Abstract
Purpose
To determine the effectiveness of salvage radiation therapy (RT) in patients with loco-regional recurrences (LRR) following initial complete resection of non-small cell lung cancer (NSCLC) and assess prognostic factors affecting survivals.
Materials and Methods
Between 1994 and 2007, 64 patients with LRR after surgery of NSCLC were treated with high dose RT alone (78.1%) or concurrent chemo-radiation therapy (CCRT, 21.9%) at Samsung Medical Center. Twenty-nine patients (45.3%) had local recurrence, 26 patients (40.6%) had regional recurrence and 9 patients (14.1%) had recurrence of both components. The median RT dose was 54 Gy (range, 44-66 Gy). The radiation target volume included the recurrent lesions only.
Results
The median follow-up time from the start of RT in survivors was 32.0 months. The rates of in-field failure free survival, intra-thoracic failure free survival and extra-thoracic failure free survival at 2 years were 52.3%, 33.9% and 59.4%, respectively. The median survival after RT was 18.5 months, and 2-year overall survival (OS) rate was 47.9%. On both univariate and multivariate analysis, the interval from surgery till recurrence and CCRT were significant prognostic factors for OS.
Conclusion
The current study demonstrates that involved field salvage RT is effective for LRR of NSCLC following surgery.
Keywords: Concurrent chemo-radiation therapy, locoregional recurrence, non-small cell lung cancer, radiation therapy, salvage treatment
INTRODUCTION
In the management of non-small cell lung cancer (NSCLC), surgery is the treatment of choice with the highest curative potential for the patients with stage I/II and selective stage III disease.1 Unfortunately, however, a significant proportion of the patients, following curative surgery, develop recurrence or metastasis during their clinical course. Distant metastases (DM) are more common than loco-regional recurrences (LRR) after complete surgical resection, and the frequency of LRR as the first site of failure ranges from 10-20% for stage I, and up to 50% for stage III patients.2-6 The overall LRR in the mediastinum, the bronchial stump and the chest wall occurs with the incidence of 30% among all recurrences without clinical evidence of DM.7
There have been a few reports on aggressive repeat surgery with the curative aim for the patients with resectable LRR or the second primary cancer.8-11 Even with the promising survival outcomes, reoperation, however may be limited only to those with adequate cardio-pulmonary functional reserves. There have been several reports on salvage radiation therapy (RT) as an alternative to repeated surgery, as RT can be widely applied.6,12-22 The survival outcomes were quite variable of 10-40% at 2-year because 1970's and 1980's and most studies had only a small number of patients, with diverse RT dose schedules being used. Among these, few studies reported that post-resection recurrent NSCLC patients treated with salvage RT have survival comparable to that of newly diagnosed patients treated with radical RT.14,17,22 Therefore, salvage RT would be actively considered in patients with inoperable disease.
We report herein our experience with high dose salvage RT for patients with LRR after curative resection for NSCLC to determine the effectiveness of salvage RT and assess prognostic factors affecting survivals.
MATERIALS AND METHODS
We retrospectively reviewed the hospital charts of the patients with completely resected NSCLC between 1994 and 2007 at Samsung Medical Center, and found 64 patients with LRR without evidence of DM and inoperable status, assessed by a trained surgeon, who were given high dose salvage RT. The clinical assessments at the time of diagnosing LRR included complete history and physical examination, complete blood cell count, chemistry panel, simple chest X-ray, computed tomography (CT) of the chest and the upper abdomen, bronchoscopy (when clinically indicated), fluoro-deoxy-glucose (FDG) positron emission tomography-computed tomography (PET-CT) scan, magnetic resonance imaging of brain, and whole body bone scan. In 46 patients, biopsy seemed technically difficult or very risky, and, therefore, the clinical diagnosis of LRR was made based either on high FDG uptake on PET-CT scan without other possible explainable causes (28 patients, 43.8%), or on evidently progressive lesion by at least two consecutive chest CT scans (18 patients, 28.1%). Biopsy confirmation of the recurrent lesion was possible in 18 patients (28.1%).
The initial types of surgery were pneumonectomy in 9 patients (14.1%), lobectomy in 53 (82.8%), and wedge resection in 2 (3.1%). The pathologic stages at the time of surgery were I in 38 patients (59.4%), II in 16 (25.0%) and III in 10 (15.6%). Following the initial surgery, adjuvant chemotherapy was given in 11 patients (17.2%), and none received adjuvant RT. Among 10 patients with pathologic stage III disease, 4 were recommended to receive postoperative RT because of pN2 disease. However, postoperative RT was not given to these 4 patients due to either postoperative morbidity (3 patients) or patient's refusal (1 patient).
There were 53 males (82.8%) and 11 females (17.2%), and the age at diagnosis of LRR ranged from 45 to 80 years (median 66 years). Eastern Cooperative Oncology Group performance scores were 0 in 10 (15.6%), 1 in 44 (68.8%), 2 in 9 (14.1%) and 3 in 1 (1.5%). Squamous cell carcinoma (39 patients, 60.9%) and adenocarcinoma (17 patients, 26.6%) were the most common histologic types. The median time from the date of initial surgery to LRR was 10 months (range, 1-72 months). The sites of LRR were local recurrence only at the bronchial stump or the chest wall in 29 patients (45.3%), regional recurrence only at the mediastinal lymph node in 26 (40.6%), and combined local and regional recurrence in the bronchial stump and the mediastinal lymph node in 9 (14.1%). All patients were re-staged as if the patients had been newly diagnosed according to the sixth Edition of the AJCC Cancer Staging and the stages were I in 3 patients (4.7%), II in 27 (42.2%) and III in 34 (53.1%).23
Salvage RT was given using 6-15 MV photon beams from linear accelerators. Three-dimensional conformal RT after individual CT-based simulation was done in all patients. The radiation target volume included the visible recurrent lesions on axial CT images with 1-2 cm margins according to normal tissue constraints, and no attention was given to include the regional lymphatics electively. Three or four beam arrangements were typically used usually to adequately cover the target volumes and to minimize the dose to the normal tissues (e.g. lung, spinal cord and esophagus). All patients received high dose RT for curative purpose. Four different fractionation schedules were employed considering the expected morbidity, based on the target volume, the patients' performance, and the concurrent chemotherapy. In 14 patients who received concurrent chemotherapy with salvage RT (CCRT), daily 1.8 or 2.0 Gy was used: 54-63 Gy by 1.8 Gy per fraction in 4 patients (6.3%); and 44-66 Gy by 2 Gy per fraction in 10 (15.6%). In the remaining patients receiving salvage RT alone, higher daily doses were used to shorten the overall treatment duration: 50-60 Gy by 2.5 Gy per fraction in 3 (4.7%); and 40-66 Gy by 3 Gy per fraction in 47 (73.4%). The total RT doses were converted into biologically equivalent dose (BED) for the comparison purpose. When α/β was assumed to be 10 Gy, the median total BED was 70.2 Gy10 (51.5-85.8 Gy10).
The chemotherapy was concurrently added as radiosensitizer, and the regimens had changed with time. During 1990's, two patients received cisplatin (100 mg/m2) plus etoposide (50 mg/m2) every four weeks. During 2000's, 12 patients received paclitaxel (50 mg/m2) plus cisplatin (25 mg/m2) weekly. The median number of chemotherapy cycles was 6 cycles (2-6 cycles). The decision to add chemotherapy to RT was individually made based on performance status of patients. The characteristics of the patients are summarized in Table 1.
Table 1.
Patients' Characteristics at Diagnosis of Post-Resection Recurrent NSCLC
NSCLC, non-small cell lung cancer; BED, biologically equivalent dose.
Disease-free interval, time interval from initial surgery till postoperative recurrence. Recurrent stage, restaging according to the sixth edition of the AJCC Cancer Staging.
Radiographic tumor response after salvage RT was evaluated using the RECIST criteria by chest CT taken 1 month post completion of salvage RT.24 Radiation pneumonitis and esophagitis were assessed by clinical symptoms, correlated with the radiographic findings, in the absence of other cause of symptoms, and estimated by the Radiation Therapy Oncology Group Acute and Late Lung Morbidity Scoring Criteria.
As only the involved lesions were the targets for salvage RT in this study, the further recurrences following salvage RT were classified as in-field failure (IFF), intra-thoracic failure (ITF) and extra-thoracic failure (ETF). ITF was defined as any loco-regional recurrence regardless of the current RT volume and included IFF and recurrence at the supraclavicular fossa or the pleura-pericardial seeding. ETF was any recurrence outside ITF.
Survival rates were estimated with the Kaplan-Meier method, and the comparisons between the groups were determined using the log-rank test.25 Multivariate analysis was performed to assess the relationships between the outcomes and the possible prognostic variables using the Cox proportional hazards model. Statistical analyses were performed using SAS software (SAS for Windows, version 9.0, SAS Institute, Cary, NC, USA).
RESULTS
Radiographic tumor response
The radiographic tumor responses were evaluable by chest CT in all patients. Complete response was achieved in 4 patients (6.2%), partial response in 40 (62.5%) and stable diseases in 12 (18.8%). Eight patients (12.5%) showed progressive disease: increased size of treated tumor in 3; new lesions in 5.
Patterns of failure
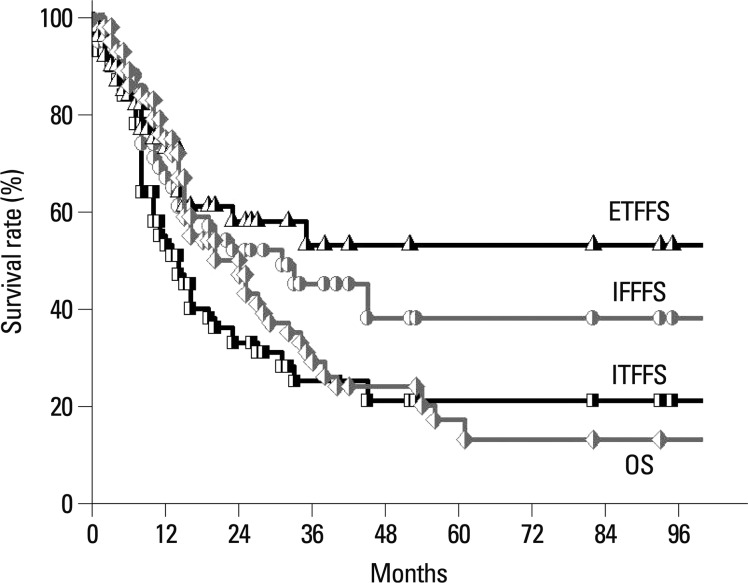
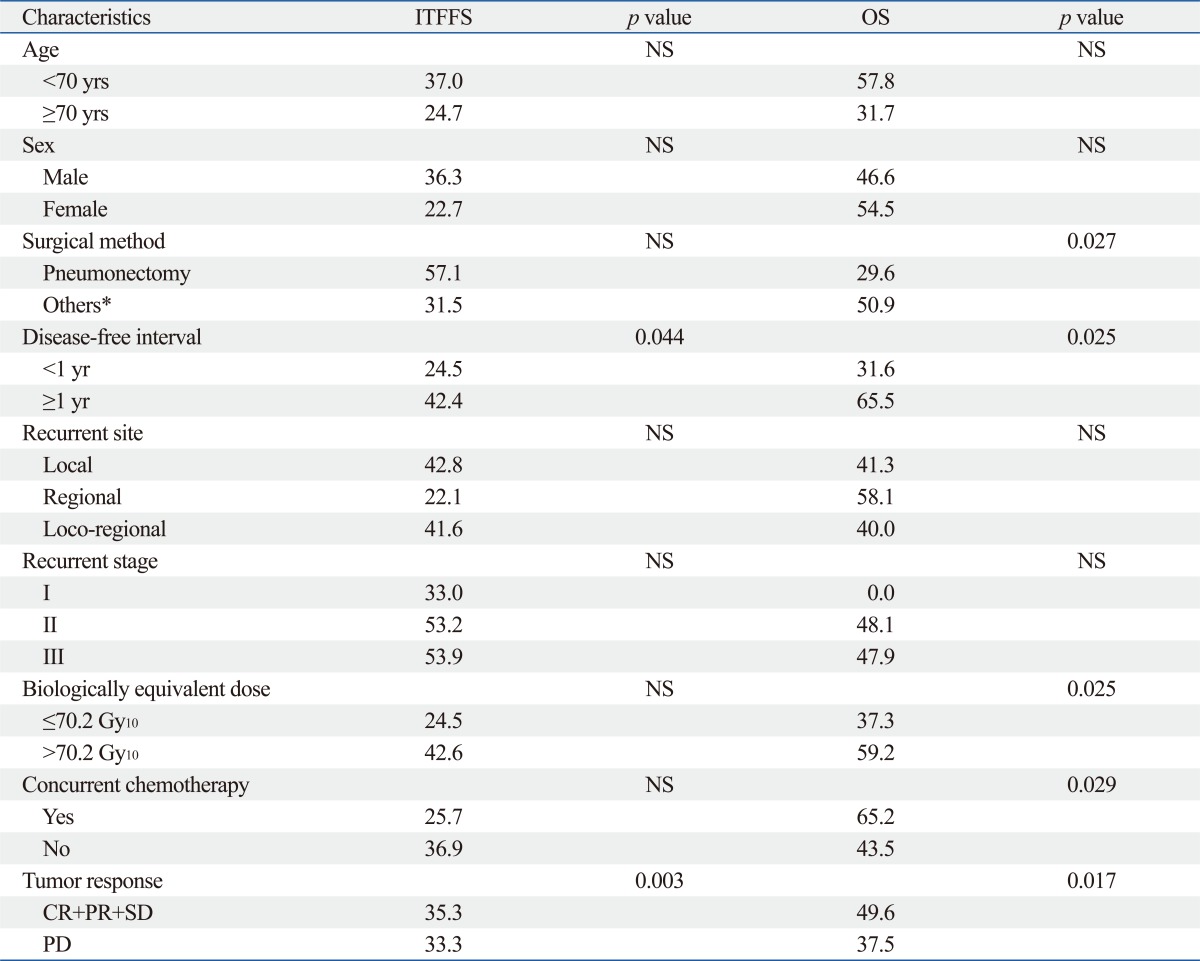
Three-fourth of the patients (48/64, 75.0%) experienced further recurrence following salvage RT: 29 (45.3%) had IFF; 40 (62.5%) had ITF; and 22 (34.4%) had ETF. The rates of IFF-free survival, ITF-free survival, and ETF-free survival at 2 years were 52.3%, 33.9%, and 59.4%, respectively (Fig. 1). On univariate analysis, there was no statistically significant factor affecting the IFF-free survival. On both univariate analysis and multivariate Cox regression analysis, the radiographic tumor response to salvage RT proved to be the most important prognostic factor for ITF-free survival and ETF-free survival (p<0.05). The significances of the prognostic factors are summarized in Table 2.
Fig. 1.
In-field failure free survival (circle, IFFFS), intra-thoracic failure free survival (square, ITFFS), extra-thoracic failure free survival (triangle, ETFFS) and overall survival (diamond, OS) rates with salvage treatment in loco-regional recurrence of resected non-small cell lung cancer.
Table 2.
2-Year Survival Rates according to Clinico-Pathological Parameters on Univariate Analysis
ITFFS, intra-thoracic failure-free survival; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Disease-free interval, time interval from initial surgery till postoperative recurrence.
Recurrent stage, restaging according to the sixth edition of the AJCC Cancer Staging.
*Includes wedge resection and lobectomy.
Survival
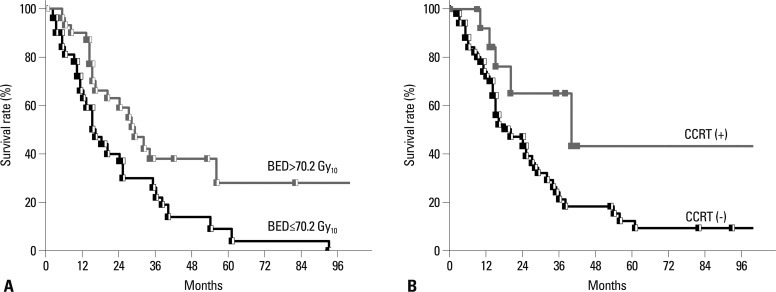
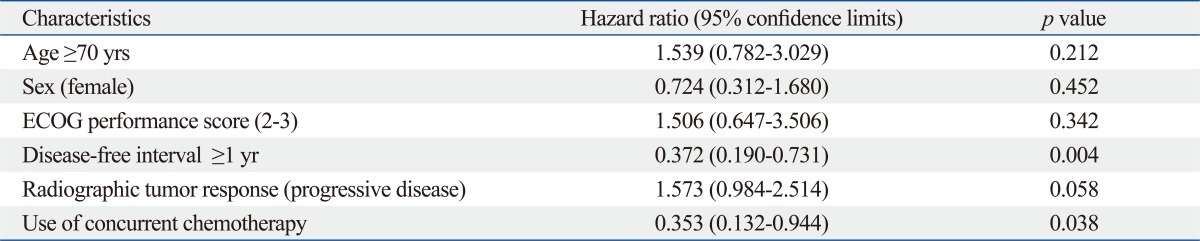
The median follow-up time from the start of salvage RT in survivors was 32.0 months (range, 2-102 months). The median survival was 18.5 months and the overall survival (OS) rates at 2- and 3-years were 47.9% and 29.5%, respectively (Fig. 1). On univariate analysis, the factors that favorably correlated with the OS with statistical significance were the initial surgery type other than pneumonectomy (p=0.027), the disease-free interval till postoperative LRR longer than 1 year (p=0.025), the BED10 higher than 70.2 Gy10 (p=0.025), the application of CCRT (p=0.029), and the radiographic tumor response other than progressive disease (p=0.017) (Table 2). Fig. 2 shows difference in the OS according to the BED10 and the treatment modality. Multivariate Cox regression analysis of the possible prognostic factors on the OS revealed that the disease-free interval till postoperative LRR longer than 1 year (hazard ratio=0.372, p=0.004) and the application of CCRT (hazard ratio=0.353, p=0.038) were favorable factors (Table 3).
Fig. 2.
Overall survival rate according to biologically equivalent dose (BED, A) and concurrent chemo-radiation therapy (CCRT, B). BED more than 70.2 Gy10 (p=0.018) and CCRT (p=0.029) were statistically significant prognostic factors in univariate analysis.
Table 3.
Multivariate Cox Regression Analysis of Risk Factors Affecting Overall Survival
ECOG, Eastern Cooperative Oncology Group.
Disease-free interval, time interval from initial surgery till postoperative recurrence.
Treatment-related toxicity
All patients tolerated the salvage RT course well and there was no incidence of treatment interruption due to acute toxicity. Forty-three patients (82.3%) experienced acute radiation-induced esophagitis with grade 1 or 2. However, there was no grade 3 or higher acute esophagitis. Six patients (9.4%) had grade 3 radiation pneumonitis and were given corticosteroids, and one (1.6%) was hospitalized for grade 4 radiation pneumonitis. All patients with radiation pneumonitis were given RT alone without concurrent chemotherapy. There was no incidence of severe chronic toxicity or treatment-related death.
DISCUSSION
Before the wide clinical use of PET-CT in the oncology practice, the diagnosis of local or regional recurrence depended mainly on the histopathologic confirmation (bronchoscopic or mediastinoscopic biopsy) when an unusual and new soft tissue lesion shows up on the follow-up CT scans. If the lesion suspected of recurrence was located at an inaccessible position by either bronchoscopy or mediastinoscopy, the usual way of confirming the recurrence used to be repeated CT scans at a few months' interval to verify that the lesion had the evidence of progression, which might lead to the delay in the start of salvage therapy. Several studies recently reported the diagnostic accuracy of PET-CT for mediastinal lymph node staging. The overall sensitivity, specificity, positive and negative predictive values, and accuracy of PET-CT were reported as 45-86%, 64-100%, 47-100%, 86-95% and 67-90%, respectively.26-30 The proportions of the diagnostic methods of biopsy, PET-CT, and repeated CT were 28.1%, 43.8%, and 28.1%, respectively, and the 2-year OS rates were 50.0%, 59.6% and 27.7%, respectively, in the current study. Though not statistically significant, the patients who had to wait a few months before initiating salvage RT and were mostly diagnosed in the earlier study period, showed relatively poorer survival. Even though the decision based on PET-CT alone may have the risk of overestimation of recurrence, its positive contribution to the improved outcomes is worthy of mention.
The role of elective regional lymph node irradiation in salvage RT is unclear. For many years, the standard target volume of radical RT for primary NSCLC usually included regional nodes electively based on high incidence of hilar and mediastinal lymph node metastasis. Hence, consideration of elective regional lymph node irradiation in salvage treatment setting seems instinctively logical, and most studies, including the latest study by Kelsey, et al.,6 employed the elective regional nodal irradiation. However, recent clinical data on RT only to the involved sites in primary NSCLC showed no correlation between RT field size and outcomes, and low incidence of nodal failure at the unirradiated lymphatics (6.4%).31,32 Tada, et al.21 reported patterns of failure after salvage RT in relation to the RT field in patients with LRR after surgery. They individualized the radiation target volume from only recurrent lesion to elective hilar and mediastinum and found that a narrow RT field did not cause frequent marginal relapse. In our study, only recurrent sites were treated without elective regional lymph node irradiation, and 45.3% of patients had IFF and 62.5% had ITF, which is comparable with other series (50-60%). This implies that high dose RT which is focused only to the involved lesion would be effective in the salvage setting. A few factors might have contributed to the rationales of applying the minimal RT volume: the accurate re-staging by PET-CT; absence of recurrence at other sites after a certain disease-free interval; possible changes in the lymphatic circulation following initial surgery; the use of high conformal radiation dose with the addition of chemotherapy; and improved RT technique.
Most studies did not report prognostic factors associated with the survival, because of small sample size. Until now, known prognostic factors are as follows; gender, disease-free interval, recurrent site, initial stage, recurrent stage, radiation dose and CCRT.6,15,16,20,22 We also found some prognostic factors on survival; the initial surgical method, the interval till the postoperative recurrence, the radiographic tumor response, radiation dose and the addition of chemotherapy. With respect to the radiation dose, Jeremic, et al.20 showed that there was a significant difference in the median survival time (18 vs. 7 months) and the 5-year survival rates (14 vs. 0%) (p=0.000) between high-dose and low-dose RT groups. This concurred with our results, which showed that the low dose group with BED ≤70.2 Gy10 had 2-years survival rates of 36.2% and high dose group with BED >70.2 Gy10 had 59.2% (p=0.018). In addition, it is thought that post-resection recurrent NSCLC patients treated with salvage RT have similar survivals to primary NSCLC patients treated with radical RT.22 Considering that the currently recommended dose for primary NSCLC is 60-70 Gy with conventional fractionation, we suggest higher dose than BED >70.2 Gy10 for salvage treatment. However, further study for the dose escalation may be needed. With respect to the addition of chemotherapy, a few reports on salvage RT combined with chemotherapy have been published. Shaw, et al.15 and Kelsey, et al.6 found no survival improvement by the addition of systemic chemotherapy to RT, whereas Cai, et al.22 observed that the addition of chemotherapy to RT proved prognostic factor significantly for the progression-free survival compared with RT alone (p=0.022), although the difference in the OS did not reach a statistical significance. On the other hand, our study showed that significantly better 2-year survival was achieved with CCRT when compared with RT alone (65.2% vs. 43.5%, p=0.029 by univariate analysis and p=0.038 by multivariate analysis). It should be noted, however, that the patients' age in the current study was younger and the average RT dose was higher in those who received CCRT than those who received RT alone.
It is generally thought that patients with bronchial stump recurrence only do better than patients with non-stump recurrence.33 There were 14 patients with bronchial stump only recurrence in the current study. However, there was no difference in survivals between bronchial stump recurrence only and other LRR. We speculate that there are several reasons for this difference. First, 3 patients in this study accounted for true stage I and other 11 patients had more extensive bronchial component of disease. Law, et al.34 reported that patients with bronchial stump recurrence only had better survival than patients with more extensive bronchial stump component of disease. In addition, the median time from the date of initial surgery to LRR was 6 months (range, 3-9 months) in these 3 patients. Considering the fact that the interval from surgery till recurrence is a significant prognostic factor, these adverse factors might negatively affect the survival in patients with bronchial stump recurrence only. Second, among other LRR, 14 patients had only single lymph node metastasis in the current study. Rea, et al.35 reported that the involvement of main bronchial nodes has a prognostic significance similar to that of N2 single station and should be considered as an early N2 disease. Recently, it is thought that pathologic N2 disease has heterogeneous nature and necessitates sub-classification: the best outcome in the case of single nodal chain.36 Since proximity of bronchial stump recurrence to main bronchus is classified as cT 3 or 4 and advanced T stage above 3 has similar survival to pN1, patients with single N2 lymph node recurrence might have similar survival to the patients with more extensive bronchial stump recurrence. Consequently, further study is needed to determine the prognosis of bronchial stump recurrence.
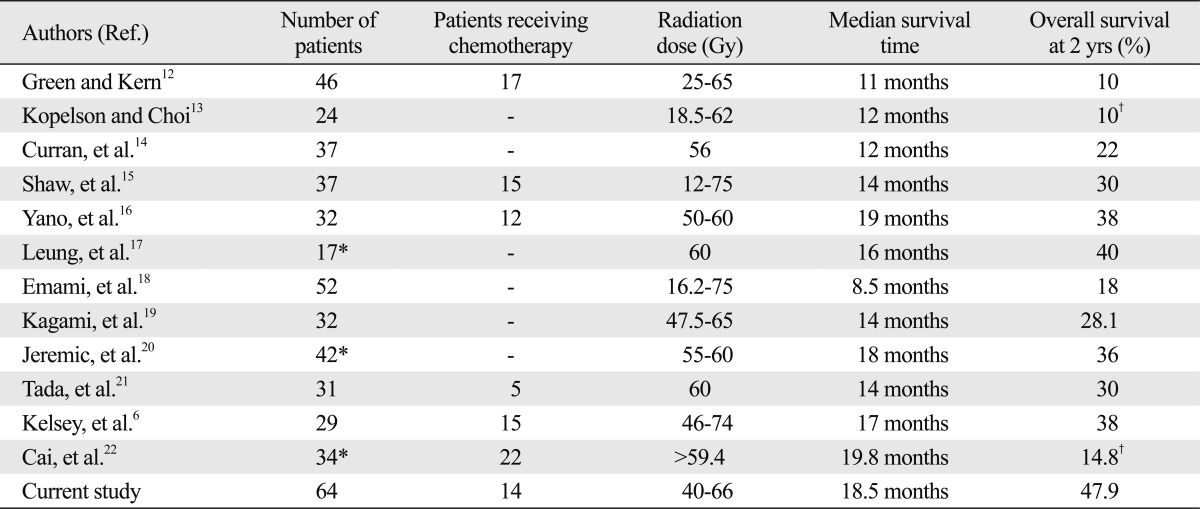
Several studies that were based on rather small number of patients, usually in a single institution have shown the benefits of salvage RT for LRR of NSCLC (Table 4). The reported median survival ranged from 11 to 19.8 months, and the 2-year OS rates did 10-40%. To our knowledge, the present study, which included 64 patients, is the largest series in the literature with favorable outcomes when compared to the previous reports: the median survival time was 18.5 months; and the 2-year OS rate was 47.9%. Favorable outcomes could have been possible because of relatively less tumor burden in the patients with LRR using improved diagnostic tools, and accordingly relatively smaller radiation target volume while delivering high conformal radiation dose. Based on these findings, the patients with LRR following surgical resection alone could be effectively salvaged by high dose RT.
Table 4.
Results of Salvage Radiation Therapy for Loco-Regional Recurrent NSCLC
NSCLC, non-small cell lung cancer.
*Includes only those treated with curative aim.
†5-years overall survival rate.
Our study has some limitations. First, this study was a retrospectively analysis. It had heterogeneous patient's group and radiation dose. The results may be affected by selection biases. Second, biopsy confirmation of the recurrent lesion was possible in 18 patients (28.1%). Because most patients were diagnosed with imaging method, it might have the risk of overestimation of recurrence and affect the result of salvage treatment. Third, we evaluated radiographic tumor response by chest CT taken 1 month post completion of salvage RT. This might be too early, since response can sometimes be further prolonged, thus leading to under-estimation of response to salvage RT. To confirm the effectiveness of involved field RT without elective regional lymph node irradiation and usefulness of CCRT, prospective trial including large number of patients might be needed.
In conclusion, the current study showed favorable survival rates when compared to other published studies of salvage RT. The involved field RT without elective regional lymph node irradiation seemed to be effective as a salvage treatment for LRR after complete resection of NSCLC. Aggressive treatment such as CCRT is strongly encouraged for improvement of salvage treatment efficacy as long as patients can tolerate such treatment.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Pearson FG. Non-small cell lung cancer: role of surgery for stages I-III. Chest. 1999;116(6 Suppl):500S–503S. doi: 10.1378/chest.116.suppl_3.500s. [DOI] [PubMed] [Google Scholar]
- 2.Matthews MJ, Kanhouwa S, Pickren J, Robinette D. Frequency of residual and metastatic tumor in patients undergoing curative surgical resection for lung cancer. Cancer Chemother Rep 3. 1973;4:63–67. [PubMed] [Google Scholar]
- 3.Lafitte JJ, Ribet ME, Prévost BM, Gosselin BH, Copin MC, Brichet AH. Postresection irradiation for T2 N0 M0 non-small cell carcinoma: a prospective, randomized study. Ann Thorac Surg. 1996;62:830–834. doi: 10.1016/s0003-4975(96)00507-3. [DOI] [PubMed] [Google Scholar]
- 4.al-Kattan K, Sepsas E, Fountain SW, Townsend ER. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg. 1997;12:380–384. doi: 10.1016/s1010-7940(97)00198-x. [DOI] [PubMed] [Google Scholar]
- 5.Weisenburger TH. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. LCSG 773. Chest. 1994;106(6 Suppl):297S–301S. [PubMed] [Google Scholar]
- 6.Kelsey CR, Clough RW, Marks LB. Local recurrence following initial resection of NSCLC: salvage is possible with radiation therapy. Cancer J. 2006;12:283–288. doi: 10.1097/00130404-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 8.Gabler A, Liebig S. Reoperation for bronchial carcinoma. Thorax. 1980;35:668–670. doi: 10.1136/thx.35.9.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe Y, Shimizu J, Oda M, Tatsuzawa Y, Hayashi Y, Iwa T. Second surgical intervention for recurrent and second primary bronchogenic carcinomas. Scand J Thorac Cardiovasc Surg. 1992;26:73–78. doi: 10.3109/14017439209099057. [DOI] [PubMed] [Google Scholar]
- 10.Voltolini L, Paladini P, Luzzi L, Ghiribelli C, Di Bisceglie M, Gotti G. Iterative surgical resections for local recurrent and second primary bronchogenic carcinoma. Eur J Cardiothorac Surg. 2000;18:529–534. doi: 10.1016/s1010-7940(00)00572-8. [DOI] [PubMed] [Google Scholar]
- 11.Yamaoka N, Uchiyama Y, Nakamura A, Muraoka M, Kondou M, Yamauchi H, et al. [Reoperation for lung cancer: indications and operation methods in cardiopulmonary function] Kyobu Geka. 1995;48:18–23. [PubMed] [Google Scholar]
- 12.Green N, Kern W. The clinical course and treatment results of patients with postresection locally recurrent lung cancer. Cancer. 1978;42:2478–2482. doi: 10.1002/1097-0142(197811)42:5<2478::aid-cncr2820420551>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Kopelson G, Choi NC. Radiation therapy for postoperative local-regionally recurrent lung cancer. Int J Radiat Oncol Biol Phys. 1980;6:1503–1506. doi: 10.1016/0360-3016(80)90007-3. [DOI] [PubMed] [Google Scholar]
- 14.Curran WJ, Jr, Herbert SH, Stafford PM, Sandler HM, Rosenthal SA, McKenna WG, et al. Should patients with post-resection locoregional recurrence of lung cancer receive aggressive therapy? Int J Radiat Oncol Biol Phys. 1992;24:25–30. doi: 10.1016/0360-3016(92)91016-g. [DOI] [PubMed] [Google Scholar]
- 15.Shaw EG, Brindle JS, Creagan ET, Foote RL, Trastek VF, Buskirk SJ. Locally recurrent non-small-cell lung cancer after complete surgical resection. Mayo Clin Proc. 1992;67:1129–1133. doi: 10.1016/s0025-6196(12)61141-0. [DOI] [PubMed] [Google Scholar]
- 16.Yano T, Hara N, Ichinose Y, Asoh H, Yokoyama H, Ohta M, et al. Local recurrence after complete resection for non-small-cell carcinoma of the lung. Significance of local control by radiation treatment. J Thorac Cardiovasc Surg. 1994;107:8–12. [PubMed] [Google Scholar]
- 17.Leung J, Ball D, Worotniuk T, Laidlaw C. Survival following radiotherapy for post-surgical locoregional recurrence of non-small cell lung cancer. Lung Cancer. 1995;13:121–127. doi: 10.1016/0169-5002(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 18.Emami B, Graham MV, Deedy M, Shapiro S, Kucik N. Radiation therapy for intrathoracic recurrence of non-small cell lung cancer. Am J Clin Oncol. 1997;20:46–50. doi: 10.1097/00000421-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kagami Y, Nishio M, Narimatsu N, Mjoujin M, Sakurai T, Hareyama M, et al. Radiotherapy for locoregional recurrent tumors after resection of non-small cell lung cancer. Lung Cancer. 1998;20:31–35. doi: 10.1016/s0169-5002(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 20.Jeremic B, Shibamoto Y, Milicic B, Milisavljevic S, Nikolic N, Dagovic A, et al. External beam radiation therapy alone for loco-regional recurrence of non-small-cell lung cancer after complete resection. Lung Cancer. 1999;23:135–142. doi: 10.1016/s0169-5002(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 21.Tada T, Fukuda H, Nakagawa K, Matsui K, Hosono M, Takada Y, et al. Non-small cell lung cancer: radiation therapy for locoregional recurrence after complete resection. Int J Clin Oncol. 2005;10:425–428. doi: 10.1007/s10147-005-0526-5. [DOI] [PubMed] [Google Scholar]
- 22.Cai XW, Xu LY, Wang L, Hayman JA, Chang AC, Pickens A, et al. Comparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1100–1105. doi: 10.1016/j.ijrobp.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 23.American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. pp. 167–184. [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80:1207–1213. doi: 10.1016/j.athoracsur.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Kim BT, Lee KS, Shim SS, Choi JY, Kwon OJ, Kim H, et al. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT--a prospective study. Radiology. 2006;241:501–509. doi: 10.1148/radiol.2412051173. [DOI] [PubMed] [Google Scholar]
- 28.Lee BE, von Haag D, Lown T, Lau D, Calhoun R, Follette D. Advances in positron emission tomography technology have increased the need for surgical staging in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;133:746–752. doi: 10.1016/j.jtcvs.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Melek H, Gunluoglu MZ, Demir A, Akin H, Olcmen A, Dincer SI. Role of positron emission tomography in mediastinal lymphatic staging of non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33:294–299. doi: 10.1016/j.ejcts.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Billé A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36:440–445. doi: 10.1016/j.ejcts.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Dosoretz DE, Galmarini D, Rubenstein JH, Katin MJ, Blitzer PH, Salenius SA, et al. Local control in medically inoperable lung cancer: an analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys. 1993;27:507–516. doi: 10.1016/0360-3016(93)90373-4. [DOI] [PubMed] [Google Scholar]
- 32.Rosenzweig KE, Sim SE, Mychalczak B, Braban LE, Schindelheim R, Leibel SA. Elective nodal irradiation in the treatment of non-small-cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:681–685. doi: 10.1016/s0360-3016(01)01482-1. [DOI] [PubMed] [Google Scholar]
- 33.Jeremic B, Bamberg M. External beam radiation therapy for bronchial stump recurrence of non-small-cell lung cancer after complete resection. Radiother Oncol. 2002;64:251–257. doi: 10.1016/s0167-8140(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 34.Law MR, Henk JM, Lennox SC, Hodson ME. Value of radiotherapy for tumour on the bronchial stump after resection for bronchial carcinoma. Thorax. 1982;37:496–499. doi: 10.1136/thx.37.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rea F, Marulli G, Callegaro D, Zuin A, Gobbi T, Loy M, et al. Prognostic significance of main bronchial lymph nodes involvement in non-small cell lung carcinoma: N1 or N2? Lung Cancer. 2004;45:215–220. doi: 10.1016/j.lungcan.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Wang LM, Bao F, Jiang GN, Xie HK, Ding JA, et al. Re-appraisal of N2 disease by lymphatic drainage pattern for non-small-cell lung cancers: by terms of nodal stations, zones, chains, and a composite. Lung Cancer. 2011;74:497–503. doi: 10.1016/j.lungcan.2011.03.020. [DOI] [PubMed] [Google Scholar]