Abstract
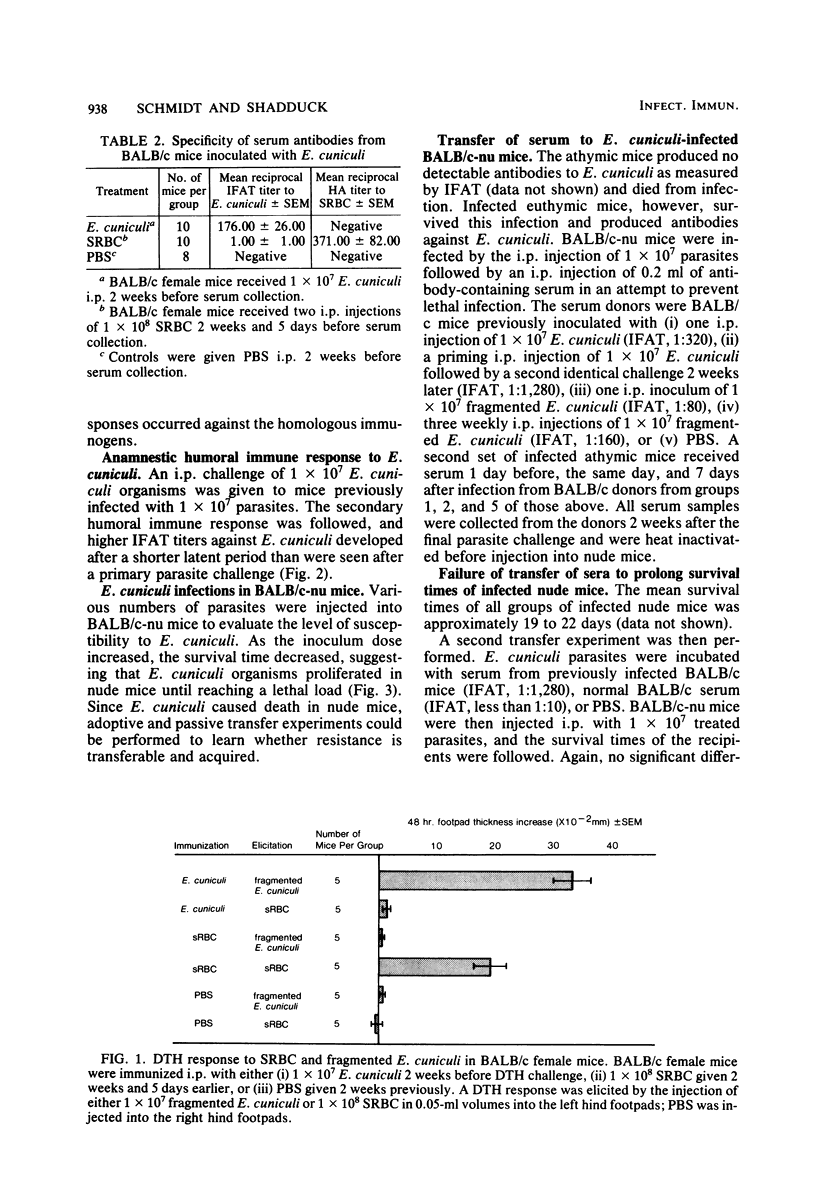
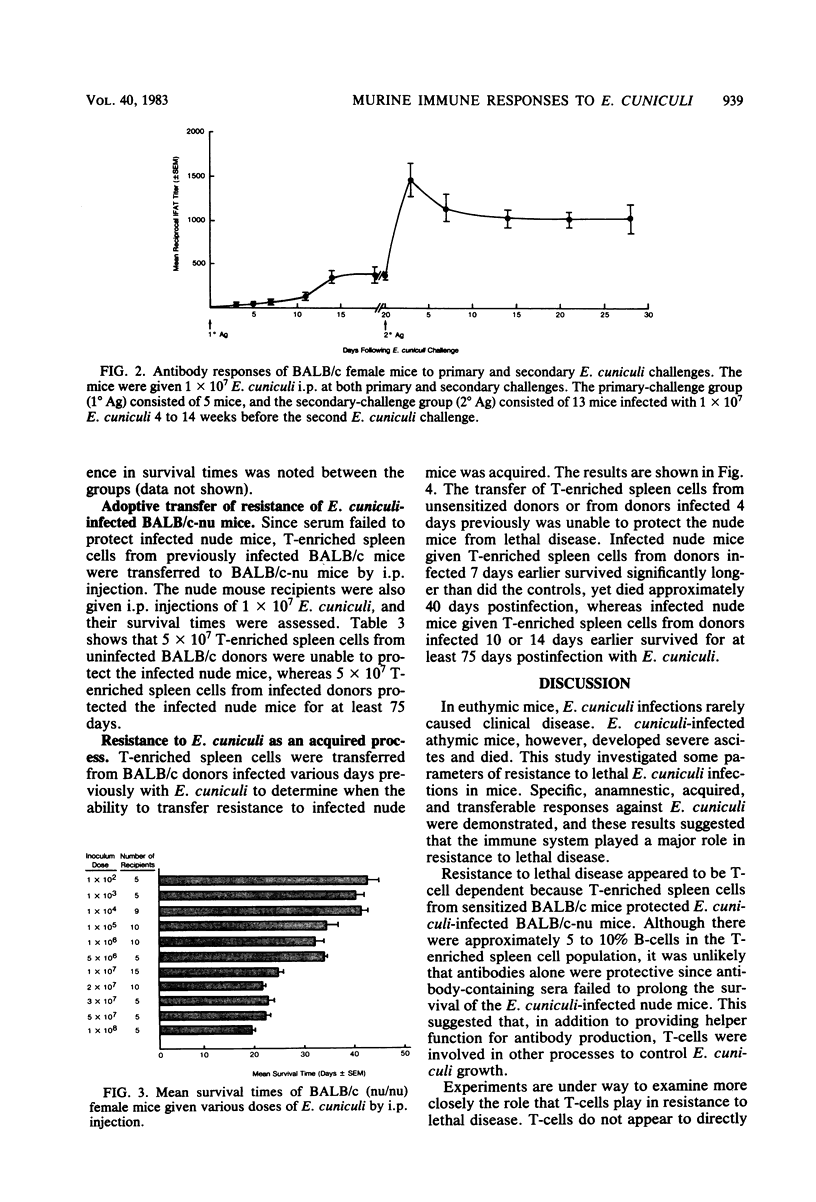
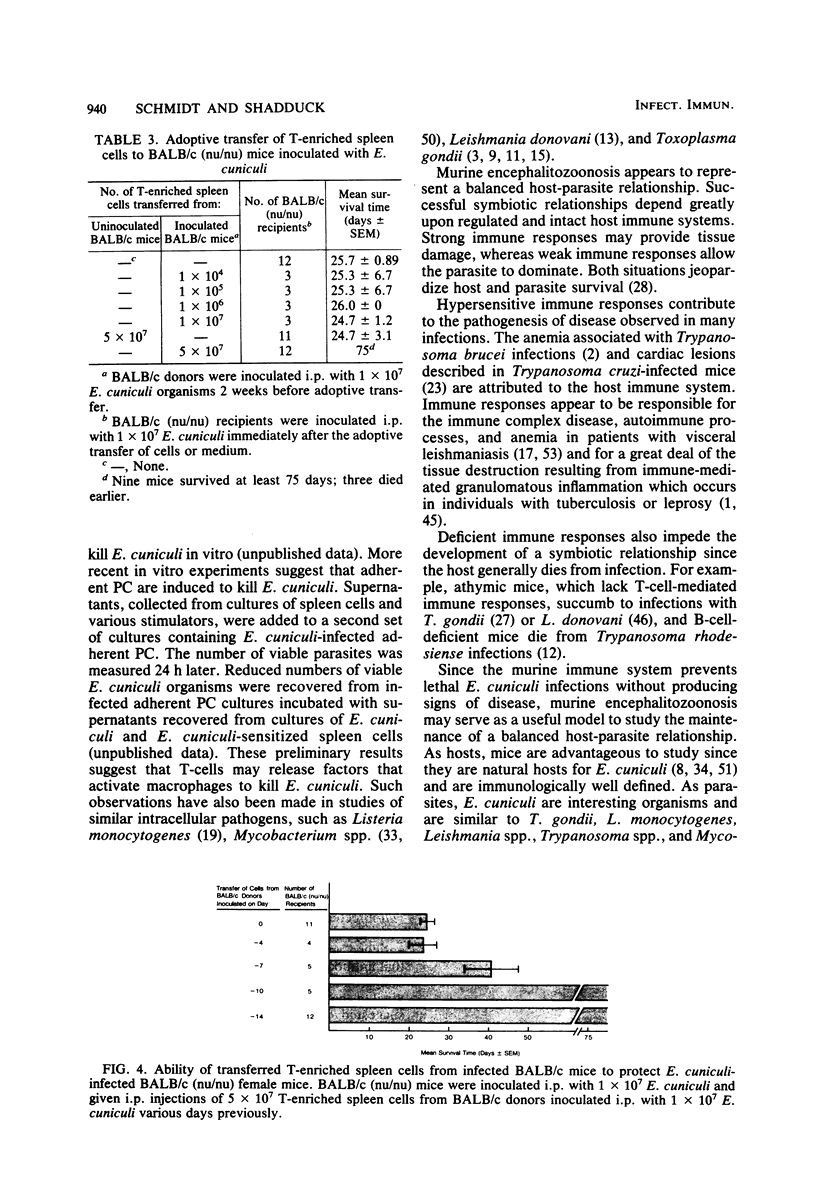
Encephalitozoon cuniculi caused chronic nonlethal infections in euthymic BALB/cAnN mice, whereas athymic BALB/cAnN-nu mice died from infection. Specific, anamnestic, transferable, and acquired responses against E. cuniculi were expressed by infected euthymic mice. Resistance to lethal disease appears to be T-cell dependent. Immune serum failed to protect infected athymic mice, whereas the transfer of T-enriched spleen cells from E. cuniculi-sensitized euthymic donors prevented lethal E. cuniculi infections in athymic mice. These findings indicate that murine encephalitozoonosis may be an excellent system for studies of a chronic infection in an immunologically well-characterized host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Amole B. O., Clarkson A. B., Jr, Shear H. L. Pathogenesis of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1982 Jun;36(3):1060–1068. doi: 10.1128/iai.36.3.1060-1068.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E., Bautista S., Remington J. S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976 Aug;117(2):381–387. [PubMed] [Google Scholar]
- Arison R. N., Cassaro J. A., Pruss M. P. Studies on a murine ascites-producing agent and its effect on tumor development. Cancer Res. 1966 Sep;26(9):1915–1920. [PubMed] [Google Scholar]
- Behin R., Mauel J., Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979 Aug;48(1):81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Bismanis J. E. Detection of latent murine nosematosis and growth of Nosema cuniculi in cell cultures. Can J Microbiol. 1970 Apr;16(4):237–242. doi: 10.1139/m70-044. [DOI] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Bywater J. E., Kellett B. S. Encephalitozoon cuniculi antibodies in a specific-pathogen-free rabbit unit. Infect Immun. 1978 Aug;21(2):360–364. doi: 10.1128/iai.21.2.360-364.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. H., Esser K. M., Weinbaum F. I. Trypanosoma rhodesiense infection in B-cell-deficient mice. Infect Immun. 1977 Nov;18(2):434–438. doi: 10.1128/iai.18.2.434-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Chiao J. W. Cellular immunity of mice to Leishmania donovani in vitro: lymphokine-mediated killing of intracellular parasites in macrophages. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7083–7087. doi: 10.1073/pnas.78.11.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla M., Frenkel J. K. Mediation of immunity to intracellular infection (Toxoplasma and Besnoitia) within somatic cells. Infect Immun. 1978 Mar;19(3):999–1012. doi: 10.1128/iai.19.3.999-1012.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. J., Hall J. G., Targett G. A., Murray M. The biological significance of the immune response with special reference to parasites and cancer. J Parasitol. 1980 Oct;66(5):705–721. [PubMed] [Google Scholar]
- Djoko-Tamnou J., Leclerc C., Modabber F., Chedid L. Studies on visceral Leishmania tropica infection in BALB/c mice. I. Clinical features and cellular changes. Clin Exp Immunol. 1981 Dec;46(3):493–498. [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M. Comparison of the development of avirulent and virulent strains of Toxoplasma gondii in the peritoneal exudate of mice. Ann Trop Med Parasitol. 1981 Oct;75(5):539–546. doi: 10.1080/00034983.1981.11687478. [DOI] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. A survey of Encephalitozoon cuniculi in laboratory animal colonies in the United Kingdom. Lab Anim. 1980 Apr;14(2):91–94. doi: 10.1258/002367780780942917. [DOI] [PubMed] [Google Scholar]
- Gannon J. The course of infection of Encephalitozoon cuniculi in immunodeficient and immunocompetent mice. Lab Anim. 1980 Jul;14(3):189–192. doi: 10.1258/002367780780937652. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971 Dec;124(6):587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- Hudson L. Immunobiology of Trypanosoma cruzi infection and Chagas's disease. Trans R Soc Trop Med Hyg. 1981;75(4):493–498. doi: 10.1016/0035-9203(81)90184-x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Modabber F., Deriaud E., Cheddid L. Systemic infection of Leishmania tropica (major) in various strains of mice. Trans R Soc Trop Med Hyg. 1981;75(6):851–854. doi: 10.1016/0035-9203(81)90430-2. [DOI] [PubMed] [Google Scholar]
- Lefford M. J., Patel P. J., Poulter L. W., Mackaness G. B. Induction of cell-mediated immunity to Mycobacterium lepraemurium in susceptible mice. Infect Immun. 1977 Dec;18(3):654–659. doi: 10.1128/iai.18.3.654-659.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Toxoplasmosis in nude mice. J Parasitol. 1977 Apr;63(2):219–221. [PubMed] [Google Scholar]
- Mitchell G. F. Effector cells, molecules and mechanisms in host-protective immunity to parasites. Immunology. 1979 Oct;38(2):209–223. [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J. Y., Shadduck J. A., Schmidt E. C. Susceptibility of selected inbred strains of mice to Encephalitozoon cuniculi. J Infect Dis. 1981 Sep;144(3):249–253. doi: 10.1093/infdis/144.3.249. [DOI] [PubMed] [Google Scholar]
- Nordstoga K., Westbye K. Polyarteritis nodosa associated with nosematosis in blue foxes. Acta Pathol Microbiol Scand A. 1976 May;84(3):291–296. doi: 10.1111/j.1699-0463.1976.tb00102.x. [DOI] [PubMed] [Google Scholar]
- PLOWRIGHT W. An encephalitis-nephritis syndrome in the dog probably due to congenital encephalitozoon infection. J Comp Pathol. 1952 Apr;62(2):83–92. doi: 10.1016/s0368-1742(52)80008-6. [DOI] [PubMed] [Google Scholar]
- Pakes S. P., Shadduck J. A., Olsen R. G. A diagnostic skin test for encephalitozoonosis (nosematosis) in rabbits. Lab Anim Sci. 1972 Dec;22(6):870–877. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G. Adoptive transfer of resistance to acute Trypanosoma cruzi infection with T-lymphocyte-enriched spleen cells. Infect Immun. 1980 May;28(2):404–410. doi: 10.1128/iai.28.2.404-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Resistance to virus challenge in mice infected with protozoa or bacteria. Proc Soc Exp Biol Med. 1969 Sep;131(4):1184–1188. doi: 10.3181/00379727-131-34066. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Pelster B., Suzuki N., Piekarski G., Brandis H. Immunity to Toxoplasma gondii induced in vitro in non-immune mouse macrophages with specifically immune lymphocytes. J Immunol. 1975 Oct;115(4):1151–1158. [PubMed] [Google Scholar]
- Shadduck J. A., Bendele R., Robinson G. T. Isolation of the causative organism of canine encephalitozoonosis. Vet Pathol. 1978 Jul;15(4):449–460. doi: 10.1177/030098587801500402. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A., Pakes S. P. Encephalitozoonosis (nosematosis) and toxoplasmosis. Am J Pathol. 1971 Sep;64(3):657–672. [PMC free article] [PubMed] [Google Scholar]
- Shadduck J. A., Watson W. T., Pakes S. P., Cali A. Animal infectivity of Encephalitozoon cuniculi. J Parasitol. 1979 Feb;65(1):123–129. [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A. Phenotypic expression of genetically controlled host resistance to Listeria monocytogenes. Infect Immun. 1979 Jul;25(1):345–351. doi: 10.1128/iai.25.1.345-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Skinsnes O. K. Infectious granulomas: exposit from the leprosy model. Annu Rev Med. 1982;33:47–67. doi: 10.1146/annurev.me.33.020182.000403. [DOI] [PubMed] [Google Scholar]
- Smrkovski L. L., Larson C. L., Reed S. G. Effect of visceral leishmaniasis on congenitally athymic mice. Infect Immun. 1979 Sep;25(3):1078–1080. doi: 10.1128/iai.25.3.1078-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischmann T. M., Bloom B. R. Trypanosoma cruzi: ability of T-cell-enriched and -depleted lymphocyte populations to passively protect mice. Exp Parasitol. 1980 Apr;49(2):225–232. doi: 10.1016/0014-4894(80)90119-8. [DOI] [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavra J., Blazek K. Nosematosis in carnivores. J Parasitol. 1971 Aug;57(4):923–924. [PubMed] [Google Scholar]
- WEISER J. NOSEMA MURIS N. SP., A NEW MICROSPORIDIAN PARASITE OF THE WHITE MOUSE (MUS MUSCULUS L.). J Protozool. 1965 Feb;12:78–83. doi: 10.1111/j.1550-7408.1965.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Wosu N. J., Shadduck J. A., Pakes S. P., Frenkel J. K., Todd K. S., Jr, Conroy J. D. Diagnosis of encephalitozoonosis in experimentally infected rabbits by intradermal and immunofluorescence tests. Lab Anim Sci. 1977 Apr;27(2):210–216. [PubMed] [Google Scholar]
- Zuckerman A. Current status of the immunology of blood and tissue Protozoa. I. Leishmania. Exp Parasitol. 1975 Dec;38(3):370–400. doi: 10.1016/0014-4894(75)90123-x. [DOI] [PubMed] [Google Scholar]



