Abstract
Indian children often present with atypical forms of pityriasis rosea (PR). We describe a female infant with acrally located eruptions consistent with a clinical diagnosis of PR. This is an extremely rare presentation of the disease.
Keywords: Acral, atypical, herald plaque, infant, pityriasis rosea, palms and soles
INTRODUCTION
In a classic case of pityriasis rosea (PR), a herald plaque (mother patch) followed by the distribution of scaly oval eruptions typically on the trunk and proximal extremities along the Langer's lines of cleavage gives the characteristic “Christmas tree appearance”. Uncharacteristic morphology and or distribution in a case of PR refer to a category of ‘Atypical PR’. Atypical clinical presentation of PR are seen in approximately 20% of patients.[1,2] In our experience, Indian children are possibly more predisposed than adults to present with atypical features of PR, especially those with atopic background. Atypical variants generally denote the morphology and distribution of secondary eruptions that follow a herald plaque.
Herein, we describe an Indian infant with atypical presentation of acral distribution of primary as well as secondary eruptions on distal extremities.
CASE REPORT
A 4-month-old healthy girl, born to a farmer couple after normal delivery, suffered from a transient episode of mild fever, slight irritability and loose motions of 2-days duration, which settled without any treatment. After 1 week, she presented to a pediatrician with sudden onset of an erythematous, mildly pruritic plaque on her left wrist, which he thought as urticaria possibly due to insect bite and prescribed cetrizine syrup and calamine lotion. The lesion gradually flattened but increased horizontally in size and began scaling from centre outwards in next few days. After 8 days, the child presented with sudden onset of multiple scaly plaques on hands and feet and was referred for dermatological opinion. On examination, the most initial lesion on left wrist was seen as an annular scaly plaque of irregular shape with relatively clear centre and a peripheral rim of collarette scale. [Figure 1] Multiple bilateral scaly plaques were seen on the anterior and lateral aspects of her lower legs and feet [Figure 2]. A few scaly eruptions were also present on soles, wrists and palms. A few dusky erythematous lesions were also seen on the palms and soles. [Figure 3] All the scaly lesions displayed annular morphology with central clearing and peripheral collarette scaling. Other anatomical areas including trunk, neck, face, flexures, genitalia and mucous membranes of nose, mouth, genitals and anus were uninvolved. There were no target lesions. There was no lymphadenopathy. Apart from cutaneous lesions, nothing abnormal was revealed in general and systemic examination of the child. There was no history of skin disease in her past or in family members of this patient especially tinea infection, psoriasis and atopic eczema. There was no history of asthma, urticaria, drug allergies, chronic rhinitis and genital ulceration in her parents.
Figure 1.
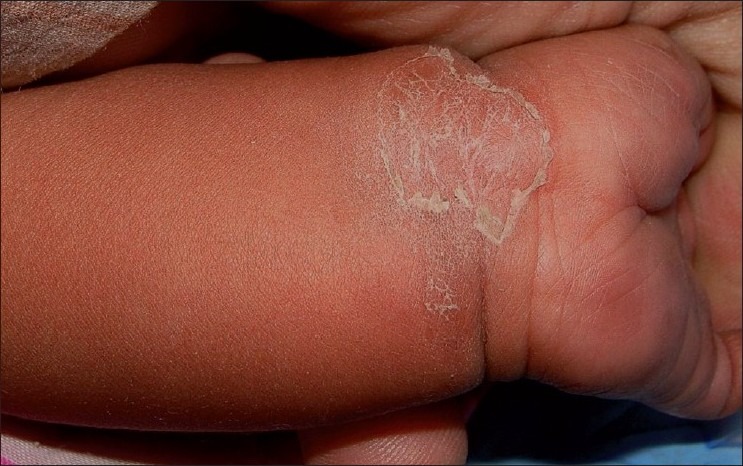
A large scaly annular herald plaque on left wrist of an infant with central clearing and peripheral rim of collarette scales. Dusky erythematous lesions on the left palm are also seen
Figure 2.
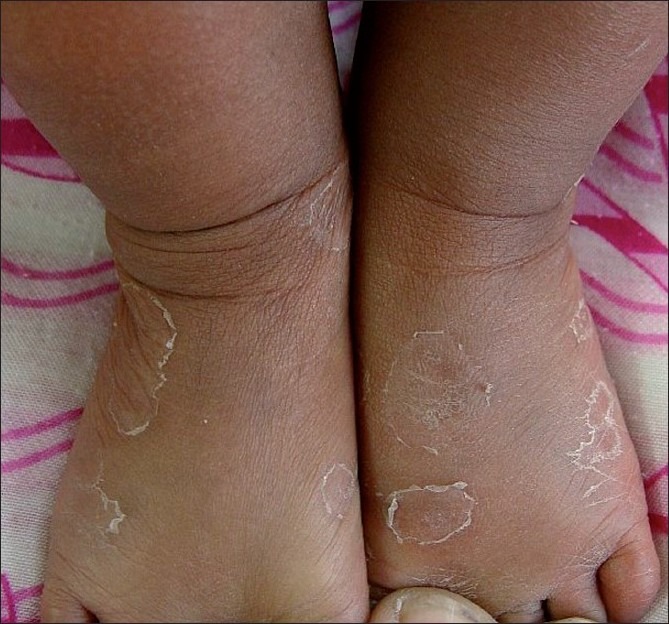
Smaller annular scaly plaques on the anterior aspect of lower legs and feet
Figure 3.
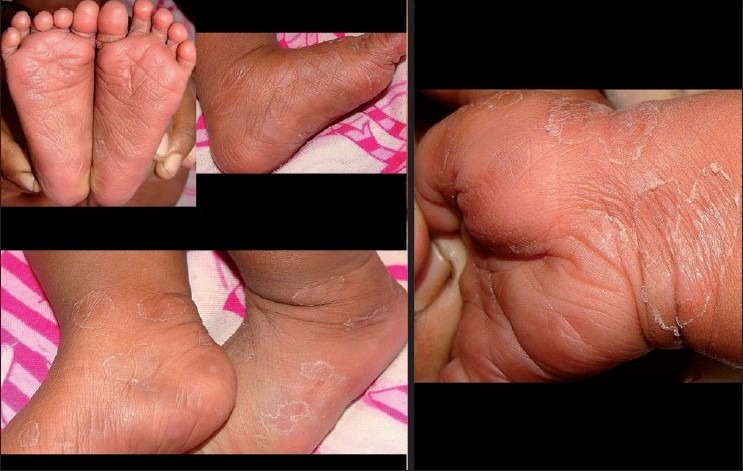
This picture clearly demonstrates the acral location of the eruptions in child, including the palmoplantar scaly lesions
Skin scrapings and KOH mount from the initial and subsequent lesions were negative for fungus. Complete blood counts and blood sugar level were normal. VDRL test and HIV antibodies were negative. Urinalysis and stool microscopy and culture were unremarkable. The skin biopsy revealed acute spongiotic dermatitis with sparse inflammatory mononuclear dermal infiltrate with no specific diagnostic clue.
A presumptive diagnosis of PR was made. The patient was treated with white petrolatum locally. Cetrizine syrup was continued for another 1 week. All the scaly lesions completely regressed over next 2 weeks, leaving slight hypopigmentation. There was no recurrence for the next 1 year of observation.
DISCUSSION
Considering the clinical events in this patient including the prodrome, clinical features and follow-up, we believe that PR was an appropriate diagnostic label for this patient, fulfilling the diagnostic criteria of PR.[3]
A finding of sudden onset of acrally distributed annular scaly plaques with palmoplantar involvement in such setting should prompt a clinician for considering dermatophytosis, psoriasis, secondary syphilis, drug eruption, erythema multiforme, acral necrolytic erythema, erythema annulare centrifugum and occasionally, atopic dermatitis. However, after careful evaluation, all these diagnoses seem unlikely in our patient.
Gastrointestinal symptoms presenting as prodrome is unusual as against the commoner upper respiratory system prodrome. Predominant acral and flexural (involvement of axillae, groins and face) is termed as PR inversa.[4] However, in our patient, the flexures were spared. Hence, it cannot be called as PR inversa. Thus, the presentation in our case was only acral.
Acrally distributed PR is itself a very rare variety of PR. One case of acral PR has been described in an adult Indian female patient.[5] Palmoplantar involvement is also rarely reported in literature and all reported patients were adults.[6–8] It is extremely unusual for a very young child to present with PR. One case of classical PR in such young infant has been reported in 1960.[9]
Very young age for presentation of PR, herald plaque as well as secondary eruptions on acral location and palmo-plantar involvement are atypical features of the case. Combined all three features together, possibly represents a novel presentation of the disease, hitherto unreported. To the best of our knowledge, we could not find publication on pubmed in English language searching strings ′acral, hands, feet, infant, palms, soles and PR’.
Amer et al.[10] pointed in their study of black American children that the natural history of PR is different from that mentioned in the textbooks. Our findings support his views.
Human herpes viruses are said to be currently favorite viral culprits for PR, though not conclusively proven.[11] Considering a hypothesis of viral origin in PR, it may be speculated that our patient may have had a viral gastrointestinal infection and may have resulted in the clinical syndrome of acral PR. Enteroviruses were claimed to have caused PR according to one study.[12] However, it was unfeasible for us to investigate this patient in depth from virological perspectives owing to inadequate resources.
This case of PR is presented here for its unique clinical features of exclusive acral lesions in an infant with palmo-plantar involvement, as a rare clinical entity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chuh A, Zawar V, Lee A. Atypical presentations of pityriasis rosea: case presentations. J Eur Acad Dermatol Venereol. 2005;19:120–6. doi: 10.1111/j.1468-3083.2004.01105.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez L, Allen R, Janniger C, Schwartz R. Pityriasis rosea: An important papulo-squamous disorder. Int J Dermatol. 2005;44:757–64. doi: 10.1111/j.1365-4632.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- 3.Chuh AA. Diagnostic criteria for pityriasis rosea-a prospective case control study for assessment of validity. J Eur Acad Dermatol. 2003;17:101–3. doi: 10.1046/j.1468-3083.2003.00519_4.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibney MD, Leonardi CL. Acute papulo-squamous eruptions of the extremities demonstrating isomorphic response.Inverse pityriasis rosea. Arch Dermatol. 1997;133:654. doi: 10.1001/archderm.133.5.651. [DOI] [PubMed] [Google Scholar]
- 5.Zawar V. Acral pityriasis rosea. 2010. Jul 25, [last cite on 2010 Jul 25]. http://wwwdermatlascom/derm/resultNoCachecfm .
- 6.Robati RM, Toossi P. Plantar herald patch in pityriasis rosea. Clin Exp Dermatol. 2009;34:269–70. doi: 10.1111/j.1365-2230.2008.02772.x. [DOI] [PubMed] [Google Scholar]
- 7.Bukhari I. Pityriasis rosea with Palmoplantar plaque lesions. Dermatol Online J. 2005;11:27. [PubMed] [Google Scholar]
- 8.Deng Y, Li H, Chen X. Palmoplantar pityriasis rosea: two case reports. J Eur Acad Dermatol Venereol. 2007;21:406–7. doi: 10.1111/j.1468-3083.2006.01891.x. [DOI] [PubMed] [Google Scholar]
- 9.Hyatt HW., Sr Pityriasis rosea in a three month old infant. Arch Pediatr. 1960;77:364–8. [PubMed] [Google Scholar]
- 10.Amer A, Fischer H, Li X. The natural history of pityriasis rosea in black American children: how correct is the “classic” description? Arch Pediatr Adolesc Med. 2007;161:503–6. doi: 10.1001/archpedi.161.5.503. [DOI] [PubMed] [Google Scholar]
- 11.Drago F, Broccolo F, Rebora A. Pityriasis rosea: an update with a critical appraisal of its possible herpesviral etiology. J Am Acad Dermatol. 2009;61:303–18. doi: 10.1016/j.jaad.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Chia JK, Shitabata P, Wu J, Chia AY. Enterovirus infection as a possible cause of pityriasis rosea: demonstration by immunochemical staining. Arch Dermatol. 2006;142:942–3. doi: 10.1001/archderm.142.7.942. [DOI] [PubMed] [Google Scholar]


