Abstract
Cannabidiol (CBD) is a major phytocannabinoid present in the Cannabis sativa plant. It lacks the psychotomimetic and other psychotropic effects that the main plant compound Δ9-tetrahydrocannabinol (THC) being able, on the contrary, to antagonize these effects. This property, together with its safety profile, was an initial stimulus for the investigation of CBD pharmacological properties. It is now clear that CBD has therapeutic potential over a wide range of non-psychiatric and psychiatric disorders such as anxiety, depression and psychosis. Although the pharmacological effects of CBD in different biological systems have been extensively investigated by in vitro studies, the mechanisms responsible for its therapeutic potential are still not clear. Here, we review recent in vivo studies indicating that these mechanisms are not unitary but rather depend on the behavioural response being measured. Acute anxiolytic and antidepressant-like effects seem to rely mainly on facilitation of 5-HT1A-mediated neurotransmission in key brain areas related to defensive responses, including the dorsal periaqueductal grey, bed nucleus of the stria terminalis and medial prefrontal cortex. Other effects, such as anti-compulsive, increased extinction and impaired reconsolidation of aversive memories, and facilitation of adult hippocampal neurogenesis could depend on potentiation of anandamide-mediated neurotransmission. Finally, activation of TRPV1 channels may help us to explain the antipsychotic effect and the bell-shaped dose-response curves commonly observed with CBD. Considering its safety profile and wide range of therapeutic potential, however, further studies are needed to investigate the involvement of other possible mechanisms (e.g. inhibition of adenosine uptake, inverse agonism at CB2 receptor, CB1 receptor antagonism, GPR55 antagonism, PPARγ receptors agonism, intracellular (Ca2+) increase, etc.), on CBD behavioural effects.
Keywords: cannabidiol, anxiety, depression, psychosis, serotonin, anandamide
1. History
Cannabidiol (CBD) is the main non-psychotropic phytocannabinoid present in the Cannabis sativa plant, constituting up to 40 per cent of its extract. The chemical characterization of the main cannabinoids present in this plant by Mechoulam's group in the 1960s [1] originated the first wave of scientific interest in this compound. With the discovery of the endocannabinoid (eCB) system in the early 1990s and the rise, in the words of Bill Devane [2], of the new dawn of cannabinoid pharmacology, there was a renewed interest in CBD, with the number of related published studies growing exponentially since then.
Recent comprehensive reviews suggest that this compound is one of the most promising candidates for a therapeutic tool in a wide range of disorders [3,4]. In the present paper, we will review the evidence that supports its use in psychiatric disorders and the proposal mechanisms that try to explain it.
2. Cannabidiol and anxiety
Early reports describing the effects of CBD in animal models of anxiety were inconsistent. Silveira Filho & Tufik [5] did not find any effect of CBD (100 mg kg−1) in rats tested in the classical Geller-Seifter conflict model of anxiety, whereas Zuardi & Karniol [6] described that a much lower CBD dose (10 mg kg−1) attenuated conditioned emotional responses. These apparent contradictory results were subsequently explained by Guimarães et al. [7]. Using an ethologically based model of anxiety, the elevated plus maze, they showed that CBD promotes anxiolytic-like effects with an inverted U-shaped dose-response curve, higher doses (more than 20 mg kg−1 in rats) being ineffective (table 1).
Table 1.
Preclinical and clinical studies investigating the anxiolytic properties of CBD. ↓, anxiolytic effect; ↑, anxiogenic effect; CFC, contextual fear conditioning; EPM, elevated plus maze; ETM, elevated T maze; GAD, generalized anxiety patients; OF, open field; VCT, Vogel conflict test; i.p., intraperitoneal injection; i.c.v., intracerebroventricular injection; DPAG, dorsal periaqueductal grey; BNST, bed nucleus of the stria terminalis; PL, prelimbic cortex; IL, infralimbic cortex; CeA, central amygdala.
| model | species | effective doses | CBD effect | references |
|---|---|---|---|---|
| studies with laboratory animals | ||||
| Geller-Seifter conflict model | rat | 100 mg kg−1 i.p. | no effect | [5] |
| conditioned emotional responses | rat | 10 mg kg−1 i.p. | ↓ | [6] |
| EPM | rat | 2.5–10 mg kg−1 i.p | ↓ | [7] |
| EPM | mouse | 1–10 mg kg−1 i.p. | ↓ | [8] |
| VCT | rat | 10 mg kg−1 | ↓ | [9] |
| CFC | rat | 10 mg kg−1 i.p. | ↓; decreased autonomic responses | [10] |
| predator exposure (Cat)+ EPM | rat | 5 mg kg−1 | ↓ | [11] |
| restraint stress + EPM | rat | 30 nmol intra-cisterna magna | decreased autonomic and delayed anxiogenic effect | [12] |
| predator exposure (snake) | mouse | 3–30 mg kg−1 i.p. | panicolytic | [13] |
| marble burying | mouse | 30 mg kg−1 i.p | decreased compulsive behaviour | [14] |
| CFC/fear memory extinction | rat | 6.35 nmol i.c.v. | ↓; facilitated extinction | [15] |
| ETM/DPAG electric stimulation | rat | 30–60 nmol intra-DPAG | ↓, panicolytic | [16] |
| restraint stress + EPM | rat | 10 mg kg−1 i.p. | decreased autonomic and delayed anxiogenic effect | [17] |
| EPM/VCT | rat | 15–30 nmol intra-DPAG | ↓ | [18] |
| EPM/VCT | rat | 30–60 nmol intra-BNST | ↓ | [19] |
| CFC | rat | 30–60 nmol intra-BNST | ↓, decreased autonomic responses | [20] |
| CFC | rat | 30 nmol intra-PL | ↓ | [21] |
| CFC | rat | 30 nmol intra-IL | ↑ | [21] |
| CFC | rat | 10 mg kg−1 i.p./daily/14 days | ↑ | [22] |
| CFC/fear reconsolidation | rat | 10 mg kg−1 i.p. | ↓; memory reconsolidation impairment | [23] |
| model/measures | subjects | dose (mg, p.o.) | CBD effect | references |
| clinical studies | ||||
| simulated public-speaking | healthy volunteers | 400 mg | ↓ | [24] |
| neuroimaging study | healthy volunteers | 400 mg | ↓ | [25] |
| fearful facial stimuli | healthy volunteers | 600 mg | ↓ | [26] |
| fearful facial stimuli | healthy volunteers | 600 mg | ↓ | [27] |
| anxiety symptoms (visual analogue mood scale) | GAD | 400 mg | ↓ | [28] |
| simulated public speaking | social phobics | 400 mg | ↓ | [29] |
The anti-anxiety properties of CBD in rats were later confirmed in different species (mice) and animal models, including the Vogel conflict test and contextual fear conditioning [8–10]. More recently, CBD was shown to decrease defensive behaviours evoked by predator exposure, a proposed model of panic attacks and posttraumatic stress disorder (PTSD) [11,13]. CBD also reduces marble burying behaviour in mice, suggesting that this compound could be effective in obsessive-compulsive disorder (OCD) [14]. Moreover, CBD can interfere in learning and/or memory of aversive events, processes that have been associated with PTSD pathophysiology [30]. Intracerebroventricular administration of CBD facilitates extinction in a contextual aversive conditioning model [15]. In this same paradigm, it can also impair reconsolidation, resulting in the attenuation of the aversive memory trace. In this study, the impairment of contextual fear memory did not show reinstatement and was long-lasting (22 days) [23]. Contrasting with these results, Elbatsh et al. [22] have recently reported that repeated (14 days) administration of CBD increases freezing in a contextual fear conditioning test. The reasons for this difference is unknown, but may involve the distinct conditioning protocols and drug administration regime (chronic versus acute) used compared with other studies that investigated the effects of CBD in this model [15,21,23]. Moreover, in this study, it is also possible that CBD could have interfered in learning/memory mechanisms, because the animals were conditioned under the drug effect [22].
(a). Clinical anxiolytic effects of cannabidiol
In agreement with the results obtained in animal models, clinical studies confirmed that CBD has anxiolytic properties (table 1). Following the initial report that it blocks the anxiogenic effects of high doses of the main psychoactive compound present in the Cannabis sativa plant, Δ9-tetrahydrocannabinol (THC) [31], it was demonstrated that CBD can also reduce anxiety in healthy volunteers during a neuroimaging study or after a simulated public-speaking procedure [24,25]. More recently, using the latter procedure, Bergamaschi et al. [29] showed that CBD (600 mg p.o.) decreases anxiety in treatment-naive social phobic patients.
(b). Brain sites of cannabidiol anxiolytic effects
Neuroimaging studies show that CBD changes the activity of brain regions related to the control of emotional process [25,27]. It attenuates blood oxygenation in the amygdala and the anterior and posterior cingulate cortex in subjects exposed to fearful faces [27], impairs the connectivity between the pre-frontal and subcortical regions [27] and decreases the activation of left-amygdala–hippocampal complex and left posterior cingulate gyrus [25].
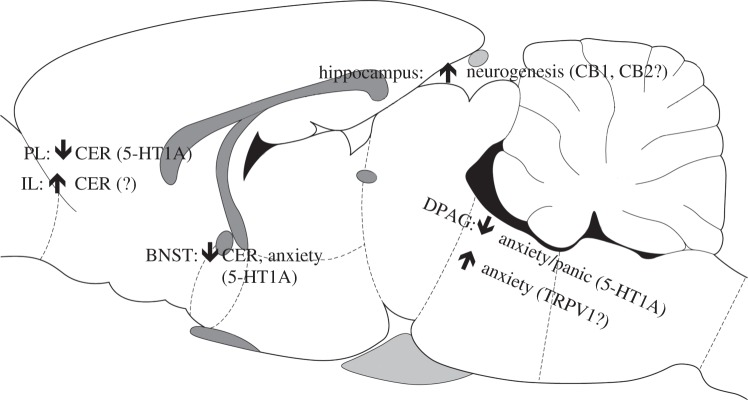
These clinical findings were complemented by studies in rodents, using direct administration into brain sites related to anxiety- or panic-like responses (figure 1). Microinjection of CBD into the dorsal portions of the periaqueductal grey (DPAG) promoted anxiolytic-like effects in the elevated plus maze, elevated T maze and Vogel conflict tests. It also decreased escape latency in a model of panic attacks, electrical stimulation of the DPAG [16,18]. Anxiolytic effects were also found after CBD injection into the bed nucleus of the stria terminalis (BNST) in rats tested in the elevated plus maze, Vogel conflict test and contextual fear conditioning [19,20]. This latter effect corroborates results showing that the effects of CBD in a contextual fear conditioning model is associated with decreased neuronal activation (measure by cFos expression) in this area [21]. This same treatment attenuated the activation of the pre- and infra-limbic cortical regions. In these two brain areas, however, CBD produced opposite effects, decreasing and facilitating, respectively, conditioned emotional responses [10]. Recently, Hsiao et al. [26] showed anxiolytic effects of CBD injection into the central nucleus of the amygdala. Other possible brain sites of CBD anxiolytic effect have not yet been investigated (e.g. the hippocampus).
Figure 1.
Possible brain sites and mechanisms of CBD effects on anxiety. BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CER, conditioned emotional response; DPAG, dorsal periaqueductal grey; IL, infralimbic prefrontal cortex; PL, prelimbic prefrontal cortex.
(c). Mechanisms of the anxiolytic effects of cannabidiol
CBD can produce multiple pharmacological actions over a wide range of drug concentrations (table 2) [3,4]. CBD is proposed to activate or modify the function of several receptors in the central nervous system (CNS), including CB1, CB2, GPR55, TRPV1 and 5-HT1A receptors (table 2) [32,33,35,42]. Moreover, it could inhibit the anandamide hydrolysing enzyme (fatty acid amide hydrolase, FAAH) and the adenosine transporter [33,43,50], indirectly increasing the levels of these neurotransmitters.
Table 2.
Possible mechanisms of CBD behaviour effects. Evidence from in vitro studies. CHO, Chinese hamster ovary cells.
| biological system | mechanism | biological system | concentration range | references |
|---|---|---|---|---|
| endocannabinoid/endovanilloid related mechanisms | CB1 receptor antagonist | mouse brain membranes | 4.9 µM (Ki)a | [32] |
| CB2 receptor inverse agonist | CHO transfected cells | 4.2 µM (Ki)b | [32] | |
| TRPV1 agonist | HEK-293 transfected cells | 3.2 µM (EC50) | [33] | |
| FAAH/anandamide transporter inhibition | HEK-293 transfected cells, rat brain membranes | 7.5–8.6/22 µM (IC50) | [33,34] | |
| serotonin-related mechanisms | 5-HT1A receptor agonist | CHO transfected cells | 16 µM (increases receptor response by 67%) | [35] |
| 5-HT2A receptor agonist | CHO transfected cells | 32 µM (IC50) | [35] | |
| 5-HT3 receptor antagonist | Xenopus laevis oocytes | 10–30 µM | [36] | |
| suppression of mitogen-induced IDO activity (decreasing tryptophan metabolism) | human blood cells | 8.9 µM (IC50) | [37] | |
| others | intracellular (Ca2+) increase | hippocampal cell cultures/hippocampal preparations | approximately 1 µM (effective concentration) | [38,39] |
| allosteric modulation of µ and δ opioid receptors | cerebral cortex preparations | 100 µM | [40] | |
| PPArγ receptors agonist | aorta preparations | 5 µM (IC50) | [41] | |
| GPR55 antagonist | cell membranes of transfected cells | 445 nM (IC50) | [42] | |
| blockade of adenosine uptake/indirect A2 agonist | microglia and macrophages cell cultures | less than 250 nM (Ki)/500 nM (effective concentration) | [43,44] | |
| TRPV2 agonist | HEK-293 transfected cells/rat dorsal root ganglia (DRG) sensory neurons | 3.7 µM (EC50) | [45] | |
| TRPM8 antagonist | HEK-293 transfected cells/rat dorsal root ganglia (DRG) sensory neurons | 80–140 nM(IC50) | [46] | |
| TRPA1 agonist | HEK-293 transfected cells/rat dorsal root ganglia (DRG) sensory neurons | 96 nM(EC50) | [46] | |
| P38 MAPKinase inhibition | PC12 cells | 10−6–10−4 M (effective concentrations) | [47] | |
| NF-κB activation | PC12 cells | 10−6–10−4 M (effective concentrations) | [47] | |
| inhibition of mitochondrial superoxide production | vascular endotelial cells | 4 µM (effective concentration) | [48] | |
| inhibition of inducible nitric oxide synthase (iNOS) expression | kidney | 10 mg kg−1 | [49] |
aCBD was able to antagonize the effects of the CB1 agonist CP55940-induced stimulation of [35S]GTPgS binding to mouse brain membranes at a much lower concentration (KB = 79 nM) than the Ki for displacement of the CB1 ligand.
bCBD acts as an inverse agonist with a lower concentration (KB = 65 nM) than the Ki for displacement of the CB2 ligand.
Some of CBD effects involve intracellular pathways that play fundamental roles in neuronal physiology. For example, in hippocampal neurons, CBD increases intracellular calcium concentrations via mitochondrial uptake and release and/or activation of type-L voltage-gated calcium channels [38,39]. CBD has also a potent action in inhibiting oxidative and nitrosative stress, a mechanism that has been related to its neuroprotective effects with implications for the treatment of Alzheimer's, Huntington's and Parkinson's diseases. It decreases the neuronal damage promoted by β-amyloid protein deposit [47,51] and attenuates the depletion of tyrosine hydroxylase, dopamine and GABA levels by modulating the expression of the inducible nitric oxide synthase and reducing the production of reactive oxygen species (ROS)-generating NADPH oxidases [47,52–54]. Moreover, CBD pretreatment attenuated high-glucose-induced mitochondrial superoxide generation and NF-κB activation, along with the expression of adhesion molecules ICAM-1 and VCAM-1 [48]. Together, these results suggest that CBD can exert CB1- and CB2-independent neuroprotective/antioxidant/anti-inflammatory effects [48].
The large majority of these possible mechanisms have been unveiled by in vitro studies. Their association to the behaviour effects of CBD is still not clear, a topic that is further complicated by the common bell-shaped dose-response curves produced by this compound in distinct biological systems [4].
In the last decade, however, several in vivo studies have helped us to understand the mechanisms of CBD central effects.
(d). In vivo mechanisms of cannabidiol anxiolytic effects: 5-HT1A receptors
Russo et al. [35] were the first to suggest that CBD could act as a 5HT1A receptor agonist. They observed that, at µM range, this drug displaces 8-OH-DPAT, a 5-HT1A receptor agonist, from cloned human 5-HT1A receptors expressed in cultured cells obtained from Chinese hamster ovary. In vivo experiments gave further support to the involvement of 5-HT1A receptors in the effects of CBD [18–20,55]. For instance, the neuroprotective effects of CBD in hepatic encephalopathy or cerebral infarction are mediated by these receptors [55,56]. Regarding the behavioural studies, the effects of CBD in a PTSD model (predator exposure) were prevented by WAY100635, a 5HT1A receptor antagonist [11]. This same antagonist prevented the anxiolytic- and panicolytic-like effects of CBD after injections into the DPAG [16,18], bed nucleus of the stria terminalis [19,20] and prefrontal cortex (M. V. Fogaça & F. S. Guimarães 2012, unpublished data; figure 1). In humans, although no study so far has investigated the involvement of 5-HT1A mechanisms in CBD effects, the anxiolytic profile of this drug in the public speaking model was remarkably similar to the positive control ipsapirone, a 5HT1A receptor partial agonist [24].
Other CBD effects also involve 5-HT1A receptors. It decreases nausea and vomiting probably by an indirect agonism at these receptors. Although the mechanism of this indirect action is unclear, it may involve interactions with allosteric sites or changes in different systems that would result in a facilitation of 5-HT1A-mediated responses [57]. Adding to the evidence that the interaction of CBD with 5-HT1A receptors could be complex, it was recently shown that this compound antagonizes food intake induced by 8-OH-DPAT [58]. Therefore, additional research is clearly needed to clarify how CBD facilitates 5-HT1A-mediated neurotransmission.
(e). In vivo mechanisms of cannabidiol effects: the endocannabinoid system
CBD could facilitate eCB-mediated neurotransmission by blocking the metabolism and uptake of anandamide [33]. However, AM251, a CB1 receptor antagonist, failed to prevent the anxiolytic effects of CBD injected into the DPAG observed in the elevated plus maze at the same dose that antagonized the anxiolytic effects of anandamide [18,59].
On the other hand, CB1, but not 5-HT1A, receptor antagonism was able to prevent CBD effects on both extinction and reconsolidation, indicating that its interference on aversive memories involves eCB-mediated mechanisms [15,23]. These results agree with the well-described facilitation of extinction by endogenous cannabinoids [60], suggesting that CBD interferes with aversive memories by facilitating the effects of eCBs [45].
Finally, AM251 blocked CBD effects in the marble burying model [14], whereas 5-HT1A-receptor antagonism was ineffective. This result corroborates the proposal that anxiety and OCD models engage distinct brain mechanisms, with the marble burying behaviour being related to repetitive behaviours instead of anxiety [61].
How facilitation of eCB-mediated neurotransmission decreases repetitive behaviour is unknown, but may involve attenuation of glutamate-mediated neurotransmission. eCBs can reduce the release of several neurotransmitters, including glutamate [62], a major neurotransmitter of the cortico-striato-thalamo-cortical circuitry that has been implicated in the pathophysiology of OCD [63]. Anti-glutamatergic drugs such as riluzole and memantine decrease marble burying behaviour [64,65] and are proposed to be clinically useful for OCD treatment [66,67].
An indirect anti-glutamatergic action via increased eCB neurotransmission may also be involved in other central effects of CBD such as anticonvulsant [3], an effect that could also be related to indirect CB1-mediated inhibition of glutamate release. Corroborating this proposal, anticonvulsant effects of other inhibitors of anandamide metabolism/uptake have recently been described [3]. Moreover, epileptic patients present a significant reduction in the fraction of CB1-positive glutamatergic, but not GABAergic, axon terminals, probably resulting in increased neural excitability [68].
(f). In vivo mechanisms of cannabidiol effects: adult hippocampal neurogenesis
Impairment in adult hippocampal neurogenesis has been associated with the pathogenesis of anxiety disorders and depression [69] and at least some of the behavioural effects of prototype antidepressant drugs depend on facilitation of this process [70]. CBD can also increase adult hippocampal neurogenesis, as first demonstrated by Wolf et al. [71]. They also showed that the proneurogenic effect of CBD was absent in CB1-knockout mice [71]. Because CBD is not a CB1-receptor agonist, this result suggested that CBD effect was mediated by an indirect activation of these receptors, possibly by inhibition of anandamide metabolism/uptake [33]. Corroborating this latter possibility, recent results from our group showed that CBD increases proliferation of hippocampal progenitor cells in culture, an effect mimicked by CB1 or CB2 receptor agonists and prevented by antagonists of these receptors [72]. Moreover, CBD effects were also inhibited by overexpression of the FAAH, reinforcing the proposal of anandamide involvement. These results agree with those previously reported by Jiang et al. [73] showing that a synthetic CB1 agonist is able to promote embryonic and adult hippocampus neurogenesis, an effect associated with the anxiolytic and antidepressant properties of the drug. Similar to prototype antidepressants, the anxiolytic effect of repeated administered CBD (30 mg/daily for 14 days) in mice submitted to a chronic unpredictable stress model disappeared when hippocampal neurogenesis was inhibited [72], suggesting a causal link between its proneurogenic and anxiolytic effect after repeated administration (figure 1).
Other mechanisms could also be involved in CBD effects on adult hippocampal neurogenesis—for example, activation of peroxisome proliferator-activated receptors. This particular mechanism seems to be important during neuroinflammation and neurodegenerative process related to β-amyloid protein deposits in CNS [51]. Although the pro-neurogenic effect of CBD has not yet been studied in rats, it could help us to explain the recent report that repeated CBD treatment enhances contextual fear conditioning [22]. Immature newborn neurons are selectively activated by this task [74], and neurogenesis suppression impairs contextual fear memory [75]. Considering the important role proposed for hippocampal neurogenesis in several brain functions [76,77], the effects and mechanisms of CBD on this process is another important research venue to be pursued.
(g). Cannabidiol and the vanilloid system
CBD can activate transient receptor potential (TRP) channels [33]. These channels comprise a family of over 50 members and are present in different species, including yeast, worms, insects, fish and mammals [78]. The vanilloid receptor 1 or TRPV1 is one of the first identified members of the family, being a non-selective cation channel with a preference for calcium. It is activated by noxious stimuli, heat, protons (pH < 5.9) and various, mostly noxious, natural products such as capsaicin [79]. TRPV1 receptors are present in the brain, where anandamide has been proposed to act as an endogenous agonist or an endovanilloid [80]. These receptors can facilitate the release of glutamate [81], a neurotransmitter that induces defensive responses in brain areas such as the DPAG [82]. On the basis of these pieces of evidence, we hypothesized that TRPV1 activation could be at least partially responsible for the inverted U-shaped dose-response curves commonly observed with CBD. Accordingly, using intra-DPAG injections, we showed that local pretreatment with an ineffective dose of the TRPV1 antagonist capsazepine turned a higher, ineffective dose (60 nmol) of CBD into an anxiolytic one [83]. TRPV1 receptors are also involved in the bell-shaped dose responses curves of anandamide analogues [84,85].
In addition to TRPV1, CBD could also interfere with other members of the TRP family, activating TRPV2 and ankyrin type 1 (TRPA1) channels and antagonizing melastatin type 8 (TRPM8) channels [45,46]. The role played by these mechanisms on CBD behavioural effects, however, is unknown at the moment.
3. Cannabidiol and psychosis
Initial studies with laboratory animals suggested that CBD prevents some of the effects produced by THC [86]. Similar antagonism was also found in humans, where CBD attenuated the impairment of time production tasks and the euphoria induced by THC in healthy volunteers [87,88]. Confirming and extending these results, Zuardi et al. [31] demonstrated that CBD inhibits THC-induced anxiety and psychotic-like symptoms such as disconnected thoughts, perceptual disturbance, depersonalization and resistance to communication. In the same year, it was observed that patients admitted to a psychiatric hospital in South Africa after the use of a variety of Cannabis virtually devoid of CBD showed a much higher frequency of acute psychotic episodes than in other countries [89], suggesting that the presence of CBD in Cannabis samples protects users against the occurrence of THC-induced acute psychotic episodes. Because experimental evidence indicates that the antagonistic effect of CBD did not result from a pharmacokinetic interaction between the two cannabinoids [90], these initial observations led to the hypothesis that CBD could possess antipsychotic properties.
An initial study in rats investigated whether this compound could attenuate stereotypies induced by the dopaminergic agonist apomorphine. CBD effects were compared with those of haloperidol [91]. Both drugs reduced apomorphine-induced stereotyped behaviour in a dose-related manner. Even though they increased plasma levels of prolactin, CBD had much lower potency, with significant increases only seen after the doses of 120 and 240 mg kg−1. Moreover, contrary to haloperidol, CBD did not induce catalepsy, even at doses as high as 480 mg kg−1. These results suggest that CBD exhibits a profile similar to atypical antipsychotic drugs. In another study, CBD was compared with haloperidol and clozapine, an atypical antipsychotic drug. The drug inhibited the hyperlocomotion induced by amphetamine and ketamine, an NMDA receptor antagonist, in mice [92]. As expected, while both haloperidol and clozapine inhibited hyperlocomotion, only haloperidol induced catalepsy. These results extend CBD antipsychotic-like effects to a glutamate-based model. In agreement with these results, CBD, similar to clozapine, reversed the disruption of prepulse inhibition (PPI) in mice and the hyperactivity and reduction of social interaction in rats caused by MK-801, another NMDA receptor antagonist [93,94]. Typical antipsychotics, on the other hand, are usually unable to restore the deficits in PPI and social interaction induced by NMDA receptor antagonists [95,96].
Extending findings from studies using single drug administration, chronic treatment with CBD attenuated amphetamine-induced hyperlocomotion [97]. Preliminary results from our group indicate that this treatment regime is also able to decrease the impairments in PPI and object recognition induced by repeated administration of MK-801 (F. V. Gomes, E. A. Del Bel & F. S. Guimarães, unpublished data). Despite these findings, there are also negative results regarding the possible antipsychotic effects of CBD. Chronic treatment with this drug failed to change behavioural changes such as locomotor hyperactivity and PPI deficits observed in transmembrane domain neuregulin 1 mutant (Nrg1) mice, a proposed model for a schizophrenia susceptibility gene [98]. A summary of the studies investigating the antipsychotic-like effects of CBD in animal models can be seen in table 3.
Table 3.
Preclinical and clinical studies investigating the antipsychotic properties of CBD. ↓, antipsychotic-like effects; BPRS, brief psychiatric rating scale; CADSS, clinician administered dissociative states scale; PANSS, positive and negative syndrome scale; PPQ, Parkinson psychosis questionnaire.
| model | species | effective doses | CBD effects | references |
|---|---|---|---|---|
| studies with laboratory animals | ||||
| apomorphine-induced stereotyped behaviour | rat | 60 mg kg−1 | ↓ | [91] |
| d-amphetamine- and ketamine-induced hyperlocomotion | mouse | 15–60 mg kg−1 | ↓ | [92] |
| MK-801-induced disruption of PPI | mouse | 5 mg kg−1 | ↓ | [93] |
| d-amphetamine-induced hyperlocomotion | mouse | 50 mg kg−1 (chronic – 21 days) | ↓ | [97] |
| MK-801-induced social withdrawal and isruption of PPI | rat | 3–30 mg kg−1 | ↓ | [99] |
| MK-801-induced hyperlocomotion and deficits in social interaction and | rat | 3 mg kg−1 | ↓ | [94] |
| locomotor hyperactivity and PPI | Nrg 1 mutant mouse | 1, 50 and 100 mg kg−1 | no effects | [98] |
| model/measures | subjects (n) | doses | CBD effects | references |
| clinical studies | ||||
| THC-induced impairment of time production task | healthy male volunteers (40) | 15–60 mg (acute) | ↓ | [87] |
| THC-induced euphoria | healthy male volunteers (15) | 0.15 mg kg−1 (inhalation; acute) | ↓ | [88] |
| THC-induced psychotic symptoms | healthy male volunteers (eight) | 1 mg kg−1 (acute) | ↓ | [31] |
| nabilone-induced impairment of perception of binocular depth inversion | healthy male volunteers (nine) | 200 mg (acute) | ↓ | [100] |
| THC-induced psychotic symptoms (PANSS) | healthy male and female volunteers (six) | 5 mg (iv, acute) | ↓ | [101] |
| ketamine-induced psychotic symptoms (BPRS and CADSS) | healthy male volunteers (10) | 600 mg (acute) | ↓ (trend) | [102] |
| psychotic symptoms (BPRS) | schizophrenic female patient (one) | increasing oral doses of CBD, reaching 1500 mg d−1 (four weeks) | ↓ | [103] |
| psychotic symptoms (BPRS) | male patients with treatment-resistant schizophrenia (three) | increased from 40 up to 1280 mg d−1 (30 days) | one patient showed mild improvement | [104] |
| l-dopa-induced psychosis (BPRS and PPQ) | Parkinson's disease patients (six) | increased from 150 up to 600 mg d−1 depending on the clinical response (four weeks) | ↓ | [105] |
| psychotic symptoms (BPRS and PANSS) | acute paranoid schizophrenia patients (42) | 600 mg d−1 (four weeks) | ↓ | [34] |
| Stroop Colour Word Test | schizophrenic patients (28) | 300 and 600 mg (acute) | no effect | [106] |
(a). Cannabidiol and psychosis: clinical studies
The antipsychotic-effects of CBD have also been demonstrated in humans (table 3). In healthy volunteers, Leweke et al. [100] observed that the decrease of the perception of illusory image induced by nabilone, a synthetic cannabinoid drug with THC-like properties, was reduced by CBD. Another model used to evaluate antipsychotic-like activity of drugs in humans is the administration of sub-anaesthetic doses of ketamine. This drug induces psychotic symptoms that mimic both positive and negative symptoms of schizophrenia. An initial investigation in healthy subjects showed that CBD induced a non-significant trend to reduce ketamine-induced dissociative effects, but, at the same time, augmented the activating effects of ketamine [102]. Because only a single dose of CBD was used, additional studies are needed to characterize the effects of CBD in this model [102].
The therapeutic use of CBD in psychotic patients was tested for the first time in 1995. In an open, case-report study, a 19-year-old black schizophrenic female patient, who presented serious side effects after treatment with conventional antipsychotics, received increasing oral doses of CBD (up to 1500 mg d−1) for four weeks [103]. A significant improvement with no side effects was observed in all items of the standard brief psychiatric rating scale (BPRS) during CBD treatment, with an efficacy similar to that of haloperidol. Symptom worsening was observed when the administration was interrupted. In another case study, CBD was administered to three 22- or 23-year-old male schizophrenic patients who had not responded to typical antipsychotic drugs for 30 days [104]. The dose of CBD was increased from 40 up to 1280 mg d−1. One patient showed mild improvement, but only slight or no change was observed in the other two, suggesting that CBD may not be effective for the treatment of resistant schizophrenia. Moreover, CBD had no beneficial effects on the performance of schizophrenic patients in the Stroop colour word test, which can be used to assess attentional processes frequently impaired in schizophrenia [106]. It is still unknown whether chronic administration of CBD could improve the cognitive deficits in the disorder. No significant side effects were observed during CBD treatment in these clinical studies, suggesting that CBD is safe and well tolerated in schizophrenic patients.
In an open-label study evaluating CBD effects on psychotic symptoms associated with L-dopa use in Parkinson's patients [105], the drug decreased scores of a questionnaire developed to assess psychotic symptoms in Parkinson's disease (Parkinson psychosis questionnaire), improved total BPRS scores as well as scores specifically related to positive and negative symptoms. Confirming the lack of motor effects observed in animal studies, CBD did not affect motor function. On the contrary, it decreased the total scores of the unified Parkinson's disease rating scale, suggesting an improvement of this function.
Overall, therefore, even if there are negative results, most clinical studies with normal subjects or schizophrenic patients suggest that CBD has antipsychotic properties. Corroborating this possibility, a four-week double-blind controlled clinical trial in 42 acute schizophrenic and schizophreniform psychosis patients comparing the effects of CBD with those of amisulpride, an atypical antipsychotic, showed that both treatments were equally effective in reducing acute psychotic symptoms after two and four weeks of treatment [34]. Moreover, compared with amisulpride, CBD caused a lower incidence of extrapyramidal symptoms and increases in prolactin and weight gain.
The presence of antipsychotic properties in CBD is also supported by convergent evidence linking the habitual use of Cannabis to the risk of developing schizophrenia or schizophrenia-like psychosis, especially in vulnerable subjects [107]. This effect has been attributed to THC. In agreement with the initial reports showing antagonism of THC-induced psychotomimetic effects [31,87,88], the presence of CBD in Cannabis strains has been shown to be protective against the occurrence of psychotic-like reactions and cognitive impairment [108–110]. In this context, Di Forti et al. [111] found that the use of Cannabis containing high THC- and low CBD concentration was associated with a higher risk of a first psychotic episode. Furthermore, the presence of CBD protects from Cannabis-associated decrease in hippocampal volume [112]. This neuroprotective effect of CBD has also been reported in the human basal ganglia, where there was a strong positive correlation between N-acetylaspartate/total creatine ratio and the amount of CBD (as measured by its presence in hair samples) in the putamen/globus pallidum of recreational Cannabis users. This finding could reflect a CBD-induced enhancement of neuronal and axonal integrity in these regions [113]. More recently, Bhattacharyya et al. [114], investigating the effects of THC and CBD during attentional salience processing, have also showed that these two cannabinoids produce opposite effects on prefrontal, striatal and hippocampal functions.
(b). Brain sites and mechanisms of cannabidiol antipsychotic effects
Few studies in laboratory animals have investigated the possible brain sites and mechanisms of CBD antipsychotic effects. Consistent with the behavioural data described earlier, both CBD and clozapine, but not haloperidol, increased neuronal activation (measured by cFos-protein expression) in the prefrontal cortex. Probably reflecting its motor side effects, only haloperidol increased cFos in the dorsal striatum. CBD, and, in addition, increased cFos in the nucleus accumbens [115], an effect shared by typical and atypical antipsychotic drugs [116]. Intracerebroventricular administration of CBD (10 μg) also enhanced cFos expression in waking-related brain areas such as hypothalamus and dorsal raphe nucleus [117], but the relation between this finding and its antipsychotic properties is unclear.
A number of neuroimaging studies in healthy volunteers have compared the effects of CBD and high doses of THC. Consistent with the behavioural findings in humans and rodents, these drugs caused opposite effects on brain activity in the striatum, anterior cingulate, prefrontal cortex, parahippocampal gyrus, amygdala, right posterior superior temporal gyrus, middle occipital gyrus and cerebellum [27,101,114,118–120]. Behavioural measurements in these studies indicated that some of these changes (decreased activation of ventral and dorsal striatum, anterior cingulate gyrus, right temporal lobe) are associated with the psychotic-like effects of THC, suggesting that these regions could be possible brain suites of CBD action [121]. No study using direct injections into key brain regions associated with the pathophysiology of schizophrenia, such as the prefrontal cortex and nucleus accumbens, has been performed so far to investigate CBD antipsychotic properties.
Regarding the pharmacological mechanisms, intracerebroventricular administration of CBD enhanced extracellular levels of dopamine in the nucleus accumbens [122]. A similar finding was found after microdialysis perfusion of CBD (30, 60 or 90 nM) into the rat lateral hypothalamus [117], a procedure that also enhanced alertness. It is unclear how this effect would relate to the antipsychotic properties of CBD because usual antipsychotic drugs act by antagonizing dopamine-2 receptors [123]. Moreover, studies with animal models that involve dopaminergic stimulation suggest that the antipsychotic-like doses of CBD (60–120 mg kg−1) are higher than those needed to induce anxiolytic-like effects [7,91,92] and reverse behavioural deficits induced by the NMDA receptor antagonist MK-801 [93,94,99]. These findings indicate that the mechanisms of CBD effects on glutamate- or dopamine-based models could be at least partially distinct. This possibility needs to be further explored.
Recently, Leweke et al. [34] showed that schizophrenic patients treated with CBD present higher anandamide serum levels compared with those receiving the antipsychotic amisulpride. Moreover, in the CBD group, there was a significant association between anandamide levels and improvement of psychotic symptoms. In the same study, they confirmed, in vitro, that CBD inhibits FAAH activity in a concentration (10 μM) that does not interact with receptors (dopamine, GABA, serotonin and glutamate) commonly associated with schizophrenia. However, by indirectly activating CB1 receptors via increased levels of anandamide, CBD could potentially modulate neurotransmitters systems related to these receptors [121,124]. Moreover, as previously discussed, facilitation of CB1-mediated neurotransmission by CBD also increases adult hippocampal neurogenesis, a mechanism that could be related to the cognitive deficits found in schizophrenic patients [124].
As discussed already, CBD and anandamide can also activate TRPV1 channels. This mechanism, probably by facilitating the pre-synaptic release of glutamate [80], is involved in the ability of CBD to reverse the disruption of PPI induced by the NMDA receptor antagonist MK-801 [93].
Other mechanisms that could also help us to explain CBD anti-psychotic effects are facilitation of 5-HT1A-mediated neurotransmission, an effect shared by the atypical anti-psychotic aripiprazole, which acts as a partial agonist at these receptors, and anti-inflammatory/neuroprotective action [124].
4. Cannabidiol and depression
Cannabis sativa exerts significant effects upon humour, which include euphoria and mood elevation [125]. THC may account for most of these effects through activation of CB1 receptors. Considering these observations, as well as the effects of synthetic cannabinoids and drugs that increase eCB levels, a putative role for the eCB system in mood disorders has been proposed [126]. The effects of CBD, however, have been scarcely investigated (table 4). The fact that this compound, in addition to facilitating eCB activity [33], may facilitate the activation of 5-HT1A receptors [35] suggests that it might also have antidepressant-like properties. 5HT1A receptors modulate responses to stressful stimuli and are proposed to mediate the effects of antidepressant drugs [129].
Table 4.
Antidepressant-like effects of CBD. Studies with laboratory animals. FST, forced swimming test; TST, tail suspension test.
| model | species | effective doses | CBD effects | references |
|---|---|---|---|---|
| restraint stress | rat | 10–20 mg kg−1 | ↓ cardiovascular and behavioural effects of stress | [17] |
| FST | mouse | 200 mg kg−1 | ↓ immobility | [127] |
| TST | mouse | 20–200 mg kg−1 | no effect | [127] |
| FST | mouse | 30 mg kg−1 | ↓ immobility | [128] |
| chronic unpredictable stress | mouse | 30 mg kg−1 (daily, chronic treatment) | ↓ the behavioural consequences of stress through enhancement of hippocampal neurogenesis | [72] |
Stress exposure is a key aetiological factor in depression [130] and animal models used to study antidepressant-like effects are generally based on acute responses to inescapable aversive stimuli, which are prevented by antidepressants [131]. Alternatively, considering the nature of depression as a chronic psychiatric disorder, some models investigate drug effects upon the diverse consequences of chronic stress, including anhedonia and changes in exploratory activity [132].
One of the first studies that indicate the presence of antidepressant-like properties in CBD focused on its ability to prevent the autonomic and behavioural consequences of inescapable stress [17]. Rats were submitted to restraint stress during 60 min, which increases heart rate and blood pressure and caused anxiogenic-like responses in rats exposed to the elevated plus maze 24 h later. CBD was injected 30 min before the stress at the doses of 1, 10 or 20 mg kg−1. The doses of 10 and 20 mg kg−1 attenuated the changes in autonomic parameters, whereas at 10 mg kg−1 CBD also prevented the late anxiogenic-like effect of stress. There were no changes in motor activity or basal cardiovascular parameters, discarding any possible confounding factor [17]. Similar effects were observed after intra-cisterna magna administration of CBD [12], thus suggesting that these effects are centrally mediated.
Another behavioural model widely used to assess antidepressant-like effects, mainly due to its pharmacological predictability, is the forced swim test. In this assay, rats or mice exposed to inescapable swimming assume a posture of immobility, which is reversed by antidepressants [131]. We tested in mice the effects of CBD (3–100 mg kg−1) injected 30 min prior to the test [128]. The drug produced an inverted U-shaped dose-response curve. At the dose of 30 mg kg−1, it reduced immobility similar to the tricyclic antidepressant imipramine (30 mg kg−1). Our behavioural findings in the forced swimming test were confirmed by another study, published in the same year. CBD was tested at the doses of 20, 100 and 200 mg kg−1 [127], with the higher dose being effective. The drug had no effect, however, in the mouse tail suspension test. One drawback common to all these studies is that the animals received only acute injections. Depression, however, is a chronic disorder that requires long-lasting drug treatment [130]. CBD has been recently tested against the consequences of chronic unpredictable stress, which includes anhedonia and anxiety-like behaviour [130]. Chronic treatment with CBD was able to prevent these behavioural changes, an effect that depends on hippocampal neurogenesis, similar to antidepressant drugs [72]. This observation further strengthens the notion that this natural cannabinoid should be considered as a potential approach for the treatment of mood disorders.
Despite this body of evidence, no clinical study has investigated whether CBD can decrease depressive symptoms in patients. This compound has been tested, however, in patients suffering from bipolar disorders, a subtype of mood disorder, in whom it was not effective in treating manic episodes [133]. Actually, this is in line with animal models, in which CBD failed to prevent hyperactivity in rodents [134].
To summarize, although the data are scarce, preclinical studies so far do provide evidence that this compound could induce antidepressant-like effects. Clinical studies are important to confirm this possibility.
(a). Mechanisms of cannabidiol antidepressant effects
Similar to findings with animal models of anxiety, the attenuation of the behavioural consequences of restraint stress and the antidepressant-like effects of CBD in the forced swimming test were attenuated by a 5-HT1A receptor antagonist [17,128]. In the latter model, despite the association between increased expression of neurotrophic factors and antidepressant activity [130], CBD failed to modify brain-derived neurotrophic factor hippocampal levels [128].
As discussed earlier, CBD can also facilitate hippocampal neurogenesis, probably by facilitation of eCB neurotransmission [72]. The involvement of this mechanism on its antidepressant-like properties after repeated administration remains to be investigated.
5. Conclusions
CBD is a safe compound with a wide range of therapeutic applications, including the treatment of psychiatric disorders [3,4]. These findings make this drug an attractive candidate for future clinical use. Its therapeutic use, however, has some limiting factors. In addition to its low and variable oral bioavailability in humans [135], it causes bell-shaped dose-response curves and, judging from the studies with laboratory animals, possesses a narrow therapeutic dose range. A clear target of future research, therefore, is to try to develop compounds with similar safety and clinical profile but with larger effective dose ranges. To this aim, a better understanding of the mechanisms responsible for the unique properties of CBD is essential. The behaviour studies reviewed here clearly indicate that more than one mechanism is involved, depending on the effects being measured (anxiolytic, anti-compulsive, antidepressant or antipsychotic-like) and the drug regime (single versus repeated administration). Facilitation of 5-HT1A-mediated neurotransmission in key brain areas related to defensive responses, including the dorsal periaqueductal grey, bed nucleus of the stria terminalis and medial prefrontal cortex, seems responsible for CBD acute anxiolytic-like effects. Other CBD effects, such as anti-compulsive, increased extinction and impaired reconsolidation of aversive memories, facilitation of adult hippocampal neurogenesis and blockade of the anxiogenic consequences of chronic unpredictable stress could depend on potentiation of anandamide-mediated neurotransmission. Finally, activation of TRPV1 channels may help us to explain the antipsychotic effect and the bell-shaped dose-response curves commonly observed with CBD. In addition to these mechanisms, CBD can interfere in several other important biological processes (e.g. inhibition of adenosine uptake, inverse agonism at CB2 receptor, CB1 receptor antagonism, GPR55 antagonism, PPARγ receptors agonism, intracellular (Ca2+) increase, etc.). Additional in vivo studies are clearly needed to investigate their possible involvement on CBD behavioural effects.
Acknowledgements
This work was supported by grants from CNPq, CAPES, NAPNA-USP and FAPESP.
References
- 1.Gaoni Y., Mechoulam R. 1964. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646. 10.1021/ja01062a046 (doi:10.1021/ja01062a046) [DOI] [Google Scholar]
- 2.Devane W. A. 1994. New dawn of cannabinoid pharmacology. Trends Pharmacol. Sci. 15, 40–41 10.1016/0165-6147(94)90106-6 (doi:10.1016/0165-6147(94)90106-6) [DOI] [PubMed] [Google Scholar]
- 3.Hill A. J., Williams C. M., Whalley B. J., Stephens G. J. 2012. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 133, 79–97 10.1016/j.pharmthera.2011.09.002 (doi:10.1016/j.pharmthera.2011.09.002) [DOI] [PubMed] [Google Scholar]
- 4.Izzo A. A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. 2009. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30, 515–527 10.1016/j.tips.2009.07.006 (doi:10.1016/j.tips.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 5.Silveira Filho N. G., Tufik S. 1981. Comparative effects between cannabidiol and diazepam on neo-phobia, food intake and conflict behavior. Res. Commun. Psychol. Psychiatr. Behav. 6, 251–266 [Google Scholar]
- 6.Zuardi A. W., Karniol I. G. 1983. Changes in the conditioned emotional response of rats induced by Δ9-THC, CBD and mixture of the two cannabinoids. Braz. Arch. Biol. Technol. 26, 391–397 [Google Scholar]
- 7.Guimarães F. S., Chiaretti T. M., Graeff F. G., Zuardi A. W. 1990. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl.) 100, 558–559 10.1007/BF02244012 (doi:10.1007/BF02244012) [DOI] [PubMed] [Google Scholar]
- 8.Onaivi E. S., Green M. R., Martin B. R. 1990. Pharmacological characterization of cannabinoids in the elevated plus maze. J. Pharmacol. Exp. Ther. 253, 1002–1009 [PubMed] [Google Scholar]
- 9.Moreira F. A., Aguiar D. C., Guimarães F. S. 2006. Anxiolytic- like effect of cannabidiol in the rat Vogel conflict test. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1466–1471 10.1016/j.pnpbp.2006.06.004 (doi:10.1016/j.pnpbp.2006.06.004) [DOI] [PubMed] [Google Scholar]
- 10.Resstel L. B., Joca S. R., Moreira F. A., Correa F. M., Guimarães F. S. 2006. Effects of cannabidiol and diazepam on behavioural and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain Res. 172, 294–298 10.1016/j.bbr.2006.05.016 (doi:10.1016/j.bbr.2006.05.016) [DOI] [PubMed] [Google Scholar]
- 11.Campos A. C., Guimarães F. S. 2009. Activation of 5HT1A receptors mediates the anxiolytic effects of cannabidiol in a PTSD model. Behav. Pharmacol. 20, S54 [Google Scholar]
- 12.Granjeiro E. M., Gomes F. V., Guimarães F. S., Correa F. M., Resstel L. B. 2011. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol. Biochem. Behav. 99, 743–748 10.1016/j.pbb.2011.06.027 (doi:10.1016/j.pbb.2011.06.027) [DOI] [PubMed] [Google Scholar]
- 13.Uribe-Marino A., Francisco A., Castiblanco-Urbina M. A., Twardowschy A., Salgado-Rohner C. J., Crippa J. A., Hallak J. E., Zuardi A. W., Coimbra N. C. 2012. Anti-aversive effects of cannabidiol on innate fear-induced behaviors evoked by an ethological model of panic attacks based on a prey vs the wild snake Epicrates cenchria crassus confrontation paradigm. Neuropsychopharmacology 37, 412–421 10.1038/npp.2011.188 (doi:10.1038/npp.2011.188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casarotto P. C., Gomes F. V., Resstel L. B., Guimarães F. S. 2010. Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav. Pharmacol. 21, 353–358 10.1097/FBP.0b013e32833b33c5 (doi:10.1097/FBP.0b013e32833b33c5) [DOI] [PubMed] [Google Scholar]
- 15.Bitencourt R. M., Pamplona F. A., Takahashi R. N. 2008. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur. Neuropsychopharmacol. 18, 849–859 10.1016/j.euroneuro.2008.07.001 (doi:10.1016/j.euroneuro.2008.07.001) [DOI] [PubMed] [Google Scholar]
- 16.Soares V. de P., Campos A. C., Bortoli V. C., Zangrossi H., Jr, Guimarães F. S., Zuardi A. W. 2010. Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behav. Brain Res. 213, 225–229 10.1016/j.bbr.2010.05.004 (doi:10.1016/j.bbr.2010.05.004) [DOI] [PubMed] [Google Scholar]
- 17.Resstel L. B., Tavares R. F., Lisboa S. F., Joca S. R., Corrêa F. M., Guimarães F. S. 2009. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 156, 181–188 10.1111/j.1476-5381.2008.00046.x (doi:10.1111/j.1476-5381.2008.00046.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campos A. C., Guimarães F. S. 2008. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl.) 199, 223–230 10.1007/s00213-008-1168-x (doi:10.1007/s00213-008-1168-x) [DOI] [PubMed] [Google Scholar]
- 19.Gomes F. V., Resstel L. B., Guimarães F. S. 2011. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl.) 213, 465–473 10.1007/s00213-010-2036-z (doi:10.1007/s00213-010-2036-z) [DOI] [PubMed] [Google Scholar]
- 20.Gomes F. V., Reis D. G., Alves F. H., Correa F. M., Guimarães F. S., Resstel L. B. 2012. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J. Psychopharmacol. 26, 104–113 10.1177/0269881110389095 (doi:10.1177/0269881110389095) [DOI] [PubMed] [Google Scholar]
- 21.Lemos J. I., Resstel L. B., Guimarães F. S. 2010. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav. Brain Res. 207, 105–111 10.1016/j.bbr.2009.09.045 (doi:10.1016/j.bbr.2009.09.045) [DOI] [PubMed] [Google Scholar]
- 22.Elbatsh M. M., Assareh N., Marsden C. A., Kendall D. A. 2012. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology (Berl.) 221, 239–247 10.1007/s00213-011-2566-z (doi:10.1007/s00213-011-2566-z) [DOI] [PubMed] [Google Scholar]
- 23.Stern C. A. J., Gazarini L., Takahashi R. N., Guimarães F. S., Bertoglio L. J. 2012. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology 37, 2132–2142 10.1038/npp.2012.63 (doi:10.1038/npp.2012.63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuardi A. W., Cosme R. A., Graeff F. G., Guimarães F. S. 1993. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 7, 82–88 [DOI] [PubMed] [Google Scholar]
- 25.Crippa J. A., et al. 2004. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29, 417–426 10.1038/sj.npp.1300340 (doi:10.1038/sj.npp.1300340) [DOI] [PubMed] [Google Scholar]
- 26.Hsiao Y. T., Yi P. L., Li C. L., Chang F. C. 2012. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology 62, 373–384 10.1016/j.neuropharm.2011.08.013 (doi:10.1016/j.neuropharm.2011.08.013) [DOI] [PubMed] [Google Scholar]
- 27.Fusar-Poli P., et al. 2010. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int. J. Neuropsychopharmacol. 13, 421–432 10.1017/S1461145709990617 (doi:10.1017/S1461145709990617) [DOI] [PubMed] [Google Scholar]
- 28.Crippa J. A., et al. 2011. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J. Psychopharmacol. 25, 121–130 10.1177/0269881110379283 (doi:10.1177/0269881110379283) [DOI] [PubMed] [Google Scholar]
- 29.Bergamaschi M. M., et al. 2011. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36, 1219–1226 10.1038/npp.2011.6 (doi:10.1038/npp.2011.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yehuda R., Joëls M., Morris R. G. 2010. The memory paradox. Nat. Rev. Neurosci. 11, 837–839 10.1038/nrn2957 (doi:10.1038/nrn2957) [DOI] [PubMed] [Google Scholar]
- 31.Zuardi A. W., Shirakawa I., Finkelfarb E., Karniol I. G. 1982. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl.) 76, 245–250 10.1007/BF00432554 (doi:10.1007/BF00432554) [DOI] [PubMed] [Google Scholar]
- 32.Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. 2007. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623 10.1038/sj.bjp.0707133 (doi:10.1038/sj.bjp.0707133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisogno T., et al. 2001. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852 10.1038/sj.bjp.0704327 (doi:10.1038/sj.bjp.0704327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leweke F. M., Piomelli D., Pahlisch F., Muhl D., Gerth C. W., Hoyer C., Klosterkötter J., Hellmich M., Koethe D. 2012. Cannabidiol enhances anadamide signilling and alliviates psychotic symptoms of schizoprenia. Transl. Psychiatry 2, e94. 10.108/tp.2012.15 (doi:10.108/tp.2012.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo E. B., Burnett A., Hall B., Parker K. K. 2005. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043 10.1007/s11064-005-6978-1 (doi:10.1007/s11064-005-6978-1) [DOI] [PubMed] [Google Scholar]
- 36.Xiong W., Koo B. N., Morton R., Zhang L. 2011. Psychotropic and nonpsychotropic cannabis derivatives inhibit human 5-HT(3A) receptors through a receptor desensitization-dependent mechanism. Neuroscience 184, 28–37 10.1016/j.neuroscience.2011.03.066 (doi:10.1016/j.neuroscience.2011.03.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenny M., Santer E., Pirich E., Schennach H., Fuchs D. 2009. Δ9-tetrahydrocannabinol and cannabidiol modulate mitogen-induced tryptophan degradation and neopterin formation in peripheral blood mononuclear cells in vitro. J. Neuroimmunol. 207, 75–82 10.1016/j.jneuroim.2008.12.004 (doi:10.1016/j.jneuroim.2008.12.004) [DOI] [PubMed] [Google Scholar]
- 38.Drysdale A. J., Ryan D., Pertwee R. G., Platt B. 2006. Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology 50, 621–631 10.1016/j.neuropharm.2005.11.008 (doi:10.1016/j.neuropharm.2005.11.008) [DOI] [PubMed] [Google Scholar]
- 39.Ryan D., Drysdale A. J., Pertwee R. G., Platt B. 2007. Interactions of cannabidiol with endocannabinoid signalling in hippocampal tissue. Eur. J. Neurosci. 25, 2093–2102 10.1111/j.1460-9568.2007.05448.x (doi:10.1111/j.1460-9568.2007.05448.x) [DOI] [PubMed] [Google Scholar]
- 40.Kathmann M., Flau K., Redmer A., Tränkle C., Schlicker E. 2006. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch. Pharmacol. 372, 354–361 10.1007/s00210-006-0033-x (doi:10.1007/s00210-006-0033-x) [DOI] [PubMed] [Google Scholar]
- 41.O'Sullivan S. E., Sun Y., Bennett A. J., Randall M. D., Kendall D. A. 2009. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 612, 61–68 10.1016/j.ejphar.2009.03.010 (doi:10.1016/j.ejphar.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 42.Ryberg E., et al. 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101 10.1038/sj.bjp.0707460 (doi:10.1038/sj.bjp.0707460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrier E. J., Auchampach J. A., Hillard C. J. 2006. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc. Natl Acad. Sci. USA 103, 7895–7900 10.1073/pnas.0511232103 (doi:10.1073/pnas.0511232103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liou G. I., Auchampach J. A., Hillard C. J., Zhu G., Yousufzai B., Mian S., Khan S., Khalifa Y. 2008. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest. Ophthalmol. Vis. Sci. 49, 5526–5531 10.1167/iovs.08-2196 (doi:10.1167/iovs.08-2196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin N., Neeper M. P., Liu Y., Hutchinson T. L., Lubin M. L., Flores C. M. 2008. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 28, 6231–6238 10.1523/JNEUROSCI.0504-08.2008 (doi:10.1523/JNEUROSCI.0504-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Petrocellis L., Vellani V., Schiano-Moriello A., Marini P., Magherini P. C., Orlando P., Di Marzo V. 2008. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 325, 1007–1015 10.1124/jpet.107.134809 (doi:10.1124/jpet.107.134809) [DOI] [PubMed] [Google Scholar]
- 47.Esposito G., De Filippis D., Maiuri M. C., De Stefano D., Carnuccio R., Iuvone T. 2006. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci. Lett. 399, 91–95 10.1016/j.neulet.2006.01.047 (doi:10.1016/j.neulet.2006.01.047) [DOI] [PubMed] [Google Scholar]
- 48.Rajesh M., Mukhopadhyay P., Bátkai S., Haskó G., Liaudet L., Drel V. R., Obrosova I. G., Pacher P. 2007. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart. Circ. Physiol. 293, H610–H619 10.1152/ajpheart.00236.2007 (doi:10.1152/ajpheart.00236.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan H., Mukhopadhyay P., Rajesh M., Patel V., Mukhopadhyay B., Gao B., Haskó G., Pacher P. 2009. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 328, 708–714 10.1124/jpet.108.147181 (doi:10.1124/jpet.108.147181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandolfo P., Silveirinha V., dos Santos-Rodrigues A., Venance L., Ledent C., Takahashi R. N., Cunha R. A., Köfalvi A. 2011. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur. J. Pharmacol. 655, 38–45 10.1016/j.ejphar.2011.01.013 (doi:10.1016/j.ejphar.2011.01.013) [DOI] [PubMed] [Google Scholar]
- 51.Esposito G., et al. 2011. Cannabidiol reduces a beta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS ONE 6, e28668. 10.1371/journal.pone.0028668 (doi:10.1371/journal.pone.0028668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esposito G., Scuderi C., Savani C., Steardo L., Jr, De Filippis D., Cottone P., Iuvone T., Cuomo V., Steardo L. 2007. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 151, 1272–1279 10.1038/sj.bjp.0707337 (doi:10.1038/sj.bjp.0707337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagredo O., Pazos M. R., Satta V., Ramos J. A., Pertwee R. G., Fernandez-Ruiz J. 2011. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J. Neurosci. Res. 89, 1509–1518 10.1002/jnr.22682 (doi:10.1002/jnr.22682) [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Arencibia M., González S., de Lago E., Ramos J. A., Mechoulam R., Fernández-Ruiz J. 2007. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 1134, 162–170 10.1016/j.brainres.2006.11.063 (doi:10.1016/j.brainres.2006.11.063) [DOI] [PubMed] [Google Scholar]
- 55.Mishima K., Hayakawa K., Abe K., Ikeda T., Egashira N., Iwasaki K., Fujiwara M. 2005. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke 36, 1077–1082 10.1161/01.STR.0000163084.16505.e3 (doi:10.1161/01.STR.0000163084.16505.e3) [DOI] [PubMed] [Google Scholar]
- 56.Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry E. M. 2010. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 159, 950–957 10.1111/j.1476-5381.2009.00589.x (doi:10.1111/j.1476-5381.2009.00589.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rock E. M., Bolognini D., Limebeer C. L., Cascio M. G., Anavi-Goffer S., Fletcher P. J., Mechoulam R., Pertwee R. G., Parker L. A. 2012. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic: autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165, 2620–2634 10.1111/j.1476-5381.2011.01621.x (doi:10.1111/j.1476-5381.2011.01621.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scopinho A. A., Guimarães F. S., Correa F. M., Resstel L. B. 2011. Cannabidiol inhibits the hyperphagia induced by cannabinoid-1 or serotonin-1A receptor agonists. Pharmacol. Biochem. Behav. 98, 268–272 10.1016/j.pbb.2011.01.007 (doi:10.1016/j.pbb.2011.01.007) [DOI] [PubMed] [Google Scholar]
- 59.Moreira F. A., Aguiar D. C., Guimarães F. S. 2007. Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaqueductal gray. Neuropharmacology 52, 958–965 10.1016/j.neuropharm.2006.10.013 (doi:10.1016/j.neuropharm.2006.10.013) [DOI] [PubMed] [Google Scholar]
- 60.Marsicano G., et al. 2002. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534 10.1038/nature00839 (doi:10.1038/nature00839) [DOI] [PubMed] [Google Scholar]
- 61.Thomas A., Burant A., Bui N., Graham D., Yuva-Paylor L. A., Paylor R. 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl.) 204, 361–373 10.1007/s00213-009-1466-y (doi:10.1007/s00213-009-1466-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piomelli D. 2003. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 4, 873–884 10.1038/nrn1247 (doi:10.1038/nrn1247) [DOI] [PubMed] [Google Scholar]
- 63.Pittenger C., Krystal J. H., Coric V. 2006. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. Neurotherapeutics 3, 69–81 10.1016/j.nurx.2005.12.006 (doi:10.1016/j.nurx.2005.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egashira N., Okuno R., Harada S., Matsushita M., Mishima K., Iwasaki K., Nishimura R., Oishi R., Fujiwara M. 2008. Effects of glutamate-related drugs on marble-burying behavior in mice: implications for obsessive-compulsive disorder. Eur. J. Pharmacol. 586, 164–170 10.1016/j.ejphar.2008.01.035 (doi:10.1016/j.ejphar.2008.01.035) [DOI] [PubMed] [Google Scholar]
- 65.Iijima M., Kurosu S., Chaki S. 2010. Effects of agents targeting glutamatergic systems on marble-burying behavior. Neurosci. Lett. 471, 63–65 10.1016/j.neulet.2009.12.048 (doi:10.1016/j.neulet.2009.12.048) [DOI] [PubMed] [Google Scholar]
- 66.Aboujaoude E., Barry J. J., Gamel N. 2009. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. J. Clin. Psychopharmacol. 29, 51–55 10.1097/JCP.0b013e318192e9a4 (doi:10.1097/JCP.0b013e318192e9a4) [DOI] [PubMed] [Google Scholar]
- 67.Grant P., Lougee L., Hirschtritt M., Swedo S. E. 2007. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 17, 761–767 10.1089/cap.2007.0021 (doi:10.1089/cap.2007.0021) [DOI] [PubMed] [Google Scholar]
- 68.Ludányi A., et al. 2008. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 28, 2976–2990 10.1523/JNEUROSCI.4465-07.2008 (doi:10.1523/JNEUROSCI.4465-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samuels B. A., Hen R. 2011. Neurogenesis and affective disorders. Eur. J. Neurosci. 33, 1152–1159 10.1111/j.1460-9568.2011.07614.x (doi:10.1111/j.1460-9568.2011.07614.x) [DOI] [PubMed] [Google Scholar]
- 70.Santarelli L., et al. 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 10.1126/science.1083328 (doi:10.1126/science.1083328) [DOI] [PubMed] [Google Scholar]
- 71.Wolf S. A., et al. 2010. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 8, 12. 10.1186/1478-811X-8-12 (doi:10.1186/1478-811X-8-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campos A. C., Palazuelos J., Aguado T., Aguiar D. C., Guzmán M., Galve-Roperh I., Guimarães F. S. 2011. Hippocampal proliferative processes are needed for behavioral effects of cannabidiol. Revista de Neurosciências 19(Suppl.), 81 [Google Scholar]
- 73.Jiang W., Zhang Y., Xiao L., Van Cleemput J., Ji S. P., Bai G., Zhang X. 2005. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 115, 3104–3116 10.1172/JCI25509 (doi:10.1172/JCI25509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirby E. D., Friedman A. R., Covarrubias D., Ying C., Sun W. G., Goosens K. A., Sapolsky R. M., Kaufer D. 2012. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol. Psychiatry 17, 527–536 10.1038/mp.2011.71 (doi:10.1038/mp.2011.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saxe M. D., et al. 2006. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl Acad. Sci. USA 103, 17 501–17 506 10.1073/pnas.0607207103 (doi:10.1073/pnas.0607207103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder J. S., Soumier A., Brewer M., Pickel J., Cameron H. A. 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 10.1038/nature10287 (doi:10.1038/nature10287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahay A., Scobie K. N., Hill A. S., O'Carroll C. M., Kheirbek M. A., Burghardt N. S., Fenton A. A., Dranovsky A., Hen R. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 10.1038/nature09817 (doi:10.1038/nature09817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilius B., Voets T. 2005. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 451, 1–10 10.1007/s00424-005-1462-y (doi:10.1007/s00424-005-1462-y) [DOI] [PubMed] [Google Scholar]
- 79.Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 10.1016/S0896-6273(00)80564-4 (doi:10.1016/S0896-6273(00)80564-4) [DOI] [PubMed] [Google Scholar]
- 80.Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. 1999. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 10.1038/22761 (doi:10.1038/22761) [DOI] [PubMed] [Google Scholar]
- 81.Xing J., Li J. 2007. TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dlPAG) neurons. J. Neurophysiol. 97, 503–511 10.1152/jn.01023.2006 (doi:10.1152/jn.01023.2006) [DOI] [PubMed] [Google Scholar]
- 82.Molchanov M. L., Guimarães F. S. 2002. Anxiolytic-like effects of AP7 injected into the dorsolateral or ventrolateral columns of the periaqueductal gray of rats. Psychopharmacology (Berl.) 160, 30–38 10.1007/s00213-001-0941-x (doi:10.1007/s00213-001-0941-x) [DOI] [PubMed] [Google Scholar]
- 83.Campos A. C., Guimarães F. S. 2009. Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1517–1521 10.1016/j.pnpbp.2009.08.017 (doi:10.1016/j.pnpbp.2009.08.017) [DOI] [PubMed] [Google Scholar]
- 84.Rubino T., et al. 2008. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb. Cortex 18, 1292–1301 10.1093/cercor/bhm161 (doi:10.1093/cercor/bhm161) [DOI] [PubMed] [Google Scholar]
- 85.Casarotto P. C., Terzian A. L., Aguiar D. C., Zangrossi H., Guimarães F. S., Wotjak C. T., Moreira F. A. 2012. Opposing roles for cannabinoid receptor type-1 (CB1) and transient receptor potential vanilloid type-1 channel (TRPV1) on the modulation of panic-like responses in rats. Neuropsychopharmacology 37, 478–486 10.1038/npp.2011.207 (doi:10.1038/npp.2011.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karniol I. G., Carlini E. A. 1973. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia 33, 53–70 10.1007/BF00428793 (doi:10.1007/BF00428793) [DOI] [PubMed] [Google Scholar]
- 87.Karniol I. G., Shirakawa I., Kasinski N., Pfeferman A., Carlini E. A. 1974. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur. J. Pharmacol. 28, 172–177 10.1016/0014-2999(74)90129-0 (doi:10.1016/0014-2999(74)90129-0) [DOI] [PubMed] [Google Scholar]
- 88.Dalton W. S., Martz R., Lemberger L., Rodda B. E., Forney R. B. 1976. Influence of cannabidiol on Δ-9-tetrahydrocannabinol effects. Clin. Pharmacol. Ther. 19, 300–309 [DOI] [PubMed] [Google Scholar]
- 89.Rottanburg D., Robins A. H., Ben-Arie O., Teggin A., Elk R. 1982. Cannabis-associated psychosis with hypomanic features. Lancet 2, 1364–1366 10.1016/S0140-6736(82)91270-3 (doi:10.1016/S0140-6736(82)91270-3) [DOI] [PubMed] [Google Scholar]
- 90.Hunt C. A., Jones R. T., Herning R. I., Bachman J. 1981. Evidence that cannabidiol does not significantly alter the pharmacokinetics of tetrahydrocannabinol in man. J. Pharmacokinet. Biopharm. 9, 245–260 [DOI] [PubMed] [Google Scholar]
- 91.Zuardi A. W., Rodrigues J. A., Cunha J. M. 1991. Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl.) 104, 260–264 10.1007/BF02244189 (doi:10.1007/BF02244189) [DOI] [PubMed] [Google Scholar]
- 92.Moreira F. A., Guimarães F. S. 2005. Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur. J. Pharmacol. 512, 199–205 10.1016/j.ejphar.2005.02.040 (doi:10.1016/j.ejphar.2005.02.040) [DOI] [PubMed] [Google Scholar]
- 93.Long L. E., Malone D. T., Taylor D. A. 2006. Cannabidiol reverses MK-801-induced disruption of prepulse inhibition in mice. Neuropsychopharmacology 31, 795–803 10.1038/sj.npp.1300838 (doi:10.1038/sj.npp.1300838) [DOI] [PubMed] [Google Scholar]
- 94.Gurujan A., Taylor D. A., Malone D. T. 2012. Cannabidiol and clozapine reverse MK-801-induced deficits in social interaction and hyperactivity in Sprague-Dawle rats. J. Psychopharmacol. 26, 1317–1332 (doi:10.1177/0269881112441865) [DOI] [PubMed] [Google Scholar]
- 95.Curzon P., Decker M. W. 1998. Effects of phencyclidine (PCP) and (+)MK-801 on sensorimotor gating in CD-1 mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 22, 129–146 10.1016/S0278-5846(97)00184-X (doi:10.1016/S0278-5846(97)00184-X) [DOI] [PubMed] [Google Scholar]
- 96.Geyer M. A., Krebs-Thomson K., Braff D. L., Swerdlow N. R. 2001. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl.) 156, 117–154 10.1007/s002130100811 (doi:10.1007/s002130100811) [DOI] [PubMed] [Google Scholar]
- 97.Long L. E., Chesworth R., Huang X. F., McGregor I. S., Arnold J. C., Karl T. 2010. A behavioural comparison of acute and chronic Δ9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int. J. Neuropsychopharmacol. 13, 861–876 10.1017/S1461145709990605 (doi:10.1017/S1461145709990605) [DOI] [PubMed] [Google Scholar]
- 98.Long L. E., Chesworth R., Huang X. F., Wong A., Spiro A., McGregor I. S., Arnold J. C., Karl T. 2012. Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuroregulin 1 mutante mice. PLoS ONE 7, e34129. 10.1371/journal.pone.0034129 (doi:10.1371/journal.pone.0034129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gururajan A., Taylor D. A., Malone D. T. 2011. Effect of cannabidiol in a MK-801-rodent model of aspects of schizophrenia. Behav. Brain Res. 222, 299–308 10.1016/j.bbr.2011.03.053 (doi:10.1016/j.bbr.2011.03.053) [DOI] [PubMed] [Google Scholar]
- 100.Leweke F. M., Schneider U., Radwan M., Schmidt E., Emrich H. M. 2000. Different effects of nabilone and cannabidiol on binocular depth inversion in man. Pharmacol. Biochem. Behav. 66, 175–181 10.1016/S0091-3057(00)00201-X (doi:10.1016/S0091-3057(00)00201-X) [DOI] [PubMed] [Google Scholar]
- 101.Bhattacharyya S., et al. 2010. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35, 764–774 10.1038/npp.2009.184 (doi:10.1038/npp.2009.184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hallak J. E., et al. 2011. The interplay of cannabinoid and NMDA glutamate receptor systems in humans: preliminary evidence of interactive effects of cannabidiol and ketamine in healthy human subjects. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 198–202 10.1016/j.pnpbp.2010.11.002 (doi:10.1016/j.pnpbp.2010.11.002) [DOI] [PubMed] [Google Scholar]
- 103.Zuardi A. W., Morais S. L., Guimarães F. S., Mechoulam R. 1995. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry 56, 485–486 [PubMed] [Google Scholar]
- 104.Zuardi A. W., Hallak J. E., Dursun S. M., Morais S. L., Sanches R. F., Musty R. E., Crippa J. A. 2006. Cannabidiol monotherapy for treatment-resistant schizophrenia. J. Psychopharmacol. 20, 683–686 10.1177/0269881106060967 (doi:10.1177/0269881106060967) [DOI] [PubMed] [Google Scholar]
- 105.Zuardi A. W., Crippa J. A., Hallak J. E., Pinto J. P., Chagas M. H., Rodrigues G. G., Dursun S. M., Tumas V. 2009. Cannabidiol for the treatment of psychosis in Parkinson's disease. J. Psychopharmacol. 23, 979–983 10.1177/0269881108096519 (doi:10.1177/0269881108096519) [DOI] [PubMed] [Google Scholar]
- 106.Hallak J. E., Machado-de-Sousa J. P., Crippa J. A., Sanches R. F., Trzesniak C., Chaves C., Bernardo S. A., Regalo S. C., Zuardi A. W. 2010. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev. Bras. Psiquiatr. 32, 56–61 10.1590/S1516-44462010000100011 (doi:10.1590/S1516-44462010000100011) [DOI] [PubMed] [Google Scholar]
- 107.Murray R. M., Morrison P. D., Henquet C., Di Forti M. 2007. Cannabis, the mind and society: the hash realities. Nat. Rev. Neurosci. 8, 885–895 10.1038/nrn2253 (doi:10.1038/nrn2253) [DOI] [PubMed] [Google Scholar]
- 108.Morgan C. J., Curran H. V. 2008. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br. J. Psychiatry 192, 306–307 10.1192/bjp.bp.107.046649 (doi:10.1192/bjp.bp.107.046649) [DOI] [PubMed] [Google Scholar]
- 109.Morgan C. J., et al. 2012. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol. Med. 42, 391–400 10.1017/S0033291711001322 (doi:10.1017/S0033291711001322) [DOI] [PubMed] [Google Scholar]
- 110.Schubart C. D., Sommer I. E., van Gastel W. A., Goetgebuer R. L., Kahn R. S., Boks M. P. 2011. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr. Res. 130, 216–221 10.1016/j.schres.2011.04.017 (doi:10.1016/j.schres.2011.04.017) [DOI] [PubMed] [Google Scholar]
- 111.Di Forti M., et al. 2009. High-potency cannabis and the risk of psychosis. Br. J. Psychiatry 195, 488–491 10.1192/bjp.bp.109.064220 (doi:10.1192/bjp.bp.109.064220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Demirakca T., Sartorius A., Ende G., Meyer N., Welzel H., Skopp G., Mann K., Hermann D. 2011. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. 114, 242–245 10.1016/j.drugalcdep.2010.09.020 (doi:10.1016/j.drugalcdep.2010.09.020) [DOI] [PubMed] [Google Scholar]
- 113.Hermann D., Sartorius A., Welzel H., Walter S., Skopp G., Ende G., Mann K. 2007. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol. Psychiatry 61, 1281–1289 10.1016/j.biopsych.2006.08.027 (doi:10.1016/j.biopsych.2006.08.027) [DOI] [PubMed] [Google Scholar]
- 114.Bhattacharyya S., et al. 2012. Induction of psychosis by delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch. Gen. Psychiatry 69, 27–36 10.1001/archgenpsychiatry.2011.161 (doi:10.1001/archgenpsychiatry.2011.161) [DOI] [PubMed] [Google Scholar]
- 115.Guimarães V. M., Zuardi A. W., Del Bel E. A., Guimarães F. S. 2004. Cannabidiol increases Fos expression in the nucleus accumbens but not in the dorsal striatum. Life Sci. 75, 633–638 10.1016/j.lfs.2004.01.015 (doi:10.1016/j.lfs.2004.01.015) [DOI] [PubMed] [Google Scholar]
- 116.Robertson G. S., Fibiger H. C. 1992. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience 46, 315–328 10.1016/0306-4522(92)90054-6 (doi:10.1016/0306-4522(92)90054-6) [DOI] [PubMed] [Google Scholar]
- 117.Murillo-Rodríguez E., Palomero-Rivero M., Millán-Aldaco D., Mechoulam R., Drucker-Colín R. 2011. Effects on sleep and dopamine levels of microdialysis perfusion of cannabidiol into the lateral hypothalamus of rats. Life Sci. 88, 504–511 10.1016/j.lfs.2011.01.013 (doi:10.1016/j.lfs.2011.01.013) [DOI] [PubMed] [Google Scholar]
- 118.Borgwardt S. J., et al. 2008. Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition . Biol. Psychiatry 64, 966–967 10.1016/j.biopsych.2008.05.011 (doi:10.1016/j.biopsych.2008.05.011) [DOI] [PubMed] [Google Scholar]
- 119.Bhattacharyya S., et al. 2009. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch. Gen. Psychiatry 66, 442–451 10.1001/archgenpsychiatry.2009.17 (doi:10.1001/archgenpsychiatry.2009.17) [DOI] [PubMed] [Google Scholar]
- 120.Winton-Brown T. T., et al. 2011. Modulation of auditory and visual processing by Δ-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology 36, 1340–1348 10.1038/npp.2011.17 (doi:10.1038/npp.2011.17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zuardi A. W., Crippa J. A., Hallak J. E., Bhatacharyya S., Atakan Z., Martin-Santos R., McGuire P. K., Fusar-Poli P., Guimarães F. S. In press A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr. Pharmac. Des . [DOI] [PubMed] [Google Scholar]
- 122.Murillo-Rodríguez E., Millán-Aldaco D., Palomero-Rivero M., Mechoulam R., Drucker-Colín R. 2006. Cannabidiol, a constituent of Cannabis sativa, modulates sleep in rats. FEBS Lett. 580, 4337–4345 10.1016/j.febslet.2006.04.102 (doi:10.1016/j.febslet.2006.04.102) [DOI] [PubMed] [Google Scholar]
- 123.Kapur S. 2004. How antipsychotics become anti-‘psychotic’—from dopamine to salience to psychosis. Trends Pharmacol. Sci. 25, 402–406 10.1016/j.tips.2004.06.005 (doi:10.1016/j.tips.2004.06.005) [DOI] [PubMed] [Google Scholar]
- 124.Zuardi W. A., Guimarães F. S., Hallak J. E. C., Crippa J. A. 2011. Is the highest density of CB1 receptors in paranoid schizophrenia a correlate of endocannabinoid system functioning? Exp. Rev. Neurother. 11, 1111–1114 10.1586/ERN.11.89 (doi:10.1586/ERN.11.89) [DOI] [PubMed] [Google Scholar]
- 125.Hall W., Degenhardt L. 2009. Adverse health effects of non-medical cannabis use. Lancet 374, 1383–1391 10.1016/S0140-6736(09)61037-0 (doi:10.1016/S0140-6736(09)61037-0) [DOI] [PubMed] [Google Scholar]
- 126.Hill M. N., Hillard C. J., Bambico F. R., Patel S., Gorzalka B. B., Gobbi G. 2009. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol. Sci. 30, 484–493 10.1016/j.tips.2009.06.006 (doi:10.1016/j.tips.2009.06.006) [DOI] [PubMed] [Google Scholar]
- 127.El-Alfy A. T., Ivey K., Robinson K., Ahmed S., Radwan M., Slade D., Khan I., ElSohly M., Ross S. 2010. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 95, 434–442 10.1016/j.pbb.2010.03.004 (doi:10.1016/j.pbb.2010.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zanelati T. V., Biojone C., Moreira F. A., Guimarães F. S., Joca S. R. 2010. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 159, 122–128 10.1111/j.1476-5381.2009.00521.x (doi:10.1111/j.1476-5381.2009.00521.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lowry C. A., Lightman S. L., Nutt D. J. 2009. That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. J. Psychopharmacol. 23, 392–400 10.1177/0269881108099956 (doi:10.1177/0269881108099956) [DOI] [PubMed] [Google Scholar]
- 130.Krishnan V., Nestler E. J. 2010. Linking molecules to mood: new insight into the biology of depression. Am. J. Psychiatry 167, 1305–1320 10.1176/appi.ajp.2009.10030434 (doi:10.1176/appi.ajp.2009.10030434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cryan J. F., Markou A., Lucki I. 2002. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 23, 238–245 10.1016/S0165-6147(02)02017-5 (doi:10.1016/S0165-6147(02)02017-5) [DOI] [PubMed] [Google Scholar]
- 132.Willner P. 2005. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110 10.1159/000087097 (doi:10.1159/000087097) [DOI] [PubMed] [Google Scholar]
- 133.Zuardi A. W., Crippa J., Dursun S., Morais S., Vilela J., Sanches R., Hallak J. 2010. Cannabidiol was ineffective for manic episode of bipolar affective disorder. J. Psychopharmacol. 24, 135–137 10.1177/0269881108096521 (doi:10.1177/0269881108096521) [DOI] [PubMed] [Google Scholar]
- 134.Valvassori S. S., et al. 2011. Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychopharmacol. 25, 274–280 10.1177/0269881109106925 (doi:10.1177/0269881109106925) [DOI] [PubMed] [Google Scholar]
- 135.Agurell S., Carlsson S., Lindgren J. E., Ohlsson A., Gillespie H., Hollister L. 1981. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia 37, 1090–1092 10.1007/BF02085029 (doi:10.1007/BF02085029) [DOI] [PubMed] [Google Scholar]