Abstract
The endocannabinoid system was revealed following the understanding of the mechanism of action of marijuana's major psychotropic principle, Δ9-tetrahydrocannabinol, and includes two G-protein-coupled receptors (GPCRs; the cannabinoid CB1 and CB2 receptors), their endogenous ligands (the endocannabinoids, the best studied of which are anandamide and 2-arachidonoylglycerol (2-AG)), and the proteins that regulate the levels and activity of these receptors and ligands. However, other minor lipid metabolites different from, but chemically similar to, anandamide and 2-AG have also been suggested to act as endocannabinoids. Thus, unlike most other GPCRs, cannabinoid receptors appear to have more than one endogenous agonist, and it has been often wondered what could be the physiological meaning of this peculiarity. In 1999, it was proposed that anandamide might also activate other targets, and in particular the transient receptor potential of vanilloid type-1 (TRPV1) channels. Over the last decade, this interaction has been shown to occur both in peripheral tissues and brain, during both physiological and pathological conditions. TRPV1 channels can be activated also by another less abundant endocannabinoid, N-arachidonoyldopamine, but not by 2-AG, and have been proposed by some authors to act as ionotropic endocannabinoid receptors. This article will discuss the latest discoveries on this subject, and discuss, among others, how anandamide and 2-AG differential actions at TRPV1 and cannabinoid receptors contribute to making this signalling system a versatile tool available to organisms to fine-tune homeostasis.
Keywords: cannabinoid, endocannabinoid, cannabinoid receptor type-1, cannabinoid receptor type-2, transient receptor potential vanilloid type-1, endovanilloid
1. Introduction
The discovery, in the 1990s, of cannabinoid receptor type-1 (CB1) and -2 (CB2) [1,2], two specific G-protein-coupled receptors (GPCR) for Δ9-tetrahydrocannabinol (THC), the psychotropic ingredient of Cannabis sativa, raised at least three important questions: (i) since there are GPCRs for THC, are there endogenous ligands for these receptors? (ii) are there other receptors for THC other than CB1 and CB2 but homologous to these two proteins? (iii) do other cannabinoids in C. sativa, such as, for example, the widely studied and potentially therapeutically important, non-psychotropic compound, cannabidiol, also have specific receptors? Studies carried out in the last 20 years have now largely answered these questions, in as much as we now know that: (i) yes, there are endogenous CB1 and CB2 ligands [3–5], named ‘endocannabinoids’ [6]; (ii) no, there are no CB1 and CB2 homologues in mammals that are activated by THC; and (iii) probably not, since, for example, cannabidiol exhibits activity, in vitro and in vivo, towards a plethora of previously identified molecular targets, be they ion channels or previously identified GPCRs, which underlie most of its pharmacological actions [7]. It has been discovered also that the two most studied endocannabinoids so far, 2-arachidonoylglycerol (2-AG) [4,5], and, particularly, N-arachidonoyl-ethanolamine (anandamide) [3], do not interact with only CB1 and CB2, and exhibit instead a degree of ‘promiscuity’ more similar to cannabidiol than THC. This promiscuity applies, to some extent, also to the less-studied and less-well-established arachidonic acid-derived endocannabinoids, such as N-arachidonoyl-dopamine (NADA), noladin ether and virodhamine [8–10], and implies that probably, in a not so distant future, the definition of ‘cannabinoid receptors’ will have to be enlarged to also encompass some of the many ‘endocannabinoid receptors’ proposed so far [11]. Perhaps more importantly and as will be discussed in this article, the functional and metabolic flexibility of endocannabinoids, allowing them to regulate both directly and through other molecular targets the activity of cannabinoid receptors, helps explain why the latter, unlike most GPCRs, have more than one endogenous ligand.
2. Endocannabinoid biochemistry and functional activity and selectivity at cannabinoid receptors
Since their discovery as high (anandamide) and low-to-moderate (2-AG) affinity ligands for CB1 receptors [3–5], it also became clear that the two major endocannabinoids exhibit varying efficacy as CB1 agonists. Anandamide was described as a partial agonist in most functional assays [12–14]. However, 2-AG, the activity of which may be decreased in vitro and in vivo by its non-enzymatic transformation into equal amounts of the two enantiomers, and 2-AG regio isomers, sn-1-AG and sn-3-AG [15], behaved as a full agonist in most assays [13,16]. Subsequently, it was also suggested, again based on a variety of in vitro functional assays, that anandamide is nearly inactive as a CB2 agonist, whereas 2-AG is a full agonist also at this receptor [17,18]. Thus, although the efficacy of a given agonist at a certain receptor in a given in vitro assay depends on several factors, including the expression level of the receptor and the several G proteins that may mediate its intracellular effects, and considering that different agonists may induce the trafficking of different G proteins towards the receptor in a more or less efficacious way depending on their chemical structure, it is now thought that anandamide is a high affinity, CB1-selective partial agonist, whereas 2-AG is a moderate affinity, CB1/CB2 full agonist.
The difference in efficacy at CB1 and CB2 receptors between the two most studied endocannabinoids is only one of those biochemical features that can be predictive of a different function for these compounds. Another biochemical difference between anandamide and 2-AG is represented by the diverse metabolic pathways that underlie their biosynthesis and breakdown. Although both compounds are usually produced following elevation of intracellular Ca2+ concentrations such to overcome the threshold for activation of their biosynthetic enzymes, the latter are different for anandamide and 2-AG [19]. Anandamide is obtained from the one- to three-step enzymatic hydrolysis of a family of minor membrane phospholipids, the N-arachidonoyl-phosphatidylethanolamines (NArPE). N-acyl-phosphatidylethanolamine specific phospholipase D (NAPE-PLD), an enzyme that has little in common with other phosphodiesterases [20], is Ca2+-sensitive and catalyses the hydrolysis of NArPE directly to anandamide. On the other hand, the sequential action of α,β-hydrolase-4 (ABHD4) and glycerophosphodiesterase-1 (GDE1) catalyses the conversion of NArPE into lyso-NArPE first, then glycerophosphoanandamide and, finally, anandamide [21]. Formation of lyso-NArPE can occur also through the action of a soluble phospholipase A2, followed by direct conversion into anandamide by a lyso-PLD [22]. Finally, an as-yet-unidentified phospholipase C (PLC), followed by the action of various phosphatases (such as protein tyrosine phosphatase N22 or SH2 domain-containing inositol phosphatase), can convert NArPE first into phospho-anandamide and then anandamide [23]. Of these four biosynthetic routes, only the first two and the last one were shown to occur in intact cells, and evidence was obtained in transgenic animals (double NAPE-PLD/GDE1 null mice) suggesting that the first two concomitantly participate in brain anandamide biosynthesis in vivo [24].
The biosynthesis of 2-AG is in seemingly simpler. Again, only one family of lipids, the sn-1-acyl-2-arachidonoylglycerols (DAGs), is used as biosynthetic precursors, and they can be directly converted into 2-AG through the action of either of two Ca2+-sensitive sn-2-selective DAG lipases (DAGLs), i.e. DAGL-α and DAGL-β [25]. Studies in mice lacking either of these two enzymes suggested that the former is the most important at determining 2-AG levels in the brain and 2-AG function in the regulation of synaptic strength (see below). Although DAGs acting as 2-AG precursors are mostly produced from the hydrolysis of phosphatidyl-inositols (PIs) via PI-specific PLC, phosphatidic acid was also suggested to act as a source of these lipids [19]. Thus, except perhaps for some unidentified PLC enzymes, little overlap exists between anandamide and 2-AG biosynthetic enzymes, which indicates that the levels of these two endocannabinoids can be regulated independently from each other following cell stimulation and intracellular Ca2+ elevation. Yet, DAGL-α−/− mice exhibit reduced brain levels not only of 2-AG, but also of anandamide [26–28], possibly suggesting that the phospholipid remodelling in these mice might also ultimately affect the fatty acid composition of brain precursors for NArPE [19]. In fact, NArPE is produced by the action of an as yet unidentified Ca2+-dependent N-acyl-transferase catalysing the transfer of a (usually rare) arachidonoyl moiety from the sn-1 position of nearly any phospholipid to the nitrogen atom of phosphatidylethanolamine [29].
Also, the catabolic enzymes for anandamide and 2-AG are mostly different. Both compounds are broken down inside the cell by intracellular enzymes, the former being the substrate of fatty acid hydrolase-1 (FAAH-1, expressed in all mammals) and, to a much lesser extent, of fatty acid hydrolase-2 (FAAH-2, not expressed in rodents); and the latter being hydrolysed through the action of monoacylglycerol lipase (MAGL) and, to a lesser extent, α,β-hydrolase-6 (ABHD6), α,β-hydrolase-12 (ABHD12) and FAAH-1 [30–32]. However, both compounds have been suggested to be taken up by cells through the action of a common and as yet unidentified membrane transporter (possibly involved also in the release of de novo biosynthesized anandamide and 2-AG) [33]. Yet, the intracellular trafficking of anandamide was found to be mediated by a protein specific for this compound and unable to bind 2-AG, shown to be a catalytically silent FAAH-1 splicing variant named FAAH-like anandamide transporter (FLAT) [34].
In summary, it is clear that the tissue levels of anandamide and 2-AG are usually regulated independent of each other, thus allowing the two compounds to exert different functions even in the same organ, tissue or cell. Indeed, as clearly shown in about 15 years of research on this topic [35], both physiological and pathological conditions can be accompanied, in either central or peripheral organs and tissues, by alterations in the concentrations of only one of these compounds, whereas examples of anandamide and 2-AG tissue levels undergoing opposite changes are not rare. This observation strengthens the ever-growing realization that endocannabinoids do not only regulate the activity of cannabinoid receptors, but might also fine-tune cell homeostasis via coordinated enhanced, or decreased, interactions with more than one target at once.
Perhaps the best established non-cannabinoid receptor for endocannabinoids, and for anandamide in particular, is the transient receptor potential vanilloid type-1 (TRPV1) channel [36,37], previously discovered as the receptor for the pungent active principle of hot chilli peppers, capsaicin [38]. Anandamide activates this channel (and hence behaves as an ‘endovanilloid’) with potency and efficacy that are usually lower than those exhibited at CB1 receptors, but which vary depending on the assay and cell type used and increase under certain pathological (e.g. inflammatory) conditions that alter TRPV1 expression in tissues and sensitivity to agonists ([39] for review).
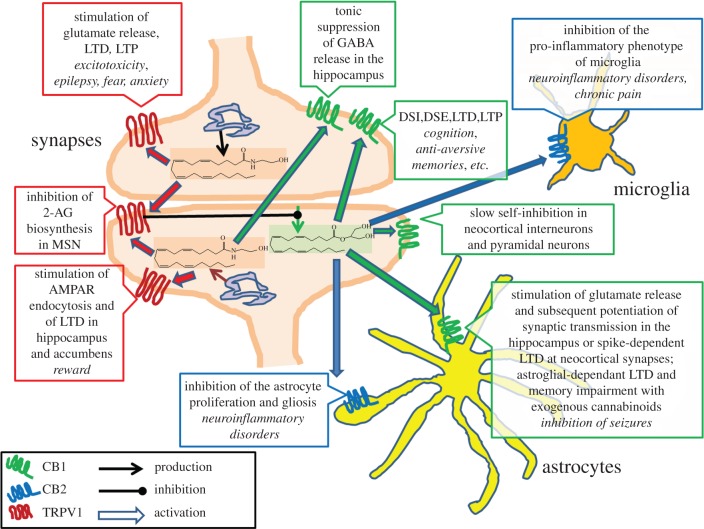
Importantly, it is not only the metabolic enzymes for anandamide and 2-AG that differ, but also their anatomical distribution, or at least of those which have been studied in the brain so far, and that of their proposed molecular targets [19]. Thus, the finding in several brain areas of DAGLα in post-synaptic dendrites and somata, and of CB1 and MAGL in pre-synaptic terminals, allows 2-AG to be produced from post-synaptic neurons, act as a ‘retrograde’ signal at pre-synaptic fibres (see below) and be inactivated near its site of action at CB1. On the other hand, the fact that: (i) NAPE-PLD is located both pre- and post-synaptically, (ii) FAAH-1 is predominantly found in post-synaptic neurons, where TRPV1 is also more frequently found, and (iii) these enzymes are mostly concentrated in intracellular membranes, allows the hypothesis of a role for anandamide also as intracellular, ‘anterograde’ or autocrine mediator through this channel (figure 1). Some aspects of the biological importance of anandamide's ‘dual’ nature as endocannabinoid and ‘endovanilloid’ will be discussed in the next sections.
Figure 1.
Different functions at different receptors for brain anandamide and 2-AG. Anandamide (structure highlighted in pink) and 2-AG (structure highlighted in light green) are depicted as being produced (thin brown arrows) from both pre- and post-synaptic intracellular membranes and from post-synaptic plasma membranes, respectively. Anandamide, by acting at pre-synaptic CB1 receptors, may participate in ‘tonic’ suppression of GABAergic signalling in organotypic hippocampal cultures [40], whereas at pre-synaptic TRPV1 it stimulates glutamate release, thereby participating in some pathological conditions (shown in italics; see text). By acting at post-synaptic TRPV1, anandamide either reduces glutamate signalling and produces long-term depression (LTD) by stimulating AMPA receptor (AMPAR) endocytosis [41,42] or, as shown in MSNs of the striatum [43], it inhibits 2-AG biosynthesis and retrograde action at CB1 receptors [44], with potential consequences on endocannabinoid-mediated retrograde control of DSE and DSI, LTD and LTP. 2-AG can also act at post-synaptic CB1 receptors, thereby mediating ‘slow self-inhibition’ of neocortical interneurons [45]. Finally, 2-AG is the likely agonist at the CB1 receptor on astrocytes, which is recently emerging as the possible mediator of a series of biological actions listed in the figure [46–49], and at CB2 receptors in the same cells as well as in microglia, with strong implications for the inhibition of neuroinflammation and potential therapeutic use in several neuroinflammatory disorders [50,51]. MSN, medium spiny neurons; LTP, long-term potentiation; AMPA, 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid; DSE, depolarization-induced suppression of excitation; DSI, depolarization-induced suppression of inhibition.
3. Co-expression of CB1 or CB2 receptors and TRPV1 channels in neuronal and non-neuronal cells
Given the local (paracrine, autocrine or even intracellular) nature of the action of endocannabinoids at their receptors, any consideration on their potential biological function as multi-target mediators must take into account the possible co-expression of these receptors in the same or in neighbouring cells. In the case of TRPV1, there are numerous studies indicating that this channel is co-expressed with either CB1, CB2 or both, in neuronal and non-neuronal cells. Such studies, mostly carried out using quantitative RT-PCR or immunohistochemical techniques, can be summarized as follows: (i) TRPV1 is co-localized with CB1 in rat primary sensory neurons of the dorsal root ganglia [52–54], where the expression of the two proteins is upregulated during inflammation [55]; they are also co-localized in perivascular neurons [56], in the isolated vagus nerve [57], in the rat retina [58] and in the somata and/or axons of central neurons from several brain areas [59–61]; (ii) TRPV1 is co-localized with CB1 and CB2 in cerebromicrovascular endothelial cells [62] and with CB1 in endothelial cells from mesenteric arteries of cirrhotic rats [63,64]; (iii) TRPV1 is co-localized with both CB1 and CB2 in mouse bone-marrow-derived dendritic cells [65], and in human skeletal muscle cells [66], myometrial smooth muscle cells [67], osteoclasts [68], proximal tubular (HK2) cells of the kidney [69], keratinocytes [70,71], melanocytes [72] and dental pulp cells [73]; (iv) TRPV1 is co-localized only with CB1 in human sperm cells [74], and only with CB2 in synoviocytes from rats after intra-articular injection of mono-iodo-acetate, a model of osteoarthritis [75].
In view of the previously reported cross-talk between TRPV1 and CB1 receptors at the level of down-stream signalling events [76,77], these many examples of co-expression between the channel and one or both cannabinoid receptor sub-types offer the opportunity of widening further the range of pharmacological effects that endogenous mediators capable of activating both types of receptors may have, and, hence, the extent of ‘plasticity’ that their action can produce in a large variety of biological systems. Some examples of this ‘plasticity’ are given in the next section, with the aim of distinguishing further the potential function of the two ‘major’ endocannabinoids, anandamide and 2-AG.
4. Anandamide dual actions at CB1 and TRPV1 as a ‘plastic’ way of regulating synaptic plasticity
Perhaps two of the most exciting discoveries made in recent years regarding the physiological role of CB1 receptors, on the one hand, and TRPV1 channels, on the other hand, are concerned with the variegated functions that these two endocannabinoid-sensitive targets play in the regulation of both short- and long-term forms of synaptic plasticity [78] (figure 1). From its discovery, CB1 appeared to be the most abundant GPCR in the brain, and its role in the inhibition of the release of both excitatory (e.g. glutamate) and inhibitory (e.g. GABA) neurotransmitters was immediately clear, especially since it was found that this receptor is very often located pre-synaptically in axon terminals, and not only in GABAergic neurons, as had been initially suggested [44]. Still, it was surprising to identify endocannabinoids as perhaps the most widespread and long-sought-after ‘retrograde’ signals in the brain [44]. These are mediators produced following depolarization or metabotropic receptor-stimulation (and subsequent elevation of intracellular Ca2+) of the post-synaptic cell, and travelling ‘backwards’ to pre-synaptic CB1 receptors, thereby inhibiting either excitatory or inhibitory transmitter release onto either the same or a neighbouring synapse. This ‘retrograde’ action causes short-term depolarization-induced inhibition of excitatory (DSE) or inhibitory (DSI) transmission, or more long-lasting forms of plasticity, such as long-term depression (LTD) or potentiation (LTP) [44]. The discovery of endocannabinoids as retrograde neuromodulators, however, immediately raised new questions: which of the two most studied endocannabinoids acts as retrograde signal? If both do, when is it anandamide and when 2-AG? Do they play this role in a redundant or in a functionally distinct way?
As soon as it was possible to dissect the role of anandamide from that of 2-AG through the use of selective pharmacological or genetic tools inactivating their catabolic or biosynthetic enzymes, the answer seemed to be unanimous: 2-AG is the true ‘retrograde’ endocannabinoid, and DAGLα is the Ca2+- or metabotropic receptor-dependent enzyme that mostly, if not uniquely, initiates this signal [26–28,44,79]. However, limited to certain synapses in some brain areas, or to some special types of synaptic plasticity, anandamide also seems to play this role. In particular, ‘tonic’ (i.e. continual) endocannabinoid actions in the hippocampus appear to be mediated by anandamide and not 2-AG. In fact, in organotypic hippocampal cultures, chronic inactivity caused by TTX or experimental deafferentation decreases the tonic CB1-mediated suppression of GABA release through the reduction in the continuous availability of an endocannabinoid, which, based on experiments with inhibitors of anandamide cellular uptake or FAAH, was suggested to be anandamide [40]. The same inhibitors did not affect DSI in non-activity-deprived, normal neurons. The authors suggested that chronic neuronal inactivity increases anandamide uptake and degradation, thereby decreasing the ‘tonic’ (as opposed to ‘phasic’, i.e. stimulus-induced) CB1-mediated suppression of GABA release [40]. On the other hand, in those less frequent cases in which anandamide was suggested to also participate in the stimulus-induced regulation of synaptic activity, this endocannabinoid was shown to do so not (or not only) in a ‘retrograde’ manner via CB1 receptors, but rather through a pre- or post-synaptic, likely intracellular, mechanism mediated by TRPV1, a function that, clearly, 2-AG is less likely to play given its very low potency and efficacy at this channel.
Although still somehow controversial [80], the presence and functional activity of TRPV1 in the brain has been now shown in an overwhelming number of studies (see, [81,82] for reviews). Studies employing TRPV1 null mice or the inhibitor of anandamide inactivation by FAAH-1, URB597, to increase the endogenous levels of anandamide, suggested that this compound influences synaptic plasticity by acting at both post- and pre-synaptic TRPV1 channels. Post-synaptic TRPV1 activation by anandamide hyperpolarizes neurons by: (i) reducing the post-synaptic DAGLα-mediated biosynthesis of 2-AG, thereby counteracting the metabotropic (glutamatergic and cholinergic)-induced and CB1-mediated retrograde inhibition of GABA release onto striatal medium spiny neurons (MSNs) [43,83]; or (ii) stimulating post-synaptic AMPA receptor endocytosis, thus impairing glutamate signalling and inducing LTD [41,42]. A recent study showed that LTD in the bed nucleus of the stria terminalis of the extended amygdala is also mediated by post-synaptic mGluR5-dependent production of anandamide acting on post-synaptic TRPV1 receptors [84], whereas, in the same neurons, 2-AG mediates both short-term depression and LTD through a retrograde action at CB1 receptors. Instead, as shown through the use of mice with genetically impaired expression of FAAH, pre-synaptic TRPV1 activation by anandamide directly facilitates glutamatergic signalling in striatal MSN [85]. Finally, two other studies showing that TRPV1 mediates LTD did not identify the ‘endovanilloid’ involved. LTD at hippocampal interneurons [86], a phenomenon that is exerted pre-synaptically and is mediated by calcineurin and potentiation of GABA signalling [87,88], might be due to either 12-hydroperoxyeicosatetranoic acid (12-HPETE) or anandamide, both possibilities being supported by the finding of NAPE-PLD and 12-lipoxygenase (the enzyme converting arachidonic acid into 12-HPETE) in CA1 pyramidal cells [89]. In the developing superior colliculus, again either 12-HPETE or anandamide might cause TRPV1-mediated LTD, possibly through a pre-synaptic mechanism occurring in glutamatergic retinal afferents to GABAergic interneurons [90].
In summary, the fact that some endocannabinoids, such as anandamide (and the less studied and less abundant in brain, NADA) activate both CB1 and TRPV1 receptors, not only differentiates these mediators from other endocannabinoids, such as 2-AG or noladin ether, but also creates the possibility of a ‘dual-target and multiple mechanism-mediated’ regulation of synaptic plasticity; in a way, a more ‘plastic’ manner to regulate plasticity, which probably underlies the proposed role not only of CB1 [91], but also of TRPV1 in stress, emotionality and cognition [92,93].
5. Anandamide dual actions at CB1 and TRPV1 as a ‘plastic’ way of regulating pain
The opposing roles of cannabinoid receptors and TRPV1 channels in pain are well established and have been reviewed in numerous articles (see, [94–96] for up-to-date examples). Recent evidence suggests, however, that one should move away from the ‘old’ concept that selective activation of CB1 or TRPV1 attenuate or worsen nociception, respectively. It is now clear that the former receptor can also participate in supra-spinal pro-nociceptive [97] or spinal pain-sensitization [98] mechanisms, which can be activated following elevation of endocannabinoid levels. Conversely, activation of TRPV1 can enhance the activity of supra-spinal pathways of pain control [97,99] or be easily followed by desensitization and hence produce analgesia as efficaciously as its inactivation [100]. Thus, anandamide can both inhibit and stimulate calcitonin gene-related peptide release from primary nociceptive mouse and rat neurons, at low and high concentrations, via CB1 and TRPV1, respectively [101]. The stimulatory effect was followed by desensitization to heat responses, suggesting that anandamide may inhibit pain and inflammation by activating and subsequently desensitizing TRPV1 channels [101]. When administered intrathecally, or when its spinal levels are increased through inhibition of FAAH-1 with URB597, anandamide reduces hyperalgesia and allodynia in rats with neuropathic pain caused by chronic constriction injury of the sciatic nerve. This effect is mediated by either CB1 or TRPV1 depending on whether the endogenously elevated spinal levels of anandamide are low or high respectively [102]. In the ventro-lateral periaqueductal grey (vl-PAG), tonic pre- and post-synaptic TRPV1 activation or pre-synaptic CB1 activation by anandamide or anandamide and 2-AG, respectively, result in stimulation of output excitatory neurons via enhancement of depolarization/glutamate release or disinhibition from tonic GABAergic control, respectively [97,103]. This results in glutamate release in the rostral ventromedial medulla (RVM) and activation of OFF neurons in this area, with subsequent anti-nociception. Furthermore, pre-synaptic TRPV1 activation by endovanilloids stimulates glutamate release also in the PAG [104], which results in the facilitation of metabotropic glutamate receptor-induced impairment of GABA release via endocannabinoids acting in a retrograde and CB1-mediated manner, and again stimulates the descending anti-nociceptive pathway [105].
The concept that, when expressed in the same or in neighbouring cells, and especially if activated by the same mediator, such as anandamide, CB1 and TRPV1 can also cross-talk, with different impacts on pain perception, is now emerging. The co-expression of CB1 and TRPV1 in: (i) both DRG and spinal cord neurons [52–55], which are crucial for the ascending transmission of painful stimuli; (ii) neurons of the vl-PAG and RVM, which participate in the descending anti-nociceptive pathway [97]; and (iii) neurons of the peri-and infra-limbic cortex [106], or of the hippocampus and thalamus [107], in neuropathic rodents, strongly suggest that cross-talk between the two receptors is crucial in pain signalling. Based on the previous observations that: (i) activation of CB1 can either or enhance counteract TRPV1 stimulation by anandamide, depending on whether it stimulates intracellular activation of the PLC-protein kinase C or inhibition of adenylate cyclase-protein kinase A pathways, respectively [76], and that (ii) TRPV1 is sensitized to the action of agonists by protein kinase C and cAMP-dependent protein kinase A ([108] for review), Fioravanti and colleagues investigated the effect of CB1 knockout or pharmacological blockade on the TRPV1-mediated pro-nociceptive effects of capsaicin. The authors reported that constitutive activity at the cannabinoid CB1 receptor and subsequent activation of the PLC pathway are required for behavioural response to noxious chemical stimulation of TRPV1 in mice [109]. In the medicinal leech, where the presence of CB1 and TRPV1 orthologues is still somewhat controversial, LTD in the monosynaptic connection between the nociceptive sensory neuron and the longitudinal motor neuron was found to be dependent on a retrograde action by post-synaptically produced 2-AG [110]. Interestingly, this form of LTD was also inhibited by pre-synaptic injection of two TRPV1 antagonists and hence suggested to be mediated by pre-synaptic TRPV1-like channels. Considering that 2-AG is not efficacious at TRPV1 (although things could be different for the leech orthologue of this channel), these findings suggest the occurrence of TRPV1-CB1 cross-talk in determining LTD in this invertebrate.
Stress may play a role in CB1-TRPV1 cross-talk and its consequences on pain perception. In rats that underwent a water-avoidance stress for 10 days, and subsequent elevation of circulating corticosterone levels and visceral hyperalgesia, anandamide levels in dorsal root ganglia (DRG) were elevated together with the expression and phosphorylation of TRPV1, whereas CB1 expression was concomitantly reduced [111]. In vitro, DRG treatment with anandamide reproduced these mutual changes in CB1 and TRPV1 expression, thus suggesting that TRPV1 over-activation may have caused CB1 downregulation. In contrast, treatment of control DRGs in vitro with the CB1-selective agonist WIN 55,212-2 decreased the levels of TRPV1 and TRPV1 phosphorylation. Treatment of stressed rats in situ with WIN 55,212-2 or the TRPV1 antagonist capsazepine prevented the development of visceral hyperalgesia and blocked the upregulation of TRPV1 [111]. Thus, mutual regulation of expression between CB1 and TRPV1 in DRG might underlie stress-induced visceral hyperalgesia.
CB1 and TRPV1 also seem to cross-talk in keratinocytes, thereby reducing their proliferation, a phenomenon that, given the capability of these cells to produce inflammatory cytokines, might contribute to counteract some inflammatory skin disorders, which can be accompanied by peripheral sensitization, neurogenic inflammation and pain. Tóth et al. [71] presented evidence suggesting that the sequential stimulation by anandamide of CB1 and TRPV1 triggers Ca2+ influx, concomitant elevation of intracellular Ca2+ concentrations and keratinocyte death.
6. Anandamide dual actions at CB2 and TRPV1 and osteoclast activity
Another cell type that is emerging as a potential site of cannabinoid receptor–TRPV1 interaction is the osteoclast, which is responsible, among others, for bone resorption and calcium homeostasis. This cell type, once differentiated, expresses both CB1 and CB2 receptors, the activation of which was reported to produce opposing effects on cell activation (see, [112,113] for reviews). Osteoclasts from non-menopausal women, in which CB2 and CB1 activation was suggested to inhibit and stimulate cell activity, respectively, also express functionally active TRPV1 channels, the activation of which strongly stimulated the expression of markers of osteoclast activity [68]. These cells also express enzymes for anandamide biosynthesis and inactivation, i.e. NAPE-PLD and FAAH-1, and produce measurable amounts of anandamide and 2-AG [68]. Importantly, when osteoclasts were prepared from post-menopausal women with osteoporosis, apart from more strongly producing markers of activity, they were found to express significantly more CB1 and TRPV1, and to contain more anandamide, than osteoclasts from post-menopausal non-osteoporotic women, whereas CB2 expression did not change [114]. Even more interestingly, despite its increased expression or perhaps because of it, TRPV1, in terms of its capability of gating Ca2+ following stimulation with capsaicin, was less functional in osteoclasts from post-menopausal osteoporotic women, an observation that was probably associated with the different TRPV1 sub-cellular distribution (intracellular, rather than on the plasma membrane) in these cells. On the other hand, TRPV1 stimulation in these cells caused reduction of their activity, instead of the activation observed in osteoclasts from non-menopausal [68] or menopausal non-osteoporotic women [114], and this effect was accompanied by a slight elevation of CB1 expression and a strong, 10- and 3-fold elevation of CB2 mRNA and protein levels, respectively. Therefore, it is possible that in post-menopausal women, osteoporosis arises as a consequence of increased CB1 activation by anandamide. On the other hand, pharmacological activation of TRPV1 in ‘osteoporotic’ osteoclasts reduces rather than increases their activity via: (i) a switch from TRPV1 stimulation of Ca2+ to TRPV1 stimulation of cannabinoid receptor expression; (ii) upregulation of CB2 versus CB1 receptors and subsequent increase of the CB2/CB1 ratio; and (iii) a switch of anandamide action from TRPV1/CB1 to CB2, which can be activated also by 2-AG, the levels of which do not change during osteoporosis. These observations can be exploited therapeutically by designing new CB2 agonists that also either activate or antagonize TRPV1, as possible treatments for osteoporosis.
In summary, the human osteoclast offers another important example of the complex interactions between cannabinoid and TRPV1 receptors and of their potential use for therapeutic purposes. Indeed, several examples of synthetic compounds that are capable of interacting with both cannabinoid receptors (either directly or indirectly, i.e. by elevating endocannabinoid levels) and TRPV1 channels have been developed (see [115] for review), and these should now be tested in models of osteoporosis as well as of other pathological conditions in which both types of receptors are involved, like neuropathic and inflammatory pain and anxiety [92].
Although no evidence exists to date for any role of TRPV1 in osteoblasts, which counterbalance osteoclast action in the bone, a recent study was carried out in odontoblasts that participate in dentin formation physiologically and developmentally in that they synthesize and secrete collagenous and non-collagenous matrix proteins and are involved in dentin mineralization by transportation and accumulation of Ca2+. The authors showed that rat odontoblasts express both CB1 and TRPV1 and suggested that in these cells TRPV1-mediated elevation of intracellular Ca2+ levels functionally couples with CB1 receptor activation via the biosynthesis of 2-AG or anandamide as intermediates, thus resulting in the activation of the protein kinase A cascade, further activation of TRPV1 and in accumulation of intracellular Ca2+ [116].
7. Emerging direct actions of 2-AG at non-cannabinoid, non-TRPV1 receptors
Alternative molecular targets, mostly among previously discovered receptors, are also emerging for 2-AG, whereas reports of anandamide directly modulating the action of non-TRPV1, non-cannabinoid receptors have been increasing for the last 10 years and have been comprehensively reviewed previously as well as recently [11,117]. Some of these interactions might distinguish even further the biological functions played by these two most studied endocannabinoids. Owing to possible non-specific effects on biological membranes of greater than 10 μM concentrations by lipophilic molecules, and to the fact that even the stimulus-induced local concentrations of 2-AG and, particularly, anandamide near their targets are not likely to be higher than this threshold value, we believe that, among the several alternative mechanisms of endocannabinoid action so far reported, only those that were shown to occur at low micromolar or sub-micromolar concentrations in vitro should be considered for now. For example, among the tens of direct effects reported so far for anandamide, (i) the inhibition of T-type Ca2+ channels [118–120] and background TASK potassium channels [121] as well as of α4β2 and α7 nicotinic acetylcholine receptors [122–124]; and (ii) the allosteric enhancement of glycine receptors, particularly at the level of the α1-subunit [125–128], are actions that have been reported most consistently and at low concentrations, and for which structure–activity relationship studies were also performed, although their physio-pathological significance in vivo has not been evaluated yet.
Once thought to be the only ‘true’ and efficacious endocannabinoid, 2-AG was recently reported to potentiate GABAA receptors at low concentrations of GABA, by binding to two amino acid residues of the transmembrane segment M4 of the β2 subunit [129]. The endocannabinoid was shown to act in a superadditive fashion with the neurosteroid 3α, 21-dihydroxy-5α-pregnan-20-one and to modulate δ-subunit-containing receptors, which are located extra-synaptically and respond to neurosteroids. Through this interaction, 2-AG was suggested to inhibit locomotor activity in CB1/CB2 double-KO mice, in agreement with the observation that β2−/− mice instead show hypermotility. The authors suggested that a 2-AG direct stimulatory effect on GABAA receptors may play a role in two of its typical in vivo actions, i.e. inhibition of locomotion and sedation [129], although they did not test the endocannabinoid in β2−/− mice.
An inhibitory effect by 2-AG was, instead, recently shown to occur at the level of the KATP channel in pancreatic β-cells, with an IC50 for 2-AG of 1 μM. The compound also inhibited other channels but at higher concentrations [130]. Pharmacological experiments showed that these effects were independent of cannabinoid receptors, and it is possible that they provide an additional mechanism for the previous suggestion that endocannabinoids increase insulin secretion [131,132]. Unfortunately, however, the authors did not test anandamide, which is known to inhibit several types of K+ channels [11,117].
Finally, the effect of 2-AG at peroxisome proliferator-activated receptor-γ (PPAR-γ) should also be mentioned, although it is not yet clear whether this action is also mediated through cannabinoid receptors [131,133], and by the endocannabinoid itself [134], or its COX-2 metabolite, 15-deoxy-delta-12,14-prostaglandin J2-glycerol ester [135]. At any rate, the relevance of this action to the anti-inflammatory effects of 2-AG has been pointed out, whereas an analogous direct effect of anandamide on PPAR-γ was instead related to its pro-adipogenic function in adipocytes [136,137].
8. Conclusions
We have discussed here the possible reasons why, unlike most GPCRs (opioid receptors perhaps being the only other exception), CB1 and CB2 receptors have more than one endogenous ligand, i.e. why there are at least two endocannabinoids, anandamide and 2-AG. Such reasons, in our opinion, are to be looked for in the different (also from the point of view of their subcellular distribution) biomolecular mechanisms that, at the cellular level: (i) regulate the levels, and (ii) underlie the biological effects of these two compounds (figure 1). Indeed, the capability of anandamide and 2-AG to be biosynthesized and inactivated independently of each other, and their ‘promiscuity’ (i.e. the fact that they, unlike most neurotransmitters, neuropeptides, hormones and cytokines, and like some other lipid mediators, do not only interact with cannabinoid receptors, but also activate or inhibit several molecular targets, ranging from GPCRs to ion channels and nuclear receptors) allows for a very high degree of differential flexibility of their actions. Although most of the ‘alternative’ endocannabinoid receptors have not yet been shown to underlie at least some of the pharmacological and biological actions of anandamide and 2-AG in vivo, they do occur in vitro at concentrations compatible with the local tissue levels that can be achieved for either compound after stimulation. One can imagine that 2-AG is produced rather selectively in those physio-pathological conditions in which stimulus-induced (‘phasic’) CB1-mediated retrograde regulation of synaptic strength is required, whereas anandamide is instead produced either during tonic control of synaptic signalling, or for the TRPV1-mediated fine-tuning of ‘phasic’ and 2-AG mediated control of synaptic strength (figure 1). Likewise, anandamide direct inhibition of T-type Ca2+ channels or TASK K+ channels might also intervene to strengthen or tone-down CB1-mediated signalling in the brain, whereas the allosteric enhancement by anandamide of glycine receptors and 2-AG of GABAA receptors might allow these ubiquitous mediators to also control neuronal excitability in those neurons that do not express CB1.
In non-neuronal cells, anandamide stimulation of TRPV1 [77] and 2-AG inhibition of KATP channels [130] might synergise with endocannabinoid CB1-mediated stimulation of insulin release from pancreatic β-cells [131,132]. In spermatozoa, anandamide activation of CB1 or TRPV1 exerts different functions, possibly in different time windows [138,139]. In several cancer cell types, both CB1/CB2 and TRPV1 expression can be increased compared to that of the corresponding non-transformed cells (see, [140] for an example], and activation of these receptors, via different molecular and cellular mechanisms, can counteract cancer growth [141] and/or reduce cancer cell invasiveness [142].
We did not discuss here the possible alternative targets of other, less studied putative endocannabinoids, such as NADA, virodhamine and noladin ether, because the biosynthesis and inactivation mechanisms of these compounds, as well as the biochemical details of their functional activity at CB1 and CB2 receptors, have been much less investigated, and as a consequence, their role as endogenous cannabinoid receptor ligands is less established. However, the little information that is known regarding these compounds suggests that they too can interact with non-cannabinoid receptors [143,144]. As to the more recently identified endogenous peptide CB1 antagonist, hemopressin, although some of its effects in vivo are clearly mediated by CB1 receptor blockade [145], evidence that it directly binds to CB1 has not been confirmed so far in laboratories other than the one that first described this activity [146,147], and very little, if anything, is known about its biosynthesis and inactivation, which makes any interpretation of its biological importance difficult.
In conclusion, it is clear that not all endocannabinoids are alike and, in the future, perhaps even the name ‘endocannabinoid’ for anandamide and 2-AG will be no longer considered appropriate. Unless strictly selective ligands for cannabinoid receptors are discovered, these proteins might eventually be regarded as part of a wider lipid-based signalling system, also involving, for example, other endogenous bioactive congeners and analogues of anandamide and, hence, other molecular signal transducers. Since non-THC cannabinoids seem to interact with some of the same non-CB1, non-CB2 receptors with which anandamide and other long-chain fatty acid amides interact [37], it is possible that even the definition of ‘cannabinoid receptors’ might be enlarged in future to encompass these proteins [11].
References
- 1.Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 10.1038/346561a0 (doi:10.1038/346561a0) [DOI] [PubMed] [Google Scholar]
- 2.Munro S., Thomas K. L., Abu-Shaar M. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 10.1038/365061a0 (doi:10.1038/365061a0) [DOI] [PubMed] [Google Scholar]
- 3.Devane W. A., et al. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 10.1126/science.1470919 (doi:10.1126/science.1470919) [DOI] [PubMed] [Google Scholar]
- 4.Mechoulam R., et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 10.1016/0006-2952(95)00109-D (doi:10.1016/0006-2952(95)00109-D) [DOI] [PubMed] [Google Scholar]
- 5.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97 10.1006/bbrc.1995.2437 (doi:10.1006/bbrc.1995.2437) [DOI] [PubMed] [Google Scholar]
- 6.Di Marzo V., Fontana A. 1995. Anandamide, an endogenous cannabinomimetic eicosanoid: ‘killing two birds with one stone’. Prostaglandins Leukot. Essent. Fatty Acids 53, 1–11 10.1016/0952-3278(95)90077-20952-3278(95)90077-2 (doi:10.1016/0952-3278(95)90077-20952-3278(95)90077-2) [DOI] [PubMed] [Google Scholar]
- 7.Izzo A. A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. 2009. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30, 515–527 10.1016/j.tips.2009.07.006 (doi:10.1016/j.tips.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 8.Huang S. M., et al. 2002. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl Acad. Sci. USA 99, 8400–8405 10.1073/pnas.122196999 (doi:10.1073/pnas.122196999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanus L., Abu-Lafi S., Fride E., Breuer A., Vogel Z., Shalev D. E., Kustanovich I., Mechoulam R. 2001. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl Acad. Sci. USA 98, 3662–3665 10.1073/pnas.061029898 (doi:10.1073/pnas.061029898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter A. C., et al. 2002. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 301, 1020–1024 10.1124/jpet.301.3.1020 (doi:10.1124/jpet.301.3.1020) [DOI] [PubMed] [Google Scholar]
- 11.Pertwee R. G., et al. 2010. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2 . Pharmacol. Rev. 62, 588–631 10.1124/pr.110.003004 (doi:10.1124/pr.110.003004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackie K., Devane W. A., Hille B. 1993. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 44, 498–503 [PubMed] [Google Scholar]
- 13.Sugiura T., et al. 1999. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 274, 2794–2801 10.1074/jbc.274.5.2794 (doi:10.1074/jbc.274.5.2794) [DOI] [PubMed] [Google Scholar]
- 14.McAllister S. D., Griffin G., Satin L. S., Abood M. E. 1999. Cannabinoid receptors can activate and inhibit G protein-coupled inwardly rectifying potassium channels in a xenopus oocyte expression system. J. Pharmacol. Exp. Ther. 291, 618–626 [PubMed] [Google Scholar]
- 15.Rouzer C. A., Ghebreselasie K., Marnett L. J. 2002. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem. Phys. Lipids 119, 69–82 10.1016/S0009-3084(02)00068-3 (doi:10.1016/S0009-3084(02)00068-3) [DOI] [PubMed] [Google Scholar]
- 16.Savinainen J. R., Järvinen T., Laine K., Laitinen J. T. 2001. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 134, 664–672 10.1038/sj.bjp.0704297 (doi:10.1038/sj.bjp.0704297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiura T., Kondo S., Kishimoto S., Miyashita T., Nakane S., Kodaka T., Suhara Y., Takayama H., Waku K. 2000. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 275, 605–612 10.1074/jbc.275.1.605 (doi:10.1074/jbc.275.1.605) [DOI] [PubMed] [Google Scholar]
- 18.Gonsiorek W., Lunn C., Fan X., Narula S., Lundell D., Hipkin R. W. 2000. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol. Pharmacol. 57, 1045–1050 [PubMed] [Google Scholar]
- 19.Di Marzo V. 2011. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat. Neurosci. 14, 9–15 10.1038/nn.2720 (doi:10.1038/nn.2720) [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y., Morishita J., Tsuboi K., Tonai T., Ueda N. 2004. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 279, 5298–5305 10.1074/jbc.M306642200 (doi:10.1074/jbc.M306642200) [DOI] [PubMed] [Google Scholar]
- 21.Simon G. M., Cravatt B. F. 2008. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J. Biol. Chem. 283, 9341–9349 10.1074/jbc.M707807200 (doi:10.1074/jbc.M707807200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y. X., Tsuboi K., Okamoto Y., Tonai T., Murakami M., Kudo I., Ueda N. 2004. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem. J. 380, 749–756 10.1042/BJ20040031 (doi:10.1042/BJ20040031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., et al. 2008. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 54, 1–7 10.1016/j.neuropharm.2007.05.020 (doi:10.1016/j.neuropharm.2007.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon G. M., Cravatt B. F. 2010. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol. Biosyst. 6, 1411–1418 10.1039/C000237B (doi:10.1039/C000237B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisogno T., et al. 2003. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 10.1083/jcb.200305129 (doi:10.1083/jcb.200305129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y., et al. 2010. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 10.1523/JNEUROSCI.5693-09.2010 (doi:10.1523/JNEUROSCI.5693-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanimura A., et al. 2010. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 10.1016/j.neuron.2010.01.021 (doi:10.1016/j.neuron.2010.01.021) [DOI] [PubMed] [Google Scholar]
- 28.Yoshino H., et al. 2011. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J. Physiol. 589, 4857–4884 10.1113/jphysiol.2011.212225 (doi:10.1113/jphysiol.2011.212225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiura T., Kondo S., Sukagawa A., Tonegawa T., Nakane S., Yamashita A., Ishima Y., Waku K. 1996. Transacylase-mediated and phosphodiesterase-mediated synthesis of N-arachidonoylethanolamine, an endogenous cannabinoid-receptor ligand, in rat brain microsomes. Comparison with synthesis from free arachidonic acid and ethanolamine. Eur. J. Biochem. 240, 53–62 10.1111/j.1432-1033.1996.0053h.x (doi:10.1111/j.1432-1033.1996.0053h.x) [DOI] [PubMed] [Google Scholar]
- 30.Feledziak M., Lambert D. M., Marchand-Brynaert J., Muccioli G. G. 2012. Inhibitors of the endocannabinoid-degrading enzymes, or how to increase endocannabinoid's activity by preventing their hydrolysis. Recent Pat. CNS Drug Discov. 7, 49–70 10.2174/157488912798842223 (doi:10.2174/157488912798842223) [DOI] [PubMed] [Google Scholar]
- 31.Savinainen J. R., Saario S. M., Laitinen J. T. 2012. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 204, 267–276 10.1111/j.1748-1716.2011.02280.x (doi:10.1111/j.1748-1716.2011.02280.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtman A. H., Blankman J. L., Cravatt B. F. 2010. Endocannabinoid overload. Mol. Pharmacol. 78, 993–995 10.1124/mol.110.069427 (doi:10.1124/mol.110.069427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fowler C. J. 2012. Anandamide uptake explained? Trends Pharmacol. Sci. 33, 181–185 10.1016/j.tips.2012.01.001 (doi:10.1016/j.tips.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 34.Fu J., et al. 2011. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat. Neurosci. 15, 64–69 10.1038/nn.2986 (doi:10.1038/nn.2986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligresti A., Petrosino S., Di Marzo V. 2009. From endocannabinoid profiling to ‘endocannabinoid therapeutics’. Curr. Opin. Chem. Biol. 13, 321–331 10.1016/j.cbpa.2009.04.615 (doi:10.1016/j.cbpa.2009.04.615) [DOI] [PubMed] [Google Scholar]
- 36.Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. 1999. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 10.1038/22761 (doi:10.1038/22761) [DOI] [PubMed] [Google Scholar]
- 37.De Petrocellis L., Di Marzo V. 2010. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharmacol. 5, 103–121 10.1007/s11481-009-9177-z (doi:10.1007/s11481-009-9177-z) [DOI] [PubMed] [Google Scholar]
- 38.Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 10.1038/39807 (doi:10.1038/39807) [DOI] [PubMed] [Google Scholar]
- 39.Starowicz K., Nigam S., Di Marzo V. 2007. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 114, 13–33 10.1016/j.pharmthera.2007.01.005 (doi:10.1016/j.pharmthera.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 40.Kim J., Alger B. E. 2010. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat. Neurosci. 13, 592–600 10.1038/nn.2517 (doi:10.1038/nn.2517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grueter B. A., Brasnjo G., Malenka R. C. 2010. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 13, 1519–1525 10.1038/nn.2685 (doi:10.1038/nn.2685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chávez A. E., Chiu C. Q., Castillo P. E. 2010. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 13, 1511–1518 10.1038/nn.2684 (doi:10.1038/nn.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maccarrone M., et al. 2008. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 11, 152–159 10.1038/nn2042 (doi:10.1038/nn2042) [DOI] [PubMed] [Google Scholar]
- 44.Alger B. E. 2012. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. J. Physiol. 590, 2203–2212 10.1113/jphysiol.2011.220855 (doi:10.1113/jphysiol.2011.220855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinelli S., Pacioni S., Bisogno T., Di Marzo V., Prince D. A., Huguenard J. R., Bacci A. 2008. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J. Neurosci. 28, 13 532–13 541 10.1523/JNEUROSCI.0847-08.2008 (doi:10.1523/JNEUROSCI.0847-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarrete M., Araque A. 2010. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 10.1016/j.neuron.2010.08.043 (doi:10.1016/j.neuron.2010.08.043) [DOI] [PubMed] [Google Scholar]
- 47.Coiret G., Ster J., Grewe B., Wendling F., Helmchen F., Gerber U., Benquet P. 2012. Neuron to astrocyte communication via cannabinoid receptors is necessary for sustained epileptiform activity in rat hippocampus. PLoS ONE 7, e37320. 10.1371/journal.pone.0037320 (doi:10.1371/journal.pone.0037320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J., et al. 2012. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 10.1016/j.cell.2012.01.037 (doi:10.1016/j.cell.2012.01.037) [DOI] [PubMed] [Google Scholar]
- 49.Min R., Nevian T. 2012. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 15, 746–753 10.1038/nn.3075 (doi:10.1038/nn.3075) [DOI] [PubMed] [Google Scholar]
- 50.Fernández-Ruiz J., Pazos M. R., García-Arencibia M., Sagredo O., Ramos J. A. 2008. Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol. Cell. Endocrinol. 286(Suppl. 1), S91–S96 10.1016/j.mce.2008.01.001 (doi:10.1016/j.mce.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 51.Stella N. 2010. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030 10.1002/glia.20983 (doi:10.1002/glia.20983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahluwalia J., Urban L., Capogna M., Bevan S., Nagy I. 2000. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 100, 685–688 10.1016/S0306-4522(00)00389-4 (doi:10.1016/S0306-4522(00)00389-4) [DOI] [PubMed] [Google Scholar]
- 53.Ahluwalia J., Urban L., Bevan S., Capogna M., Nagy I. 2002. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience 110, 747–753 10.1016/S0306-4522(01)00601-7 (doi:10.1016/S0306-4522(01)00601-7) [DOI] [PubMed] [Google Scholar]
- 54.Binzen U., Greffrath W., Hennessy S., Bausen M., Saaler-Reinhardt S., Treede R. D. 2006. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neuroscience 142, 527–539 10.1016/j.neuroscience.2006.06.020 (doi:10.1016/j.neuroscience.2006.06.020) [DOI] [PubMed] [Google Scholar]
- 55.Amaya F., Shimosato G., Kawasaki Y., Hashimoto S., Tanaka Y., Ji R. R., Tanaka M. 2006. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124, 175–183 10.1016/j.pain.2006.04.001 (doi.org/10.1016/j.pain.2006.04.001) [DOI] [PubMed] [Google Scholar]
- 56.Ralevic V., Kendall D. A. 2009. Cannabinoid modulation of perivascular sympathetic and sensory neurotransmission. Curr. Vasc. Pharmacol. 7, 15–25 10.2174/157016109787354114 (doi:10.2174/157016109787354114) [DOI] [PubMed] [Google Scholar]
- 57.Weller K., Reeh P. W., Sauer S. K. 2011. TRPV1, TRPA1, and CB1 in the isolated vagus nerve–axonal chemosensitivity and control of neuropeptide release. Neuropeptides 45, 391–400 10.1016/j.npep.2011.07.011 (doi:10.1016/j.npep.2011.07.011) [DOI] [PubMed] [Google Scholar]
- 58.Nucci C., et al. 2007. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Invest. Ophthalmol. Vis. Sci. 48, 2997–3004 10.1167/iovs.06-1355 (doi:10.1167/iovs.06-1355) [DOI] [PubMed] [Google Scholar]
- 59.Cristino L., de Petrocellis L., Pryce G., Baker D., Guglielmotti V., Di Marzo V. 2006. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139, 1405–1415 10.1016/j.neuroscience.2006.02.074 (doi:10.1016/j.neuroscience.2006.02.074) [DOI] [PubMed] [Google Scholar]
- 60.Maione S., De Petrocellis L., de Novellis V., Moriello A. S., Petrosino S., Palazzo E., Rossi F. S., Woodward D. F., Di Marzo V. 2007. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 150, 766–781 10.1038/sj.bjp.0707145 (doi:10.1038/sj.bjp.0707145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micale V., Cristino L., Tamburella A., Petrosino S., Leggio G. M., Drago F., Di Marzo V. 2009. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology 34, 593–606 10.1038/npp.2008.98 (doi:10.1038/npp.2008.98) [DOI] [PubMed] [Google Scholar]
- 62.Golech S. A., McCarron R. M., Chen Y., Bembry J., Lenz F., Mechoulam R., Shohami E., Spatz M. 2004. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 132, 87–92 10.1016/j.molbrainres.2004.08.025 (doi.org/10.1016/j.molbrainres.2004.08.025) [DOI] [PubMed] [Google Scholar]
- 63.Domenicali M., et al. 2005. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut 54, 522–527 10.1136/gut.2004.051599 (doi:10.1136/gut.2004.051599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moezi L., Gaskari S. A., Liu H., Baik S. K., Dehpour A. R., Lee S. S. 2006. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br. J. Pharmacol. 149, 898–908 10.1038/sj.bjp.0706928 (doi:10.1038/sj.bjp.0706928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu T., Newton C., Perkins I., Friedman H., Klein T. W. 2006. Role of cannabinoid receptors in delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila. Eur. J. Pharmacol. 532, 170–177 10.1016/j.ejphar.2005.12.040 (doi:10.1016/j.ejphar.2005.12.040) [DOI] [PubMed] [Google Scholar]
- 66.Cavuoto P., McAinch A. J., Hatzinikolas G., Janovská A., Game P., Wittert G. A. 2007. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 364, 105–110 10.1016/j.bbrc.2007.09.099 (doi:10.1016/j.bbrc.2007.09.099) [DOI] [PubMed] [Google Scholar]
- 67.Brighton P. J., McDonald J., Taylor A. H., Challiss R. A., Lambert D. G., Konje J. C., Willets J. M. 2009. Characterization of anandamide-stimulated cannabinoid receptor signaling in human ULTR myometrial smooth muscle cells. Mol. Endocrinol. 23, 1415–1427 10.1210/me.2009-0097 (doi:10.1210/me.2009-0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi F., et al. 2009. The endovanilloid/endocannabinoid system in human osteoclasts: possible involvement in bone formation and resorption. Bone 44, 476–484 10.1016/j.bone.2008.10.056 (doi:10.1016/j.bone.2008.10.056) [DOI] [PubMed] [Google Scholar]
- 69.Jenkin K. A., McAinch A. J., Grinfeld E., Hryciw D. H. 2010. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell. Physiol. Biochem. 26, 879–886 10.1159/000323997 (doi:10.1159/000323997) [DOI] [PubMed] [Google Scholar]
- 70.Petrosino S., et al. 2010. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 65, 698–711 10.1111/j.1398-9995.2009.02254.x (doi:10.1111/j.1398-9995.2009.02254.x) [DOI] [PubMed] [Google Scholar]
- 71.Tóth B. I., et al. 2011. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J. Invest. Dermatol. 131, 1095–1104 10.1038/jid.2010.421 (doi:10.1038/jid.2010.421) [DOI] [PubMed] [Google Scholar]
- 72.Pucci M., et al. 2012. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 287, 15 466–15 478 10.1074/jbc.M111.314880 (doi:10.1074/jbc.M111.314880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyashita K., Oyama T., Sakuta T., Tokuda M., Torii M. 2012. Anandamide induces matrix metalloproteinase-2 production through cannabinoid-1 receptor and transient receptor potential vanilloid-1 in human dental pulp cells in culture. J. Endod. 38, 786–790 10.1016/j.joen.2012.02.025 (doi:10.1016/j.joen.2012.02.025) [DOI] [PubMed] [Google Scholar]
- 74.Francavilla F., et al. 2009. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology 150, 4692–700 10.1210/en.2009-0057 (doi:10.1210/en.2009-0057) [DOI] [PubMed] [Google Scholar]
- 75.Schuelert N., Zhang C., Mogg A. J., Broad L. M., Hepburn D. L., Nisenbaum E. S., Johnson M. P., McDougall J. J. 2010. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthr. Cartilage 18, 1536–1543 10.1016/j.joca.2010.09.005 (doi:10.1016/j.joca.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 76.Hermann H., De Petrocellis L., Bisogno T., Schiano Moriello A., Lutz B., Di Marzo V. 2003. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell. Mol. Life Sci. 60, 607–616 10.1007/s000180300052 (doi:10.1007/s000180300052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Petrocellis L., Marini P., Matias I., Moriello A. S., Starowicz K., Cristino L., Nigam S., Di Marzo V. 2007. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma beta-cells. Exp. Cell Res. 313, 2993–3004 10.1016/j.yexcr.2007.05.012 (doi:10.1016/j.yexcr.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 78.Di Marzo V. 2010. Anandamide serves two masters in the brain. Nat. Neurosci. 13, 1446–1448 10.1038/nn1210-1446 (doi:10.1038/nn1210-1446) [DOI] [PubMed] [Google Scholar]
- 79.Szabo B., Urbanski M. J., Bisogno T., Di Marzo V., Mendiguren A., Baer W. U., Freiman I. 2006. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J. Physiol. 577, 263–280 10.1113/jphysiol.2006.119362 (doi:10.1113/jphysiol.2006.119362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cavanaugh D. J., et al. 2011. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 31, 5067–5077 10.1523/JNEUROSCI.6451-10.2011 (doi:10.1523/JNEUROSCI.6451-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kauer J. A., Gibson H. E. 2009. Hot flash: TRPV channels in the brain. Trends Neurosci. 32, 215–224 10.1016/j.tins.2008.12.006 (doi:10.1016/j.tins.2008.12.006) [DOI] [PubMed] [Google Scholar]
- 82.Matta J. A., Ahern G. P. 2011. TRPV1 and synaptic transmission. Curr. Pharm. Biotechnol. 12, 95–101 10.2174/138920111793937925 (doi:10.2174/138920111793937925) [DOI] [PubMed] [Google Scholar]
- 83.Musella A., De Chiara V., Rossi S., Cavasinni F., Castelli M., Cantarella C., Mataluni G., Bernardi G., Centonze D. 2010. Transient receptor potential vanilloid 1 channels control acetylcholine/2-arachidonoylglicerol coupling in the striatum. Neuroscience 167, 864–871 10.1016/j.neuroscience.2010.02.058 (doi:10.1016/j.neuroscience.2010.02.058) [DOI] [PubMed] [Google Scholar]
- 84.Puente N., Cui Y., Lassalle O., Lafourcade M., Georges F., Venance L., Grandes P., Manzoni O. J. 2011. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 14, 1542–1547 10.1038/nn.2974 (doi:10.1038/nn.2974) [DOI] [PubMed] [Google Scholar]
- 85.Musella A., De Chiara V., Rossi S., Prosperetti C., Bernardi G., Maccarrone M., Centonze D. 2009. TRPV1 channels facilitate glutamate transmission in the striatum. Mol. Cell. Neurosci. 40, 89–97 10.1016/j.mcn.2008.09.001 (doi:10.1016/j.mcn.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 86.Gibson H. E., Edwards J. G., Page R. S., Van Hook M. J., Kauer J. A. 2008. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57, 746–759 10.1016/j.neuron.2007.12.027 (doi:10.1016/j.neuron.2007.12.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bennion D., et al. 2011. Transient receptor potential vanilloid 1 agonists modulate hippocampal CA1 LTP via the GABAergic system. Neuropharmacology 61, 730–738 10.1016/j.neuropharm.2011.05.018 (doi:10.1016/j.neuropharm.2011.05.018) [DOI] [PubMed] [Google Scholar]
- 88.Jensen T., Edwards J. G. 2012. Calcineurin is required for TRPV1-induced long-term depression of hippocampal interneurons. Neurosci. Lett. 510, 82–87 10.1016/j.neulet.2012.01.006 (doi:10.1016/j.neulet.2012.01.006) [DOI] [PubMed] [Google Scholar]
- 89.Cristino L., Starowicz K., De Petrocellis L., Morishita J., Ueda N., Guglielmotti V., Di Marzo V. 2008. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience 151, 955–968 10.1016/j.neuroscience.2007.11.047 (doi:10.1016/j.neuroscience.2007.11.047) [DOI] [PubMed] [Google Scholar]
- 90.Maione S., Cristino L., Migliozzi A. L., Georgiou A. L., Starowicz K., Salt T. E., Di Marzo V. 2009. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J. Physiol. 587, 2521–2535 10.1113/jphysiol.2009.171900 (doi:10.1113/jphysiol.2009.171900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Häring M., Guggenhuber S., Lutz B. 2012. Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience 204, 145–158 10.1016/j.neuroscience.2011.12.035 (doi:10.1016/j.neuroscience.2011.12.035) [DOI] [PubMed] [Google Scholar]
- 92.Starowicz K., Cristino L., Di Marzo V. 2008. TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr. Pharm. Des. 14, 42–54 10.2174/138161208783330790 (doi:10.2174/138161208783330790) [DOI] [PubMed] [Google Scholar]
- 93.Chahl L. A. 2011. TRP channels and psychiatric disorders. Adv. Exp. Med. Biol. 704, 987–1009 10.1007/978-94-007-0265-3_51 (doi:10.1007/978-94-007-0265-3_51) [DOI] [PubMed] [Google Scholar]
- 94.Talwar R., Potluri V. K. 2011. Cannabinoid 1 (CB1) receptor–pharmacology, role in pain and recent developments in emerging CB1 agonists. CNS Neurol. Disord. Drug Targets 10, 536–544 10.2174/187152711796235005 (doi:10.2174/187152711796235005) [DOI] [PubMed] [Google Scholar]
- 95.Murineddu G., Asproni B., Pinna G. A. A. 2012. survey of recent patents on CB2 agonists in the management of pain. Recent Pat. CNS Drug Discov. 7, 4–24 10.2174/157488912798842214 (doi:10.2174/157488912798842214) [DOI] [PubMed] [Google Scholar]
- 96.Jara-Oseguera A., Simon S. A., Rosenbaum T. 2008. TRPV1: on the road to pain relief. Curr. Mol. Pharmacol. 1, 255–269 10.2174/1874467210801030255 (doi:10.2174/1874467210801030255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maione S., et al. 2006. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J. Pharmacol. Exp. Ther. 316, 969–982 10.1124/jpet.105.093286 (doi:10.1124/jpet.105.093286) [DOI] [PubMed] [Google Scholar]
- 98.Pernía-Andrade A. J., et al. 2009. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325, 760–764 10.1126/science.1171870 (doi:10.1126/science.1171870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palazzo E., Luongo L., de Novellis V., Berrino L., Rossi F., Maione S. 2010. Moving towards supraspinal TRPV1 receptors for chronic pain relief. Mol. Pain 6, 66. 10.1186/1744-8069-6-66 (doi:10.1186/1744-8069-6-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kissin I., Szallasi A. 2011. Therapeutic targeting of TRPV1 by resiniferatoxin, from preclinical studies to clinical trials. Curr. Top. Med. Chem. 11, 2159–2170 10.2174/156802611796904924 (doi:10.2174/156802611796904924) [DOI] [PubMed] [Google Scholar]
- 101.Engel M. A., Izydorczyk I., Mueller-Tribbensee S. M., Becker C., Neurath M. F., Reeh P. W. 2011. Inhibitory CB1 and activating/desensitizing TRPV1-mediated cannabinoid actions on CGRP release in rodent skin. Neuropeptides 45, 229–237 10.1016/j.npep.2011.03.005 (doi:10.1016/j.npep.2011.03.005). [DOI] [PubMed] [Google Scholar]
- 102.Starowicz K., Makuch W., Osikowicz M., Piscitelli F., Petrosino S., Di Marzo V., Przewlocka B. 2012. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology 62, 1746–1755 10.1016/j.neuropharm.2011.11.021 (doi:10.1016/j.neuropharm.2011.11.021) [DOI] [PubMed] [Google Scholar]
- 103.Starowicz K., Maione S., Cristino L., Palazzo E., Marabese I., Rossi F., de Novellis V., Di Marzo V. 2007. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J. Neurosci. 27, 13 739–13 749 10.1523/JNEUROSCI.3258-07.2007 (doi:10.1523/JNEUROSCI.3258-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawahara H., Drew G. M., Christie M. J., Vaughan C. W. 2011. Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey. Br. J. Pharmacol. 163, 1214–1222 10.1111/j.1476-5381.2010.01157.x (doi:10.1111/j.1476-5381.2010.01157.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao H. T., Lee H. J., Ho Y. C., Chiou L. C. 2011. Capsaicin in the periaqueductal gray induces analgesia via metabotropic glutamate receptor-mediated endocannabinoid retrograde disinhibition. Br. J. Pharmacol. 163, 330–345 10.1111/j.1476-5381.2011.01214.x (doi:10.1111/j.1476-5381.2011.01214.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giordano C., et al. In press. TRPV1-dependent and-independent alterations in the limbic cortex of neuropathic mice: impact on glial caspases and pain perception. Cereb. Cortex. 10.1093/cercor/bhr328 (doi:10.1093/cercor/bhr328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knerlich-Lukoschus F., Noack M., von der Ropp-Brenner B., Lucius R., Mehdorn H. M., Held-Feindt J. 2011. Spinal cord injuries induce changes in CB1 cannabinoid receptor and C–C chemokine expression in brain areas underlying circuitry of chronic pain conditions. J. Neurotrauma 28, 619–634 10.1089/neu.2010.1652 (doi:10.1089/neu.2010.1652) [DOI] [PubMed] [Google Scholar]
- 108.De Petrocellis L., Di Marzo V. 2005. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci. 77, 1651–1666 10.1016/j.lfs.2005.05.021 (doi:10.1016/j.lfs.2005.05.021) [DOI] [PubMed] [Google Scholar]
- 109.Fioravanti B., et al. 2008. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J. Neurosci. 28, 11 593–11 602 10.1523/JNEUROSCI.3322-08.2008 (doi:10.1523/JNEUROSCI.3322-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan S., Burrell B. D. 2010. Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J. Neurophysiol. 104, 2766–2777 10.1152/jn.00491.2010 (doi:10.1152/jn.00491.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong S., Fan J., Kemmerer E. S., Evans S., Li Y., Wiley J. W. 2009. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 58, 202–210 10.1136/gut.2008.157594 (doi:10.1136/gut.2008.157594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bab I., Zimmer A. 2008. Cannabinoid receptors and the regulation of bone mass. Br. J. Pharmacol. 153, 182–188 10.1038/sj.bjp.0707593 (doi:10.1038/sj.bjp.0707593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Idris A. I., Ralston S. H. 2010. Cannabinoids and bone: friend or foe? Calcif. Tissue Int. 87, 285–297 10.1007/s00223-010-9378-8 (doi:10.1007/s00223-010-9378-8) [DOI] [PubMed] [Google Scholar]
- 114.Rossi F., et al. 2011. The endovanilloid/endocannabinoid system: a new potential target for osteoporosis therapy. Bone 48, 997–1007 10.1016/j.bone.2011.01.001 (doi:10.1016/j.bone.2011.01.001) [DOI] [PubMed] [Google Scholar]
- 115.Di Marzo V., De Petrocellis L. 2010. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 17, 1430–1449 10.2174/092986710790980078 (doi:10.2174/092986710790980078) [DOI] [PubMed] [Google Scholar]
- 116.Tsumura M., Sobhan U., Muramatsu T., Sato M., Ichikawa H., Sahara Y., Tazaki M., Shibukawa Y. 2012. TRPV1-mediated calcium signal couples with cannabinoid receptors and sodium–calcium exchangers in rat odontoblasts. Cell Calcium 52, 124–136 10.1016/j.ceca.2012.05.002 (doi:10.1016/j.ceca.2012.05.002). [DOI] [PubMed] [Google Scholar]
- 117.Oz M. 2006. Receptor-independent effects of endocannabinoids on ion channels. Curr. Pharm. Des. 12, 227–239 10.2174/138161206775193073 (doi:10.2174/138161206775193073) [DOI] [PubMed] [Google Scholar]
- 118.Chemin J., Monteil A., Perez-Reyes E., Nargeot J., Lory P. 2001. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 20, 7033–7040 10.1093/emboj/20.24.7033 (doi:10.1093/emboj/20.24.7033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chemin J., Nargeot J., Lory P. 2007. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J. Biol. Chem. 282, 2314–2323 10.1074/jbc.M610033200 (doi:10.1074/jbc.M610033200) [DOI] [PubMed] [Google Scholar]
- 120.Lambert R. C., Bessaih T., Leresche N. 2006. Modulation of neuronal T-type calcium channels. CNS Neurol. Disord. Drug Targets 5, 611–627 10.2174/187152706779025544 (doi:10.2174/187152706779025544) [DOI] [PubMed] [Google Scholar]
- 121.Maingret F., Patel A. J., Lazdunski M., Honoré E. 2001. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 20, 47–54 10.1093/emboj/20.1.47 (doi:10.1093/emboj/20.1.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oz M., Ravindran A., Diaz-Ruiz O., Zhang L., Morales M. 2003. The endogenous cannabinoid anandamide inhibits alpha7 nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J. Pharmacol. Exp. Ther. 306, 1003–1010 10.1124/jpet.103.049981 (doi:10.1124/jpet.103.049981) [DOI] [PubMed] [Google Scholar]
- 123.Zaniewska M., McCreary A. C., Przegaliński E., Filip M. 2006. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur. J. Pharmacol. 540, 96–106 10.1016/j.ejphar.2006.04.034 (doi:10.1016/j.ejphar.2006.04.034) [DOI] [PubMed] [Google Scholar]
- 124.Spivak C. E., Lupica C. R., Oz M. 2007. The endocannabinoid anandamide inhibits the function of alpha4beta2 nicotinic acetylcholine receptors. Mol. Pharmacol. 72, 1024–1032 10.1124/mol.107.036939 (doi:10.1124/mol.107.036939) [DOI] [PubMed] [Google Scholar]
- 125.Hejazi N., Zhou C., Oz M., Sun H., Ye J. H., Zhang L. 2006. Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol. Pharmacol. 69, 991–997 10.1124/mol.105.019174 (doi:10.1124/mol.105.019174) [DOI] [PubMed] [Google Scholar]
- 126.Yang Z., Aubrey K. R., Alroy I., Harvey R. J., Vandenberg R. J., Lynch J. W. 2008. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem. Pharmacol. 76, 1014–1023 10.1016/j.bcp.2008.07.037 (doi:10.1016/j.bcp.2008.07.037) [DOI] [PubMed] [Google Scholar]
- 127.Yévenes G. E., Zeilhofer H. U. 2011. Molecular sites for the positive allosteric modulation of glycine receptors by endocannabinoids. PLoS ONE 6, e23886. 10.1371/journal.pone.0023886 (doi:10.1371/journal.pone.0023886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiong W., Wu X., Lovinger D. M., Zhang L. 2012. A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. J. Neurosci. 32, 5200–5208 10.1523/JNEUROSCI.6347-11.2012 (doi:10.1523/JNEUROSCI.6347-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sigel E., Baur R., Rácz I., Marazzi J., Smart T. G., Zimmer A., Gertsch J. 2011. The major central endocannabinoid directly acts at GABA(A) receptors. Proc. Natl Acad. Sci. USA 108, 18 150–18 155 10.1073/pnas.1113444108 (doi:10.1073/pnas.1113444108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spivak C. E., Kim W., Liu Q.-R., Lupica C. R., Doyle M. E. 2012. Blockade of β-cell K(ATP) channels by the endocannabinoid, 2-arachidonoylglycerol. Biochem. Biophys. Res. Comm. 423, 13–16 10.1016/j.bbrc.2012.05.042 (doi:10.1016/j.bbrc.2012.05.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matias I., et al. 2006. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 91, 3171–3180 10.1210/jc.2005-2679 (doi:10.1210/jc.2005-2679) [DOI] [PubMed] [Google Scholar]
- 132.Li C., Bowe J. E., Jones P. M., Persaud S. J. 2010. Expression and function of cannabinoid receptors in mouse islets. Islets 2, 293–302 10.4161/isl.2.5.12729 (doi:10.4161/isl.2.5.12729) [DOI] [PubMed] [Google Scholar]
- 133.Du H., Chen X., Zhang J., Chen C. 2011. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-γ. Br. J. Pharmacol. 163, 1533–1549 10.1111/j.1476-5381.2011.01444.x (doi:10.1111/j.1476-5381.2011.01444.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rockwell C. E., Snider N. T., Thompson J. T., Vanden Heuvel J. P., Kaminski N. E. 2006. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol. Pharmacol. 70, 101–111 10.1124/mol.105.019117 (doi:10.1124/mol.105.019117) [DOI] [PubMed] [Google Scholar]
- 135.Raman P., Kaplan B. L., Thompson J. T., Vanden Heuvel J. P., Kaminski N. E. 2011. 15-Deoxy-delta12,14-prostaglandin J2-glycerol ester, a putative metabolite of 2-arachidonyl glycerol, activates peroxisome proliferator activated receptor gamma. Mol. Pharmacol. 80, 201–209 10.1124/mol.110.070441 (doi:10.1124/mol.110.070441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouaboula M., Hilairet S., Marchand J., Fajas L., Le Fur G., Casellas P. 2005. Anandamide induced PPAR-γ transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur. J. Pharmacol. 517, 174–181 10.1016/j.ejphar.2005.05.032 (doi:10.1016/j.ejphar.2005.05.032) [DOI] [PubMed] [Google Scholar]
- 137.Karaliota S., Siafaka-Kapadai A., Gontinou C., Psarra K., Mavri-Vavayanni M. 2009. Anandamide increases the differentiation of rat adipocytes and causes PPAR-γ and CB1 receptor upregulation. Obesity (Silver Spring) 17, 1830–1838 10.1038/oby.2009.177 (doi:10.1038/oby.2009.177) [DOI] [PubMed] [Google Scholar]
- 138.Maccarrone M., Barboni B., Paradisi A., Bernabò N., Gasperi V., Pistilli M. G., Fezza F., Lucidi P., Mattioli M. 2005. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J. Cell Sci. 118, 4393–4404 10.1242/jcs.02536 (doi:10.1242/jcs.02536) [DOI] [PubMed] [Google Scholar]
- 139.Gervasi M. G., Osycka-Salut C., Caballero J., Vazquez-Levin M., Pereyra E., Billi S., Franchi A., Perez-Martinez S. 2011. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS ONE 6, e16993. 10.1371/journal.pone.0016993 (doi:10.1371/journal.pone.0016993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Czifra G., Varga A., Nyeste K., Marincsák R., Tóth B. I., Kovács I., Kovács L., Bíró T. 2009. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 135, 507–514 10.1007/s00432-008-0482-3 (doi:10.1007/s00432-008-0482-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melck D., De Petrocellis L., Orlando P., Bisogno T., Laezza C., Bifulco M., Di Marzo V. 2000. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 141, 118–126 10.1210/en.141.1.118 (doi:10.1210/en.141.1.118) [DOI] [PubMed] [Google Scholar]
- 142.Ramer R., Hinz B. 2008. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl Cancer Inst. 100, 59–69 10.1093/jnci/djm268 (doi:10.1093/jnci/djm268) [DOI] [PubMed] [Google Scholar]
- 143.Ross H. R., Gilmore A. J., Connor M. 2009. Inhibition of human recombinant T-type calcium channels by the endocannabinoid N-arachidonoyl dopamine. Br. J. Pharmacol. 156, 740–750 10.1111/j.1476-5381.2008.00072.x (doi:10.1111/j.1476-5381.2008.00072.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O'Sullivan S. E., Kendall D. A. 2010. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology 215, 611–616 10.1016/j.imbio.2009.09.007 (doi:10.1016/j.imbio.2009.09.007) [DOI] [PubMed] [Google Scholar]
- 145.Dodd G. T., Mancini G., Lutz B., Luckman S. M. 2010. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J. Neurosci. 30, 7369–7376 10.1523/JNEUROSCI.5455-09.2010 (doi:10.1523/JNEUROSCI.5455-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heimann A. S., et al. 2007. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc. Natl Acad. Sci. USA 104, 20 588–20 593 10.1073/pnas.0706980105 (doi:10.1073/pnas.0706980105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomes I., et al. 2009. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 23, 3020–3029 10.1096/fj.09-132142 (doi:10.1096/fj.09-132142) [DOI] [PMC free article] [PubMed] [Google Scholar]