Abstract
The role of endocannabinoids as inhibitory retrograde transmitters is now widely known and intensively studied. However, endocannabinoids also influence neuronal activity by exerting neuroprotective effects and regulating glial responses. This review centres around this less-studied area, focusing on the cellular and molecular mechanisms underlying the protective effect of the cannabinoid system in brain ageing. The progression of ageing is largely determined by the balance between detrimental, pro-ageing, largely stochastic processes, and the activity of the homeostatic defence system. Experimental evidence suggests that the cannabinoid system is part of the latter system. Cannabinoids as regulators of mitochondrial activity, as anti-oxidants and as modulators of clearance processes protect neurons on the molecular level. On the cellular level, the cannabinoid system regulates the expression of brain-derived neurotrophic factor and neurogenesis. Neuroinflammatory processes contributing to the progression of normal brain ageing and to the pathogenesis of neurodegenerative diseases are suppressed by cannabinoids, suggesting that they may also influence the ageing process on the system level. In good agreement with the hypothesized beneficial role of cannabinoid system activity against brain ageing, it was shown that animals lacking CB1 receptors show early onset of learning deficits associated with age-related histological and molecular changes. In preclinical models of neurodegenerative disorders, cannabinoids show beneficial effects, but the clinical evidence regarding their efficacy as therapeutic tools is either inconclusive or still missing.
Keywords: oxidative stress, macromolecule clearance, neuroinflammation, glial regulation, neurogenesis, neurodegenerative diseases
1. Functional, histological and molecular changes in the ageing brain
Ageing is associated with a decline of motor coordination [1], sensory abilities [2], attention and cognitive performance [3], which together are responsible for the increasing deficits in learning and memory tasks. However, as with all age-related health issues, there is a wide spectrum of potential outcomes: While many senior citizens still enjoy their cognitive abilities at an advanced age, others, especially those who suffer from neurodegenerative disorders such as Alzheimer's disease (AD), may show signs of cognitive impairment early in their life. Thus, a better understanding of the molecular and cellular processes that contribute to, or protect against, cognitive decline, may offer novel routes for therapy and prevention.
The mitotic activity within the central nervous system is low and the newly generated cells are mostly glia cells. Neurogenesis is restricted to the subventricular zone and to the subgranular zone of the hippocampus in mammals. Only a small percentage of the new neurons integrate to the existing neuronal networks, the majority undergo apoptosis and die. Although the brain is mostly a post-mitotic organ, the onset and progression of age-related changes is independent from the chronological age of the neurons but correlates with the lifespan of the species: neurons from 3-year-old mice show signs of accelerated senescence, whereas neurons from the brain of a 3-year-old dog are free from these changes. At present, the reason for this huge interspecies difference, despite identical mechanisms influencing the process of brain ageing, is not fully understood.
The onset and progression of age-related decline in brain functions also differs strongly between the cognitive domains in humans [3] and in animals from primates [4] and rodents [5] to zebrafish [6]. The reason for this large variance is partly the differences in the ability to recruit additional brain areas for task solving, which can partially counterbalance the effect of functional decline [7]. Another factor, which may contribute to the differences in the effect of ageing on cognitive functions, is the large variance in the sensitivity to age-related changes between brain areas and neuronal types [8,9]. Nevertheless, it is generally assumed that age-related progression in synaptic dysfunction and neuronal plasticity impairment are the direct causes of the alterations in neuronal connectivity [10] and thus functional deficits in ageing [11].
Probably the most consistent change during healthy ageing in the brain structure in humans is the shrinkage in brain volume and the expansion of the ventricular system. The frontal and parietal cortex together with the putamen, thalamus and accumbens are the most affected areas, whereas the volume of the brain stem remains largely unchanged in ageing [12]. Atrophic changes in white matter [13] play a major role in the reduction of brain volume. The white matter, of which the integrity is crucial for the communication between brain areas, contains mostly large myelinated axons. Probably both degeneration and loss of nerve fibres, and deterioration of myelin sheets with age, contribute to the reduction of white matter volume [14,15]. Although it is tempting to speculate that the degeneration of white matter contributes to the development of age-related cognitive deficits, the correlation between these structural and functional changes is weak [16]. In normal healthy ageing, the global number of microglia [17] or neurons [18] does not decrease significantly. However, a significant decline in neuronal number was detected in several brain regions such as the subiculum and hilus regions of the hippocampus [19] and in the entorhinal cortex [20]. These changes correlate well with the severity of declarative memory decline [21]. Although the intensity of adult neurogenesis strongly decreases in ageing, it is improbable that this effect contributes to the reduction of neuronal numbers in the hippocampus [22]. However, because the newly generated neurons participate in pattern separation involving the generation of new neuronal networks [23], this effect can contribute to age-related cognitive deficits. The extent and branching of dendrites is largely preserved during ageing [24] (but see also [25]) with a region-specific pattern [26,27], which suggests that during healthy ageing it is probably functional rather than structural alterations that are responsible for the progression of cognitive ageing.
Although detailed stereological analyses of the ageing brain revealed that the histological structure of the brain is largely preserved, the expression and composition of neurotransmitter receptors, the amount of trophic factor and their receptors characteristically changes in normal ageing and in neurodegenerative disorders [28]. Not surprising therefore that the intracellular signalling systems where the receptor signals converge also undergo characteristic changes during ageing in both neurons and glial cells. Substantial evidence from a variety of species indicates that cAMP response element-binding protein (CREB) is a molecular switch that converts alterations in receptor activity into transcriptional changes leading to long-lasting adaptation [29]. A crucial role of CREB signalling in memory formation was found in both invertebrates and vertebrates [30]. Generally, decreased CREB activity was associated with learning impairments in healthy aged animals [31,32] and with cognitive deficits in animal models of neurodegenerative disorders [33–35]. Importantly, the level of phosphorylated CREB and the activity-induced increase in CREB phosphorylation is diminished in ageing [36,37], and this itself may influence the ageing process [38,39]. Altered calcium signalling in ageing neurons significantly contributes to diminished CREB activity. It is suggested that the calcium homeostasis is disturbed in ageing, because the amplitude of the calcium-dependent after-hyperpolarising potential is increased in aged neurons [40], which makes them less excitable. The reason for this phenomenon is that the expression of L-type calcium channels increases with age [41], whereas the expression of proteins involved in the termination of calcium signal such as calcium extrusion ATPases [42,43] and calcium-binding proteins [44] is diminished in old neurons. These changes may lead to an enhanced calcium signal after activation and elevated activity of calcium-dependent signalling pathways. Age-dependent alterations in the intracellular signalling system have a huge impact on the transcriptional activity of the cells [30,38,45].
Change in the expression profile during ageing in the brain is a characteristic phenomenon [46] observed from humans [47] and mice [48] to Drosophila [49]. The differences in expression patterns are more prominent between age groups than between sexes, ethnicities or individuals in humans [50]. Generally, among the most affected genes are members of the calcium signalling and the CREB pathway [24], genes playing central roles in synaptic plasticity, stress responses and inflammation [47,51]. The measure of age-dependent changes in gene expression correlated with memory performance [52,53] and could be partially reversed by techniques known to positively influence the process of brain ageing [51,54,55]. It should nevertheless be noted that the detected changes in gene expression could be responsible for the neuronal deficits but they could also be adaptive, helping to maintain the structural integrity of neuronal networks in the ageing environment [56]. In neurodegenerative disorders, an acceleration of expression changes is observed in genes involved in inflammatory and apoptotic processes; thus, there is partially a continuum between age-related and neuropathological expression changes. On the other hand, there is a group of genes where the expression change is specific for the disease and the expression pattern significantly differs from the pattern observed in age-matched healthy controls [57–59]. The reason for the altered gene expression is, besides the changes in the intracellular signalling system, also an alteration of the epigenetic structure. During ageing, there is a characteristic change in chromatin structure owing to histone modifications [60,61], which can profoundly influence the accessibility of genes to transcription factors and thus the cognitive functions [62].
2. Processes contributing to brain ageing
The balance between the generation and clearance of toxic metabolic by-products and damaged macromolecules crucially influences the progression of ageing. Evolutionarily highly conserved homeostatic mechanisms such as anti-oxidant, DNA maintenance, and proteosome systems and autophagic processes, keep control over the continuously generated toxic or non-functional by-products and damaged macromolecules. Thus, the expression of genes encoding elements of these systems significantly influences the onset and course of the ageing process. Dividing cells can further reduce the intracellular concentration of non-degradable particles by each division. However, neurons are practically fully post-mitotic cells, therefore they are especially sensitive to the accumulation of damaged, oxidized macromolecules. Moreover, production of reactive oxygen species (ROS) is tightly connected to energy production and thus to mitochondrial activity [63]. Because the brain has one of the highest energy demands in higher organisms, the balance between mitochondrial ROS production and activity of anti-oxidative systems also has a high impact on the progression of its ageing [64,65]. Similar molecular mechanisms contribute to the pathogenesis of neurodegenerative diseases as involved in brain ageing. The selective and significant loss of neurons in neurodegenerative disorders reflects the differences in the vulnerability of neuronal populations to different forms of cellular stress such as protein misfolding, high biosynthetic or secretory demands, oxidative stress, alterations in the dynamics of calcium signalling, etc. [66].
The mitochondrial activity is tightly regulated, because reduced activity leads to a diminished energy support, whereas increased activity results in an overproduction of ROS [67]. In ageing, a reduction in the expression of mitochondrial genes is observed [47,52], which could be a compensatory change to reduce ROS load. Furthermore, as mentioned earlier, one of the largest class of genes upregulated in ageing are involved in oxidative defence and DNA repair [47,68]. Nevertheless, an increase in the amount of oxidized macromolecules in ageing is a generally observed phenomenon in a wide range of species [69,70]. Enhanced oxidative stress in the brain generally correlates with cognitive decline [46,71] and with an enhanced risk for the development of neurodegenerative diseases [72,73]. Evidence of increased oxidative damage was found in the brain of AD patients [74,75]. This damage was present in the early stage of AD in humans [76] suggesting that it plays a causal role in the pathogenesis of the disease. In mouse models of AD, signs of oxidative stress also preceded the appearance of learning and memory deficits [77–79] further supporting the hypothesis about the potential causal role of oxidative damage in AD. Enhanced oxidative stress was found in the dopaminergic neurons in the ageing substantia nigra of humans suffering from Parkinson's disease [80,81] and in the noradrenergic neurons in the locus coeruleus of AD patients [82]. The selective loss of neurons in these cases is probably the result of the increased ROS production owing to the metabolism of catecholamines [83].
As a result, catecholaminergic neurons have depleted anti-oxidant levels making them vulnerable to oxidative stress. Not surprising therefore that anti-oxidants were intensively tested as potential therapeutic agents against the negative consequences of brain ageing and neurodegenerative disorders [84–86]. Generally, it was found that anti-oxidant treatment or activation of anti-oxidative pathways improve brain functions and partially restores age-dependent changes in gene expression both in normal ageing [87,88] and in models of accelerated ageing [89,90]. Clinical data suggest that dietary anti-oxidants have some protective effects against AD, Parkinson's disease and amyotrophic lateral sclerosis [91] and also in pharmacological and genetic models of neurodegenerative disorders [92–94]. We have to note that ROS have an important intracellular signalling function: oxidative stress responses may regulate autophagy and induce synaptic growth [95,96] suggesting that long-lasting diminished ROS production by pharmacological treatment could have a negative impact on brain functions.
The increasing concentration of toxic metabolic end products, misfolded macromolecules and non-functional organelles, is a typical characteristic of the ageing brain and it is thought to significantly contribute to cognitive decline [46]. Neurodegenerative diseases such as AD [97], Parkinson's [98] and Huntington's [99] disease, are each associated with the accumulation of specific protein aggregates, which play a key role in the pathogenesis of the disease. Thus, the intensity and efficacy of clearance of the damaged molecules largely determines the progression of neurodegenerative disorders [100–102] but also the pace of brain ageing in healthy individuals [46,103,104]. The significance of autophagic processes in normal ageing is shown by a genome-wide association study which found a significant association between genetic variance in autophagy associated genes and survival in healthy aged humans [105]. It is generally observed that improving clearance with the induction of autophagy [10,106] or proteasome activity [107,108] slows down the ageing process and improves cognitive functions. Moreover, it was shown that reduced autophagy causes ageing-like decrements [109,110] and facilitates the development of neurodegenerative diseases [111]. Not surprising therefore that induction of autophagy was intensively tested in various models of neurodegenerative diseases. Although it is known that autophagy per se contributes to neuronal death [112–114], it was concluded that facilitation of clearance processes is a potential strategy in the treatment of neurodegenerative disorders [115].
The increasing amount of altered macromolecules and release of signal molecules from ageing damaged neurons activate the immune system of the brain [116,117]. The major cell types of the immune system in the brain are astrocytes and microglial cells. Astrocytes, which are the most numerous glial cell population of the brain, secrete cytokines and chemokines after induction and therefore can contribute to an inflammatory environment in the brain. Microglial cells originate from a macrophage lineage and they are the main form of active immune defence within the central nervous system. Glial cells and neurons are in a mutual interaction: astrocytes but also microglia control neuronal activity providing trophic factors [118,119], energy [120,121] and regulation of synaptic transmission [122] as well as neurogenesis [123]. Neurons exert an inhibitory control on the immune activity of glial cells [124]. Normal, healthy brain ageing is associated with an increased number of activated microglia [125–127] and with an enhanced level of pro-inflammatory cytokines. The increased pro-inflammatory environment in the brain accelerates brain ageing [128,129], aggravates cognitive deficits impairing neuronal functions [130–132] and reduces neurogenesis [133,134]. Activated microglia can be both anti-inflammatory (which supports the neurons) and pro-inflammatory (which damages neurons). It is generally observed that in the ageing brain microglial activation and the resulting pro-inflammatory environment contributes to cognitive impairments [135,136] and promotes neurodegeneration [137]. In the brain of patients suffering from neurodegenerative diseases [138–140] and in animal models of these disorders [141–144], signs of massive neuroinflammation are detected. Importantly, the phenotype of both astrocytes and microglia changes in ageing. During ageing, a characteristic secretory phenotype was observed in different cell types [145], as well as in astrocytes in the brain [146]. Senescent astrocytes are dysfunctional and provide a reduced support for neurons [147]. Microglia in the brain of old individuals show activated, amoeboid-like morphology, their cytoplasm contains lipofuscin granules, and they are primed for exaggerated immune response. Moreover, senescent microglia are less functional because they have serious autophagic dysfunction [148]. It is thought the reduced autophagy combined with an enhanced pro-inflammatory character leads to enhanced neurotoxicity and contributes to neurodegenerative changes [149,150]. On the basis of significant contribution of immune system activity to the development of cognitive deficits, it was suggested that rejuvenation of immunity could reverse age-related cognitive deficits [151].
3. The cannabinoid system modulates age-related molecular and cellular processes
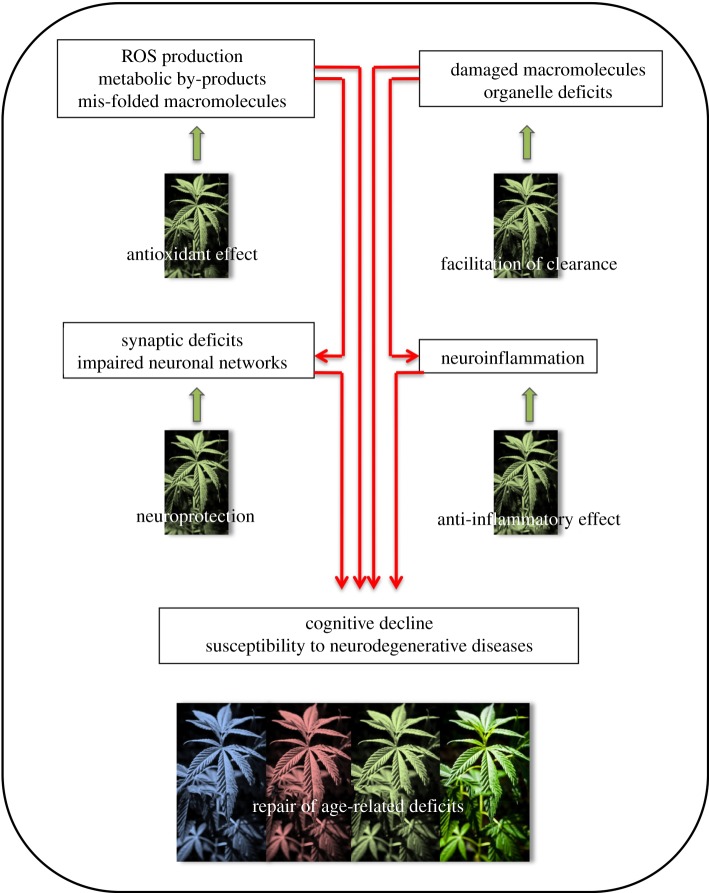
There are several lines of evidence that cannabinoid system activity modulates critical molecular and cellular processes influencing the pace of the ageing process (figure 1).
Figure 1.
Accumulation of damaged macromolecules, organelle deficits lead to impairment in neuronal functions and neuroinflammation. These mutually interacting processes are the major driving forces of brain ageing. Activity of cannabinoid system antagonizes these changes and thus decreases the progression of brain aging.
(a). Oxidative stress
Phytocannabinoids and structurally related synthetic compounds are known to possess anti-oxidant properties [152,153]. Although the structurally unrelated endogenous cannabinoid 2-AG is able to inhibit ROS formation in vitro [154], when considering its in vivo anti-oxidant properties one should keep in mind that it is metabolized by the prostaglandin/leukotriene pathway [155] resulting indirectly in pro-oxidant actions. It was suggested that besides the chemical structure of the cannabinoids, their metal-ion chelating property could also contribute to their anti-oxidant capacity [156]. The protective effect of cannabinoids against oxidative stress in vitro in neurons is dependent on their anti-oxidant properties as cannabinoid receptors are not involved in this process [157,158]. However, in astrocytes, the CB1 receptor mediates the protective effect of cannabinoids, because cannabinoids prevented H2O2-induced loss of viability of astrocytes in cell culture in a CB1-receptor-dependent manner [159]. For the neuroprotective effect of cannabinoids both their anti-oxidant and receptor binding properties contribute, as was shown in a rodent model of Parkinson's disease [160,161]. The neuroprotective effect of the CB1 receptor agonists Δ9-THC and WIN55,212-2 was blocked by the CB1 antagonist rimonabant on hippocampal neurons in culture [162] suggesting further the importance of cannabinoid receptor activity in neuroprotection. It is important to note that the cannabinoids modulate oxidative load in a cell-type-specific manner: in glioma [163] and leukaemia cell lines [164] but also in hepatic cells [165,166], cannabinoids induce cell death by increasing oxidative stress.
(b). Clearance of damaged macromolecules
The cannabinoid system can reduce oxidative load besides reducing the amount of ROS by influencing the removal of damaged macromolecules. It has been recently shown that the majority of CB1 receptors do not reach the cell surface but instead show an intracellular localization. A significant part of the intracellular CB1 receptors is present at lysosomes and late endosomes [167,168]. Cannabinoids at physiological concentrations increase lysosomal stability and integrity [169]. On the other hand, Δ9-THC in high concentration can increase lysosomal permeability through CB1 receptor binding, which may contribute to the neurotoxic effect of the drug [170]. Whether decreased CB1 receptor activity owing to genetic variation or epigenetic changes in humans influences lysosomal function and thus contributes to the development of neurodegenerative diseases is not known.
Cannabinoids have been previously implicated in inducing autophagy in various cancer cell types: glioma [171] hepatocellular carcinoma [172], pancreatic adenocarcinoma [173], and these effects are at least, in part, dependent on the CB1 receptor. We have found an altered autophagosomal and lysosomal turnover in Cnr1–/− mice (A. Piyanova 2012, unpublished results), suggesting a regulatory role for endocannabinoid signalling in autophagy also in non-cancerous tissues. This hypothesis was recently supported by Redlich et al. [174], who showed that the endocannabinoid palmitoylethanolamide increased the phagocytosis of murine microglial cells. The autophagy dysregulation owing to reduced CB1 receptor signalling is probably more important in ageing, as there is a general increase in oxidative load as well as formation of lipofuscin-like aggregates in the neurons and microglial cells.
(c). Mitochondrial activity
Cannabinoid system activity influences the amount of intracellular ROS not only via their anti-oxidant buffering capacity but also by regulating mitochondrial activity [175]. It has been recently shown that CB1 receptors are also present on mitochondrial membranes and regulate the activity of mitochondria [176]. Whether cannabinoids decrease or increase mitochondrial activity is not fully known: an early study showed that cannabinoids can decrease oxidative metabolism of isolated mitochondria [177], which was later supported by showing that CB1 agonists decrease oxygen consumption, ROS production, membrane potential [175] and oxidative phosphorylation [178]. On the other hand, an increase in brain mitochondrial oxidative phosphorylation was shown ex vivo in anandamide or Δ9-THC treated rats, which was antagonized by the CB1 receptor blocker SR141716A [179]. Under cellular stress, cannabinoids seems to be protective for the mitochondria: the cannabinoid receptor agonist CP55,940 and JWH-015 both attenuated mitochondrial damage against paraquat-induced oxidative stress [180] and the endogenous cannabinoid 2-AG decreased calcium-induced cytochrome c release from mitochondria [181].
(d). Regulation of glial activity
The cannabinoid system also influences ROS levels indirectly through the regulation of glial activity [182]. Both astrocytes and microglia express CB1 and CB2 cannabinoid receptors in an activity-dependent manner [183–185]. In the microglia, the expression of CB2 receptor exceeds the expression of CB1 receptors and correlates with microglial phenotype and activity [186]. Activation of glial CB2 receptors attenuates glial activation [187] and prevents neurodegeneration and reduces symptoms in mouse model of Huntington's disease [188]. These data suggest that cannabinoids regulate glial activity primarily through CB2 receptors. Glial cells not only receive cannabinoid signals but can themselves produce cannabinoids such as anandamide [189], 2-AG [190] and palmitoylethanolamide [191], and also express the enzymes involved in the synthesis and degradation of endocannabinoids [192–194]. Because both neurons and glia express elements of the cannabinoid system, it was hypothesized that the cannabinoid system plays an important role in neuron–glia communication. In support of this hypothesis, it was shown that cannabinoids modulate glial activity by directly binding to the glial cannabinoid receptors [185,195,196] and indirectly by modulating neuronal activities [197]. During central nervous system inflammation, as in multiple sclerosis [195], AD [198] or HIV encephalitis [199], a general upregulation of cannabinoid system activity is observed. Increased activity of the cannabinoid system is generally anti-inflammatory: elevation of anandamide levels [200,201] or activation of the cannabinoid receptor by synthetic receptor agonists [202,203] inhibits the production of pro-inflammatory mediators and reduces microglial migration in vitro. This effect may contribute to the beneficial effect of the cannabinoid system against neurodegeneration [204]. On the other hand, increased 2-AG levels increase inhibitory signalling and impair the control of retrograde neurotransmission thus contributing to the synaptic impairments in AD [198].
(e). Synaptic plasticity and neurogenesis
The age-dependent decline in brain-derived neurotrophic factor (BDNF) expression plays a crucial role in the chain of events leading to functional deficits. BDNF signalling regulates morphological and physiological synaptic plasticity [205], and restoration of BDNF levels was crucial for the rescue of synaptic plasticity in aged animals [206]. Application of CB1 receptor agonists increased BDNF expression both in vitro and in vivo [207,208], which may significantly contribute to the neuroprotective effect of the cannabinoids. This hypothesis was supported by Marsicano showing that induction of BDNF expression contributes to the protective effect of CB1 receptor activity against excitotoxicity [209]. CB1 receptor activity can enhance TrkB signalling partly by activating MAP kinase/ERK kinase pathways [210] but also by directly transactivating the TrkB receptors [211]. The fact that genetic deletion of CB1 receptors leads to a decreased BDNF expression suggests that endogenous cannabinoids exert a tonic control over BDNF expression [212]. Clinical data testing the BDNF levels in marijuana users and control individuals [213] also support the role of CB1 receptor activity on BDNF expression.
Besides influencing synaptic plasticity, cannabinoid system activity also facilitates embryonic and adult neurogenesis. Neural progenitor cells express the elements of the cannabinoid system, and thus actively use endocannabinoids as signalling molecules [214]. Activation of cannabinoid receptors by agonists [215,216] or by elevation of endocannabinoid levels [215,217,218] promotes cell proliferation, neurogenesis and neuronal diversification [219]. It was shown that CB1 receptor activity is necessary for the upregulation of neurogenesis and proliferation after excitotoxic stress [220]. In good agreement with these findings, genetic deletion of CB1 receptors leads to defective neurogenesis [221]. The consequence of this effect on the learning phenotype is unclear but it is unrelated to the early loss of cognitive abilities in this strain [197].
4. Cannabinoid system may change during ageing in the brain
Because cannabinoid system activity regulates mechanisms underlying normal and pathological ageing, age-dependent change in the activity of the cannabinoid system might contribute to the process of ageing. Although the results of different groups are sometimes conflicting, a decline in cannabinoid system activity in ageing is probable. Berrendero, in one of his earlier works, found a lower level of CB1 receptor level in rats in a brain-region-specific manner: the reduction was most prominent in the cerebellum and cerebral cortex, whereas it was less pronounced but still significant in the limbic and hypothalamic structures as well as in the hippocampus [222]. A similar decrease in both CB1 receptor binding and mRNA levels was found in most of the basal ganglia in rats during ageing [223]. Also, in isolated rat hippocampal synaptosomes, a reduction in CB1 receptor densities in ageing was described [224]. On the contrary, Liu et al. [225] found no differences in CB1 receptor protein levels between four- and 24-month-old rats in the hippocampus. They found, however, a significant difference in the CB1 receptor levels in the adjacent structures: they were reduced in the postrhinal, whereas elevated in the entorhinal and temporal cortices in the old animals. Wang et al. [226], testing C57BL/6J mice, did not find differences in CB1 densities in the hippocampus, limbic forebrain, amygdala and cerebellum. On the other hand, he reported a significantly reduced coupling between the receptors and Gi proteins in the limbic forebrain during ageing, which could be responsible for a reduced CB1 receptor signalling even when receptor levels are unchanged. In humans, a sex-dependent increase in CB1 receptor binding during ageing in the basal ganglia, lateral temporal cortex and in limbic areas was reported [227]. Whether the level of endogenous cannabinoids changes in ageing is unclear: some studies have reported diminished anandamide levels during ageing in CB1-receptor-deficient mice [228,229], whereas others found no significant differences in the endocannabinoid levels during ageing in different brain regions in WT or CB1-receptor-deficient mice [226]. There are also considerable differences in the endogenous cannabinoid concentrations reported in those earlier studies compared with more recent work (for review, see Buczynski & Parsons [230]). It is now known that multiple factors can influence the endogenous cannabinoid levels, such as post-mortem tissue handling and sample extraction methods, thus contributing to discrepancies between the studies from different groups.
5. Age-dependent effect of the cannabinoid system on learning and memory
Considering that the cannabinoid system regulates processes involved in ageing one would expect that (i) reduced activity of the cannabinoid system in genetically modified animals is accompanied by accelerated progression of ageing, and (ii) cannabinoid receptor agonists have different effects in young and old individuals. And in reality, genetic deletion of CB1 receptors leads to an age-related change in the learning and memory abilities of mice. Young CB1 receptor knockout (CB1−/−) mice showed a superior performance in a broad range of models including object [231] and social recognition tests, in skill and operant learning models [232] as well as enhanced long-term potentiation [233]. The learning ability of 12-month-old CB1−/− mice is, however, impaired and this impairment was accompanied by a loss of principal neurons in the hippocampus. Importantly, the early onset of age-related changes was specific for the cognitive functions in the brain, because neither the motor nor sensory abilities were affected [5]. The peripheral organs also did not show signs of early ageing except in the skin, where an atrophy of the subdermal fat layer was present in 12-month-old CB1−/− but not in wild-type mice [5]. A following study showed that the early onset of cognitive decline and neuronal loss was accompanied by neuroinflammatory changes in the hippocampus, but not in the cortex or striatum [197]. It was suggested that CB1 receptors on the GABAergic neurons play a principal role in the regulation of glial activity and thus in protection against age-related changes: deletion of CB1 receptors from the forebrain GABAergic but not glutamatergic neurons resulted in a similar ageing phenotype as found in the constitutive knockouts [197]. Interestingly, similar neuroinflammatory changes were not present in the cortex and striatum, brain areas having high expression of CB1 receptors and containing abundant GABAergic neurons. Thus, we can conclude that CB1 receptor activity plays an important role in anti-ageing defence. On the other hand, genetic deletion of CB2 receptors does not lead to similar enhanced lipofuscin accumulation or glial activation in the brain (A. Bilkei-Gorzo 2012, unpublished observation), therefore this receptor type is probably not involved in age-related processes. The question of whether 2-AG or anandamide is responsible for the anti-ageing activity of CB1 receptor is not known: although animals with genetic deletion of key enzymes of endocannabinoid metabolism are available [234], their age-related phenotype has not yet been published. On the basis of previous results, one would expect a delayed or slowed ageing in animals having increased CB receptor signalling. In good accordance with this assumption, animals lacking the anandamide degrading enzyme fatty acid amino hydrolase (FAAH) show an attenuated ageing associated decline in cardiac function and decreased expression of inflammation-associated genes [235]. Whether genetic variation in genes encoding the elements of the cannabinoid system in humans contributes to the variation in the progression of age-related cognitive decline is an open question.
The age dependency of cannabinoids on cognitive functions was demonstrated by a number of studies showing that adolescents are more sensitive to the adverse long-term effects of cannabinoid receptor agonists on cognitive abilities as adults [236–238]. On the basis of biological effects of cannabinoids, it was suggested that in old individuals cannabinoid receptor ligands may have even beneficial effects against age-related cognitive deficits [239]. Only a low number of publications exists focusing on the influence of cannabinoids on brain functions in healthy aged animals, but their results support this hypothesis: the CB1 receptor agonist WIN-55212-2 attenuated spatial memory impairment, reduced the number of activated microglia [240] and triggered neurogenesis [216] in aged rats. Similar to CB1 agonists, CB2 agonists or blockers of FAAH also enhanced proliferation of neuronal progenitor cells in old individuals [215]. These reports and the accelerated ageing phenotype of CB1 knockout animals together suggest that elevation of cannabinoid system activity ameliorates symptoms of brain ageing.
6. Potential role of cannabinoids in the treatment of neurodegenerative diseases
It was suggested that modulators of cannabinoid system activity could be a therapeutic tool for the treatment of neurodegenerative diseases [241,242]. At first sight, it is striking that cannabinoid agonists, substances known to impair cognitive functions, could be beneficial in neurodegenerative cognitive disorders. However, cannabinoid receptor activation could reduce oxidative stress and excitotoxicity, suppress neuroinflammatory processes and thus alleviate the symptoms of neurodegenerative motor [243] and cognitive diseases [244]. In vitro studies together with pharmacological experiments on animal models of AD generally supported the speculations about the therapeutic value of CB1 receptor agonists in the treatment of AD [245]. Cannabinoids protected against microglia-mediated neurotoxicity elicited by amyloid beta protein in rat cortical coculture [246]. In vivo, chronic treatment with CB1 agonists in the pre-symptomatic and early symptomatic phases ameliorated cognitive deficits and reduced microglial activity and cortical amyloid β-protein levels in APP2576 and APP/PS1 transgenic mice [247,248]. In rats, CB1 receptor agonists prevented cognitive impairment and microglial activation induced by intracerebroventricular injection of amyloid β-protein [246]. Despite the promising preclinical results, the detailed clinical evaluation of cannabinoids in AD patients is still missing. One pilot study, however, reported a significant reduction in nocturnal motor activity and agitation after dronabinol treatment in patients in late stages of dementia [249]. Cannabinoid treatment effectively attenuated the loss of dopaminergic neurons in rat models of Parkinson's disease [160,161] and proved to be neuroprotective in human neuronal cell culture exposed to Parkinson's disease relevant toxins [250]. The authors suggested that the anti-oxidative effect and suppression of microglial activity by cannabinoids together play a major role in these models [161,251]. The three published clinical trials in Parkinson's disease patients did not provide a clear answer whether cannabinoids modify the progression or the outcome of the disease. A double-blind, placebo-controlled crossover study that tested the symptom relieving effect of cannabis testing nine patients reported a significant reduction in levodopa-induced dyskinesia [252]. The following two clinical trials using a larger number of patients could not find any improvement in levodopa treatment induced dyskinesia or parkinsonian motor disability using cannabis [253] or the CB1 receptor antagonist rimonabant [254]. The situation is similar in the case of Huntington's disease and amyotrophic lateral sclerosis: a limited number of promising preclinical results using animal models of the diseases are reported but the supporting clinical data are missing [255–258]. Both cannabinoid receptor agonists and elevation of cannabinoid levels led to a significant improvement in disease progression and alleviation of symptoms in rodent models of multiple sclerosis [259,260]. Moreover, a case–control human genetic study reported an association between the genetic variance of CNR1 and primary progressive multiple sclerosis [261]. Unlike with other neurodegenerative disorders, numerous clinical studies were carried out testing different cannabis plant preparations and synthetic cannabinoids on patients with multiple sclerosis. It is clear from these studies that cannabinoid treatment alleviates the symptoms of the disease, reducing pain and sleep disturbances and improving general well being [262]. There are some conflicting results published regarding the effect of cannabis plant extracts on spasticity [263,264], but in a recent meta-analysis a trend for reduced spasticity was reported [265]. Generally, the treatment was well tolerated and maintained its efficacy after long-lasting administration [266], which is a prerequisite in the treatment of a chronic progressive disease. The recently completed CUPID study (http://sites.pcmd.ac.uk/cnrg/cupid.php) focused on the effect of THC on the progression of multiple sclerosis. In this large study, which involved 493 patients and ran for 8 years, the researchers found beneficial effects in patients in the initial phase of the diseases but no evidence for slowing down the progression of the disease generally.
7. Conclusion
Experimental evidences show that cannabinoid system activity is neuroprotective regulating critical homeostatic processes and that cannabinoid signalling is possibly decreasing in ageing. Thus, elevation of cannabinoid receptor activity either by pharmacological blockade of the degradation of cannabinoids or by receptor agonists could be a promising strategy for slowing down the progression of brain ageing and for alleviating the symptoms of neurodegenerative disorders.
Acknowledgements
This work was supported by German Research Council (grant no. FOR926 SP2). Many thanks for the suggestions and corrections to Ildiko Racz, to Andreas Zimmer for supporting the project and discussing the topic in detail, and to Önder Albayram who gave valuable input writing the manuscript and created the illustration.
References
- 1.Seidler R. D., Bernard J. A., Burutolu T. B., Fling B. W., Gordon M. T., Gwin J. T., Kwak Y., Lipps D. B. 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 34, 721–733 10.1016/j.neubiorev.2009.10.005 (doi:10.1016/j.neubiorev.2009.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltes P. B., Lindenberger U. 1997. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol. Aging 12, 12–21 10.1037/0882-7974.12.1.12 (doi:10.1037/0882-7974.12.1.12) [DOI] [PubMed] [Google Scholar]
- 3.Glisky E. L. 2007. Changes in cognitive function in human aging. In Brain aging models, methods, and mechanisms (ed. Riddle D. R.). New York, NY: CRC Press; [PubMed] [Google Scholar]
- 4.Rapp P. R., Amaral D. G. 1989. Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci. 9, 3568–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilkei-Gorzo A., et al. 2012. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1(−/−) mice. Neurobiol. Aging 33, e11–e22 10.1016/j.neurobiolaging.2010.07.009 (doi:10.1016/j.neurobiolaging.2010.07.009) [DOI] [PubMed] [Google Scholar]
- 6.Yu L., Tucci V., Kishi S., Zhdanova I. V. 2006. Cognitive aging in zebrafish. PLoS ONE 1, e14. 10.1371/journal.pone.0000014 (doi:10.1371/journal.pone.0000014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyler L. T., Sherzai A., Kaup A. R., Jeste D. V. 2011. A review of functional brain imaging correlates of successful cognitive aging. Biol. Psychiatry 70, 115–122 10.1016/j.biopsych.2010.12.032 (doi:10.1016/j.biopsych.2010.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Double K. L., Reyes S., Werry E. L., Halliday G. M. 2010. Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog. Neurobiol. 92, 316–329 10.1016/j.pneurobio.2010.06.001 (doi:10.1016/j.pneurobio.2010.06.001) [DOI] [PubMed] [Google Scholar]
- 9.Small S. A., Schobel S. A., Buxton R. B., Witter M. P., Barnes C. A. 2011. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 12, 585–601 10.1038/nrn3085 (doi:10.1038/nrn3085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bano D., Agostini M., Melino G., Nicotera P. 2011. Ageing, neuronal connectivity and brain disorders: an unsolved ripple effect. Mol. Neurobiol. 43, 124–130 10.1007/s12035-011-8164-6 (doi:10.1007/s12035-011-8164-6) [DOI] [PubMed] [Google Scholar]
- 11.Mattson M. P. 2007. Calcium and neurodegeneration. Aging Cell 6, 337–350 10.1111/j.1474-9726.2007.00275.x (doi:10.1111/j.1474-9726.2007.00275.x) [DOI] [PubMed] [Google Scholar]
- 12.Fjell A. M., Walhovd K. B. 2010. Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci. 21, 187–221 10.1515/REVNEURO.2010.21.3.187 (doi:10.1515/REVNEURO.2010.21.3.187) [DOI] [PubMed] [Google Scholar]
- 13.Jernigan T. L., Gamst A. C. 2005. Changes in volume with age-consistency and interpretation of observed effects. Neurobiol. Aging 26, 1271–1274; discussion 1275–8 10.1016/j.neurobiolaging.2005.05.016 (doi:10.1016/j.neurobiolaging.2005.05.016) [DOI] [PubMed] [Google Scholar]
- 14.Bartzokis G., Sultzer D., Lu P. H., Nuechterlein K. H., Mintz J., Cummings J. L. 2004. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical ‘disconnection’ in aging and Alzheimer's disease. Neurobiol. Aging 25, 843–851 10.1016/j.neurobiolaging.2003.09.005 (doi:10.1016/j.neurobiolaging.2003.09.005) [DOI] [PubMed] [Google Scholar]
- 15.Marner L., Nyengaard J. R., Tang Y., Pakkenberg B. 2003. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 462, 144–152 10.1002/cne.10714 (doi:10.1002/cne.10714) [DOI] [PubMed] [Google Scholar]
- 16.Salthouse T. A. 2011. Neuroanatomical substrates of age-related cognitive decline. Psychol. Bull. 137, 753–784 10.1037/a0023262 (doi:10.1037/a0023262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pakkenberg B., Pelvig D., Marner L., Bundgaard M. J., Gundersen H. J., Nyengaard J. R., Regeur L. 2003. Aging and the human neocortex. Exp. Gerontol. 38, 95–99 10.1016/S0531-5565(02)00151-1 (doi:10.1016/S0531-5565(02)00151-1) [DOI] [PubMed] [Google Scholar]
- 18.Pakkenberg B., Gundersen H. J. 1997. Neocortical neuron number in humans: effect of sex and age. J. Comp. Neurol. 384, 312–320 (doi:10.1002/(SICI)1096-9861(19970728)384:2<312::AID-CNE10>3.0.CO;2-K) [DOI] [PubMed] [Google Scholar]
- 19.West M. J. 1993. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging 14, 287–293 10.1016/0197-4580(93)90113-P (doi:10.1016/0197-4580(93)90113-P) [DOI] [PubMed] [Google Scholar]
- 20.Simic G., Bexheti S., Kelovic Z., Kos M., Grbic K., Hof P. R., Kostovic I. 2005. Hemispheric asymmetry, modular variability and age-related changes in the human entorhinal cortex. Neuroscience 130, 911–925 10.1016/j.neuroscience.2004.09.040 (doi:10.1016/j.neuroscience.2004.09.040) [DOI] [PubMed] [Google Scholar]
- 21.Kordower J. H., Chu Y., Stebbins G. T., DeKosky S. T., Cochran E. J., Bennett D., Mufson E. J. 2001. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 49, 202–213 (doi:10.1002/1531-8249(20010201)49:2<202::AID-ANA40>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 22.Couillard-Despres S., Iglseder B., Aigner L. 2011. Neurogenesis, cellular plasticity and cognition: the impact of stem cells in the adult and aging brain: a mini-review. Gerontology 57, 559–564 10.1159/000323481 (doi:10.1159/000323481) [DOI] [PubMed] [Google Scholar]
- 23.Yassa M. A., Stark C. E. 2011. Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 10.1016/j.tins.2011.06.006 (doi:10.1016/j.tins.2011.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke S. N., Barnes C. A. 2006. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40 10.1038/nrn1809 (doi:10.1038/nrn1809) [DOI] [PubMed] [Google Scholar]
- 25.Pannese E. 2011. Morphological changes in nerve cells during normal aging. Brain Struct. Funct. 216, 85–89 10.1007/s00429-011-0308-y (doi:10.1007/s00429-011-0308-y) [DOI] [PubMed] [Google Scholar]
- 26.Flood D. G. 1993. Critical issues in the analysis of dendritic extent in aging humans, primates, and rodents. Neurobiol. Aging 14, 649–654 10.1016/0197-4580(93)90058-J (doi:10.1016/0197-4580(93)90058-J) [DOI] [PubMed] [Google Scholar]
- 27.Grill J. D., Riddle D. R. 2002. Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res. 937, 8–21 10.1016/S0006-8993(02)02457-5 (doi:10.1016/S0006-8993(02)02457-5) [DOI] [PubMed] [Google Scholar]
- 28.Ginsberg S. D. 2007. Expression profile analysis of brain aging. In Brain aging models methods, and mechanisms (ed. Riddle D. R.), pp. 159–185 Boca Raton, FL: CRC Press [Google Scholar]
- 29.Lonze B. E., Ginty D. D. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 10.1016/S0896-6273(02)00828-0 (doi:10.1016/S0896-6273(02)00828-0) [DOI] [PubMed] [Google Scholar]
- 30.Saura C. A., Valero J. 2011. The role of CREB signaling in Alzheimer's disease and other cognitive disorders. Rev. Neurosci. 22, 153–169 10.1515/rns.2011.018 (doi:10.1515/rns.2011.018) [DOI] [PubMed] [Google Scholar]
- 31.Chen G., Zou X., Watanabe H., van Deursen J. M., Shen J. 2010. CREB binding protein is required for both short-term and long-term memory formation. J. Neurosci. 30, 13 066–13 077 10.1523/JNEUROSCI.2378-10.2010 (doi:10.1523/JNEUROSCI.2378-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menard C., Quirion R. 2012. Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS ONE 7, e28666. 10.1371/journal.pone.0028666 (doi:10.1371/journal.pone.0028666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ljungberg M. C., Ali Y. O., Zhu J., Wu C. S., Oka K., Zhai R. G., Lu H. C. 2012. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum. Mol. Genet. 21, 251–267 10.1093/hmg/ddr492 (doi:10.1093/hmg/ddr492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Q. L., Harris-White M. E., Ubeda O. J., Simmons M., Beech W., Lim G. P., Teter B., Frautschy S. A., Cole G. M. 2007. Evidence of Abeta- and transgene-dependent defects in ERK-CREB signaling in Alzheimer's models. J. Neurochem. 103, 1594–1607 10.1111/j.1471-4159.2007.04869.x (doi:10.1111/j.1471-4159.2007.04869.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomobe K., Okuma Y., Nomura Y. 2007. Impairment of CREB phosphorylation in the hippocampal CA1 region of the senescence-accelerated mouse (SAM) P8. Brain Res. 1141, 214–217 10.1016/j.brainres.2006.08.026 (doi:10.1016/j.brainres.2006.08.026) [DOI] [PubMed] [Google Scholar]
- 36.Monti B., Berteotti C., Contestabile A. 2005. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus 15, 1041–1049 10.1002/hipo.20099 (doi:10.1002/hipo.20099) [DOI] [PubMed] [Google Scholar]
- 37.Porte Y., Buhot M. C., Mons N. 2008. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol. Aging 29, 1533–1546 10.1016/j.neurobiolaging.2007.03.023 (doi:10.1016/j.neurobiolaging.2007.03.023) [DOI] [PubMed] [Google Scholar]
- 38.Mair W., Morantte I., Rodrigues A. P., Manning G., Montminy M., Shaw R. J., Dillin A. 2011. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470, 404–408 10.1038/nature09706 (doi:10.1038/nature09706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M., Poplawski M., Yen K., Cheng H., Bloss E., Zhu X., Patel H., Mobbs C. V. 2009. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 7, e1000245. 10.1371/journal.pbio.1000245 (doi:10.1371/journal.pbio.1000245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toescu E. C., Verkhratsky A. 2004. Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J. Cell Mol. Med. 8, 181–190 10.1111/j.1582-4934.2004.tb00273.x (doi:10.1111/j.1582-4934.2004.tb00273.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thibault O., Landfield P. W. 1996. Increase in single L-type calcium channels in hippocampal neurons during aging. Science 272, 1017–1020 10.1126/science.272.5264.1017 (doi:10.1126/science.272.5264.1017) [DOI] [PubMed] [Google Scholar]
- 42.Michaelis M. L., et al. 1996. Decreased plasma membrane calcium transport activity in aging brain. Life Sci. 59, 405–412 10.1016/0024-3205(96)00319-0 (doi:10.1016/0024-3205(96)00319-0) [DOI] [PubMed] [Google Scholar]
- 43.Zaidi A., Gao J., Squier T. C., Michaelis M. L. 1998. Age-related decrease in brain synaptic membrane Ca2+-ATPase in F344/BNF1 rats. Neurobiol. Aging 19, 487–495 10.1016/S0197-4580(98)00078-5 (doi:10.1016/S0197-4580(98)00078-5) [DOI] [PubMed] [Google Scholar]
- 44.Bu J., Sathyendra V., Nagykery N., Geula C. 2003. Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp. Neurol. 182, 220–231 10.1016/S0014-4886(03)00094-3 (doi:10.1016/S0014-4886(03)00094-3) [DOI] [PubMed] [Google Scholar]
- 45.Toescu E. C., Verkhratsky A., Landfield P. W. 2004. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 27, 614–620 10.1016/j.tins.2004.07.010 (doi:10.1016/j.tins.2004.07.010) [DOI] [PubMed] [Google Scholar]
- 46.Bishop N. A., Lu T., Yankner B. A. 2010. Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535 10.1038/nature08983 (doi:10.1038/nature08983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu T., Pan Y., Kao S. Y., Li C., Kohane I., Chan J., Yankner B. A. 2004. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 10.1038/nature02661 (doi:10.1038/nature02661) [DOI] [PubMed] [Google Scholar]
- 48.Lee C. K., Weindruch R., Prolla T. A. 2000. Gene-expression profile of the ageing brain in mice. Nat. Genet. 25, 294–297 10.1038/77046 (doi:10.1038/77046) [DOI] [PubMed] [Google Scholar]
- 49.Zhan M., Yamaza H., Sun Y., Sinclair J., Li H., Zou S. 2007. Temporal and spatial transcriptional profiles of aging in Drosophila melanogaster. Genome Res. 17, 1236–1243 10.1101/gr.6216607 (doi:10.1101/gr.6216607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang H. J., et al. 2011. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 10.1038/nature10523 (doi:10.1038/nature10523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang C. H., Tsien J. Z., Schultz P. G., Hu Y. 2001. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc. Natl Acad. Sci. USA 98, 1930–1934 10.1073/pnas.98.4.1930 (doi:10.1073/pnas.98.4.1930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blalock E. M., Chen K. C., Sharrow K., Herman J. P., Porter N. M., Foster T. C., Landfield P. W. 2003. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 23, 3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burger C., Lopez M. C., Feller J. A., Baker H. V., Muzyczka N., Mandel R. J. 2007. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol. Learn. Mem. 87, 21–41 10.1016/j.nlm.2006.05.003 (doi:10.1016/j.nlm.2006.05.003) [DOI] [PubMed] [Google Scholar]
- 54.Stranahan A. M., Lee K., Becker K. G., Zhang Y., Maudsley S., Martin B., Cutler R. G., Mattson M. P. 2010. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol. Aging 31, 1937–1949 10.1016/j.neurobiolaging.2008.10.016 (doi:10.1016/j.neurobiolaging.2008.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swanson K. S., Vester B. M., Apanavicius C. J., Kirby N. A., Schook L. B. 2009. Implications of age and diet on canine cerebral cortex transcription. Neurobiol. Aging 30, 1314–1326 10.1016/j.neurobiolaging.2007.10.017 (doi:10.1016/j.neurobiolaging.2007.10.017) [DOI] [PubMed] [Google Scholar]
- 56.Haberman R. P., Colantuoni C., Stocker A. M., Schmidt A. C., Pedersen J. T., Gallagher M. 2011. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol. Aging 32, 1678–1692 10.1016/j.neurobiolaging.2009.10.005 (doi:10.1016/j.neurobiolaging.2009.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopra O., et al. 2004. A mouse model for Finnish variant late infantile neuronal ceroid lipofuscinosis, CLN5, reveals neuropathology associated with early aging. Hum. Mol. Genet. 13, 2893–2906 10.1093/hmg/ddh312 (doi:10.1093/hmg/ddh312) [DOI] [PubMed] [Google Scholar]
- 58.Ricciarelli R., d'Abramo C., Massone S., Marinari U., Pronzato M., Tabaton M. 2004. Microarray analysis in Alzheimer's disease and normal aging. IUBMB Life 56, 349–354 10.1080/15216540412331286002 (doi:10.1080/15216540412331286002) [DOI] [PubMed] [Google Scholar]
- 59.Wu Z. L., Ciallella J. R., Flood D. G., O'Kane T. M., Bozyczko-Coyne D., Savage M. J. 2006. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol. Aging 27, 377–386 10.1016/j.neurobiolaging.2005.02.010 (doi:10.1016/j.neurobiolaging.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 60.Cheung I., Shulha H. P., Jiang Y., Matevossian A., Wang J., Weng Z., Akbarian S. 2010. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc. Natl Acad. Sci. USA 107, 8824–8829 10.1073/pnas.1001702107 (doi:10.1073/pnas.1001702107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moroz L. L., Kohn A. B. 2010. Do different neurons age differently? Direct genome-wide analysis of aging in single identified cholinergic neurons. Front. Aging Neurosci. 2, 6. 10.3389/neuro.24.006.2010 (doi:10.3389/neuro.24.006.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peleg S., et al. 2010. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328, 753–756 10.1126/science.1186088 (doi:10.1126/science.1186088) [DOI] [PubMed] [Google Scholar]
- 63.Gemma C., Vila J., Bachstetter A., Bickford P. C. 2007. Oxidative stress and the aging brain: from theory to prevention. In Brain aging: models, methods, and mechanisms (ed. D. R. Riddle), pp. 353-374. Boca Raton, FL: CRC Press. [PubMed] [Google Scholar]
- 64.Genova M. L., et al. 2004. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann. NY Acad. Sci. 1011, 86–100 10.1196/annals.1293.010 (doi:10.1196/annals.1293.010) [DOI] [PubMed] [Google Scholar]
- 65.Serrano F., Klann E. 2004. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 3, 431–443 10.1016/j.arr.2004.05.002 (doi:10.1016/j.arr.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 66.Saxena S., Caroni P. 2011. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71, 35–48 10.1016/j.neuron.2011.06.031 (doi:10.1016/j.neuron.2011.06.031) [DOI] [PubMed] [Google Scholar]
- 67.Sedensky M. M., Morgan P. G. 2006. Mitochondrial respiration and reactive oxygen species in mitochondrial aging mutants. Exp. Gerontol. 41, 237–245 10.1016/j.exger.2006.01.004 (doi:10.1016/j.exger.2006.01.004) [DOI] [PubMed] [Google Scholar]
- 68.Fraser H. B., Khaitovich P., Plotkin J. B., Paabo S., Eisen M. B. 2005. Aging and gene expression in the primate brain. PLoS Biol. 3, e274. 10.1371/journal.pbio.0030274 (doi:10.1371/journal.pbio.0030274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki T., Unno K., Tahara S., Shimada A., Chiba Y., Hoshino M., Kaneko T. 2008. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell 7, 459–469 10.1111/j.1474-9726.2008.00394.x (doi:10.1111/j.1474-9726.2008.00394.x) [DOI] [PubMed] [Google Scholar]
- 70.Yankner B. A., Lu T., Loerch P. 2008. The aging brain. Annu. Rev. Pathol. 3, 41–66 10.1146/annurev.pathmechdis.2.010506.092044 (doi:10.1146/annurev.pathmechdis.2.010506.092044) [DOI] [PubMed] [Google Scholar]
- 71.Toescu E. C. 2005. Normal brain ageing: models and mechanisms. Phil. Trans. R. Soc. B 360, 2347–2354 10.1098/rstb.2005.1771 (doi:10.1098/rstb.2005.1771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Floyd R. A., Hensley K. 2002. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 23, 795–807 10.1016/S0197-4580(02)00019-2 (doi:10.1016/S0197-4580(02)00019-2) [DOI] [PubMed] [Google Scholar]
- 73.Grimm S., Hoehn A., Davies K. J., Grune T. 2011. Protein oxidative modifications in the ageing brain: consequence for the onset of neurodegenerative disease. Free Radic. Res. 45, 73–88 10.3109/10715762.2010.512040 (doi:10.3109/10715762.2010.512040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korolainen M. A., Goldsteins G., Nyman T. A., Alafuzoff I., Koistinaho J., Pirttila T. 2006. Oxidative modification of proteins in the frontal cortex of Alzheimer's disease brain. Neurobiol. Aging 27, 42–53 10.1016/j.neurobiolaging.2004.11.010 (doi:10.1016/j.neurobiolaging.2004.11.010) [DOI] [PubMed] [Google Scholar]
- 75.Pratico D. 2008. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann. NY Acad. Sci. 1147, 70–78 10.1196/annals.1427.010 (doi:10.1196/annals.1427.010) [DOI] [PubMed] [Google Scholar]
- 76.Nunomura A., et al. 2012. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J. Neuropathol. Exp. Neurol. 71, 233–241 10.1097/NEN.0b013e318248e614 (doi:10.1097/NEN.0b013e318248e614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du H., Guo L., Yan S., Sosunov A. A., McKhann G. M., Yan S. S. 2010. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc. Natl Acad. Sci. USA 107, 18 670–18 675 10.1073/pnas.1006586107 (doi:10.1073/pnas.1006586107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H. P., Pancholi N., Esposito L., Previll L. A., Wang X., Zhu X., Smith M. A., Lee H. G. 2012. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS ONE 7, e28033. 10.1371/journal.pone.0028033 (doi:10.1371/journal.pone.0028033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McManus M. J., Murphy M. P., Franklin J. L. 2011. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 15 703–15 715 10.1523/JNEUROSCI.0552-11.2011 (doi:10.1523/JNEUROSCI.0552-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guzman J. N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P. T., Surmeier D. J. 2010. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468, 696–700 10.1038/nature09536 (doi:10.1038/nature09536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venkateshappa C., Harish G., Mythri R. B., Mahadevan A., Bharath M. M., Shankar S. K. 2012. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson's disease. Neurochem. Res. 37, 358–369 10.1007/s11064-011-0619-7 (doi:10.1007/s11064-011-0619-7) [DOI] [PubMed] [Google Scholar]
- 82.German D. C., Manaye K. F., White C. L., 3rd, Woodward D. J., McIntire D. D., Smith W. K., Kalaria R. N., Mann D. M. 1992. Disease-specific patterns of locus coeruleus cell loss. Ann. Neurol. 32, 667–676 10.1002/ana.410320510 (doi:10.1002/ana.410320510) [DOI] [PubMed] [Google Scholar]
- 83.Napolitano A., Manini P., d'Ischia M. 2011. Oxidation chemistry of catecholamines and neuronal degeneration: an update. Curr. Med. Chem. 18, 1832–1845 10.2174/092986711795496863 (doi:10.2174/092986711795496863) [DOI] [PubMed] [Google Scholar]
- 84.Head E. 2009. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem. Res. 34, 670–678 10.1007/s11064-008-9808-4 (doi:10.1007/s11064-008-9808-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lau F. C., Shukitt-Hale B., Joseph J. A. 2005. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 26(Suppl. 1), 128–132 10.1016/j.neurobiolaging.2005.08.007 (doi:10.1016/j.neurobiolaging.2005.08.007) [DOI] [PubMed] [Google Scholar]
- 86.Melov S. 2002. Therapeutics against mitochondrial oxidative stress in animal models of aging. Ann. NY Acad. Sci. 959, 330–340 10.1111/j.1749-6632.2002.tb02104.x (doi:10.1111/j.1749-6632.2002.tb02104.x) [DOI] [PubMed] [Google Scholar]
- 87.Haxaire C., Turpin F. R., Potier B., Kervern M., Sinet P. M., Barbanel G., Mothet J. P., Dutar P., Billard J. M. 2012. Reversal of age-related oxidative stress prevents hippocampal synaptic plasticity deficits by protecting d-serine-dependent NMDA receptor activation. Aging Cell 11, 336–344 10.1111/j.1474-9726.2012.00792.x (doi:10.1111/j.1474-9726.2012.00792.x) [DOI] [PubMed] [Google Scholar]
- 88.Liu R., Liu I. Y., Bi X., Thompson R. F., Doctrow S. R., Malfroy B., Baudry M. 2003. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl Acad. Sci. USA 100, 8526–8531 10.1073/pnas.1332809100 (doi:10.1073/pnas.1332809100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolosova N. G., Shcheglova T. V., Sergeeva S. V., Loskutova L. V. 2006. Long-term antioxidant supplementation attenuates oxidative stress markers and cognitive deficits in senescent-accelerated OXYS rats. Neurobiol. Aging 27, 1289–1297 10.1016/j.neurobiolaging.2005.07.022 (doi:10.1016/j.neurobiolaging.2005.07.022) [DOI] [PubMed] [Google Scholar]
- 90.Shih A. Y., Imbeault S., Barakauskas V., Erb H., Jiang L., Li P., Murphy T. H. 2005. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J. Biol. Chem. 280, 22 925–22 936 10.1074/jbc.M414635200 (doi:10.1074/jbc.M414635200) [DOI] [PubMed] [Google Scholar]
- 91.Esposito E., Rotilio D., Di Matteo V., Di Giulio C., Cacchio M., Algeri S. 2002. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 23, 719–735 10.1016/S0197-4580(02)00078-7 (doi:10.1016/S0197-4580(02)00078-7) [DOI] [PubMed] [Google Scholar]
- 92.Kumar A., Dogra S., Prakash A. 2009. Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against d-galactose induced senescence in mice. Naunyn Schmiedebergs Arch. Pharmacol. 380, 431–441 10.1007/s00210-009-0442-8 (doi:10.1007/s00210-009-0442-8) [DOI] [PubMed] [Google Scholar]
- 93.Peng Y., Sun J., Hon S., Nylander A. N., Xia W., Feng Y., Wang X., Lemere C. A. 2010. L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer's disease. J. Neurosci. 30, 8180–8189 10.1523/JNEUROSCI.0340-10.2010 (doi:10.1523/JNEUROSCI.0340-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yokota T., et al. 2001. Delayed-onset ataxia in mice lacking alpha-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl Acad. Sci. USA 98, 15 185–15 190 10.1073/pnas.261456098 (doi:10.1073/pnas.261456098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lipinski M. M., et al. 2010. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc. Natl Acad. Sci. USA 107, 14 164–14 169 10.1073/pnas.1009485107 (doi:10.1073/pnas.1009485107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.West R. J., Sweeney S. T. 2012. Oxidative stress and autophagy: mediators of synapse growth? Autophagy 8, 284–285 10.4161/auto.8.2.18981 (doi:10.4161/auto.8.2.18981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hardy J., Selkoe D. J. 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 10.1126/science.1072994 (doi:10.1126/science.1072994) [DOI] [PubMed] [Google Scholar]
- 98.Chu Y., Kordower J. H. 2007. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson's disease? Neurobiol. Dis. 25, 134–149 10.1016/j.nbd.2006.08.021 (doi:10.1016/j.nbd.2006.08.021) [DOI] [PubMed] [Google Scholar]
- 99.Lee S. J., Lim H. S., Masliah E., Lee H. J. 2011. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci. Res. 70, 339–348 10.1016/j.neures.2011.05.008 (doi:10.1016/j.neures.2011.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casarejos M. J., Solano R. M., Rodriguez-Navarro J. A., Gomez A., Perucho J., Castano J. G., Garcia de Yebenes J., Mena M. A. 2009. Parkin deficiency increases the resistance of midbrain neurons and glia to mild proteasome inhibition: the role of autophagy and glutathione homeostasis. J. Neurochem. 110, 1523–1537 10.1111/j.1471-4159.2009.06248.x (doi:10.1111/j.1471-4159.2009.06248.x) [DOI] [PubMed] [Google Scholar]
- 101.Nakanishi H. 2003. Neuronal and microglial cathepsins in aging and age-related diseases. Ageing Res. Rev. 2, 367–381 10.1016/S1568-1637(03)00027-8 (doi:10.1016/S1568-1637(03)00027-8) [DOI] [PubMed] [Google Scholar]
- 102.Pandey U. B., et al. 2007. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 10.1038/nature05853 (doi:10.1038/nature05853) [DOI] [PubMed] [Google Scholar]
- 103.Caballero B., Coto-Montes A. 2012. An insight into the role of autophagy in cell responses in the aging and neurodegenerative brain. Histol. Histopathol. 27, 263–275 [DOI] [PubMed] [Google Scholar]
- 104.Ling D., Salvaterra P. M. 2011. Brain aging and Abeta neurotoxicity converge via deterioration in autophagy-lysosomal system: a conditional Drosophila model linking Alzheimer's neurodegeneration with aging. Acta Neuropathol. 121, 183–191 10.1007/s00401-010-0772-0 (doi:10.1007/s00401-010-0772-0) [DOI] [PubMed] [Google Scholar]
- 105.Walter S., et al. 2011. A genome-wide association study of aging. Neurobiol. Aging 32, 2109 e15–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubinsztein D. C., Marino G., Kroemer G. 2011. Autophagy and aging. Cell 146, 682– 695 10.1016/j.cell.2011.07.030 (doi:10.1016/j.cell.2011.07.030) [DOI] [PubMed] [Google Scholar]
- 107.Crowe E., Sell C., Thomas J. D., Johannes G. J., Torres C. 2009. Activation of proteasome by insulin-like growth factor-I may enhance clearance of oxidized proteins in the brain. Mech. Ageing Dev. 130, 793–800 10.1016/j.mad.2009.10.005 (doi:10.1016/j.mad.2009.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Medina D. X., Caccamo A., Oddo S. 2011. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 21, 140–149 10.1111/j.1750-3639.2010.00430.x (doi:10.1111/j.1750-3639.2010.00430.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cooper J. D., Messer A., Feng A. K., Chua-Couzens J., Mobley W. C. 1999. Apparent loss and hypertrophy of interneurons in a mouse model of neuronal ceroid lipofuscinosis: evidence for partial response to insulin-like growth factor-1 treatment. J. Neurosci. 19, 2556–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poulose S. M., Bielinski D. F., Carrihill-Knoll K., Rabin B. M., Shukitt-Hale B. 2011. Exposure to 16O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat. Res. 176, 761–769 10.1667/RR2605.1 (doi:10.1667/RR2605.1) [DOI] [PubMed] [Google Scholar]
- 111.Komatsu M., et al. 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 10.1038/nature04723 (doi:10.1038/nature04723) [DOI] [PubMed] [Google Scholar]
- 112.Qin A. P., Zhang H. L., Qin Z. H. 2008. Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci. Bull. 24, 117–123 10.1007/s12264-008-0117-3 (doi:10.1007/s12264-008-0117-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y., Dong X. X., Cao Y., Liang Z. Q., Han R., Wu J. C., Gu Z. L., Qin Z. H. 2009. p53 induction contributes to excitotoxic neuronal death in rat striatum through apoptotic and autophagic mechanisms. Eur. J. Neurosci. 30, 2258–2270 10.1111/j.1460-9568.2009.07025.x (doi:10.1111/j.1460-9568.2009.07025.x) [DOI] [PubMed] [Google Scholar]
- 114.Wen Y. D., Sheng R., Zhang L. S., Han R., Zhang X., Zhang X. D., Han F., Fukunaga K., Qin Z. H. 2008. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4, 762–769 [DOI] [PubMed] [Google Scholar]
- 115.Harris H., Rubinsztein D. C. 2011. Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 8, 108–117 10.1038/nrneurol.2011.200 (doi:10.1038/nrneurol.2011.200) [DOI] [PubMed] [Google Scholar]
- 116.Kaunzner U. W., Miller M. M., Gottfried-Blackmore A., Gal-Toth J., Felger J. C., McEwen B. S., Bulloch K. 2012. Accumulation of resident and peripheral dendritic cells in the aging CNS. Neurobiol. Aging 33, 681–693 10.1016/j.neurobiolaging.2010.06.007 (doi:10.1016/j.neurobiolaging.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 117.Schuitemaker A., et al. 2010. Microglial activation in healthy aging. Neurobiol. Aging 33, 1067–1072 10.1016/j.neurobiolaging.2010.09.016 (doi:10.1016/j.neurobiolaging.2010.09.016) [DOI] [PubMed] [Google Scholar]
- 118.Agulhon C., Petravicz J., McMullen A. B., Sweger E. J., Minton S. K., Taves S. R., Casper K. B., Fiacco T. A., McCarthy K. D. 2008. What is the role of astrocyte calcium in neurophysiology? Neuron 59, 932–946 10.1016/j.neuron.2008.09.004 (doi:10.1016/j.neuron.2008.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Streit W. J. 2005. Microglia and neuroprotection: implications for Alzheimer's disease. Brain Res. Brain Res. Rev. 48, 234–239 10.1016/j.brainresrev.2004.12.013 (doi:10.1016/j.brainresrev.2004.12.013) [DOI] [PubMed] [Google Scholar]
- 120.Castro M. A., Beltran F. A., Brauchi S., Concha I. I. 2009. A metabolic switch in brain: glucose and lactate metabolism modulation by ascorbic acid. J. Neurochem. 110, 423–440 10.1111/j.1471-4159.2009.06151.x (doi:10.1111/j.1471-4159.2009.06151.x) [DOI] [PubMed] [Google Scholar]
- 121.Petzold G. C., Murthy V. N. 2011. Role of astrocytes in neurovascular coupling. Neuron 71, 782–797 10.1016/j.neuron.2011.08.009 (doi:10.1016/j.neuron.2011.08.009) [DOI] [PubMed] [Google Scholar]
- 122.Bacci A., Verderio C., Pravettoni E., Matteoli M. 1999. The role of glial cells in synaptic function. Phil. Trans. R. Soc. Lond. B 354, 403–409 10.1098/rstb.1999.0393 (doi:10.1098/rstb.1999.0393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferron S. R., et al. 2011. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature 475, 381–385 10.1038/nature10229 (doi:10.1038/nature10229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gemma C., Bachstetter A. D., Bickford P. C. 2010. Neuron-microglia dialogue and hippocampal neurogenesis in the aged brain. Aging Dis. 1, 232–244 [PMC free article] [PubMed] [Google Scholar]
- 125.Hanisch U. K., Kettenmann H. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 10.1038/nn1997 (doi:10.1038/nn1997) [DOI] [PubMed] [Google Scholar]
- 126.Lucin K. M., Wyss-Coray T. 2009. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64, 110–122 10.1016/j.neuron.2009.08.039 (doi:10.1016/j.neuron.2009.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tremblay M. E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. 2011. The role of microglia in the healthy brain. J. Neurosci. 31, 16 064–16 069 10.1523/JNEUROSCI.4158-11.2011 (doi:10.1523/JNEUROSCI.4158-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Finch C. E. 2010. Evolution in health and medicine Sackler colloquium: evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107(Suppl. 1), 1718–1724 10.1073/pnas.0909606106 (doi:10.1073/pnas.0909606106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Villeda S. A., et al. 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 10.1038/nature10357 (doi:10.1038/nature10357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Katsel P., Tan W., Haroutunian V. 2009. Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS ONE 4, e7642. 10.1371/journal.pone.0007642 (doi:10.1371/journal.pone.0007642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lynch A. M., Murphy K. J., Deighan B. F., O'Reilly J. A., Gun'ko Y. K., Cowley T. R., Gonzalez-Reyes R. E., Lynch M. A. 2010. The impact of glial activation in the aging brain. Aging Dis. 1, 262–278 [PMC free article] [PubMed] [Google Scholar]
- 132.Naert G., Rivest S. 2011. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 6208–6220 10.1523/JNEUROSCI.0299-11.2011 (doi:10.1523/JNEUROSCI.0299-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moriyama M., et al. 2011. Complement receptor 2 is expressed in neural progenitor cells and regulates adult hippocampal neurogenesis. J. Neurosci. 31, 3981–3989 10.1523/JNEUROSCI.3617-10.2011 (doi:10.1523/JNEUROSCI.3617-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Russo I., Barlati S., Bosetti F. 2011. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J. Neurochem. 116, 947–956 10.1111/j.1471-4159.2010.07168.x (doi:10.1111/j.1471-4159.2010.07168.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lynch M. A. 2010. Age-related neuroinflammatory changes negatively impact on neuronal function. Front. Aging Neurosci. 1, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tha K. K., Okuma Y., Miyazaki H., Murayama T., Uehara T., Hatakeyama R., Hayashi Y., Nomura Y. 2000. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 885, 25–31 10.1016/S0006-8993(00)02883-3 (doi:10.1016/S0006-8993(00)02883-3) [DOI] [PubMed] [Google Scholar]
- 137.Perry V. H., Nicoll J. A., Holmes C. 2010. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 6, 193–201 10.1038/nrneurol.2010.17 (doi:10.1038/nrneurol.2010.17) [DOI] [PubMed] [Google Scholar]
- 138.Lue L. F., Kuo Y. M., Beach T., Walker D. G. 2010. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol. Neurobiol. 41, 115–128 10.1007/s12035-010-8106-8 (doi:10.1007/s12035-010-8106-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sardi F., Fassina L., Venturini L., Inguscio M., Guerriero F., Rolfo E., Ricevuti G. 2011. Alzheimer's disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun. Rev. 11, 149–153 10.1016/j.autrev.2011.09.005 (doi:10.1016/j.autrev.2011.09.005) [DOI] [PubMed] [Google Scholar]
- 140.Simpson J. E., Ince P. G., Lace G., Forster G., Shaw P. J., Matthews F., Savva G., Brayne C., Wharton S. B. 2010. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging 31, 578–590 10.1016/j.neurobiolaging.2008.05.015 (doi:10.1016/j.neurobiolaging.2008.05.015) [DOI] [PubMed] [Google Scholar]
- 141.Bradford J., Shin J. Y., Roberts M., Wang C. E., Li X. J., Li S. 2009. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl Acad. Sci. USA 106, 22 480–22 485 10.1073/pnas.0911503106 (doi:10.1073/pnas.0911503106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katsouri L., Georgopoulos S. 2011. Lack of LDL receptor enhances amyloid deposition and decreases glial response in an Alzheimer's disease mouse model. PLoS ONE 6, e21880. 10.1371/journal.pone.0021880 (doi:10.1371/journal.pone.0021880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klein R. L., Dayton R. D., Diaczynsky C. G., Wang D. B. 2010. Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol. Aging 31, 2091–2102 10.1016/j.neurobiolaging.2008.12.002 (doi:10.1016/j.neurobiolaging.2008.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tress O., et al. 2011. Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus–Merzbacher-like disease in humans. PLoS Genet. 7, e1002146. 10.1371/journal.pgen.1002146 (doi:10.1371/journal.pgen.1002146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Campisi J. 2005. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 10.1016/j.cell.2005.02.003 (doi:10.1016/j.cell.2005.02.003) [DOI] [PubMed] [Google Scholar]
- 146.Salminen A., Ojala J., Kaarniranta K., Haapasalo A., Hiltunen M., Soininen H. 2011. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci. 34, 3–11 10.1111/j.1460-9568.2011.07738.x (doi:10.1111/j.1460-9568.2011.07738.x) [DOI] [PubMed] [Google Scholar]
- 147.Garcia-Matas S., Gutierrez-Cuesta J., Coto-Montes A., Rubio-Acero R., Diez-Vives C., Camins A., Pallas M., Sanfeliu C., Cristofol R. 2008. Dysfunction of astrocytes in senescence-accelerated mice SAMP8 reduces their neuroprotective capacity. Aging Cell 7, 630–640 10.1111/j.1474-9726.2008.00410.x (doi:10.1111/j.1474-9726.2008.00410.x) [DOI] [PubMed] [Google Scholar]
- 148.Nakanishi H., Wu Z. 2009. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav. Brain Res. 201, 1–7 10.1016/j.bbr.2009.02.001 (doi:10.1016/j.bbr.2009.02.001) [DOI] [PubMed] [Google Scholar]
- 149.Luo X. G., Ding J. Q., Chen S. D. 2010. Microglia in the aging brain: relevance to neurodegeneration. Mol. Neurodegener. 5, 12. 10.1186/1750-1326-5-12 (doi:10.1186/1750-1326-5-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.von Bernhardi R., Tichauer J. E., Eugenin J. 2010. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 112, 1099–1114 10.1111/j.1471-4159.2009.06537.x (doi:10.1111/j.1471-4159.2009.06537.x) [DOI] [PubMed] [Google Scholar]
- 151.Ron-Harel N., Schwartz M. 2009. Immune senescence and brain aging: can rejuvenation of immunity reverse memory loss? Trends Neurosci. 32, 367–375 10.1016/j.tins.2009.03.003 (doi:10.1016/j.tins.2009.03.003) [DOI] [PubMed] [Google Scholar]
- 152.Hampson R. E., Simeral J. D., Kelly E. J., Deadwyler S. A. 2003. Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus 13, 543–556 10.1002/hipo.10081 (doi:10.1002/hipo.10081) [DOI] [PubMed] [Google Scholar]
- 153.Mechoulam R., Fride E., Di M.arzo V. 1998. Endocannabinoids. Eur. J. Pharmacol. 359, 1–18 10.1016/S0014-2999(98)00649-9 (doi:10.1016/S0014-2999(98)00649-9) [DOI] [PubMed] [Google Scholar]
- 154.Gallily R., Breuer A., Mechoulam R. 2000. 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice. Eur. J. Pharmacol. 406, R5–R7 10.1016/S0014-2999(00)00653-1 (doi:10.1016/S0014-2999(00)00653-1) [DOI] [PubMed] [Google Scholar]
- 155.Nomura D. K., et al. 2011. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334, 809–813 10.1126/science.1209200 (doi:10.1126/science.1209200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kessiova M., Alexandrova A., Georgieva A., Kirkova M., Todorov S. 2006. In vitro effects of CB1 receptor ligands on lipid peroxidation and antioxidant defense systems in the rat brain. Pharmacol. Rep. 58, 870–875 [PubMed] [Google Scholar]
- 157.Bobrov M. Y., Lizhin A. A., Andrianova E. L., Gretskaya N. M., Frumkina L. E., Khaspekov L. G., Bezuglov V. V. 2008. Antioxidant and neuroprotective properties of N-arachidonoyldopamine. Neurosci. Lett. 431, 6–11 10.1016/j.neulet.2007.11.010 (doi:10.1016/j.neulet.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 158.Marsicano G., Moosmann B., Hermann H., Lutz B., Behl C. 2002. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J. Neurochem. 80, 448–456 10.1046/j.0022-3042.2001.00716.x (doi:10.1046/j.0022-3042.2001.00716.x) [DOI] [PubMed] [Google Scholar]
- 159.Carracedo A., Geelen M. J., Diez M., Hanada K., Guzman M., Velasco G. 2004. Ceramide sensitizes astrocytes to oxidative stress: protective role of cannabinoids. Biochem. J. 380, 435–440 10.1042/BJ20031714 (doi:10.1042/BJ20031714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia C., Palomo-Garo C., Garcia-Arencibia M., Ramos J., Pertwee R., Fernandez-Ruiz J. 2011. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ-THCV in animal models of Parkinson's disease. Br. J. Pharmacol. 163, 1495–1506 10.1111/j.1476-5381.2011.01278.x (doi:10.1111/j.1476-5381.2011.01278.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Garcia-Arencibia M., Gonzalez S., de Lago E., Ramos J. A., Mechoulam R., Fernandez-Ruiz J. 2007. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 1134, 162–170 10.1016/j.brainres.2006.11.063 (doi:10.1016/j.brainres.2006.11.063) [DOI] [PubMed] [Google Scholar]
- 162.Kim H. J., Waataja J. J., Thayer S. A. 2008. Cannabinoids inhibit network-driven synapse loss between hippocampal neurons in culture. J. Pharmacol. Exp. Ther. 325, 850–858 10.1124/jpet.107.131607 (doi:10.1124/jpet.107.131607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goncharov I., Weiner L., Vogel Z. 2005. Δ9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience 134, 567–574 10.1016/j.neuroscience.2005.04.042 (doi:10.1016/j.neuroscience.2005.04.042) [DOI] [PubMed] [Google Scholar]
- 164.McKallip R. J., Jia W., Schlomer J., Warren J. W., Nagarkatti P. S., Nagarkatti M. 2006. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 70, 897–908 10.1124/mol.106.023937 (doi:10.1124/mol.106.023937) [DOI] [PubMed] [Google Scholar]
- 165.Giuliano M., Calvaruso G., Pellerito O., Portanova P., Carlisi D., Vento R., Tesoriere G. 2006. Anandamide-induced apoptosis in Chang liver cells involves ceramide and JNK/AP-1 pathway. Int. J. Mol. Med. 17, 811–819 [PubMed] [Google Scholar]
- 166.Siegmund S. V., Qian T., de Minicis S., Harvey-White J., Kunos G., Vinod K. Y., Hungund B., Schwabe R. F. 2007. The endocannabinoid 2-arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. FASEB J. 21, 2798–2806 10.1096/fj.06-7717com (doi:10.1096/fj.06-7717com) [DOI] [PubMed] [Google Scholar]
- 167.Brailoiu G. C., Oprea T. I., Zhao P., Abood M. E., Brailoiu E. 2011. Intracellular cannabinoid type 1 (CB1) receptors are activated by anandamide. J. Biol. Chem. 286, 29 166–29 174 10.1074/jbc.M110.217463 (doi:10.1074/jbc.M110.217463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rozenfeld R., Devi L. A. 2008. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 22, 2311–2322 10.1096/fj.07-102731 (doi:10.1096/fj.07-102731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Noonan J., Tanveer R., Klompas A., Gowran A., McKiernan J., Campbell V. A. 2010. Endocannabinoids prevent beta-amyloid-mediated lysosomal destabilization in cultured neurons. J. Biol. Chem. 285, 38 543–38 554 10.1074/jbc.M110.162040 (doi:10.1074/jbc.M110.162040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gowran A., Campbell V. A. 2008. A role for p53 in the regulation of lysosomal permeability by Δ9-tetrahydrocannabinol in rat cortical neurones: implications for neurodegeneration. J. Neurochem. 105, 1513–1524 10.1111/j.1471-4159.2008.05278.x (doi:10.1111/j.1471-4159.2008.05278.x) [DOI] [PubMed] [Google Scholar]
- 171.Salazar M., et al. 2009. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119, 1359–1372 10.1172/JCI37948 (doi:10.1172/JCI37948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vara D., Salazar M., Olea-Herrero N., Guzman M., Velasco G., Diaz-Laviada I. 2011. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 18, 1099–1111 10.1038/cdd.2011.32 (doi:10.1038/cdd.2011.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Donadelli M., et al. 2011. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2, e152. 10.1038/cddis.2011.36 (doi:10.1038/cddis.2011.36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Redlich S., Ribes S., Schutze S., Czesnik D., Nau R. 2012. Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 and Streptococcus pneumoniae R6 by microglial cells. J. Neuroimmunol. 244, 32–34 10.1016/j.jneuroim.2011.12.013 (doi:10.1016/j.jneuroim.2011.12.013) [DOI] [PubMed] [Google Scholar]
- 175.Athanasiou A., et al. 2007. Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 364, 131–137 10.1016/j.bbrc.2007.09.107 (doi:10.1016/j.bbrc.2007.09.107) [DOI] [PubMed] [Google Scholar]
- 176.Benard G., et al. 2012. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat. Neurosci. 15, 558–564 10.1038/nn.3053 (doi:10.1038/nn.3053) [DOI] [PubMed] [Google Scholar]
- 177.Chiu P., Karler R., Craven C., Olsen D. M., Turkanis S. A. 1975. The influence of Δ9-tetrahydrocannabinol, cannabinol and cannabidiol on tissue oxygen consumption. Res. Commun. Chem. Pathol. Pharmacol. 12, 267–286 [PubMed] [Google Scholar]
- 178.Zaccagnino P., Corcelli A., Baronio M., Lorusso M. 2011. Anandamide inhibits oxidative phosphorylation in isolated liver mitochondria. FEBS Lett. 585, 429–434 10.1016/j.febslet.2010.12.032 (doi:10.1016/j.febslet.2010.12.032) [DOI] [PubMed] [Google Scholar]
- 179.Costa B., Colleoni M. 2000. Changes in rat brain energetic metabolism after exposure to anandamide or Δ(9)-tetrahydrocannabinol. Eur. J. Pharmacol. 395, 1–7 10.1016/S0014-2999(00)00170-9 (doi:10.1016/S0014-2999(00)00170-9) [DOI] [PubMed] [Google Scholar]
- 180.Velez-Pardo C., Jimenez-Del-Rio M., Lores-Arnaiz S., Bustamante J. 2010. Protective effects of the synthetic cannabinoids CP55,940 and JWH-015 on rat brain mitochondria upon paraquat exposure. Neurochem. Res. 35, 1323–1332 10.1007/s11064-010-0188-1 (doi:10.1007/s11064-010-0188-1) [DOI] [PubMed] [Google Scholar]
- 181.Zaccagnino P., D'Oria S., Romano L. L., Di Venere A., Sardanelli A. M., Lorusso M. 2012. The endocannabinoid 2-arachidonoylglicerol decreases calcium induced cytochrome c release from liver mitochondria. J. Bioenerg. Biomembr. 44, 273–280 10.1007/s10863-012-9431-6 (doi:10.1007/s10863-012-9431-6) [DOI] [PubMed] [Google Scholar]
- 182.Germain N., Boichot E., Advenier C., Berdyshev E. V., Lagente V. 2002. Effect of the cannabinoid receptor ligand, WIN 55,212-2, on superoxide anion and TNF-α production by human mononuclear cells. Int. Immunopharmacol. 2, 537–543 10.1016/S1567-5769(01)00200-4 (doi:10.1016/S1567-5769(01)00200-4) [DOI] [PubMed] [Google Scholar]
- 183.Atwood B. K., Mackie K. 2010. CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160, 467–479 10.1111/j.1476-5381.2010.00729.x (doi:10.1111/j.1476-5381.2010.00729.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lou Z. Y., Chen C., He Q., Zhao C. B., Xiao B. G. 2011. Targeting CB(2) receptor as a neuroinflammatory modulator in experimental autoimmune encephalomyelitis. Mol. Immunol. 49, 453–461 10.1016/j.molimm.2011.09.016 (doi:10.1016/j.molimm.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 185.Stella N. 2009. Endocannabinoid signaling in microglial cells. Neuropharmacology 56(Suppl. 1), 244–253 10.1016/j.neuropharm.2008.07.037 (doi:10.1016/j.neuropharm.2008.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stella N. 2010. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030 10.1002/glia.20983 (doi:10.1002/glia.20983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Racz I., et al. 2008. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 28, 12 125–12 135 10.1523/JNEUROSCI.3400-08.2008 (doi:10.1523/JNEUROSCI.3400-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Palazuelos J., et al. 2009. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 132, 3152–3164 10.1093/brain/awp239 (doi:10.1093/brain/awp239) [DOI] [PubMed] [Google Scholar]
- 189.Walter L., Franklin A., Witting A., Moller T., Stella N. 2002. Astrocytes in culture produce anandamide and other acylethanolamides. J. Biol. Chem. 277, 20 869–20 876 10.1074/jbc.M110813200 (doi:10.1074/jbc.M110813200) [DOI] [PubMed] [Google Scholar]
- 190.Witting A., Walter L., Wacker J., Moller T., Stella N. 2004. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl Acad. Sci. USA 101, 3214–3219 10.1073/pnas.0306707101 (doi:10.1073/pnas.0306707101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muccioli G. G., Stella N. 2008. Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology 54, 16–22 10.1016/j.neuropharm.2007.05.015 (doi:10.1016/j.neuropharm.2007.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carrier E. J., Kearn C. S., Barkmeier A. J., Breese N. M., Yang W., Nithipatikom K., Pfister S. L., Campbell W. B., Hillard C. J. 2004. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol. Pharmacol. 65, 999–1007 10.1124/mol.65.4.999 (doi:10.1124/mol.65.4.999) [DOI] [PubMed] [Google Scholar]
- 193.Muccioli G. G., Xu C., Odah E., Cudaback E., Cisneros J. A., Lambert D. M., Lopez Rodriguez M. L., Bajjalieh S., Stella N. 2007. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J. Neurosci. 27, 2883–2889 10.1523/JNEUROSCI.4830-06.2007 (doi:10.1523/JNEUROSCI.4830-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Walter L., Dinh T., Stella N. 2004. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J. Neurosci. 24, 8068–8074 10.1523/JNEUROSCI.2419-04.2004 (doi:10.1523/JNEUROSCI.2419-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eljaschewitsch E., et al. 2006. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49, 67–79 10.1016/j.neuron.2005.11.027 (doi:10.1016/j.neuron.2005.11.027) [DOI] [PubMed] [Google Scholar]
- 196.Navarrete M., Araque A. 2008. Endocannabinoids mediate neuron-astrocyte communication. Neuron 57, 883–893 10.1016/j.neuron.2008.01.029 (doi:10.1016/j.neuron.2008.01.029) [DOI] [PubMed] [Google Scholar]
- 197.Albayram O., et al. 2011. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl Acad. Sci. USA 108, 11 256–11 261 10.1073/pnas.1016442108 (doi:10.1073/pnas.1016442108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mulder J., et al. 2011. Molecular reorganization of endocannabinoid signalling in Alzheimer's disease. Brain 134, 1041–1060 10.1093/brain/awr046 (doi:10.1093/brain/awr046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cosenza-Nashat M. A., Bauman A., Zhao M. L., Morgello S., Suh H. S., Lee S. C. 2011. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol. Appl. Neurobiol. 37, 464–483 10.1111/j.1365-2990.2011.01177.x (doi:10.1111/j.1365-2990.2011.01177.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tham C. S., Whitaker J., Luo L., Webb M. 2007. Inhibition of microglial fatty acid amide hydrolase modulates LPS stimulated release of inflammatory mediators. FEBS Lett. 581, 2899–2904 10.1016/j.febslet.2007.05.037 (doi:10.1016/j.febslet.2007.05.037) [DOI] [PubMed] [Google Scholar]
- 201.Ortega-Gutierrez S., Molina-Holgado E., Guaza C. 2005. Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia 52, 163–168 10.1002/glia.20229 (doi:10.1002/glia.20229) [DOI] [PubMed] [Google Scholar]
- 202.Sheng W. S., Hu S., Min X., Cabral G. A., Lokensgard J. R., Peterson P. K. 2005. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia 49, 211–219 10.1002/glia.20108 (doi:10.1002/glia.20108) [DOI] [PubMed] [Google Scholar]
- 203.Romero-Sandoval E. A., Horvath R., Landry R. P., DeLeo J. A. 2009. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol. Pain 5, 25. 10.1186/1744-8069-5-25 (doi:10.1186/1744-8069-5-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.van der Stelt M., Veldhuis W. B., Maccarrone M., Bar P. R., Nicolay K., Veldink G. A., Di Marzo V., Vliegenthart J. F. 2002. Acute neuronal injury, excitotoxicity, and the endocannabinoid system. Mol. Neurobiol. 26, 317–346 10.1385/MN:26:2-3:317 (doi:10.1385/MN:26:2-3:317) [DOI] [PubMed] [Google Scholar]
- 205.Tapia-Arancibia L., Aliaga E., Silhol M., Arancibia S. 2008. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res. Rev. 59, 201–220 10.1016/j.brainresrev.2008.07.007 (doi:10.1016/j.brainresrev.2008.07.007) [DOI] [PubMed] [Google Scholar]
- 206.Zeng Y., Tan M., Kohyama J., Sneddon M., Watson J. B., Sun Y. E., Xie C.-W. 2011. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J. Neurosci. 31, 17 800–17 810 10.1523/JNEUROSCI.3878-11.2011 (doi:10.1523/JNEUROSCI.3878-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 207.Grigorenko E., et al. 2002. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem. Phys. Lipids 121, 257–266 10.1016/S0009-3084(02)00161-5 (doi:10.1016/S0009-3084(02)00161-5) [DOI] [PubMed] [Google Scholar]
- 208.Butovsky E., Juknat A., Goncharov I., Elbaz J., Eilam R., Zangen A., Vogel Z. 2005. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Δ-tetrahydrocannabinol. J. Neurochem. 93, 802–811 10.1111/j.1471-4159.2005.03074.x (doi:10.1111/j.1471-4159.2005.03074.x) [DOI] [PubMed] [Google Scholar]
- 209.Marsicano G., et al. 2003. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 10.1126/science.1088208 (doi:10.1126/science.1088208) [DOI] [PubMed] [Google Scholar]
- 210.Derkinderen P., Valjent E., Toutant M., Corvol J. C., Enslen H., Ledent C., Trzaskos J., Caboche J., Girault J. A. 2003. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J. Neurosci. 23, 2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Berghuis P., et al. 2005. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl Acad. Sci. USA 102, 19 115–19 120 10.1073/pnas.0509494102 (doi:10.1073/pnas.0509494102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aso E., Ozaita A., Valdizan E. M., Ledent C., Pazos A., Maldonado R., Valverde O. 2008. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 105, 565–572 10.1111/j.1471-4159.2007.05149.x (doi:10.1111/j.1471-4159.2007.05149.x) [DOI] [PubMed] [Google Scholar]
- 213.D'Souza D. C., Pittman B., Perry E., Simen A. 2009. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology (Berl.) 202, 569–578 10.1007/s00213-008-1333-2 (doi:10.1007/s00213-008-1333-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Aguado T., et al. 2005. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 19, 1704–1706 [DOI] [PubMed] [Google Scholar]
- 215.Goncalves M. B., et al. 2008. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol. Cell Neurosci. 38, 526–536 10.1016/j.mcn.2008.05.001 (doi:10.1016/j.mcn.2008.05.001) [DOI] [PubMed] [Google Scholar]
- 216.Marchalant Y., Brothers H. M., Wenk G. L. 2009. Cannabinoid agonist WIN-55,212-2 partially restores neurogenesis in the aged rat brain. Mol. Psychiatry 14, 1068–1069 10.1038/mp.2009.62 (doi:10.1038/mp.2009.62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hill M. N., Kambo J. S., Sun J. C., Gorzalka B. B., Galea L. A. 2006. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur. J. Neurosci. 24, 1845–1849 10.1111/j.1460-9568.2006.05061.x (doi:10.1111/j.1460-9568.2006.05061.x) [DOI] [PubMed] [Google Scholar]
- 218.Oudin M. J., Hobbs C., Doherty P. 2011. DAGL-dependent endocannabinoid signalling: roles in axonal pathfinding, synaptic plasticity and adult neurogenesis. Eur. J. Neurosci. 34, 1634–1646 10.1111/j.1460-9568.2011.07831.x (doi:10.1111/j.1460-9568.2011.07831.x) [DOI] [PubMed] [Google Scholar]
- 219.Mulder J., et al. 2008. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl Acad. Sci. USA 105, 8760–8765 10.1073/pnas.0803545105 (doi:10.1073/pnas.0803545105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Aguado T., Romero E., Monory K., Palazuelos J., Sendtner M., Marsicano G., Lutz B., Guzman M., Galve-Roperh I. 2007. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J. Biol. Chem. 282, 23 892–23 898 10.1074/jbc.M700678200 (doi:10.1074/jbc.M700678200) [DOI] [PubMed] [Google Scholar]
- 221.Jin K., Xie L., Kim S. H., Parmentier-Batteur S., Sun Y., Mao X. O., Childs J., Greenberg D. A. 2004. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 66, 204–208 10.1124/mol.66.2.204 (doi:10.1124/mol.66.2.204) [DOI] [PubMed] [Google Scholar]
- 222.Berrendero F., Romero J., Garcia-Gil L., Suarez I., De la Cruz P., Ramos J. A., Fernandez-Ruiz J. J. 1998. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta 1407, 205–214 10.1016/S0925-4439(98)00042-8 (doi:10.1016/S0925-4439(98)00042-8) [DOI] [PubMed] [Google Scholar]
- 223.Romero J., Berrendero F., Garcia-Gil L., de la Cruz P., Ramos J. A., Fernandez-Ruiz J. J. 1998. Loss of cannabinoid receptor binding and messenger RNA levels and cannabinoid agonist-stimulated [35S]guanylyl-5′O-(thio)-triphosphate binding in the basal ganglia of aged rats. Neuroscience 84, 1075–1083 10.1016/S0306-4522(97)00552-6 (doi:10.1016/S0306-4522(97)00552-6) [DOI] [PubMed] [Google Scholar]
- 224.Canas P. M., Duarte J. M., Rodrigues R. J., Kofalvi A., Cunha R. A. 2009. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging 30, 1877–1884 10.1016/j.neurobiolaging.2008.01.003 (doi:10.1016/j.neurobiolaging.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 225.Liu P., Bilkey D. K., Darlington C. L., Smith P. F. 2003. Cannabinoid CB1 receptor protein expression in the rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: regional variations and age-related changes. Brain Res. 979, 235–239 10.1016/S0006-8993(03)02872-5 (doi:10.1016/S0006-8993(03)02872-5) [DOI] [PubMed] [Google Scholar]
- 226.Wang L., Liu J., Harvey-White J., Zimmer A., Kunos G. 2003. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl Acad. Sci. USA 100, 1393–1398 10.1073/pnas.0336351100 (doi:10.1073/pnas.0336351100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Van Laere K., Goffin K., Casteels C., Dupont P., Mortelmans L., de Hoon J., Bormans G. 2008. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage 39, 1533–1541 10.1016/j.neuroimage.2007.10.053 (doi:10.1016/j.neuroimage.2007.10.053) [DOI] [PubMed] [Google Scholar]
- 228.Maccarrone M., Attina M., Bari M., Cartoni A., Ledent C., Finazzi-Agro A. 2001. Anandamide degradation and N-acylethanolamines level in wild-type and CB1 cannabinoid receptor knockout mice of different ages. J. Neurochem. 78, 339–348 10.1046/j.1471-4159.2001.00413.x (doi:10.1046/j.1471-4159.2001.00413.x) [DOI] [PubMed] [Google Scholar]
- 229.Maccarrone M., Valverde O., Barbaccia M. L., Castane A., Maldonado R., Ledent C., Parmentier M., Finazzi-Agro A. 2002. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur. J. Neurosci. 15, 1178–1186 10.1046/j.1460-9568.2002.01957.x (doi:10.1046/j.1460-9568.2002.01957.x) [DOI] [PubMed] [Google Scholar]
- 230.Buczynski M. W., Parsons L. H. 2010. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br. J. Pharmacol. 160, 423–442 10.1111/j.1476-5381.2010.00787.x (doi:10.1111/j.1476-5381.2010.00787.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Reibaud M., Obinu M. C., Ledent C., Parmentier M., Bohme G. A., Imperato A. 1999. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur. J. Pharmacol. 379, R1–R2 10.1016/S0014-2999(99)00496-3 (doi:10.1016/S0014-2999(99)00496-3) [DOI] [PubMed] [Google Scholar]
- 232.Bilkei-Gorzo A., Racz I., Valverde O., Otto M., Michel K., Sastre M., Zimmer A. 2005. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc. Natl Acad. Sci. USA 102, 15 670–15 675 10.1073/pnas.0504640102 (doi:10.1073/pnas.0504640102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bohme G. A., Laville M., Ledent C., Parmentier M., Imperato A. 2000. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience 95, 5–7 10.1016/S0306-4522(99)00483-2 (doi:10.1016/S0306-4522(99)00483-2) [DOI] [PubMed] [Google Scholar]
- 234.Di Marzo V. 2011. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat. Neurosci. 14, 9–15 10.1038/nn.2720 (doi:10.1038/nn.2720) [DOI] [PubMed] [Google Scholar]
- 235.Batkai S., Rajesh M., Mukhopadhyay P., Hasko G., Liaudet L., Cravatt B. F., Csiszar A., Ungvari Z., Pacher P. 2007. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart Circ. Physiol. 293, H909–H918 10.1152/ajpheart.00373.2007 (doi:10.1152/ajpheart.00373.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Moore N. L., Greenleaf A. L., Acheson S. K., Wilson W. A., Swartzwelder H. S., Kuhn C. M. 2010. Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J. Pharmacol. Exp. Ther. 335, 294–301 10.1124/jpet.110.169359 (doi:10.1124/jpet.110.169359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Trezza V., Cuomo V., Vanderschuren L. J. 2008. Cannabis and the developing brain: insights from behavior. Eur. J. Pharmacol. 585, 441–452 10.1016/j.ejphar.2008.01.058 (doi:10.1016/j.ejphar.2008.01.058) [DOI] [PubMed] [Google Scholar]
- 238.Nahas G., Latour C. 1992. The human toxicity of marijuana. Med. J. Austr. 156, 495–497 [PubMed] [Google Scholar]
- 239.Paradisi A., Oddi S., Maccarrone M. 2006. The endocannabinoid system in ageing: a new target for drug development. Curr. Drug Targets 7, 1539–1552 [DOI] [PubMed] [Google Scholar]
- 240.Marchalant Y., Brothers H. M., Wenk G. L. 2008. Inflammation and aging: can endocannabinoids help? Biomed. Pharmacother. 62, 212–217 10.1016/j.biopha.2008.02.004 (doi:10.1016/j.biopha.2008.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bisogno T., Di Marzo V. 2010. Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 9, 564–573 [DOI] [PubMed] [Google Scholar]
- 242.Micale V., Mazzola C., Drago F. 2007. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 56, 382–392 10.1016/j.phrs.2007.09.008 (doi:10.1016/j.phrs.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 243.Fernandez-Ruiz J. 2009. The endocannabinoid system as a target for the treatment of motor dysfunction. Br. J. Pharmacol. 156, 1029–1040 10.1111/j.1476-5381.2008.00088.x (doi:10.1111/j.1476-5381.2008.00088.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fernandez-Ruiz J., Garcia C., Sagredo O., Gomez-Ruiz M., de Lago E. 2010. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin. Ther. Targets 14, 387–404 10.1517/14728221003709792 (doi:10.1517/14728221003709792) [DOI] [PubMed] [Google Scholar]
- 245.Karl T., Cheng D., Garner B., Arnold J. C. 2012. The therapeutic potential of the endocannabinoid system for Alzheimer's disease. Expert Opin. Ther. Targets 16, 407–420 10.1517/14728222.2012.671812 (doi:10.1517/14728222.2012.671812) [DOI] [PubMed] [Google Scholar]
- 246.Ramirez B. G., Blazquez C., Gomez del Pulgar T., Guzman M., de Ceballos M. L. 2005. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J. Neurosci. 25, 1904–1913 10.1523/JNEUROSCI.4540-04.2005 (doi:10.1523/JNEUROSCI.4540-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Martin-Moreno A. M., et al. 2012. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflammation 9, 8. 10.1186/1742-2094-9-8 (doi:10.1186/1742-2094-9-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Aso E., Palomer E., Juves S., Maldonado R., Munoz F. J., Ferrer I. 2012. CB1 agonist ACEA protects neurons and reduces the cognitive impairment of AbetaPP/PS1 mice. J. Alzheimer's Dis. 30, 439–459 [DOI] [PubMed] [Google Scholar]
- 249.Walther S., Mahlberg R., Eichmann U., Kunz D. 2006. Δ-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl.) 185, 524–528 10.1007/s00213-006-0343-1 (doi:10.1007/s00213-006-0343-1) [DOI] [PubMed] [Google Scholar]
- 250.Carroll C. B., Zeissler M. L., Hanemann C. O., Zajicek J. P. 2012. Δ(9)-THC exerts a direct neuroprotective effect in a human cell culture model of Parkinson's disease. Neuropathol. Appl. Neurobiol. 10.1111/j.1365-2990.2011.01248.x (doi:10.1111/j.1365-2990.2011.01248.x) [DOI] [PubMed] [Google Scholar]
- 251.Chung E. S., Bok E., Chung Y. C., Baik H. H., Jin B. K. 2012. Cannabinoids prevent lipopolysaccharide-induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res. 1451, 110–116 10.1016/j.brainres.2012.02.058 (doi:10.1016/j.brainres.2012.02.058) [DOI] [PubMed] [Google Scholar]
- 252.Sieradzan K. A., Fox S. H., Hill M., Dick J. P., Crossman A. R., Brotchie J. M. 2001. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology 57, 2108–2111 10.1212/WNL.57.11.2108 (doi:10.1212/WNL.57.11.2108) [DOI] [PubMed] [Google Scholar]
- 253.Carroll C. B., et al. 2004. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 63, 1245–1250 10.1212/01.WNL.0000140288.48796.8E (doi:10.1212/01.WNL.0000140288.48796.8E) [DOI] [PubMed] [Google Scholar]
- 254.Mesnage V., et al. 2004. Neurokinin, B., neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin. Neuropharmacol. 27, 108–110 10.1097/00002826-200405000-00003 (doi:10.1097/00002826-200405000-00003) [DOI] [PubMed] [Google Scholar]
- 255.Bilsland L. G., Greensmith L. 2008. The endocannabinoid system in amyotrophic lateral sclerosis. Curr. Pharm. Des. 14, 2306–2316 10.2174/138161208785740081 (doi:10.2174/138161208785740081) [DOI] [PubMed] [Google Scholar]
- 256.Pazos M. R., Sagredo O., Fernandez-Ruiz J. 2008. The endocannabinoid system in Huntington's disease. Curr. Pharm. Des. 14, 2317–2325 10.2174/138161208785740108 (doi:10.2174/138161208785740108) [DOI] [PubMed] [Google Scholar]
- 257.Sagredo O., Pazos M. R., Satta V., Ramos J. A., Pertwee R. G., Fernandez-Ruiz J. 2011. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J. Neurosci. Res. 89, 1509–1518 10.1002/jnr.22682 (doi:10.1002/jnr.22682) [DOI] [PubMed] [Google Scholar]
- 258.Blazquez C., et al. 2011. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington's disease. Brain 134, 119–136 10.1093/brain/awq278 (doi:10.1093/brain/awq278) [DOI] [PubMed] [Google Scholar]
- 259.Arevalo-Martin A., Vela J. M., Molina-Holgado E., Borrell J., Guaza C. 2003. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 23, 2511–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bilsland L. G., Dick J. R., Pryce G., Petrosino S., Di Marzo V., Baker D., Greensmith L. 2006. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J. 20, 1003–1005 10.1096/fj.05-4743fje (doi:10.1096/fj.05-4743fje) [DOI] [PubMed] [Google Scholar]
- 261.Ramil E., et al. 2010. The cannabinoid receptor 1 gene (CNR1) and multiple sclerosis: an association study in two case-control groups from Spain. Mult. Scler. 16, 139–146 10.1177/1352458509355071 (doi:10.1177/1352458509355071) [DOI] [PubMed] [Google Scholar]
- 262.Rog D. J., Nurmikko T. J., Friede T., Young C. A. 2005. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 65, 812–819 10.1212/01.wnl.0000176753.45410.8b (doi:10.1212/01.wnl.0000176753.45410.8b) [DOI] [PubMed] [Google Scholar]
- 263.Zajicek J., Fox P., Sanders H., Wright D., Vickery J., Nunn A., Thompson A. 2003. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet 362, 1517–1526 10.1016/S0140-6736(03)14738-1 (doi:10.1016/S0140-6736(03)14738-1) [DOI] [PubMed] [Google Scholar]
- 264.Collin C., Davies P., Mutiboko I. K., Ratcliffe S. 2007. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 14, 290–296 10.1111/j.1468-1331.2006.01639.x (doi:10.1111/j.1468-1331.2006.01639.x) [DOI] [PubMed] [Google Scholar]
- 265.Lakhan S. E., Rowland M. 2009. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 9, 59–10 10.1186/1471-2377-9-59 (doi:10.1186/1471-2377-9-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wade D. T., Makela P. M., House H., Bateman C., Robson P. 2006. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult. Scler. 12, 639–645 10.1177/1352458505070618 (doi:10.1177/1352458505070618) [DOI] [PubMed] [Google Scholar]