Abstract
A 27-year-old man presented at the emergency room with hemoptysis. His blood pressure was 180/100 mm Hg, and he had no history of hypertension. Chest radiographs showed bilateral infiltration, suggestive of alveolar hemorrhage. His laboratory data were consistent with acute kidney injury. His serum creatinine level increased abruptly; therefore, renal biopsy was performed. Steroid pulse therapy was administered because of a strong suspicion of immune-mediated pulmonary renal syndrome. Renal biopsy showed proliferative endarteritis, fibrinoid necrosis, and intraluminal thrombi in the vessels without crescent formation or necrotizing lesions. Steroid pulse therapy rapidly tapered and stopped. His serum creatinine level gradually decreased with strict blood pressure control. Ten months after discharge, his blood pressure was approximately 120/80 mm Hg with a serum creatinine level of 1.98 mg/dL. Pulmonary renal syndrome is generally caused by an immune-mediated mechanism. However, malignant hypertension accompanying renal insufficiency and heart dysfunction causing end-organ damage can create a pulmonary hemorrhage, similar to pulmonary renal syndrome caused by an immune-mediated mechanism. The present case shows that hypertension, a common disease, can possibly cause pulmonary renal syndrome, a rare condition.
Keywords: Malignant hypertension, hemoptysis, pulmonary renal syndrome
INTRODUCTION
Acute renal failure accompanying pulmonary hemorrhage, known as pulmonary renal syndrome, is generally caused by an immune-mediated mechanism. However, malignant hypertension with left ventricular dysfunction may cause alveolar hemorrhage followed by pulmonary edema. Such a condition can mimic immune-mediated pulmonary renal syndrome.1 Here, we report an unusal case with malignant hypertension and pulmonary hemorrhage as the initial manifestation, which was confirmed by renal biopsy.
CASE REPORT
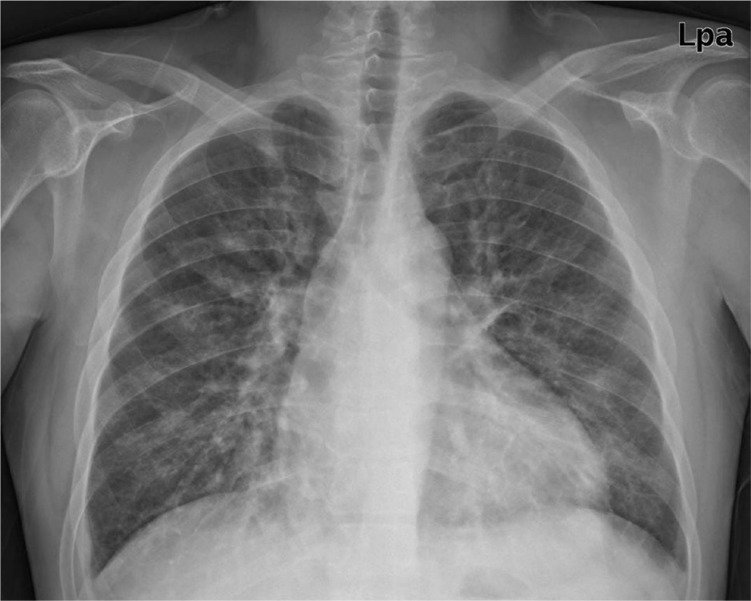
A 27-year-old man visited the emergency room with hemoptysis. His blood pressure was 180/100 mm Hg, and he had tachycardia (110/min) and tachypnea (24/min). He had been a heavy smoker, but had never been diagnosed with hypertension. Laboratory data was as follows: white blood cell count 13150/µL; hemoglobin level, 11.3 g/dL; platelet count, 110×103/µL; serum urea nitrogen level, 32 mg/dL; and serum creatinine level, 3.11 mg/dL. His serum electrolyte levels were as follows: sodium, 120 mEq/L; potassium, 2.5 mEq/L; chloride, 80 mEq/L; calcium, 7.5 mg/dL; and phosphorus, 3.2 mg/dL. Urinalysis showed microscopic hematuria and proteinuria (+++). Total protein to creatinine ratio in spot urine was 6.08 mg/g. Pro-brain natriuretic peptide level was markedly increased. Chest radiograph (Fig. 1) and computed tomography showed bilateral infiltration, suggesting alveolar hemorrhage. Renal ultrasound using a Doppler scan showed increased echogenicity in both normal-sized kidneys without renal artery stenosis. Goodpasture's syndrome, SLE, microscopic polyangiitis or PAN was strongly suspected.
Fig. 1.
Chest radiograph showing infiltration in both lower lobes and cardiomegaly.
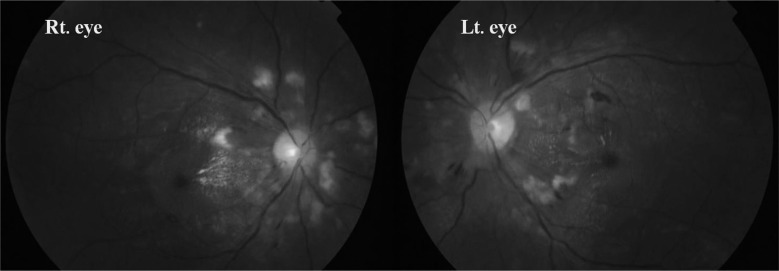
On the second hospital day, his serum creatinine level increased to 4.65 mg/dL. Anti-nuclear antibody test was negative, complement levels were normal, plasma renin activity was 6.27 ng/L·s (reference, 0.28-0.69 ng/L·s), serum aldosterone level was 6.54 nmol/L (reference, 0.11-0.28 nmol/L), and serum parathyroid hormone level was 159.1 pg/mL (reference, 14-72 pg/mL). Peripheral blood smear (PBS) showed some helmet cells suggestive of micrcoangiopathic hemolysis. Fundoscopic examination showed cotton wool spots and splinter hemorrhage with papillary edema, consistent with hypertensive changes (Fig. 2).
Fig. 2.
Fundoscopic examination showing multiple cotton wool spots and a splinter hemorrhage.
On the third day, his serum creatinine level increased to 4.91 mg/dL. Steroid pulse therapy was administered because of a strong suspicion of immune-mediated pulmonary renal syndrome.
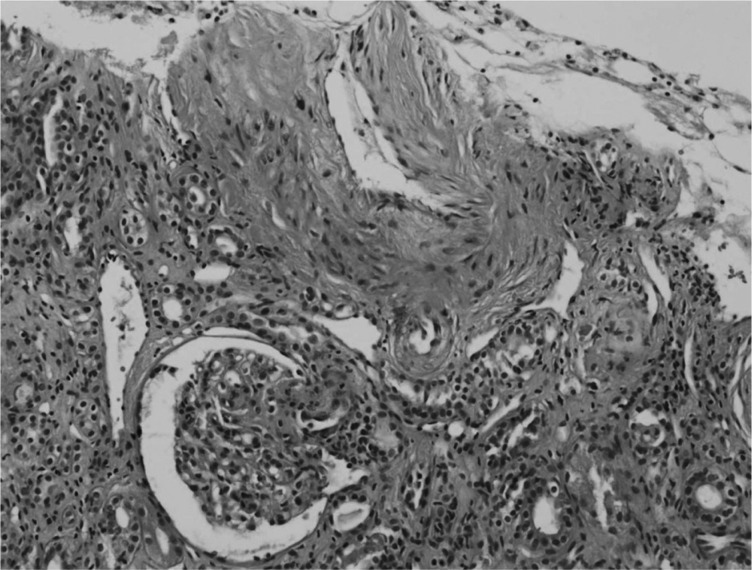
A renal biopsy and bronchoscopy were promptly performed. Bronchoscopic examination showed mild hyperemia in both bronchi, with red bronchial alveolar lavage fluid. Light microscopic examination of the renal biopsy showed proliferative endarteritis in interlobular arteries and fibrinoid necrosis and intramural thrombi of small arterioles without crescent formation or necrotizing lesions, consistent with hypertensive nephrosclerosis (Fig. 3). Steroid therapy was rapidly decreased and stopped. A trans-bronchial lung biopsy showed macrophage infiltration into alveolar spaces and goblet cell metaplasia in bronchial mucosa generally seen in heavy smokers. Vanillyl-mandelic acid, metanephrine, epinephrine, and norepinephrine in 24-h urine samples were within normal levels. Tests for anti-glomerular basement membrane antibody and anti-neutrophil cytoplasmic antibody (ANCA) were negative. An echocardiogram showed eccentric left ventricular hypertrophy with decreased systolic and diastolic dysfunctions and a dilated left atrium. In the further reports of the patient's renal biopsy, immunofluorescent studies showed fine granular deposition of C3 and C1q only within the vessel walls and electron microscopic examination showed focal effacement of the epithelial cell foot processes, focally swollen endothelial cell with irregularly thickened capillary basement membrane and normal mesangial basement membrane without deposition.
Fig. 3.
Light microscopy examination showing intima fibrous hyperplasia, smooth muscle cell hyperplasia in the media of interlobular artery, and a collapsed glomerulus by ischemia (H&E ×200).
His serum creatinine level increased to 5.15 mg/dL on the fourth day, but he did not require dialysis. Serum creatinine level gradually decreased with strict blood pressure control and was 4.03 mg/dL at discharge. The patient last visited the outpatient clinic 10 months after discharge: his blood pressure was 120/80 mm Hg and well controlled with antihypertensive mediation, and his serum creatinine level was 1.98 mg/dL.
DISCUSSION
A patient presenting with malignant hypertension with pulmonary hemorrhage and rapidly deteriorating renal function presented at our hospital. This unusual case suggested immune-mediated pulmonary renal syndrome. Immune-mediated pulmonary renal syndrome has a poor prognosis in the absence of early appropriate immunosuppression.2 Steroid therapy was administered in our patient, however when the renal biopsy showed only thrombotic microangiopathy without crescent formation or necrotizing vasculitis, the therapy was rapidly decreased and stopped. The patient's renal function improved as his blood pressure was controlled.
Malignant hypertension is characterized by elevated blood pressure accompanying encephalopathy or acute nephropathy as target organ damage.3 In malignant hypertension, the damaged endothelium increases permeability and activates coagulation cascade, including platelet activation and fibrin deposition. Red blood cells are destroyed within vessels and helmet cells or schistocytes can be seen in PBS, resulting in end-organ ischemia.4 In contrast, in immune-mediated systemic diseases such as Goodpasture's syndrome, SLE, microscopic polyangiitis and PAN, immune complex or direct vasculitis related to autoantibodies destroys body organs, and the inflammatory cell infiltration or active necrotizing lesion is seen on histology.2
Few cases of malignant hypertension mimicking immune-mediated pulmonary renal syndrome have been reported.1,5,6 Hida, et al.1 showed that alveolar capillaries might be injured as the capillaries in systemic circulation were injured by malignant hypertension, resulting in alveolar hemorrhage. However, Sato, et al.5 and Aithal, et al.6 concluded that left ventricular dysfunction resulting from systemic hypertension can cause pulmonary edema; thus leading to hemorrhage. Pulmonary circulation is somewhat free of systemic hypertension because it is separated from the systemic circulation by the heart; however, it can be affected by systemic hypertension with left ventricular dysfunction. Renal ischemia from renal artery stenosis, malignant hypertension or renin producing tumor may cause hyponatremic-hypertensive syndrome. Secondary aldosteronism with the concomitant elevation of serum renin level and the subsequent activation of ADH caused by volume depletion from pressure natriuresis manifest hyponatremia together with hypokalemia in this syndrome.7 Therefore, the initial hyponatremia and hypokalemia in our patient can be one of the manifestations by malignant hypertension.
Rapid ELISA tests for anti-GBM antibody and ANCAs yielding results within 30 minutes may help for early differential diagnoses in the cases where immune-mediated pulmonary renal syndrome are suspected, although these rapid tests cannot be substituted for renal biopsy for confirmative diagnosis because small numbers of false negative tests exist and correct diagnosis in the cases with RPGN is essential for improving the patients' overall prognosis.8,9
Thus, we suggest that for patients with pulmonary renal syndrome, common illnesses like hypertension should not be overlooked.
ACKNOWLEDGEMENTS
This case report was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hida K, Wada J, Odawara M, Kunitomi M, Hayakawa N, Kashihara N, et al. Malignant hypertension with a rare complication of pulmonary alveolar hemorrhage. Am J Nephrol. 2000;20:64–67. doi: 10.1159/000013558. [DOI] [PubMed] [Google Scholar]
- 2.Hirayama K, Yamagata K, Kobayashi M, Koyama A. Anti-glomerular basement membrane antibody disease in Japan: part of the nationwide rapidly progressive glomerulonephritis survey in Japan. Clin Exp Nephrol. 2008;12:339–347. doi: 10.1007/s10157-008-0051-8. [DOI] [PubMed] [Google Scholar]
- 3.Slama M, Modeliar SS. Hypertension in the intensive care unit. Curr Opin Cardiol. 2006;21:279–287. doi: 10.1097/01.hco.0000231396.56738.d8. [DOI] [PubMed] [Google Scholar]
- 4.Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19:502–512. doi: 10.1111/j.1525-139X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, Hara S, Yamada K, Fujimoto S. A rare case of alveolar haemorrhage due to malignant hypertension. Nephrol Dial Transplant. 2005;20:2289–2290. doi: 10.1093/ndt/gfi017. [DOI] [PubMed] [Google Scholar]
- 6.Aithal S, Marley N, Venkat-Raman G. An unusual non-immunological cause of renal pulmonary syndrome. Clin Nephrol. 2009;72:322–325. doi: 10.5414/cnp72322. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal M, Lynn KL, Richards AM, Nicholls MG. Hyponatremic-hypertensive syndrome with renal ischemia: an underrecognized disorder. Hypertension. 1999;33:1020–1024. doi: 10.1161/01.hyp.33.4.1020. [DOI] [PubMed] [Google Scholar]
- 8.Saxena R, Isaksson B, Bygren P, Wieslander J. A rapid assay for circulating anti-glomerular basement membrane antibodies in Goodpasture syndrome. J Immunol Methods. 1989;118:73–78. doi: 10.1016/0022-1759(89)90055-0. [DOI] [PubMed] [Google Scholar]
- 9.Westman KW, Bygren PG, Eilert I, Wiik A, Wieslander J. Rapid screening assay for anti-GBM antibody and ANCAs; an important tool for the differential diagnosis of pulmonary renal syndromes. Nephrol Dial Transplant. 1997;12:1863–1868. doi: 10.1093/ndt/12.9.1863. [DOI] [PubMed] [Google Scholar]