Sir,
Nonsedating antihistamines are recommended as first-line treatment for patients with urticaria. In chronic spontaneous urticaria, wheals arise spontaneously for more than 6 weeks without external physical stimuli. The current European Academy of Allergology and Clinical Immunology/Global Allergy and Asthma European Network/European Dermatology Forum (EAACI/GA2 LEN/EDF) guidelines call for updosing of nonsedating antihistamines (up to four times the standard dose) in urticaria patients who do not respond satisfactorily to the standard doses.[1] There are a few studies carried out to assess the efficacy of such a recommendation.
Fexofenadine is one of the several second-generation H 1-antihistamines approved for the treatment of various allergic disorders; however, it shows numerous unique properties that make it an optimal choice for many patients. Fexofenadine is devoid of sedative and anticholinergic effects and may offer equivalent or greater efficacy in treating allergic disorders compared with other currently available second-generation H1-antihistamines.[2]
We tried fexofenadine in chronic spontaneous urticaria in higher doses. Thirty-seven patients (17 females and 20 males; age group, 18-60 years; mean age, 35.13 years) with chronic spontaneous urticaria for at least 6 weeks; and pruritus, wheal score of more than 2 were enrolled after obtaining an informed written consent. The exclusion criteria included physical urticaria, urticarial vasculitis, pregnant or lactating women, gastritis, a history of sensitivity to aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), hypertension, diabetes, kidney diseases and a history of aggravation of symptoms by pressure. Routine investigations like complete blood count, blood sugar, thyroid stimulating hormone (TSH) and urine examination were done to rule out infections before starting therapy. All 37 patients had chronic spontaneous urticaria of duration ranging from 3 months to 2 years (mean duration, 7.84 months).
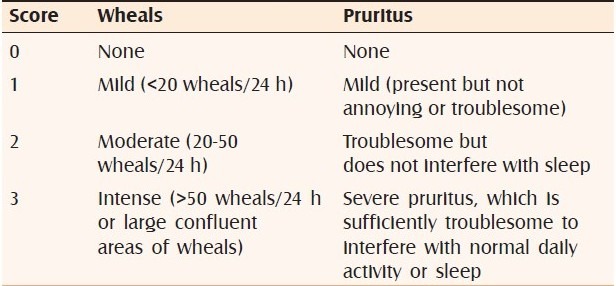
After a 1-day washout without treatment, we graded symptoms using the urticaria activity score (UAS). The UAS measures two symptoms — number of wheals and intensity of itching — each on a 0-3 scale each day. The UAS was recorded by each patient daily and was obtained from the patients weekly. The number of wheals was scored from 0 to 3: 0, no wheals; 1, less than 20 wheals; 2, 20-50 wheals; 3, >50 wheals almost covered large confluent areas of wheals. Severity of itch was scored as 0, none; 1, mild; 2, moderate; and 3, severe. One has to add both these scores, viz., for both the number of wheals and the severity of itch, on a given day for each of the days in a given week to get the weekly UAS [Table 1]. The possible weekly aggregate UAS thereby ranged from 0 to 42.[3] Sedation was graded from 0 to 3 (0, none; 1, mild; 2, moderate; and 3, severe). To monitor urticaria, we recorded UAS at the beginning and at the end of 1 week, 2 and 4 weeks of treatment.
Table 1.
Urticaria activity score
All patients were started with fexofenadine 180 mg tablet in the morning after breakfast. Patients were reviewed at weekly intervals for 4 weeks. For symptomatic patients, the dose of fexofenadine was doubled to 360 mg of fexofenadine (2 tablets) in two divided doses at the end of 1 week; and 3 tablets of fexofenadine (540 mg) in three divided doses at the end of 2 weeks. Investigations revealed microcytic anemia in 5 patients and raised thyroid-stimulating hormone (TSH) in 3 patients. Appropriate treatment was given to these patients in the form of hematinics and thyroid supplements. Average daily UAS was 3.6 at 0 weeks, which came down to 2.27 at the end of 1 week. At the end of 1 week, 26 patients out of 37 were symptomatic. We doubled the dose to 360 mg of fexofenadine in divided doses. At 2 weeks, UAS was 1.2. At the end of 2 weeks, 14 out of 26 patients were symptomatic, whose dose was tripled to 540 mg of fexofenadine in three divided doses. At the end of 4 weeks, UAS came down to 0.19. Difference between UAS at week 0 and that at week 4 was statistically significant (P value, < 0.05). At 540 mg dose of fexofenadine, 13 out of 14 patients were asymptomatic. Electrocardiographic study was done in 5 patients who were on 540 mg of fexofenadine and was within normal limits. One patient required second-line treatment in the form of methotrexate.
Sedation was recorded as 0, mild, moderate or severe. One patient with 540 mg of fexofenadine complained of sedation, which was mild. Headache was reported in 2 patients with higher doses. Eleven, 12 and 13 patients became asymptomatic when administered 180, 360 and 540 mg fexofenadine, respectively. We have reported updosing with levocetirizine to control chronic urticaria in Indian patients.[4] The cardiovascular safety of fexofenadine has also been thoroughly investigated. These studies, involving approximately 6,000 patients, have shown that fexofenadine is not associated with cardiotoxicity; it does not inhibit cardiac K+ channels and has no apparent effect on heart rate or QT interval.[5] Fexofenadine HCl at single doses up to 800 mg once daily and multiple doses up to 690 mg b.d. for 28 days in healthy volunteers resulted in no increase in QTc interval. Recommended dose range of fexofenadine is 120-180 mg daily.[6] In a study comparing fexofenadine (360mg) versus promethazine (30mg), fexofenadine was found to be free from disruptive effects on aspects of psychomotor and cognitive function. The identification of antihistamine fexofenadine as being devoid of central effects even at supraclinical doses separates it from currently available first- and second-generation drugs, with no objective evidence of CNS side effects on cognition and psychomotor function.[7]
We found fexofenadine in higher doses effectively controlled urticaria in majority of the patients. Side effects were noted in the form of headache and sedation.
REFERENCES
- 1.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Gimιnez-Arnau AM, et al. EAACI/GA2LEN/EDF guideline: management of urticaria. Allergy. 2009;64:1427–43. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Gums JG. Fexofenadine: biochemical, pharmacokinetic and pharmacodynamic properties and its unique role in allergic disorders. Expert Opin Drug Metab Toxicol. 2009;5:813–22. doi: 10.1517/17425250903044967. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Gimιnez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417–26. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 4.Godse KV. Updosing of antihistamines to improve control of chronic urticaria. Indian J Dermatol Venereol Leprol. 2010;76:61–2. doi: 10.4103/0378-6323.58684. [DOI] [PubMed] [Google Scholar]
- 5.Pratt CM, Mason J, Russell T, Reynolds R, Ahlbrandt R. Cardiovascular safety of fexofenadine HCl. Am J Cardiol. 1999;83:1451–4. doi: 10.1016/s0002-9149(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 6.Pratt C, Brown AM, Rampe D, Mason J, Russell T, Reynolds R, et al. Cardiovascular safety of fexofenadine HCl. Clin Exp Allergy. 1999;29:212–6. doi: 10.1046/j.1365-2222.1999.0290s3212.x. [DOI] [PubMed] [Google Scholar]
- 7.Hindmarch I, Shamsi Z, Kimber S. An evaluation of the effects of high-dose fexofenadine on the central nervous system: a double-blind, placebo-controlled study in healthy volunteers. Clin Exp Allergy. 2002;32:133–9. doi: 10.1046/j.0022-0477.2001.01245.x. [DOI] [PubMed] [Google Scholar]


