Abstract
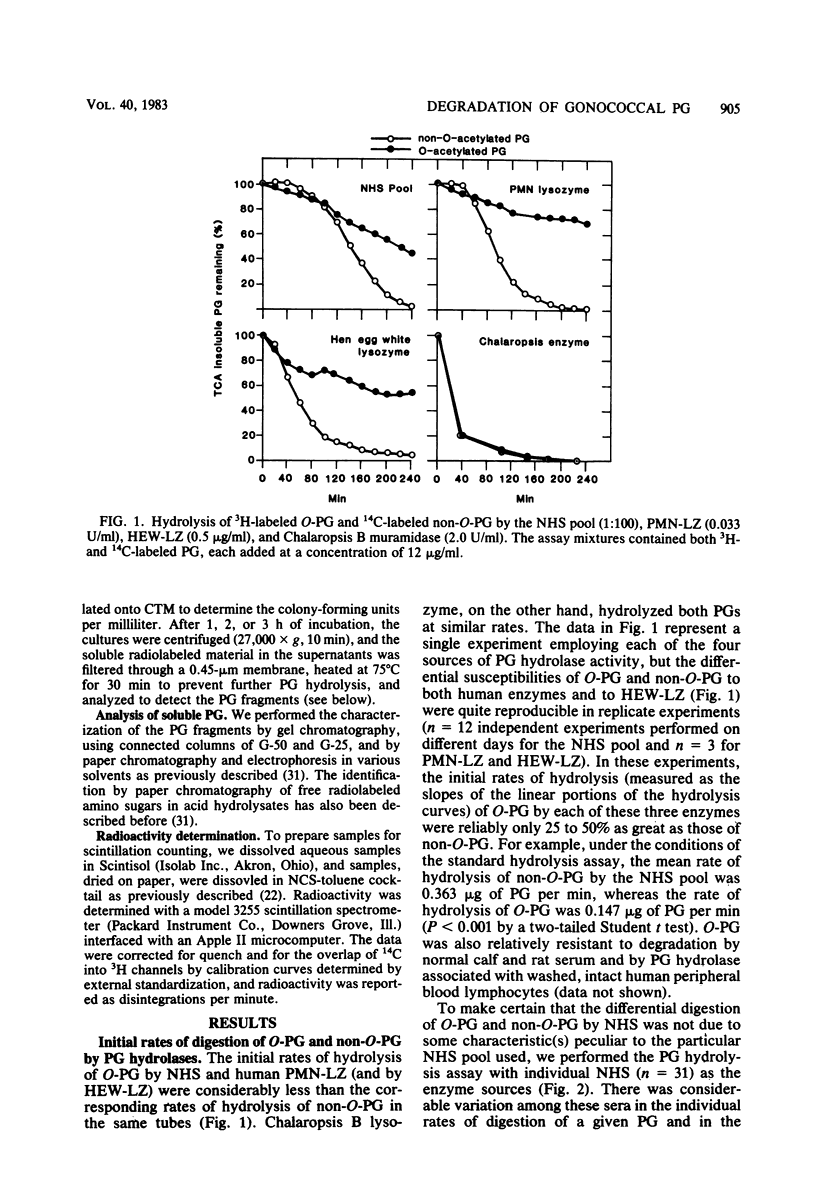
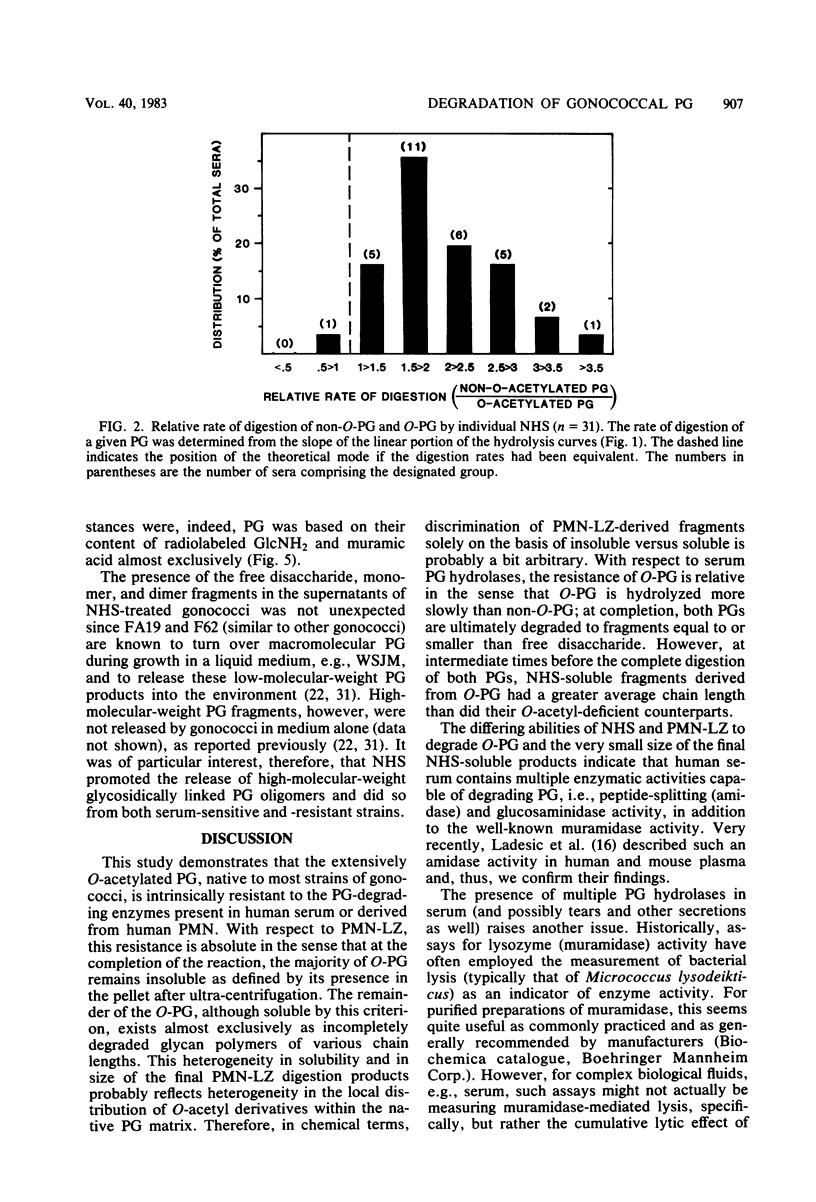
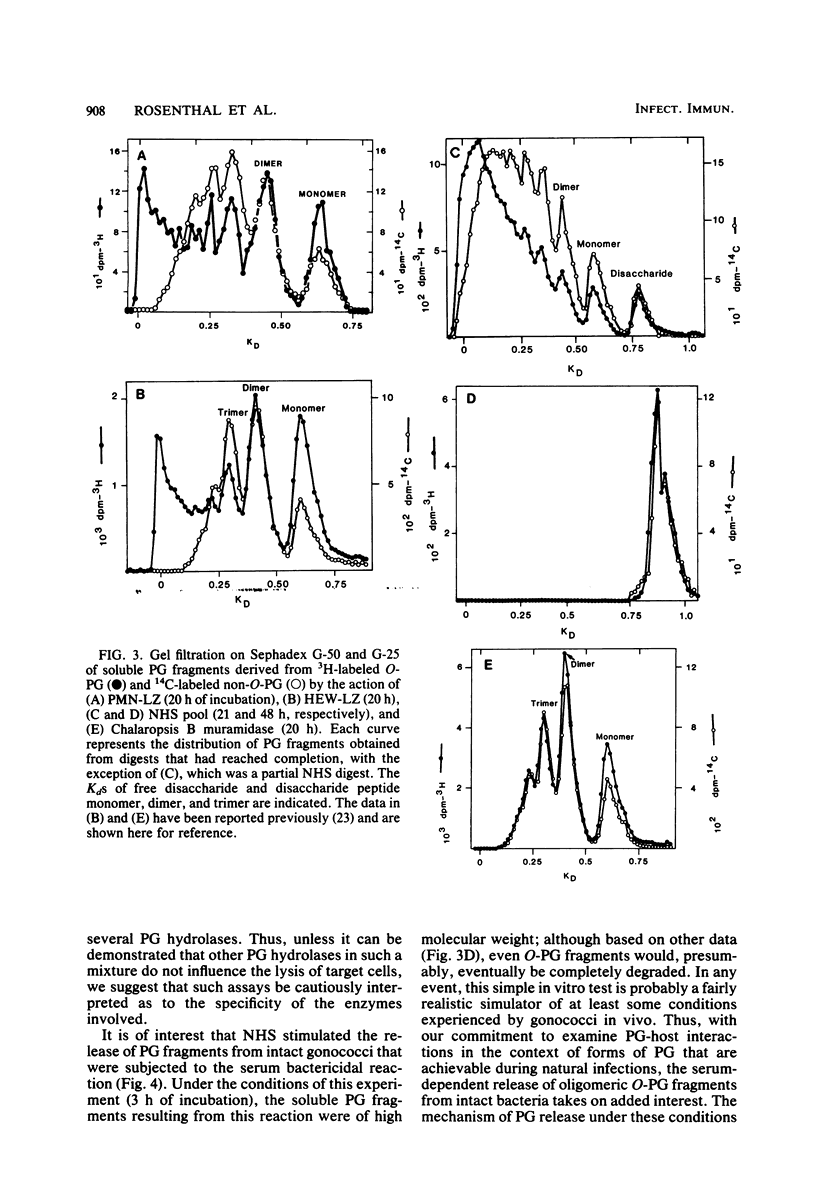
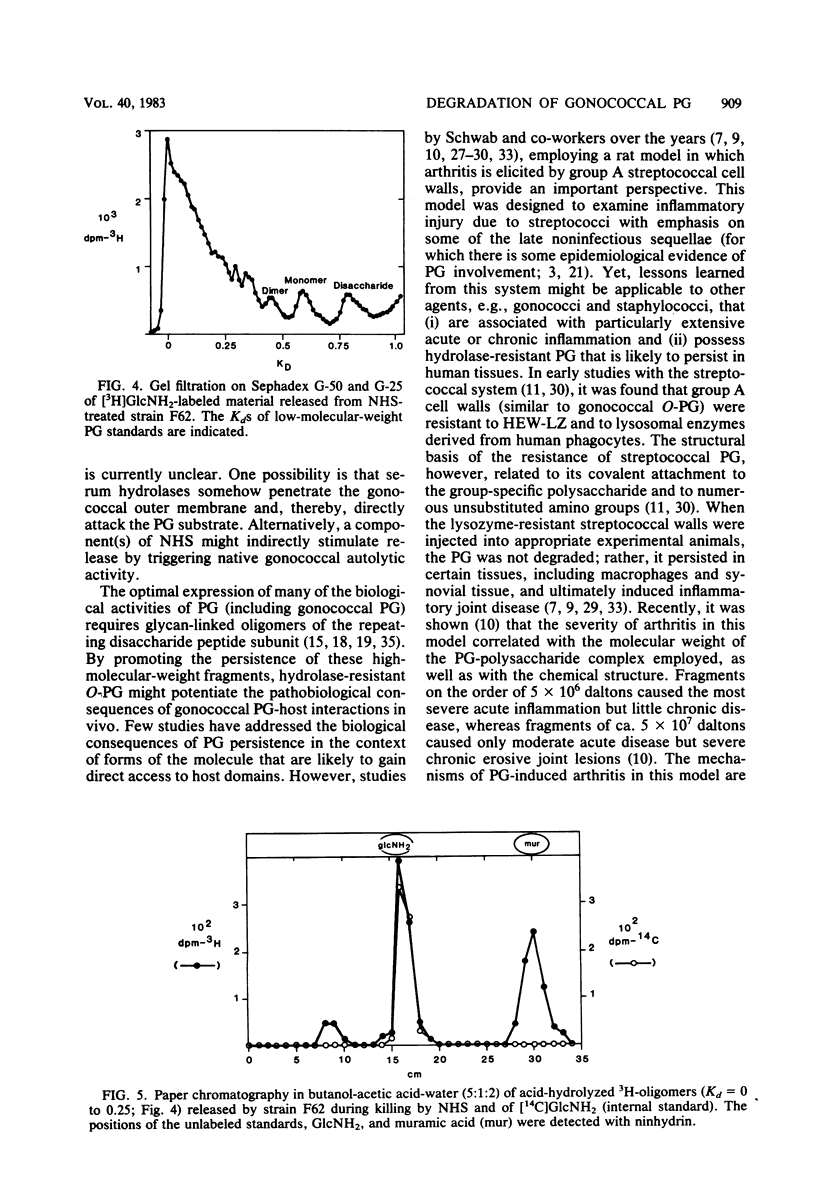
Two naturally occurring forms of gonococcal peptidoglycan (PG) were tested for their susceptibility to human PG hydrolases. Purified 3H-labeled PG substituted extensively with O-acetyl derivatives (O-PG; from Neisseria gonorrhoeae FA19) and 14C-labeled O-acetyl-deficient PG (non-O-PG; from N. gonorrhoeae RD5) were mixed together and treated with either normal human sera (NHS) or with lysozyme purified from human polymorphonuclear leukocytes (PMN-LZ). The initial rate of hydrolysis of O-PG by NHS or by PMN-LZ was two- to fourfold less than that of its non-O-PG counterpart in the same tube. When the reactions were allowed to go to completion. NHS solubilized both PGs completely, whereas PMN-LZ solubilized all of the non-O-PG and left ca. 60% of the O-PG insoluble. The PMN-LZ-soluble fraction of O-PG consisted largely of glycosidically linked fragments with molecular weights greater than ca. 10(4), whereas the corresponding non-O-PG was degraded to lower-molecular-weight fragments, exclusively. At completion, NHS hydrolyzed both PGs to fragments whose size was equal to or smaller than that of the free disaccharide unit of PG, suggesting that human sera contain a peptide-splitting (amidase) activity and a glycosidase activity, in addition to that of the well-known muramidase. NHS also promoted the release of high-molecular-weight PG fragments from intact gonococci. The persistence of human hydrolase-resistant PG in the form of soluble macromolecular fragments may potentiate the biological effects of gonococcal PG in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun D. G., Holm S. E. Streptococcal anti-group A precipitins in sera from patients with rheumatic arthritis and acute glomerulonephritis. Int Arch Allergy Appl Immunol. 1970;37(2):216–224. doi: 10.1159/000230234. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Lee T. J., Guymon L. F., Sparling P. F. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect Immun. 1981 May;32(2):547–552. doi: 10.1128/iai.32.2.547-552.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Chetty C., Klapper D. G., Schwab J. H. Soluble peptidoglycan-polysaccharide fragments of the bacterial cell wall induce acute inflammation. Infect Immun. 1982 Dec;38(3):1010–1019. doi: 10.1128/iai.38.3.1010-1019.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S. Preparation of arthritogenic hydrosoluble peptidoglycans from both arthritogenic and non-arthritogenic bacterial cell walls. Infect Immun. 1977 Jun;16(3):861–866. doi: 10.1128/iai.16.3.861-866.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladesić B., Tomasić J., Kveder S., Hrsak I. The metabolic fate of 14C-labeled immunoadjuvant peptidoglycan monomer. II. In vitro studies. Biochim Biophys Acta. 1981 Nov 18;678(1):12–17. doi: 10.1016/0304-4165(81)90042-8. [DOI] [PubMed] [Google Scholar]
- Martin J. P., Fleck J., Mock M., Ghuysen J. M. The wall peptidoglycans of Neisseria perflava, Moraxella glucidolytica, Pseudomonas alcaligenes and Proteus vulgaris strain P18. Eur J Biochem. 1973 Oct 5;38(2):301–306. doi: 10.1111/j.1432-1033.1973.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Oken M. M., Peterson P. K., Wilkinson B. J. Endogenous pyrogen production by human blood monocytes stimulated by staphylococcal cell wall components. Infect Immun. 1981 Jan;31(1):208–213. doi: 10.1128/iai.31.1.208-213.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Rosenthal R. S. Complement consumption gonococcal peptidoglycan. Infect Immun. 1982 Feb;35(2):442–448. doi: 10.1128/iai.35.2.442-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Pretzer E. Degradation of gonococcal outer membrane proteins by human neutrophil lysosomal proteases. Infect Immun. 1981 Oct;34(1):62–68. doi: 10.1128/iai.34.1.62-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolicka M., Massell B. F., Wilgram G. F. Antipeptidoglycan in rheumatic fever: agreement with carditis. Proc Soc Exp Biol Med. 1973 Dec;144(3):892–895. doi: 10.3181/00379727-144-37705. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Blundell J. K., Perkins H. R. Strain-related differences in lysozyme sensitivity and extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1982 Aug;37(2):826–829. doi: 10.1128/iai.37.2.826-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Wright R. M., Sinha R. K. Extent of peptide cross-linking in the peptidoglycan of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):867–875. doi: 10.1128/iai.28.3.867-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Allen J. B., Anderle S. K., Dalldorf F., Eisenberg R., Cromartie W. J. Relationship of complement to experimental arthritis induced in rats with streptococcal cell walls. Immunology. 1982 May;46(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Effect of penicillin G on release of peptidoglycan fragments by Neisseria gonorrhoeae: characterization of extracellular products. Antimicrob Agents Chemother. 1981 Jul;20(1):98–103. doi: 10.1128/aac.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K. Factors affecting complement activation by Staphylococcus aureus cell walls, their components, and mutants altered in teichoic acid. Infect Immun. 1981 Apr;32(1):216–224. doi: 10.1128/iai.32.1.216-224.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. P., Shockley R. K., Johnston K. H. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. J Clin Microbiol. 1980 Apr;11(4):363–369. doi: 10.1128/jcm.11.4.363-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



