitory peptide for the treatment of head and neck cancer. Cancer Res. 69, 5829–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koyanagi T., Noguchi K., Ootani A., Inagaki K., Robbins R. C., Mochly-Rosen D. (2007) Pharmacological inhibition of ϵ PKC suppresses chronic inflammation in murine cardiac transplantation model. J. Mol. Cell Cardiol. 43, 517–522 [DOI] [PubMed] [Google Scholar]
- 60. Caino M. C., Lopez-Haber C., Kim J., Mochly-Rosen D., Kazanietz M. G. (2012) Proteins kinase Cvarϵ is required for non-small cell lung carcinoma growth and regulates the expression of apoptotic genes. Oncogene 31, 2593–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Caino M. C., Lopez-Haber C., Kissil J. L., Kazanietz M. G. (2012) Non-small cell lung carcinoma cell motility, rac activation and metastatic dissemination are mediated by protein kinase C ϵ. PLoS ONE 7, e31714. [DOI] [PMC free article] [PubMed] [Google Scholar]