Abstract
Endocannabinoids (eCBs) act as modulators of synaptic transmission through activation of a number of receptors, including, but not limited to, cannabinoid receptor 1 (CB1). eCBs share CB1 receptors as a common target with Δ9-tetrahydrocannabinol (THC), the main psychoactive ingredient in marijuana. Although THC has been used for recreational and medicinal purposes for thousands of years, little was known about its effects at the cellular level or on neuronal circuits. Identification of CB1 receptors and the subsequent development of its specific ligands has therefore enhanced our ability to study and bring together a substantial amount of knowledge regarding how marijuana and eCBs modify interneuronal communication. To date, the eCB system, composed of cannabinoid receptors, ligands and the relevant enzymes, is recognized as the best-described retrograde signalling system in the brain. Its impact on synaptic transmission is widespread and more diverse than initially thought. The aim of this review is to succinctly present the most common forms of eCB-mediated modulation of synaptic transmission, while also illustrating the multiplicity of effects resulting from specializations of this signalling system at the circuital level.
Keywords: endocannabinoids, synaptic plasticity, cannabinoid receptor 1, transient receptor potential 1, anandamide, 2-arachidonoyglycerol
1. Introduction: synaptic elements responsible for triggering the release of endocannabinoids
eCBs constitute molecules of lipidic nature that, released on demand from postsynaptic domains, are known to modulate synaptic transmission in a retrograde manner. Initial identification of the synaptic events responsible for eCB release was strongly supported by the finding that postsynaptic depolarization triggered GABA-mediated suppression of inhibition. In separate publications over the course of less than two years, the laboratories of Alain Marty and Brad Alger reported that postsynaptic depolarization triggered a decrease of GABAergic transmission in cerebellum [1] and hippocampus [2], termed DSI (depolarization-induced suppression of inhibition). This effect was shown to be expressed in the presynaptic domain, suggesting the existence of a retrograde mediator [3]. However, it was not until 2001 that the subcellular distribution of components of the eCB system were characterized, allowing for the conceptual framework to describe how eCBs act as retrograde transmitters [4]. In confluence with this, two critical studies from the laboratories of Roger Nicoll and Masanobu Kano directly demonstrated that cannabinoid signalling was in fact responsible for DSI [5,6]. Alongside, work from Wade Regehr's laboratory showed that reduced transmission at excitatory synapses in Purkinje neurons due to reductions in calcium influx also involved cannabinoids, and this was correspondingly named DSE (depolarization-induced suppression of excitation) [7], indicating that the phenomenon of cannabinoid-modulated transmission was even more widespread.
Because postsynaptic activity appeared necessary to trigger either DSI or DSE, the logical experimental continuum was to look for forms of synaptic activity that could re-create the required postsynaptic depolarization. Subsequently, a variety of ionotropic and G-protein-coupled receptors were shown to be capable of triggering eCB release. For G-protein-coupled receptors, a range of Gq/11-coupled receptors have been shown to be necessary or sufficient for eCB-dependent modulation of synaptic transmission in various brain areas. For example, activation of group I metabotropic glutamate receptors (mGluR) reduced excitatory transmission onto Purkinje cells (PC) from climbing fibres [8] and inhibitory transmission on hippocampal pyramidal cells [9], both subject to CB1 activation. Gq/11-coupled muscarinic receptors have been reported to mediate eCB release in the hippocampus, resulting in the suppression of inhibitory [10,11] and autaptic excitatory transmission [12]. Also dependent on CB1 activation, in the inferior olive, excitatory transmission is suppressed owing to the activation of Gq/11-coupled 5-hydroxytryptamine receptors [13], as well as in the dorsal raphe following application of orexin-B [14]. On the other hand, regarding ionotropic receptors, it was shown that activation of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid (AMPA) receptors triggers eCB release [15], while application of N-methyl-d-aspartate (NMDA) was found to trigger eCB-dependent suppression of inhibitory transmission onto cultured hippocampal neurons in a Ca++ sensitive manner [16]. Thus, there is great diversity in the types of receptors and forms of synaptic activity related to triggering eCB synthesis and release.
2. Presynaptic and postsynaptic targets of endocannabinoid action
Although the experimental manipulations that initially evoked DSI performed by Marty and Alger in 1991 and 1992 focused on the postsynaptic domain, the results suggested the existence of a presynaptic target downstream capable of modulating neurotransmitter release [1,2,17]. At the time, however, there was not enough evidence to definitively point to the involvement of cannabinoids and the CB1 receptor in these phenomena. A few years prior to their work, the CB1 receptor had been identified [18], sequenced [19] and its global distribution in the brain characterized [20]. However, it was not until 1999 that ultra-structural characterization of the CB1 receptor demonstrated its localization to presynaptic sites [21], results that immediately preceded the electrophysiological evidence of Nicoll, Kano and Regehr, showing that pharmacological blockade of the CB1 receptor prevents DSI [5,6] and DSE [7]. The subsequent identification of endogenous ligands for the CB1 receptor, anandamide [22] and 2-arachidonoyglycerol (2-AG) [23,24] permitted investigation of their hydrolytic and synthetic enzymes, furthering the construction of a solid conceptual framework essential to what today we understand as the cannabinoid retrograde signalling system.
It has become accepted that the majority of CB1 receptors are expressed on GABAergic terminals and, to a lesser degree, at glutamatergic terminals [25–27]. However, on the basis of immuno-labelling studies combined with either confocal or electron microscopy, they have also been reported to co-localize with acetylcholine- and catecholamine-synthesizing enzymes. For example, approximately, one-third of synaptic terminals in the frontal cortex that labelled positive for the CB1 receptor were found co-labelled for the norepinephrine-synthesizing enzyme dopamine (DA)-beta-hydroxylase [28]. This finding was crucial to understanding a previous report in which systemic application of the CB1 agonist WIN55,212-2 evoked an increase in levels of norepinephrine in the frontal cortex [29,30]. Expression of CB1 receptor at DA terminals has also been reported [31], including a study done in teleost fish in which eCBs evoked DA release independently of the involvement of GABAergic mechanisms [32]. Adding even more complexity to this picture are studies demonstrating that CB1 receptors are also expressed in glial cells [33–38] and on postsynaptic domains [39–42]. In the cerebral cortex, autocrine activation of postsynaptic CB1 receptors by 2-AG evokes potassium conductance-mediated long-lasting self-inhibition of a specific subset of interneurons [42–44]. Together, these studies constitute evidence that CB1 receptor activation involves a variety of modalities that coexist with its most commonly described function as modulator of release at GABAergic and glutamatergic terminals.
Although many signalling cascades have been described resulting from its activation, the CB1 receptor is best-known for binding to members of the Gi/o group of G proteins, resulting in decreased cyclic AMP (cAMP) production and protein kinase A (PKA) activation. Ultimately, these changes trigger a decrease in calcium influx and an increase in potassium conductance at presynaptic sites, resulting in a reduction in neurotransmitter release. However, a number of studies report the existence of additional signalling cascades to which CB1 is coupled, establishing an even wider array of possible effects that can result from its activation. For example, the synthetic CB1 receptor agonist WIN55,212-2 has been shown to trigger an increase in intracellular Ca++ that is dependent on Gq/11 activation in a heterologous expression system and cultured hippocampal neurons [45]. In striatal neurons, CB1 receptor activation leads to an increase in intracellular concentrations of cAMP, mediated by coupling of CB1 to a GS protein [46]. In astrocytes, activation of CB1 by eCBs points to activation of Gq/11 and subsequent activation of phospholipase C [35,36]. Making the range of responses derived from CB1 receptor activation even more diverse, it has recently been demonstrated that co-expression of opioid and CB1 receptors can attenuate the potency at which each of these receptors would otherwise respond individually; i.e. co-activation of CB1 and µ opioid receptors in heterologous expression systems and in rat striatal membranes reduces the potency conveyed by the activation of CB1 receptor alone or µ opioid receptor alone [47]. Similar interactions have also been identified between CB1 and δ opioid receptors, ‘enhancing the repertoire of GPCR (G- protein-coupled receptor) signalling’ [48]. Additional downstream targets of the CB1 receptor also include receptor tyrosine kinases and mitogen-activated protein kinases (MAPK), among others [49–51].
Although the most remarkable target of eCB action impacting synaptic transmission is the presynaptic CB1 receptor, the development of pharmacological tools has greatly furthered the potential to elucidate the role of eCBs in modifying synaptic plasticity in a manner not related to CB1 receptors. Probably the best example of this is the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor, which is sensitive to capsaicin as well as to high temperatures. Expression of TRPV1 channels has been demonstrated in glial cells as well as on presynaptic and postsynaptic neuronal domains [52–54]; its interaction with the eCB system has received considerable attention [55–57] and a number of studies have evidenced the role of presynaptic TRPV1 and cannabinoids in the modulation of synaptic transmission [58–62]. One of the most recent and significant advances regarding eCB-mediated synaptic plasticity is the identification of transient receptor potential (TRP) channels as postsynaptic target for the endogenous cannabinoid anandamide [62–64]. The following sections of this review will offer a condensed overview on the role these different targets play in mediating the actions of eCBs on synaptic plasticity.
3. Short-term synaptic plasticity mediated by endocannabinoids
Hippocampal and cerebellar DSI and DSE as eCB-mediated modulatory events on synaptic transmission [5–7] were later reported in other brain areas as well, including basal ganglia and cerebral cortex. However, it was also shown that a feature common to DSI/DSE, namely the dependence on increased postsynaptic Ca++ concentrations, was not necessarily required for other mechanisms of induction of eCB activity. Specifically, eCB release triggered by activation of Gq/11-coupled receptors did not require increased postsynaptic calcium. Over time, DSI and DSE had become a reliable way to trigger eCB-mediated modulation of synaptic transmission as well as to identify axon terminals that might or might not be sensitive to the action of cannabinoid ligands (i.e. expressing CB1 receptor); so they had played a crucial role in identifying the molecular elements of the eCB system. However, increasing interest was developing in finding ways to evoke the release of eCBs via synaptic activity directly, as it meant coming a step closer to understanding endogenous physiological methods of triggering eCB-mediated plasticity [65].
A first step in this process involved demonstrating which eCBs were directly involved in causing DSI/DSE. As previously mentioned, two have been the most-studied (anandamide [22] and 2-AG [66–68]), and significant differences in their distribution, concentrations, and synthetic and hydrolytic pathways have been shown. By manipulating the synthetic and hydrolytic pathways for each, identification of the eCB involved in DSI/DSE was possible. Deletion of the α isoform of diacylglycerol lipase (DGL), the main enzyme involved in the synthesis of 2-AG, resulted in an inability to trigger eCB-mediated plasticity in cortex, striatum, cerebellum and hippocampus [69–71], while inhibition of the anandamide-hydrolysing enzyme fatty acid amide hydrolase (FAAH) did not affect DSI [72], suggesting that anandamide was not involved in this phenomenon.
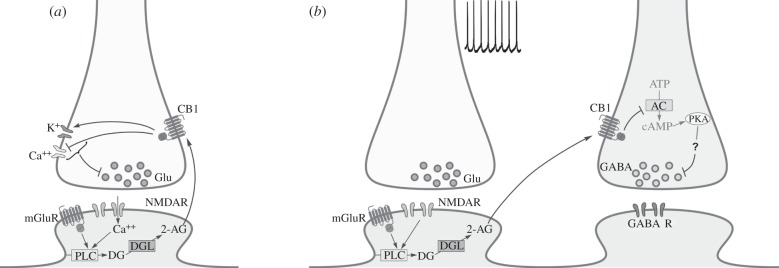
Following this, proving that eCBs could be released in an activity-dependent fashion became the key to demonstrating that neuronal activity resulting in eCB production could trigger short- or long-term influences on interneuronal communication. Given that activation of presynaptic CB1 results in reduced neurotransmitter release, an increase in the production of eCBs was expected to lead to short-term depression (STD). eCB-mediated STD (eCB-STD) is characterized by a reduction in the amplitude of postsynaptic potentials that usually manifests its maximum effect for a short time following induction, after which it recovers progressively over tens of seconds. Several stimulation paradigms for presynaptic terminals are now known to trigger short-term modulation of synaptic plasticity in an eCB-dependent manner, most of which require a repetitive pattern of stimulation known to enhance activation of metabotropic receptors (figure 1a). In the cerebellum, postsynaptic depolarization, pharmacological activation of mGluR1 and electrical stimulation of parallel fibres in the form of 50 pulses at 100 Hz have all been shown to trigger a reduction in excitatory neurotransmission from climbing fibres to PCs [8], an effect that is blocked by CB1 receptor antagonists. Note that this eCB-dependent form of STD refers to a heterosynaptic mechanism, i.e. synaptic activity from a type of afferent that ends up modulating synaptic input from a different afferent.
Figure 1.
eCB-dependent short-term depression (STD) and long-term depression (LTD). (a) eCB-mediated STD. Activation of Gq/11-coupled metabotropic receptors (mGluR) and/or Ca++-permeable ionotropic receptors (NMDAR) on the postsynaptic cell promotes the hydrolysis of membrane phospholipids into diacylglycerol (DG) by phospholipase C (PLC). Conversion of DG into 2-AG is then performed by the enzyme DGL. 2-AG binds to the presynaptic Gi/o-coupled CB1 receptor, causing decreased Ca++ conductance and increased K+ conductance. This modulation of calcium results in a decreased probability of transmitter release from the presynaptic terminal. (b) eCB-mediated LTD. Activity-dependent release of glutamate results in mGluR and NMDA receptor activation. This promotes the synthesis of 2-AG, which then travels to the presynaptic domain, where it activates the CB1 receptor on neighbouring GABAergic terminals. The resulting inhibition of adenylyl cyclase (AC) promotes a decrease in PKA activation. Decreased PKA activity is believed to reduce phosphorylation of a presynaptic regulatory messenger, resulting in a lasting reduction of neurotransmitter release.
Shortly after this study, it was also shown that electrical stimulation of parallel fibres (consisting of 10 pulses at 50 Hz) could reduce excitatory neurotransmission from parallel fibres onto PCs, and that this event was dependent on the activation of CB1-receptors [15]. This constitutes a homosynaptic mechanism. As mentioned before, this study reported not only the involvement of mGluR1, but also of AMPA receptors, and that it required increases in postsynaptic Ca++ concentration. Interestingly, this same study showed that postsynaptic changes in Ca++ concentration occur in a local manner and that modulation of presynaptic release was therefore synapse-specific [15]. Such an observation is in agreement with previous studies showing that eCB signalling occurs in a limited spatial setting in which diffusion of eCBs extend approximately 20 µm from their site of release [5].
In 2004, a form of activity-dependent eCB-mediated suppression of inhibition was reported. In this case, electrical stimulation of parallel fibres in the form of 10 pulses at 100 Hz was sufficient to trigger transient depression of spontaneous inhibitory postsynaptic currents (IPSCs) in PCs [73]. Although mGluR1 again emerged as the postsynaptic receptor involved in triggering the postsynaptic production of the eCB 2-AG, this mechanism did not require increases in Ca++ concentration. While certainly a multiplicity of factors can affect diffusion, this study reported that eCB signalling could spread to an adjacent neuron [73], which is in remarkable contrast with previous reports describing shorter distances of eCB action [5,8].
Another brain circuit in which eCB signalling has received special attention is the mesolimbic system [74]. In 2004, after demonstrating the presence of DSE in DA neurons of the ventral tegmental area (VTA) [75], this laboratory also reported that in vitro stimulation of cortical afferents (10 pulses at 5 Hz) was able to trigger suppression of excitation of VTA DA neurons in a CB1-receptor-dependent manner [76]. Even more interesting is that this same pattern of stimulation applied in vivo to the prefrontal cortex replicated such suppression of excitation. These effects on presynaptic excitatory afferents required the action of 2-AG released from DA neurons, which was shown to be Ca++-dependent and mediated by mGluR1 [76].
4. Long-term plasticity mediated by endocannabinoids
As in the case of eCB-STD, the ability to modulate synaptic strength remained an attractive possibility for the action of eCBs via their activation of the CB1 receptor. More importantly, eCB-mediated long-term effects imply, by definition, long-lasting effects on synaptic strength, meaning that postsynaptic activity could modulate synaptic inputs on a longer time scale. eCB-mediated long-term depression (eCB-LTD) was first demonstrated in the striatum, where CB1 receptors were shown to be involved in activity-dependent plasticity years before. In this ground-breaking study, stimulation of striatal afferents (using four trains of 1 s duration at 100 Hz) was able to trigger LTD that lasted for over 20 min [77]. This effect also required a paired postsynaptic depolarization at the time of afferent electrical stimulation and was dependent on increased postsynaptic Ca++ concentrations [77]. It is now known that eCB-LTD can be triggered by a diverse set of patterns of stimulation that ranges from theta burst stimulation to long trains at 1 or 100 Hz, and has been demonstrated in a wide range of brain areas, including sensory cortex, hippocampus, amygdala, dorsal and ventral striatum, cerebellum, VTA, dorsal cochlear nucleus and superior colliculus (for a review, see [78]). As a result, a few common factors became clear on eCB-LTD. First, characteristics of presynaptic stimulation did not seem strikingly different from those related to eCB-STD. Second, coincident presynaptic/postsynaptic activity was often (but not necessarily) a critical component of the long-term effect.
As previously mentioned, a crucial implication of eCB-triggered long-term plasticity is its ability to regulate interneuronal communication on a prolonged temporal scale. A recent series of studies carried out in the laboratory of Pablo Castillo has shown not only the capacity of eCBs to evoke LTD of inhibitory transmission in the hippocampus, but also furthered our understanding of the mechanisms underlying how CB1 activation leads to long-term effects on synaptic activity [79–81]. Specifically, in a 2003 study, Castillo's laboratory described how either high-frequency stimulation or theta burst stimulation of the excitatory Schaffer collaterals triggered a decrease in IPSCs at CA1 pyramidal neurons [79]. This effect was shown to be independent of ionotropic glutamate receptor activation and postsynaptic Ca++ increase, but requires mGluR group I receptor activation and synthesis of 2-AG. Also, in a key effort to clarify differences with eCB-mediated short-term plasticity, it was shown that inhibitory LTD requires continuous activation of the CB1 receptor for about 10 min [79]. In a later report, Castillo and his group reported how this eCB-induced inhibitory LTD is able to locally affect the ability of excitatory afferents to undergo long-term potentiation (LTP) [80]. Together, these results represent a novel and interesting eCB-mediated interaction between inhibitory and excitatory inputs: activation of excitatory afferents triggers LTP in a local manner while evoking inhibitory LTD in a larger area, subsequently facilitating LTP induction of nearby excitatory afferents [80]. Finally, a third article in this series characterized cAMP and PKA signalling as required to establish long-term but not short-term eCB-mediated effects on inhibitory inputs in the hippocampus and amygdala [81] (figure 1b) and that LTD requires active zone protein RIM1α [81]. Focusing on a different player, presynaptic NMDA receptors have been suggested as coincidence detector for the expression of eCB-LTD [82–84], bringing additional support to a critical role of the presynaptic domain for the consolidation of changes that result in long-term expression of eCB-mediated synaptic plasticity.
As mentioned already, TRPV1 receptors are recognized targets of eCB action. A TRPV1-mediated mechanism of LTD has been reported in both hippocampus and nucleus accumbens (NAc), two brain areas in which cannabinoid signalling is known to play a prominent role in modulation of synaptic strength. In the hippocampus, presynaptic stimulation paired with postsynaptic depolarization at dentate granular cells triggered a long-term decrease in the amplitude of excitatory inputs from the medial perforant path [63]. This effect was blocked by a TRPV1 antagonist and not seen in Trpv1 knockout mice. More interestingly yet, changes in presynaptic release were not detected, implying a postsynaptic effect that is likely to involve the removal of AMPA receptors. In addition, this form of LTD was still present while blocking 2-AG synthesis and also when a subthreshold induction protocol was used under conditions of reduced anandamide metabolism. Together, these results indicate that postsynaptic production of the eCB anandamide results in activation of postsynaptic TRPV1 receptors, which in turn promotes internalization of postsynaptic AMPA receptors in a long-term fashion [63]. Malenka's group also studied TRPV1-mediated LTD, taking advantage of selective enhanced green fluorescent protein expression in D2-positive neurons of the NAc. This enabled differentiation of DA D1-receptor-positive medium spiny neurons (MSNs) corresponding to the so-called direct pathway from D2-receptor-positive MSNs corresponding to the ‘indirect pathway’. Electrical stimulation of MSN afferents by a low-frequency protocol triggered LTD in D2+ MSNs, but not in D2– MSNs. This effect required mGluR I activation and was reduced but not abolished by application of either a CB1 antagonist alone, or a TRPV1 antagonist alone. However, co-application of the CB1 antagonist AM251 and the TRPV1 antagonist capsazepine prevented LTD [64]. As in the case of hippocampal TRPV1-mediated LTD, in this study, changes in presynaptic release were not detected when LTD was evoked under CB1 blockade, supporting a postsynaptic locus of action of TRPV1 channels. Adding up these possibilities, a form of long-lasting depression of excitatory inputs to interneurons of the stratum radiatum in the hippocampus has been recently described [85]. Strikingly, in this form of eCB-mediated plasticity, neither CB1 nor TRPV1 receptors are involved. While eCB-mediated long-term effects on synaptic transmission represent a key mechanism to influence long-lasting changes in neuronal communication, comprehensive characterization of the events involved in the presynaptic expression of LTD is still lacking.
5. Specializations of the endocannabinoid system at the circuit level
The overall role of eCB signalling could be described in terms of postsynaptic activation leading to on-demand synthesis and release of eCBs, which then mobilize to the presynaptic domain, resulting in CB1 receptor activation and reduced neurotransmitter release (either GABA or glutamate). However, there is also evidence from experimental models that in several circuits the eCB system acts as a specialized signalling system subserving precise modulatory roles. As a way to ponder these possibilities, a few examples are presented below.
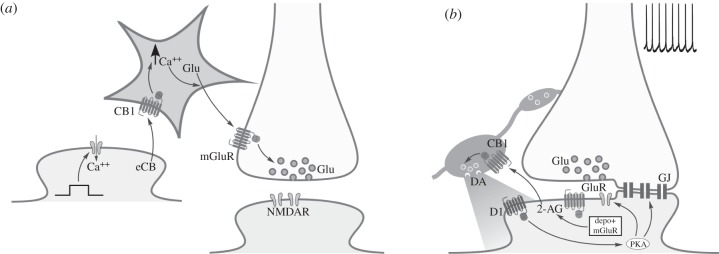
In the nervous system, glial cells were long considered passive elements, their major role involving serving as structural support to neurons and their associated networks. In recent years, this idea has received a major overhaul, as today glial cells are considered key components of and players in synaptic communication. This applies to the role of glial cells in eCB signalling as well. In particular, a recent set of studies have shown the prominent role that astrocytes play in cannabinoid signalling [35–38]. Strikingly, it was not only reported that the CB1 receptor is expressed in hippocampal astrocytes, but also that its activation results in an increase in intracellular Ca++ [35]. This effect was seen by locally applying puffs of either 2-AG, anandamide or the synthetic agonist WIN55,212-2, a result that indicates that the CB1-mediated Ca++ increase is not ligand-specific. Because hippocampal pyramidal neurons could constitute a source of eCBs, the authors also tested whether endogenous release of CB resulted in a similar effect to the one triggered by pressure puff application. They found that depolarization of pyramidal neurons did indeed evoke Ca++ increases in neighbouring astrocytes in a CB1-dependent manner, which also subsequently evoked glutamate-mediated inward currents in pyramidal neurons [35]. An extension of these results indicates a bimodal effect of eCBs on spatially segregated inputs to pyramidal neurons [36]: while eCBs depress synaptic inputs to the depolarized pyramidal neuron, they also evoke a Ca++ increase in and glutamate release from astrocytes, which then potentiates synaptic transmission acting on presynaptic mGluR1 [36] (figure 2a). Even more recently, it has been shown that astrocytic CB1-dependent increase in Ca++ concentration determines the release of glutamate from astrocytes and subsequent activation of neuronal presynaptic NMDA receptors, resulting in time-dependent LTD in the sensory barrel cortex [37]. Also, it was found that genetic deletion of astrocytic CB1 prevents hippocampal CA3-CA1 LTD and alterations in working memory induced by acute exposure to Δ9-tetrahydrocannabinol (THC), which does not occur when expression of CB1 receptors is suppressed in glutamatergic and GABAergic neurons [38]. These results confirm the importance of astrocytic function in cannabinoid signalling and its involvement in a variety of phenomenon, including synaptic depression, potentiation and even responsiveness to administration of exogenous cannabinoids.
Figure 2.
Specializations of eCB activity at the circuit level. (a) Astrocytes modulate interneuronal synaptic communication in an eCB-dependent manner. Depolarization of the postsynaptic neuron (left) promotes synthesis and release of eCBs, which in turn activate astrocytic CB1 receptors resulting in increased intracellular Ca++ levels. This stimulates glutamate release from the astrocyte, which then activates presynaptic neuronal mGluRs, enhancing glutamate release. (b) Activity-dependent long-term potentiation of electrical and chemical synaptic transmission mediated by eCBs. At mixed chemical and electrical synapses in the goldfish Mauthner cell, synaptic activity results in postsynaptic depolarization via electrical synapses and in mGluR activation. Both are required to evoke synthesis and release of 2-AG, which activates CB1 receptors on nearby dopaminergic varicosities, resulting in increased availability of DA. Subsequent activation of postsynaptic D1 receptors increases PKA activity, modulating ionotropic glutamate receptors and gap junction proteins (GJ), and resulting in a long-term enhancement of electrical and chemical synaptic transmission.
Traditionally, the discussion of interneuronal communication has been abridged to consider only chemical synaptic transmission, in part because electrical synapses were considered to be static and non-modifiable. Formed by transmembrane proteins called connexins, gap junctions (GJs) are intercellular channels that constitute the structural substrate of electrical synapses. Identification and cloning of the neuron-specific connexin-36 (Cx36) drove an increase in our understanding of the distribution and function of electrical synapses [86,87]. As such, electrical synapses are known to date to be present in several areas of the mammalian brain [87,88], to be much more plastic than initially thought [89] and to actively interact with chemical synaptic transmission [90]. Cannabinoids have been shown to modulate electrical as well as chemical synaptic communication. The first effect of cannabinoids on electrical synapses described their modulation of intercellular communication between astrocytes in a CB1-receptor-independent fashion [91]. Subsequently, they have been shown to have a potentiating effect on electrical synapses in the goldfish Mauthner cell circuit, a classic model for the study of GJ-mediated electrical synaptic transmission [33]. In this study, it was shown that electrical stimulation of auditory afferents triggers the production and release of 2-AG from the postsynaptic neuron. The subsequent activation of the CB1 receptor thought to be present on nearby dopaminergic varicosities drives an increase in the availability of DA, which in turn enhances mixed (electrical and chemical) synaptic transmission in an effect mediated by activation of DA D1 receptor and enhancement of PKA activity [32] (figure 2b). In this case, the enhancement of DA release due to CB1 activation is thought to be dependent on coupling of the CB1 receptor to a G protein other than Gi/o.
Along with the identification and characterization of CB1 receptor ligands, vanilloid receptors have been recognized as targets of eCBs such as anandamide [56,92] and N-arachidonoyl-dopamine (NADA) [93]. This fact adds even more complexity to the list of possible effects triggered by eCB action. In previous sections of this review, it was mentioned that TRPV1 participates in triggering LTD in hippocampus [63] and enhances the effect mediated by CB1 in nucleus accumbens [64]. The possibility that an eCB might target both TRPV1 and CB1 receptors and thereby evoke a multifaceted response was reported a while ago. Initially, a phenomenon was reported in which anandamide, acting through TRPV1 receptors in the substantia nigra (SN), evoked an increase in the frequency of excitatory input to dopaminergic neurons [59]. A few years later, another study analysed the anatomical distribution of TRPV1 and CB1 and the effects of NADA on these receptors. NADA constitutes a vanilloid/eCB that acts with different potency over TRPV1 and CB1 compared with anandamide. Double immunolabelling analysis of TRPV1 and CB1 distribution evidenced colocalization in close contact with SN dopaminergic neurons [58]. More importantly, it was shown that NADA increased glutamatergic synaptic transmission through activation of TRPV1 receptors, decreased it through activation of CB1 receptors and that the effects on these two types of receptors depended strongly on NADA concentration levels. The authors speculated that whereas low levels of NADA activate TRPV1 and subsequent glutamate release, an increase in neuronal excitation would lead to higher levels of NADA and thus to activation of CB1 and its consequent inhibitory effects on glutamatergic neurotransmission. As portrayed in this section, the eCB system can display a number of mechanistic adaptations, both at the cellular level and at the level of the circuit, and thus can result in specialized ways to affect both inter-neuronal and glial-neuronal transmission.
6. Cannabinoid signalling and mental illness
Cannabis sativa (marijuana) has long been used for medicinal purposes for a diversity of ailments [94]. Although marijuana is generally used for the treatment of symptoms only, a number of alterations in the eCB system have also been identified in the pathophysiology of certain mental illnesses, indicating a potential role for their involvement in these diseases as well. Alterations in tissue expression of eCBs, altered distribution of CB1 or CB2 receptors or altered sensitivity to ligands are some of the characteristics manifest in disorders of the nervous system in which the eCB system has been found to be involved. These changes could, obviously, represent either primary alterations or compensatory mechanisms in response to primary changes in other signalling systems or circuits. The eCB system has therefore been studied both as a pathophysiological mechanism and as a potential therapeutic possibility for disorders such as obesity, Parkinson's, Huntington's (HD) and Alzheimer's diseases, multiple sclerosis, Tourette's syndrome, schizophrenia, bipolar disorder, post-traumatic stress disorder, amyotrophic lateral sclerosis and epilepsy, among others. A brief discussion of the eCB systems involvement in schizophrenia and HD will be presented as an example.
The history of cannabinoids and schizophrenia is rich and full of debate, marked by the controversy over whether cannabis consumption acts primarily as a form of self-medication or as an environmental risk factor for the disease [95–97]. Identification of the CB1 receptor and other components of the eCB system has allowed not only the development of new methods to characterize the relationship between schizophrenia and cannabis use, but also to better study cannabinoid pharmacotherapy in the treatment of psychosis. The rich distribution of the CB1 receptor in brain areas known to be involved in schizophrenia has pointed to the possibility that an altered eCB system could play a major role in either its pathophysiology or therapeutics. Increases in the expression of CB1 receptors have been described in the anterior cingulate cortex [98] and the posterior cingulate cortex of schizophrenia patients [99], areas considered to play key roles in cognitive processing. Also, increases in the capacity of CB1 receptor binding have been found in the dorsolateral area of the prefrontal cortex, as determined using radioactive ligands [100]. However, this result has not been shown to correlate with mRNA and CB1 protein measurements, in which a reduction in the level of both was detected in the dorsolateral area of prefrontal cortex [101]. Clearly, the reason for these conflicting results could in part be due to the significant limitations involved with studying post-mortem human brain tissue, the most notable of which is the difficulty in standardizing and making comparable factors such as exposure to antipsychotic treatment, history of cannabis consumption, genetic predisposition and inter-individual variations in the progression of the disease.
Increases in the level of anandamide have also been found in patients, both from blood samples [102] and cerebrospinal fluid (CSF) [103]. Interestingly, the increase in anandamide levels in CSF were shown to inversely correlate with clinical expression of the symptoms of schizophrenia in non-medicated acute patients [103]. As increases in anandamide levels can be seen as a compensatory mechanism, it would support the view that cannabis use in schizophrenia corresponds to self-medicating behaviour. To this end, it has also been reported that cannabis use in patients with schizophrenia decreases levels of anandamide, which does not occur in healthy subjects [104]. Although studies in the phencyclidine (PCP) model of schizophrenia have shown increased levels of anandamide only in the NAc, increasing levels of anandamide led to amelioration of negative symptoms [105]. Specifically, treatment with the FAAH inhibitor URB597 (reducing hydrolysis of anandamide) reversed PCP-induced social withdrawal. However, treatment with the CB1 antagonist AM251 also decreased deficits in working memory evoked by PCP [105]. Numerous factors could influence potential comparisons among models, between models and findings in humans; the stage of the disease, exposure to antipsychotic treatment, specific tasks to which the animals could be exposed before sampling, all constitute additional factors contributing to population variability requiring examination prior to the extrapolation of scientific findings to the clinical setting.
HD is an inherited neurodegenerative condition in which motor control deficits coexist with impaired cognition and changes in personality and mood. Caused by a high number of CAG repeats in the Huntingtin (Htt) gene, most of the maladaptive histopathological, molecular and circuit changes affect selected cortical areas as well as MSNs in the striatum. Multiple signalling systems in the brain of HD patients are altered even before the clinical expression of the disease, including glutamate, DA, GABA, acetylcholine and eCB systems [106]. In fact, changes in CB1 receptor distribution constitute one of the earliest signs of molecular/cellular degeneration in the globus pallidus (GP) and SN of human HD brains [107]. Studies conducted in young animals (12 weeks-old) of the R6/1 mouse model of HD show reduced levels of mRNA in the striatum, together with decreased CB1 protein expression in the SN, but no differences in the GP or striatum. This same study shows decreases in CB binding in basal ganglia and increased levels of 2-AG in cerebral cortex, while anandamide levels are decreased in the hippocampus [108]. Another insightful study, in which labelling for CB1 and GABA receptors was performed in HD brains, shows that degeneration of striatal MSNs (which project to the GP) correlates with upregulation of different GABAA and GABAB receptor subunits in the GP. Thus, loss of CB1 receptors in the GP could constitute a compensatory mechanism to help increase GABAergic transmission at degenerating terminals [109]. However, studies in the R6/2 mouse model of HD show that decrease in CB1 density occurs even before changes in the levels of eCBs, suggesting that a decrease in CB1 expression might also constitute a primary event, instead of a compensatory one [110,111]. The fact that many of the HD rodent models are based on the expression of Htt with a specific number of CAG repeats likely represents a more accurate disease model of the cellular and circuit alterations occurring in the human form of the disease compared with, for example, schizophrenia. However, differences related to stage of progression, low or high number of repeats and the type of techniques used for the detection of CB1 or eCB levels still pose a difficulty when extrapolating studies to the pre-clinical setting.
7. Concluding remarks
Characterization of the eCB system has certainly been the subject of great advances in the last two decades. Our knowledge has evolved from recognizing the CB1 receptor as the target for THC and being responsible for DSI and DSE, to identifying its role as a powerful and widespread modulator of synaptic strength. To date, the eCB system is considered the best characterized form of retrograde synaptic transmission. Short-term forms of plasticity induced by cannabinoids have been described in numerous brain areas in different organisms, accounting not only for our understanding of the hippocampus and cerebellum as the major sites of action for eCBs, but also suggesting that the eCB system itself is an ancient mechanism in evolutionary terms. However, our more global understanding of the role eCBs play in regulating behaviour and mental disease is just beginning. eCB-STD is thought to be involved in regulating over-excitability and promoting synaptic homeostasis, as the expression of the CB1 receptor is localized more prominently on inhibitory than excitatory terminals. Further identification of eCBs as modulators of the processes underlying long-term plasticity have prompted interest in this system's involvement in processes such as learning and memory. While PKA activity or a potential NMDA-dependent increase in presynaptic Ca++ concentrations seem to be key players in forms of eCB-LTD, we still lack a complete mechanistic characterization of the molecules and events that describe the long-lasting influence of eCBs on synaptic depression. Expanding the traditional focus of CB1 action on interneuronal STD and LTD, numerous studies (from which for reasons of space we only mention a few) have reported eCB involvement in glia-mediated effects, plasticity of electrical synapses and concurrent activation of vanilloid receptors, among many others. These studies demonstrate that the eCB system can be considered functionally malleable and has established specializations leading to a much wider array of neuronal effects than the emblematic short- and long-term depression of glutamatergic and GABAergic transmission. It is likely that a considerable number of those eCB-mediated effects remain unknown. As expected for a neuromodulatory system with such a wide distribution in the brain, a wealth of evidence has shown that the eCB system is altered in numerous diseases and abnormal conditions, and for many of these illnesses, distribution of eCB or CB1 receptor levels implicates this system in initial, even presymptomatic, stages. To determine how these alterations end up modifying synaptic transmission and the specific factors underlying these modifications will likely be the focus of research in upcoming years.
Acknowledgements
The author acknowledges the members of the Alberto Pereda laboratory at Albert Einstein College of Medicine, and the members of the Joseph Cheer, Patricio O'Donnell and Brad Alger laboratories at University of Maryland School of Medicine for insightful discussions on eCB function. Thanks to Dr Theresa Szabo for helpful comments on the manuscript.
References
- 1.Llano I., Leresche N., Marty A. 1991. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6, 565–574 10.1016/0896-6273(91)90059-9 (doi:10.1016/0896-6273(91)90059-9) [DOI] [PubMed] [Google Scholar]
- 2.Pitler T. A., Alger B. E. 1992. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J. Neurosci. 12, 4122–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitler T. A., Alger B. E. 1994. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron 13, 1447–1455 10.1016/0896-6273(94)90430-8 (doi:10.1016/0896-6273(94)90430-8) [DOI] [PubMed] [Google Scholar]
- 4.Elphick M. R., Egertová M. 2001. The neurobiology and evolution of cannabinoid signalling. Phil. Trans. R. Soc. Lond. B 356, 381–408 10.1098/rstb.2000.0787 (doi:10.1098/rstb.2000.0787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson R. I., Nicoll R. A. 2001. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592 10.1038/35069076 (doi:10.1038/35069076) [DOI] [PubMed] [Google Scholar]
- 6.Ohno-Shosaku T., Maejima T., Kano M. 2001. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29, 729–738 10.1016/S0896-6273(01)00247-1 (doi:10.1016/S0896-6273(01)00247-1) [DOI] [PubMed] [Google Scholar]
- 7.Kreitzer A. C., Regehr W. G. 2001. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29, 717–727 10.1016/S0896-6273(01)00246-X (doi:10.1016/S0896-6273(01)00246-X) [DOI] [PubMed] [Google Scholar]
- 8.Maejima T., Hashimoto K., Yoshida T., Aiba A., Kano M. 2001. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31, 463–475 10.1016/S0896-6273(01)00375-0 (doi:10.1016/S0896-6273(01)00375-0) [DOI] [PubMed] [Google Scholar]
- 9.Varma N., Carlson G. C., Ledent C., Alger B. E. 2001. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J. Neurosci. 21, RC188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Isokawa M., Ledent C., Alger B. E. 2002. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J. Neurosci. 22, 10 182–10 191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukudome Y., et al. 2004. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur. J. Neurosci. 19, 2682–2692 10.1111/j.0953-816X.2004.03384.x (doi:10.1111/j.0953-816X.2004.03384.x) [DOI] [PubMed] [Google Scholar]
- 12.Straiker A., Mackie K. 2007. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J. Physiol. 578, 773–785 10.1113/jphysiol.2006.117499 (doi:10.1113/jphysiol.2006.117499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best A. R., Regehr W. G. 2008. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J. Neurosci. 28, 6508–6515 10.1523/JNEUROSCI.0678-08.2008 (doi:10.1523/JNEUROSCI.0678-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haj-Dahmane S., Shen R.-Y. 2005. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J. Neurosci. 25, 896–905 10.1523/JNEUROSCI.3258-04.2005 (doi:10.1523/JNEUROSCI.3258-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown S. P., Brenowitz S. D., Regehr W. G. 2003. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat. Neurosci. 6, 1048–1057 10.1038/nn1126 (doi:10.1038/nn1126) [DOI] [PubMed] [Google Scholar]
- 16.Ohno-Shosaku T., Hashimotodani Y., Ano M., Takeda S., Tsubokawa H., Kano M. 2007. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J. Physiol. 584, 407–418 10.1113/jphysiol.2007.137505 (doi:10.1113/jphysiol.2007.137505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent P., Armstrong C. M., Marty A. 1992. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J. Physiol. 456, 453–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devane W. A., Dysarz F. A., Johnson M. R., Melvin L. S., Howlett A. C. 1988. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 34, 605–613 [PubMed] [Google Scholar]
- 19.Matsuda L., Lolait S., Brownstein M., Young A. C., Bonner T. I. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 10.1038/346561a0 (doi:10.1038/346561a0) [DOI] [PubMed] [Google Scholar]
- 20.Herkenham M., Lynn A. B., Little M. D., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. 1990. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. USA 87, 1932–1936 10.1073/pnas.87.5.1932 (doi:10.1073/pnas.87.5.1932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katona I., Sperlágh B., Sík A., Käfalvi A., Vizi E. S., Mackie K., Freund T. F. 1999. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devane W. A., et al. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 10.1126/science.1470919 (doi:10.1126/science.1470919) [DOI] [PubMed] [Google Scholar]
- 23.Mechoulam R., et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 10.1016/0006-2952(95)00109-D (doi:10.1016/0006-2952(95)00109-D) [DOI] [PubMed] [Google Scholar]
- 24.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97 10.1006/bbrc.1995.2437 (doi:10.1006/bbrc.1995.2437) [DOI] [PubMed] [Google Scholar]
- 25.Domenici M. R., Azad S. C., Marsicano G., Schierloh A., Wotjak C. T., Dodt H. U., Zieglgänsberger W., Lutz B., Rammes G. 2006. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J. Neurosci. 26, 5794–5799 10.1523/JNEUROSCI.0372-06.2006 (doi:10.1523/JNEUROSCI.0372-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mátyás F., Yanovsky Y., Mackie K., Kelsch W., Misgeld U., Freund T. F. 2006. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience 137, 337–361 10.1016/j.neuroscience.2005.09.005 (doi:10.1016/j.neuroscience.2005.09.005) [DOI] [PubMed] [Google Scholar]
- 27.Kushmerick C., Price G. D., Taschenberger H., Puente N., Renden R., Wadiche J. I., Duvoisin R. M., Grandes P., von Gersdorff H. 2004. Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J. Neurosci. 24, 5955–5965 10.1523/JNEUROSCI.0768-04.2004 (doi:10.1523/JNEUROSCI.0768-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oropeza V. C., Mackie K., Van Bockstaele E. J. 2007. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 1127, 36–44 10.1016/j.brainres.2006.09.110 (doi:10.1016/j.brainres.2006.09.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oropeza V. C., Page M. E., Van Bockstaele E. J. 2005. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 1046, 45–54 10.1016/j.brainres.2005.03.036 (doi:10.1016/j.brainres.2005.03.036) [DOI] [PubMed] [Google Scholar]
- 30.Reyes B. A. S., Rosario J. C., Piana P. M. T., Van Bockstaele E. J. 2009. Cannabinoid modulation of cortical adrenergic receptors and transporters. J. Neurosci. Res. 87, 3671–3678 10.1002/jnr.22158 (doi:10.1002/jnr.22158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degroot A., Köfalvi A., Wade M. R., Davis R. J., Rodrigues R. J., Rebola N., Cunha R. A., Nomikos G. G. 2006. CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol. Pharmacol. 70, 1236–1245 10.1124/mol.106.024661 (doi:10.1124/mol.106.024661) [DOI] [PubMed] [Google Scholar]
- 32.Cachope R., Mackie K., Triller A., O'Brien J., Pereda A. E. 2007. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron 56, 1034–1047 10.1016/j.neuron.2007.11.014 (doi:10.1016/j.neuron.2007.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salio C., Doly S., Fischer J., Franzoni M. F., Conrath M. 2002. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci. Lett. 329, 13–16 10.1016/S0304-3940(02)00549-9 (doi:10.1016/S0304-3940(02)00549-9) [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez J. J., Mackie K., Pickel V. M. 2001. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. J. Neurosci. 21, 823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarrete M., Araque A. 2008. Endocannabinoids mediate neuron-astrocyte communication. Neuron 57, 883–893 10.1016/j.neuron.2008.01.029 (doi:10.1016/j.neuron.2008.01.029) [DOI] [PubMed] [Google Scholar]
- 36.Navarrete M., Araque A. 2010. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 10.1016/j.neuron.2010.08.043 (doi:10.1016/j.neuron.2010.08.043) [DOI] [PubMed] [Google Scholar]
- 37.Min R., Nevian T. 2012. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 15, 746–753 10.1038/nn.3075 (doi:10.1038/nn.3075) [DOI] [PubMed] [Google Scholar]
- 38.Han J., et al. 2012. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 10.1016/j.cell.2012.01.037 (doi:10.1016/j.cell.2012.01.037) [DOI] [PubMed] [Google Scholar]
- 39.Hohmann A. G., Briley E. M., Herkenham M. 1999. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 822, 17–25 10.1016/S0006-8993(9801321-3 (doi:10.1016/S0006-8993(9801321-3) [DOI] [PubMed] [Google Scholar]
- 40.Ong W., Mackie K. 1999. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience 92, 1177–1191 10.1016/S0306-4522(99)00025-1 (doi:10.1016/S0306-4522(99)00025-1) [DOI] [PubMed] [Google Scholar]
- 41.Nyíri G., Szabadits E., Cserép C., Mackie K., Shigemoto R., Freund T. F. 2005. GABAB and CB1 cannabinoid receptor expression identifies two types of septal cholinergic neurons. Eur. J. Neurosci. 21, 3034–3042 10.1111/j.1460-9568.2005.04146.x (doi:10.1111/j.1460-9568.2005.04146.x) [DOI] [PubMed] [Google Scholar]
- 42.Bacci A., Huguenard J. R., Prince D. A. 2004. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431, 312–316 10.1038/nature02913 (doi:10.1038/nature02913) [DOI] [PubMed] [Google Scholar]
- 43.Marinelli S., Pacioni S., Cannich A., Marsicano G., Bacci A. 2009. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci. 12, 1488–1490 10.1038/nn.2430 (doi:10.1038/nn.2430) [DOI] [PubMed] [Google Scholar]
- 44.Marinelli S., Pacioni S., Bisogno T., Di Marzo V., Prince D. A., Huguenard J. R., Bacci A. 2008. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J. Neurosci. 28, 13 532–13 541 10.1523/JNEUROSCI.0847-08.2008 (doi:10.1523/JNEUROSCI.0847-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauckner J. E., Hille B., Mackie K. 2005. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl Acad. Sci. USA 102, 19 144–19 149 10.1073/pnas.0509588102 (doi:10.1073/pnas.0509588102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glass M., Felder C. C. 1997. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 17, 5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rios C., Gomes I., Devi L. A. 2006. Mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 148, 387–395 10.1038/sj.bjp.0706757 (doi:10.1038/sj.bjp.0706757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozenfeld R., et al. 2012. Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS ONE 7, e29239. 10.1371/journal.pone.0029239 (doi:10.1371/journal.pone.0029239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalton G. D., Bass C. E., Van Horn C. G., Howlett A. C. 2009. Signal transduction via cannabinoid receptors. CNS Neurol. Disord. Drug Targets 8, 422–431 10.2174/187152709789824615 (doi:10.2174/187152709789824615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howlett A. C., Blume L. C., Dalton G. D. 2010. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 17, 1382–1393 10.2174/092986710790980023 (doi:10.2174/092986710790980023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turu G., Hunyady L. 2010. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 44, 75–85 10.1677/JME-08-0190 (doi:10.1677/JME-08-0190) [DOI] [PubMed] [Google Scholar]
- 52.Mezey E., Tóth Z. E., Cortright D. N., Arzubi M. K., Krause J. E., Elde R., Guo A., Blumberg P. M., Szallasi A. 2000. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl Acad. Sci. USA 97, 3655–3660 10.1073/pnas.060496197 (doi:10.1073/pnas.060496197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tóth A., Boczán J., Kedei N., Lizanecz E., Bagi Z., Papp Z., Edes I., Csiba L., Blumberg P. M. 2005. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 135, 162–168 10.1016/j.molbrainres.2004.12.003 (doi:10.1016/j.molbrainres.2004.12.003) [DOI] [PubMed] [Google Scholar]
- 54.Cristino L., de Petrocellis L., Pryce G., Baker D., Guglielmotti V., Di Marzo V. 2006. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139, 1405–1415 10.1016/j.neuroscience.2006.02.074 (doi:10.1016/j.neuroscience.2006.02.074) [DOI] [PubMed] [Google Scholar]
- 55.Di Marzo V., De Petrocellis L. 2010. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 17, 1430–1449 10.2174/092986710790980078 (doi:10.2174/092986710790980078) [DOI] [PubMed] [Google Scholar]
- 56.Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. 1999. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 10.1038/22761 (doi:10.1038/22761) [DOI] [PubMed] [Google Scholar]
- 57.Maccarrone M., et al. 2008. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 11, 152–159 10.1038/nn2042 (doi:10.1038/nn2042) [DOI] [PubMed] [Google Scholar]
- 58.Marinelli S., et al. 2007. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology 32, 298–308 10.1038/sj.npp.1301118 (doi:10.1038/sj.npp.1301118) [DOI] [PubMed] [Google Scholar]
- 59.Marinelli S., Di Marzo V., Berretta N., Matias I., Maccarrone M., Bernardi G., Mercuri N. B. 2003. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J. Neurosci. 23, 3136–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marinelli S., Pascucci T., Bernardi G., Puglisi-Allegra S., Mercuri N. B. 2005. Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology 30, 864–870 10.1038/sj.npp.1300615 (doi:10.1038/sj.npp.1300615) [DOI] [PubMed] [Google Scholar]
- 61.Yuan S., Burrell B. D. 2010. Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J. Neurophysiol. 104, 2766–2777 10.1152/jn.00491.2010 (doi:10.1152/jn.00491.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puente N., Cui Y., Lassalle O., Lafourcade M., Georges F., Venance L., Grandes P., Manzoni O. J. 2011. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 14, 1542–1547 10.1038/nn.2974 (doi:10.1038/nn.2974) [DOI] [PubMed] [Google Scholar]
- 63.Chávez A. E., Chiu C. Q., Castillo P. E. 2010. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 13, 1511–1518 10.1038/nn.2684 (doi:10.1038/nn.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grueter B. A., Brasnjo G., Malenka R. C. 2010. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 13, 1519–1525 10.1038/nn.2685 (doi:10.1038/nn.2685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hampson R. E., Zhuang S., Weiner J. L., Deadwyler S. A. 2003. Functional significance of cannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) in the hippocampus. J. Neurophysiol. 90, 55–64 10.1152/jn.01161.2002 (doi:10.1152/jn.01161.2002) [DOI] [PubMed] [Google Scholar]
- 66.Stella N., Schweitzer P., Piomelli D. 1997. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388, 773–778 10.1038/42015 (doi:10.1038/42015) [DOI] [PubMed] [Google Scholar]
- 67.Alger B. E., Kim J. 2011. Supply and demand for endocannabinoids. Trends Neurosci. 34, 304–315 10.1016/j.tins.2011.03.003 (doi:10.1016/j.tins.2011.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Marzo V. 2009. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 60, 77–84 10.1016/j.phrs.2009.02.010 (doi:10.1016/j.phrs.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 69.Gao Y., et al. 2010. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 10.1523/JNEUROSCI.5693-09.2010 (doi:10.1523/JNEUROSCI.5693-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshino H., et al. 2011. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J. Physiol. 589, 4857–4884 10.1113/jphysiol.2011.212225 (doi:10.1113/jphysiol.2011.212225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanimura A., et al. 2010. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 10.1016/j.neuron.2010.01.021 (doi:10.1016/j.neuron.2010.01.021) [DOI] [PubMed] [Google Scholar]
- 72.Kim J., Alger B. E. 2004. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat. Neurosci. 7, 697–698 10.1038/nn1262 (doi:10.1038/nn1262) [DOI] [PubMed] [Google Scholar]
- 73.Galante M., Diana M. A. 2004. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J. Neurosci. 24, 4865–4874 10.1523/JNEUROSCI.0403-04.2004 (doi:10.1523/JNEUROSCI.0403-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melis M., Pistis M. 2012. Hub and switches: endocannabinoid signalling in midbrain dopamine neurons. Phil. Trans. R. Soc. B 367, 3276–3285 10.1098/rstb.2011.0383 (doi:10.1098/rstb.2011.0383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melis M., Pistis M., Perra S., Muntoni A. L., Pillolla G., Gessa G. L. 2004. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 24, 53–62 10.1523/JNEUROSCI.4503-03.2004 (doi:10.1523/JNEUROSCI.4503-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melis M., Perra S., Muntoni A. L., Pillolla G., Lutz B., Marsicano G., Di Marzo V., Gessa G. L., Pistis M. 2004. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J. Neurosci. 24, 10 707–10 715 10.1523/JNEUROSCI.3502-04.2004 (doi:10.1523/JNEUROSCI.3502-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerdeman G. L., Ronesi J., Lovinger D. M. 2002. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 5, 446. [DOI] [PubMed] [Google Scholar]
- 78.Heifets B. D., Castillo P. E. 2009. Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306 10.1146/annurev.physiol.010908.163149 (doi:10.1146/annurev.physiol.010908.163149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chevaleyre V., Castillo P. E. 2003. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38, 461–472 10.1016/S0896-6273(03)00235-6 (doi:10.1016/S0896-6273(03)00235-6) [DOI] [PubMed] [Google Scholar]
- 80.Chevaleyre V., Castillo P. E. 2004. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43, 871–881 10.1016/j.neuron.2004.08.036 (doi:10.1016/j.neuron.2004.08.036) [DOI] [PubMed] [Google Scholar]
- 81.Chevaleyre V., Heifets B. D., Kaeser P. S., Südhof T. C., Purpura D. P., Castillo P. E. 2007. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron 54, 801–812 10.1016/j.neuron.2007.05.020 (doi:10.1016/j.neuron.2007.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Penzo M. A., Peña J. L. 2009. Endocannabinoid-mediated long-term depression in the avian midbrain expressed presynaptically and postsynaptically. J. Neurosci. 29, 4131–4139 10.1523/JNEUROSCI.5466-08.2009 (doi:10.1523/JNEUROSCI.5466-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sjöström P. J., Turrigiano G. G., Nelson S. B. 2003. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641–654 10.1016/S0896-6273(03)00476-8 (doi:10.1016/S0896-6273(03)00476-8) [DOI] [PubMed] [Google Scholar]
- 84.Bender V. A., Bender K. J., Brasier D. J., Feldman D. E. 2006. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 26, 4166–4177 10.1523/JNEUROSCI.0176-06.2006 (doi:10.1523/JNEUROSCI.0176-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards J. G., Gibson H. E., Jensen T., Nugent F., Walther C., Blickenstaff J., Kauer J. A. 2012. A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons. Hippocampus 22, 209–221 10.1002/hipo.20884 (doi:10.1002/hipo.20884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennett M. V. L., Zukin R. S. 2004. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41, 495–511 10.1016/S0896-6273(04)00043-1 (doi:10.1016/S0896-6273(04)00043-1) [DOI] [PubMed] [Google Scholar]
- 87.Condorelli D. 2000. Expression of Cx36 in mammalian neurons. Brain Res. Rev. 32, 72–85 10.1016/S0165-0173(99)00068-5 (doi:10.1016/S0165-0173(99)00068-5) [DOI] [PubMed] [Google Scholar]
- 88.Connors B. W., Long M. A. 2004. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 27, 393–418 10.1146/annurev.neuro.26.041002.131128 (doi:10.1146/annurev.neuro.26.041002.131128) [DOI] [PubMed] [Google Scholar]
- 89.Pereda A. E., Faber D. S. 1996. Activity-dependent short-term enhancement of intercellular coupling. J. Neurosci. 16, 983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith M., Pereda A. E. 2003. Chemical synaptic activity modulates nearby electrical synapses. Proc. Natl Acad. Sci. USA 100, 4849–4854 10.1073/pnas.0734299100 (doi:10.1073/pnas.0734299100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venance L., Piomelli D., Glowinski J., Giaume C. 1995. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature 376, 590–594 10.1038/376590a0 (doi:10.1038/376590a0) [DOI] [PubMed] [Google Scholar]
- 92.Smart D., Gunthorpe M. J., Jerman J. C., Nasir S., Gray J., Muir A. I., Chambers J. K., Randall A. D., Davis J. B. 2000. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (HVR1). Br. J. Pharmacol. 129, 227–230 10.1038/sj.bjp.0703050 (doi:10.1038/sj.bjp.0703050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang S. M., et al. 2002. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl Acad. Sci. USA 99, 8400–8405 10.1073/pnas.122196999 (doi:10.1073/pnas.122196999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zias J., Stark H., Sellgman J., Levy R., Werker E., Breuer A., Mechoulam R. 1993. Early medical use of cannabis. Nature 363, 215. 10.1038/363215a0 (doi:10.1038/363215a0) [DOI] [PubMed] [Google Scholar]
- 95.Bossong M. G., Niesink R. J. M. 2010. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog. Neurobiol. 92, 370–385 10.1016/j.pneurobio.2010.06.010 (doi:10.1016/j.pneurobio.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 96.Kolliakou A., Joseph C., Ismail K., Atakan Z., Murray R. M. 2011. Why do patients with psychosis use cannabis and are they ready to change their use? Int. J. Dev. Neurosci. 29, 335–346 10.1016/j.ijdevneu.2010.11.006 (doi:10.1016/j.ijdevneu.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 97.D'Souza D. C., Sewell R. A., Ranganathan M. 2009. Cannabis and psychosis/schizophrenia: human studies. Eur. Arch. Psychiatry Clin. Neurosci. 259, 413–431 10.1007/s00406-009-0024-2 (doi:10.1007/s00406-009-0024-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zavitsanou K., Garrick T., Huang X. F. 2004. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 355–360 10.1016/j.pnpbp.2003.11.005 (doi:10.1016/j.pnpbp.2003.11.005) [DOI] [PubMed] [Google Scholar]
- 99.Newell K. A., Deng C., Huang X.-F. 2006. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp. Brain Res. 172, 556–560 10.1007/s00221-006-0503-x (doi:10.1007/s00221-006-0503-x) [DOI] [PubMed] [Google Scholar]
- 100.Dean B., Sundram S., Bradbury R., Scarr E., Copolov D. 2001. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 103, 9–15 10.1016/S0306-4522(00)00552-2 (doi:10.1016/S0306-4522(00)00552-2) [DOI] [PubMed] [Google Scholar]
- 101.Eggan S. M., Hashimoto T., Lewis D. A. 2008. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch. Gen. Psychiatry 65, 772–784 10.1001/archpsyc.65.7.772 (doi:10.1001/archpsyc.65.7.772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Marchi N., De Petrocellis L., Orlando P., Daniele F., Fezza F., Di Marzo V. 2003. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2, 5. 10.1186/1476-511X-2-5 (doi:10.1186/1476-511X-2-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giuffrida A., Leweke F. M., Gerth C. W., Schreiber D., Koethe D., Faulhaber J., Klosterkötter J., Piomelli D. 2004. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 29, 2108–2114 10.1038/sj.npp.1300558 (doi:10.1038/sj.npp.1300558) [DOI] [PubMed] [Google Scholar]
- 104.Leweke F. M., et al. 2007. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr. Res. 94, 29–36 10.1016/j.schres.2007.04.025 (doi:10.1016/j.schres.2007.04.025) [DOI] [PubMed] [Google Scholar]
- 105.Seillier A., Advani T., Cassano T., Hensler J. G., Giuffrida A. 2010. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int. J. Neuropsychopharmacol. 13, 373–386 10.1017/S146114570999023X (doi:10.1017/S146114570999023X) [DOI] [PubMed] [Google Scholar]
- 106.Kumar P., Kalonia H., Kumar A. 2010. Huntington's disease: pathogenesis to animal models. Pharmacol. Rep. 62, 1–14 10.1016/j.physrep.2009.11.001 (doi:10.1016/j.physrep.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 107.Glass M., Dragunow M., Faull R. L. 2000. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience 97, 505–519 10.1016/S0306-4522(00)00008-7 (doi:10.1016/S0306-4522(00)00008-7) [DOI] [PubMed] [Google Scholar]
- 108.Dowie M. J., Bradshaw H. B., Howard M. L., Nicholson L. F. B., Faull R. L. M., Hannan A. J., Glass M. 2009. Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse model of Huntington's disease. Neuroscience 163, 456–465 10.1016/j.neuroscience.2009.06.014 (doi:10.1016/j.neuroscience.2009.06.014) [DOI] [PubMed] [Google Scholar]
- 109.Allen K. L., Waldvogel H. J., Glass M., Faull R. L. M. 2009. Cannabinoid (CB(1)), GABA(A) and GABA(B) receptor subunit changes in the globus pallidus in Huntington's disease. J. Chem. Neuroanat. 37, 266–281 10.1016/j.jchemneu.2009.02.001 (doi:10.1016/j.jchemneu.2009.02.001) [DOI] [PubMed] [Google Scholar]
- 110.McCaw E. A., Hu H., Gomez G. T., Hebb A. L. O., Kelly M. E. M., Denovan-Wright E. M. 2004. Structure, expression and regulation of the cannabinoid receptor gene (CB1) in Huntington's disease transgenic mice. Eur. J. Biochem. 271, 4909–4920 10.1111/j.1432-1033.2004.04460.x (doi:10.1111/j.1432-1033.2004.04460.x) [DOI] [PubMed] [Google Scholar]
- 111.Bisogno T., Martire A., Petrosino S., Popoli P., Di Marzo V. 2008. Symptom-related changes of endocannabinoid and palmitoylethanolamide levels in brain areas of R6/2 mice, a transgenic model of Huntington's disease. Neurochem. Int. 52, 307–313 10.1016/j.neuint.2007.06.031 (doi:10.1016/j.neuint.2007.06.031) [DOI] [PubMed] [Google Scholar]