Abstract
The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established. However, the side-effect profile of CB1 receptor ligands has necessitated the search for alternative cannabinoid-based approaches to analgesia. Herein, we review the current literature describing the impact of chronic pain states on the key components of the endocannabinoid receptor system, in terms of regionally restricted changes in receptor expression and levels of key metabolic enzymes that influence the local levels of the endocannabinoids. The evidence that spinal CB2 receptors have a novel role in the modulation of nociceptive processing in models of neuropathic pain, as well as in models of cancer pain and arthritis is discussed. Recent advances in our understanding of the spinal location of the key enzymes that regulate the levels of the endocannabinoid 2-AG are discussed alongside the outcomes of recent studies of the effects of inhibiting the catabolism of 2-AG in models of pain. The complexities of the enzymes capable of metabolizing both anandamide (AEA) and 2-AG have become increasingly apparent. More recently, it has come to light that some of the metabolites of AEA and 2-AG generated by cyclooxygenase-2, lipoxygenases and cytochrome P450 are biologically active and can either exacerbate or inhibit nociceptive signalling.
Keywords: pain, spinal cord, cannabinoid
1. An overview of the cannabinoid receptor system and pain pathways
The key components of the endocannabinoid system—receptors, ligands and the metabolic enzymes—are present throughout the pain pathway from peripheral nerve terminals up to supraspinal centres. CB1 receptor density is moderate to high in regions involved in pain transmission and modulation, such as dorsal root ganglion (DRG), spinal cord, thalamus, periaqueductal grey (PAG), amygdala and rostroventromedial medulla [1].
Under normal physiological conditions, potentially damaging stimuli are detected by nociceptors expressed on primary afferent fibres, which relay noxious inputs to the spinal cord prior to these inputs being relayed to supraspinal regions. Under pathological conditions, changes in the peripheral, spinal and supraspinal processing of noxious inputs can produce altered nociceptive signalling, leading to aberrant pain responses (for review see [2]).
As discussed in more detail in other chapters, the two classes of endocannabinoids have distinct synthetic pathways. N-acylethanolamines (NAEs) such as anandamide (AEA) are produced via the stimulus-dependent hydrolysis of membrane phospholipid precursors of the N-arachidonoyl-phosphatidyl-ethanolamine (NAPE) family. Initially, this process was thought to be solely mediated by a specific isoform of phospholipase D known as NAPE-PLD [3]. However, genetic deletion of NAPE-PLD does not alter brain levels of AEA [4], calling into question the relevance of this pathway in the central nervous system (CNS). Two further synthetic pathways for the NAEs have been mapped, involving PLC-PTPN22 [5] and αβ hydrolase D4 (ABHD4)-GDE1 [6]. The expression of these enzymes within the CNS is heterologous, and the relative contributions of each to NAE production have yet to be mapped. The other major endocannabinoid 2-AG is produced through a mechanism involving sequential hydrolysis of phosphatidylinositol by PLA1 and PLC [7] or PLCβ [8] to produce diacyl glycerol (DAG). DAG is then cleaved by diacylglycerol lipase α or β (DAGLα/β) [8,9] to form 2-AG. The synaptic localization of DAGLα has been mapped in various areas of the brain and, relevant to this chapter, the dorsal horn of the spinal cord [10]. The termination of endocannabinoid signalling occurs in two stages: rapid removal of endocannabinoids from the synaptic cleft, and subsequent catabolism via specific enzymes in the intracellular environment; fatty acid amide hydrolase (FAAH) for the NAEs [11], and monoacyl glycerol lipase (MAGL) for the MAGs [12,13].
The analgesic properties of cannabinoids have been extensively characterized and reviewed [14–16], but their therapeutic appeal is limited by the plethora of side-effects associated with global activation of CB1 receptors. Endocannabinoids also possess anti-nociceptive properties, and these have been thoroughly explored in animal models of pain (see references in recent studies [17–19]). Many chronic pain states have unknown aetiology and their underlying mechanisms remain unclear. As such, animal models that mimic key aspects of the disease have been developed in order to gain a better understanding of the pain states and to test the potential of therapeutic targets.
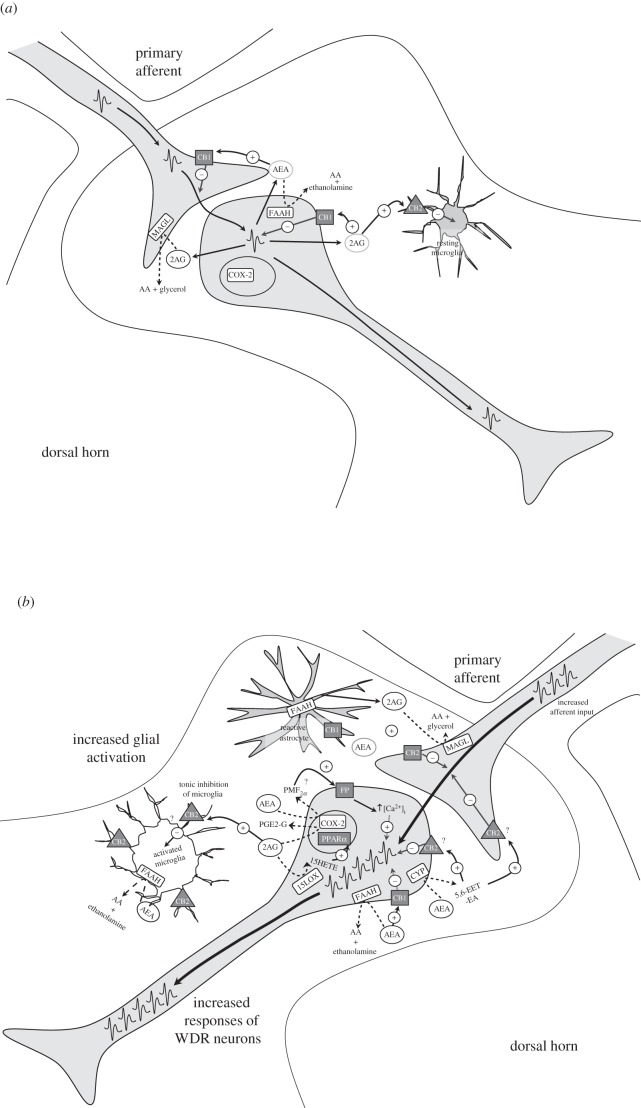
A common feature of many chronic pain states arising from a peripheral injury or lesion is the presence of primary hypersensitivity in the area of damage. This arises as a result of the sensitization of peripheral nerve terminals; the infiltration of immune cells such as monocytes, macrophages and neutrophils; and the development of inflammation (see references in McMahon et al. [20]). In addition to these peripheral events, chronic pain states are invariably characterized by the presence of central sensitization at both the level of the spinal cord and supraspinally, which leads to aberrant pain responses such as allodynia (see references in Woolf & Salter [21]). Central sensitization is also associated with the activation and/or recruitment of glial cells within the nervous system, which modulate neuronal responses through the initiation of multiple signalling cascades [22]. The activation of microglia and astrocytes in the dorsal horn of the spinal cord plays a critical role in the development of facilitated nociceptive responses and spinal hyper-excitability in chronic pain models [23]. The plasticity of the systems that impact upon nociceptive processing—in particular, the types of cell present at sites of injury, and the activation states of these cells, can have profound influence on the levels of endocannabinoids and their receptors in these discrete regions. Here, we discuss the evidence that the dynamic changes in the endocannabinoid system that are associated with chronic pain states provide new opportunities for cannabinoid-mediated modulation of nociceptive processing (figure 1).
Figure 1.
(a) Under basal conditions, endocannabinoids modulate spinal nociceptive transmission through activation of pre- [10,24] and post-synaptic [25] CB1 receptors expressed on primary afferent fibres. Increased intracellular calcium ([↑Ca2+]i) in postsynaptic neurons can stimulate AEA and 2-AG production and consequently activation of CB1 receptors. AEA and 2-AG are broken down via their respective catabolic enzymes (AEA: FAAH, 2-AG: MAGL) in the pre- and post-synaptic neurons and potentially also by resting microglia, which may express cannabinoid CB2 receptors. (b) In chronic pain states, activated spinal microglia and astrocytes release sensitizing factors, which can further facilitate the already-enhanced nociceptive signalling in the spinal cord. Under these conditions, endocannabinoid production is augmented in the spinal cord [26,27], and enzymes such as cytochrome p450 (CYP) and upregulation of cycloxygenase-2 (COX-2) and lipoxygenases (15-LOX) may provide alternative catabolic pathways for these endocannabinoids. These alternative pathways can result in the production of biologically active metabolites that modulate nociceptive transmission [28–30]. The generation of prostaglandin glycerol esters such as PGE2-G and prostaglandin ethanolamides (prostamide) such as prostamide F2α (PMF2α) can facilitate neuronal responses, although the mechanisms for these effects are currently unclear. One possibility is activation of prostaglandin FP receptors, producing increased intracellular calcium, leading to enhanced neuronal responses. Cannabinoid receptor expression is upregulated in the spinal cord; however, the exact location is not yet clear. CB1 receptors are also present on astrocytes [31], while CB2 receptors are present on microglia [32] and are thought to be upregulated on pre-synaptic terminals [33] following nerve injury. Spinal administration of CB2 receptor agonists inhibits responses of WDR neurons and thus an increase in postsynaptic expression of CB2 receptors cannot be ruled out. CB2 receptors appear to play a modulatory role in chronic pain state, with exacerbated nociceptive responses present in CB2 null mice [34]. +denotes activation;−denotes inhibition; dashed line denotes enzymatic breakdown.
2. CB1 receptor modulation of pain processing in models of chronic pain
A number of studies have investigated the effects of systemic and spinal administration of cannabinoid ligands on pain behaviour in models of neuropathic pain (table 1). Early studies demonstrated that systemic administration of WIN55, 212-2 attenuates pain behaviour in neuropathic rats [55]. Subsequently, it was reported that CB1 receptor expression is increased in the spinal cord of neuropathic rats, predominantly in the superficial laminae of the dorsal horn [56]. Increases in the expression of CB1 receptors were significant at day 4 post-injury, with levels continuing to increase until day 14. These increases were mediated in part by tyrosine kinase receptors and by ERK–MAPK signalling pathways. This upregulation of CB1 receptors appeared to be of functional relevance, because prevention of CB1 receptor upregulation diminished the inhibitory effects of WIN55,212-2 in this model [56]. In contrast to this functional enhancement of spinal CB1 receptors, a downregulation of CB1 receptors in the PAG has been described in the chronic constriction injury model of neuropathic pain [57]. Given the complex role of the descending controls in chronic pain states, and the importance of descending facilitations in driving central sensitization [58], these supra-spinal changes in CB1 receptor expression are likely to have important functional consequences.
Table 1.
Novel effects of cannabinoids—CB2-mediated effects. i.p., intraperitoneal administration; i.t., intrathecal administration; i.pl., intraplantar administration; i.a., intra-articular administration; ↑, increase; ↓, decrease.
| model | species | drug | route | effect | reference |
|---|---|---|---|---|---|
| neuropathic pain | |||||
| chemotherapy | rat | AM1241, AM1714 | i.p. | ↓mechanical allodynia | [35,36] |
| spinal nerve ligation (L5 and L6) | rat | JWH-133 | i.pl., spinal | ↓ mechanically evoked responses | [37,38] |
| rat | AM1241 | i.p. | ↓ tactile allodynia and thermal hypersensitivity | [39] | |
| mouse | AM1241 | i.p. | ↑ tactile withdrawal thresholds to pre-ligation values | [39] | |
| partial sciatic nerve ligation | rat | GW405833 | i.p. | ↓ mechanical hyperalgesia | [40] |
| mouse | GW405833 | i.p. | ↓ mechanical hyperalgesia | [41] | |
| mouse | JWH-133 | i.t. | ↓mechanical allodynia | [42] | |
| L5 nerve transection | rat | JWH-015 | i.t. | ↓mechanical allodynia | [32] |
| spared nerve injury | mouse | NES400 | i.p. | ↓ tactile allodynia and thermal hypersensitivity | [43] |
| chronic constriction injury | rat | GW405833, JWH-133 | i.t. | no effects | [44] |
| brachial plexus avulsion | mouse | JWH-015 | i.p. | ↓mechanical allodynia | [45] |
| cancer pain | |||||
| bone cancer | mouse | AM1241, | i.p., i.pl. | ↓ spontaneous and evoked pain in inoculated limb, ↓mechanical allodynia | [46,47] |
| mouse | JWH-015 | i.p., i.t. | ↓tactile allodynia and thermal hyperalgesia | [48,49] | |
| rat | WIN55,212-2 | i.t., i.pl. | ↓mechanical allodynia | [50,51] | |
| arthritis pain | |||||
| MIA-induced OA | rat | GW405833 | i.a. | ↑ peripheral neuronal responses | [52] |
| rat | A-796260 | i.p. | ↑ hind limb grip strength | [53] | |
| CFA-induced joint | rat | Δ9-THC | i.p. | ↓ mechanical hyperalgesia | [54] |
Another major pain state with an emerging therapeutic role for cannabinoids is cancer pain. Cannabinoid drugs are now used in the symptomatic treatment of pain associated with cancer [59]. Studies in models of cancer pain have revealed differing effects on the expression of cannabinoid receptors within the CNS, with some groups reporting an upregulation of CB1 receptor in the L5 DRG in mice injected with squamous cell carcinoma into the hindpaw [46], while others studying models of bone cancer report no change in expression [48,60]. Despite these differences, administration of cannabinoid agonists produces robust analgesia in all models of cancer pain via activation of both CB1 [46,50,60] and CB2 receptors [46–50]. Interestingly, antinociceptive effects of both intrathecal and systemic administration of the CB2 receptor selective agonist AM1241 were abolished by intrathecal administration of the CB2 receptor antagonist SR144528, indicating a spinal site of action [48], and the effects of systemic AM1241 were blocked by naloxone [48], indicating a role of endogenous opioids, which warrants further investigation. Such interactions have previously been demonstrated in keratinocytes in naive rats [61], and may indicate interplay between the endogenous nociceptive systems at multiple levels of the pain pathways.
3. A novel role of CB2 receptors in chronic pain states
The expression of CB2 receptors by immune cells is well-established [62]. It is now nearly 10 years since Zhang et al. [63] reported the presence of CB2 mRNA in the spinal cord of neuropathic rats, which appeared to be associated with microglia. Since then, a number of other studies have reported CB2 receptor mRNA and protein in the spinal cord in models of neuropathic pain (table 2). This upregulation of CB2 receptors has been shown to have functional consequences, as activation of spinal CB2 receptors attenuates neuronal [37] and behavioural [32,42] nociceptive responses in models of neuropathic pain. In contrast to both mixed CB1 and CB2 agonists and selective CB1 agonists, activation of spinal CB2 receptors attenuated pain responses in neuropathic rats without altering nociceptive processing per se in control rats [37]. Studies using CB2 knockout mice report that effects of spinally administered CB2 agonists are absent in these animals [42], further consolidating the evidence for novel functional effects of spinal CB2 receptors in models of neuropathic pain.
Table 2.
Changes in cannabinoid CB1 and CB2 expression in models of chronic pain. ↑ denotes increase; ↓ denotes decrease; ↔ denotes no change.
| model | species | tissue | CB1 | CB2 | reference |
|---|---|---|---|---|---|
| neuropathic pain | |||||
| brachial plexus avulsion | mice | C4-T2 DRG | ↑mRNA and protein Ipsilateral | ↑mRNA and protein | [45] |
| cervical-thoracic spinal cord | ↑mRNA and protein | ↑mRNA and protein | [45] | ||
| cingulate cortex | ↑ protein | [45] | |||
| spinal nerve ligation | rat | lumbar spinal cord | ↑ protein ipsilateral | [33] | |
| sciatic nerve section | rat | lumbar spinal cord | ↑ protein ipsilateral | [33] | |
| rat | L4-5 DRG | ↑ protein ipsilateral | [33] | ||
| chronic constriction injury | rat | spinal cord | ↑ protein | ↑ mRNA ipsilateral | [56,63] |
| rat | spinal cord | ↔ protein | [44] | ||
| L5 spinal nerve transection | rat | lumbar spinal cord | ↑ protein | [32] | |
| streptozotocin-induced diabetes | rat | DRG | ↓ protein | [64] | |
| spare nerve injury | rat | lumbar spinal cord | ↑ protein | [43] | |
| cancer pain | |||||
| bone cancer pain | mouse | lumbar spinal cord | ↔ protein | ↔ protein | [48,60] |
| mouse | L4-6 DRG | ↔ protein | [48] | ||
Neuropathic pain responses are often associated with diseases such as diabetes, and can also arise as a result of drug treatments such as chemotherapy [65]. Although less widely studied, the effects of cannabinoids on pain responses have been evaluated in animal models of these conditions. Systemic administration of WIN55, 212-2 has been shown to attenuate mechanical allodynia in a model of chemotherapy-induced neuropathy by a number of groups [35,66,67]. The inhibition of mechanical allodynia by spinal WIN55,212-2 was sensitive to both CB1 and CB2 receptor antagonists [36], despite studies showing no change in the expression of either receptor, which may indicate a change in function of CB2 under these conditions. The impact of diabetes on the expression of cannabinoid receptors and the effects of cannabinoid ligands on pain responses have been studied in the rat STZ model of diabetes and diabetic neuropathy. In this model, expression levels of cannabinoid CB1 receptors in the small diameter dorsal root ganglia cell bodies (corresponding to C- and Aδ-fibres) were decreased, which may be related to exposure to high levels of blood glucose [64]. Despite this downregulation in CB1 receptor expression, systemic administration of WIN55,212-2 has been shown to attenuate pain responses in this model [68,69].
In contrast to the previous studies described earlier (tables 1 and 2), a recent study has reported that spinal administration of the CB2 agonists GW405833 and JWH-133 does not alter mechanical allodynia at 3 or 10 days in the CCI model of neuropathy [44]. Furthermore, this study found no evidence of changes in CB2 receptor protein expression (both using Western blotting and immunohistochemistry) in the spinal cord in the CCI model of neuropathic pain, compared with naive rats [44]. On balance, there are a larger number of studies in support of a novel functional role for spinal CB2 receptors in models of neuropathic pain, although the findings reported by Brownjohn & Ashton [44] suggest that further studies with improved tools are required.
4. CB2 receptor modulation of spinal immune cell function
To date, studies of the mechanisms underlying the spinal-CB2-receptor-mediated inhibition of neuropathic pain have focused on potential interactions with spinal immune cells (microglia and astrocytes), which play a pivotal role in these chronic pain states. Ipsilateral CB2 receptor upregulation has been demonstrated within 4 days following nerve injury, with CB2 receptors present on microglia and perivascular cells in the spinal cord of neuropathic rats [32]. Spinal administration of the CB2 receptor agonist JWH015 reduced peripheral-nerve-induced hypersensitivity and levels of spinal markers of microglia activation in this model of neuropathic pain. Similarly, treatment with WIN55,212-2 attenuated the expression of markers of microglial activation in the spinal cord in a model of chemotherapy-induced neuropathy [66]. Consistent with these findings, repeated treatment with another selective CB2 ligand was also shown to be antinociceptive. NESS400 administration significantly attenuated mechanical allodynia and thermal hyperalgesia, and also decreased markers of microglia and astrocyte activation in the spinal cord of neuropathic mice at 7 days post-injury [43]. Similarly, repeated treatment with WIN55,212-2 produced a significant attenuation of pain behaviour and levels of markers of astrocytes activation in tumour-bearing mice [70] but had no effect on pain behaviour in neuropathic mice.
Interestingly, the functional upregulation of spinal CB2 receptors in models of neuropathic pain appears to provide an essential brake on the development of central sensitization, as evidenced by the exacerbation of ipsilateral touch-evoked pain (allodynia), and the novel manifestation of contralateral allodynia in CB2 null mice [34]. These changes in pain behaviour in the absence of endogenous CB2 receptors were associated with increased levels of activated microglia and astrocytes in both the ipsilateral and contralateral spinal cord—responses that were strongly attenuated in mice over-expressing CB2 receptors [34]. Gene array studies in neuropathic CB2-null mice revealed a strong interferon response following nerve injury. The involvement of interferon-gamma (IFN-γ) was further implicated by the absence of pain behaviour in neuropathic mice deficient in both IFN-γ and CB2 [71]. Collectively, these data suggest that the presence of spinal CB2 receptors plays a crucial role in dampening down spinal sensitization via modulation of IFN-γ-mediated glial cell activation in these models of neuropathic pain.
The majority of evidence that CB2 receptors modulate microglia cells comes from cell culture studies. Almost 10 years ago, 2-AG was shown to stimulate microglia migration, whereas the CB2 receptor antagonist SR144528 inhibited basal microglia migration [72]. A more recent study [73] showed that a selective CB2 receptor agonist (JWH015) reduced p-ERK1/2 protein expression in LPS-stimulated primary microglia cells, which led to a significant reduction in the expression of tumour necrosis factor-α. In addition, JWH015 inhibited LPS-stimulated microglial migration was shown to be CB2-receptor-mediated as SR144528 blocked the anti-migratory effects of JWH015. Taken together, these reports suggest that CB2 receptors may have an important role in modulating both the activity state of microglia, as well as microglia chemotaxis, particularly during inflammatory conditions.
5. Cannabinoid modulation of arthritic pain
There is increasing evidence that cannabinoid receptors may have clinical potential in other types of chronic pain states—in particular, arthritic pain. Preclinical studies have evaluated their therapeutic potential in models of both rheumatoid arthritis (RA) and osteoarthritis (OA).
RA is an autoimmune disease that is characterized by inflammation of the synovium and swelling of the joints. The cause of OA remains incompletely understood, although its development can be precipitated by damage to the joint structures or ligaments following injury. OA is primarily characterized by a loss of cartilage within the joint, as well as the development of bony outgrowths, or osteophytes, which further alter the structure of the joint. Furthermore, OA is associated with moderate inflammation. Both RA and OA are associated with reduced function of the joint and chronic pain, which dramatically reduces quality of life.
Electrophysiological studies in models of spontaneous and chemically induced arthritis have demonstrated that the facilitated nociceptive responses of peripheral nerves are attenuated in the presence of cannabinoid CB1 receptor agonists [74]. The role of CB2 receptors in modulating peripheral nerves innervating the arthritic joint appear to be complex; close arterial (peripheral) administration of CB2 receptor agonists increased vasodilatation in the inflamed knee joints of rats [75] and facilitated peripheral nerve responses in rats with OA joint damage [52]. Nevertheless, systemic administration of the CB2 receptor agonist A-796260 reversed decreases in grip strength, a surrogate measure of pain, in the monosodium iodoacetate (MIA) model of osteoarthritis pain [76]. Our current lack of knowledge about how arthritis joint pathology impacts on the expression of cannabinoid receptors, both on peripheral nerves and on local cells within the knee joint, hinders the further interpretation of these studies at this point in time. We have demonstrated the expression of cannabinoid CB1 and CB2 receptors in the synovial tissue of patients with RA and OA [77], but the extent by which peripheral cannabinoid receptors present in the synovium modulate arthritis-induced pain remains unknown.
A role of spinal cannabinoid receptors in modulating pain responses has been described in the MIA model of osteoarthritis. We have reported enhanced levels of AEA, 2-AG, PEA and OEA in the spinal cord of MIA-treated rats at 14 and 28 days, and associated elevations in protein levels of the synthetic enzymes DAGLα and NAPE-PLD [26]. These changes in the spinal endocannabinoid receptor system appeared to have functional consequences, as electrophysiological studies demonstrated that blocking spinal CB2 receptors significantly facilitated mechanically evoked responses of spinal neurons in MIA-treated rats, but not in control rats [26]. Furthermore, spinal administration of the FAAH inhibitor URB597 attenuated mechanically evoked responses, to a greater extent, in MIA-treated rats compared with control rats [26], presumably through potentiating inhibitory effects of the elevated levels of spinal endocannabinoids. Collectively, these data are indicative of a tonic modulation of spinal neuronal responses by endocannabinoids in this model of OA pain.
Inhibitory effects of cannabinoids have also been reported in models of inflammatory arthritis. Studies in rats with CFA-induced joint inflammation revealed a novel role of the CB2 receptor in mediating the effects of systemic Δ9-THC in inflamed, but not in non-inflamed rats [54]. Few papers have addressed the potential role of spinal CB2 receptors in inflammatory arthritis, although studies in models of chronic inflammatory pain may provide information pertinent to these models. The effects of peripheral inflammation on spinal CB2 receptor expression are not conclusive, with evidence both for [78] and against [54,63] changes in the expression levels of spinal CB2 receptors following chronic inflammation. The demonstration that the effects of intrathecal AM1241 were abolished when co-administered with naloxone are suggestive of an interaction between CB2 receptor mechanisms and opioid receptor systems at the level of the spinal cord [78], mirroring that seen in cancer pain models (see above).
6. Tonic control of spinal nociceptive processing by endocannabinoids
As discussed earlier, the spinal cord plays a critical role in the integration and modulation of nociceptive inputs prior to messages being sent to the higher brain centres. A number of studies have investigated the role of spinal endocannabinoids in maintaining the balance of neuronal excitability at this level, and how this may be manipulated to harness the therapeutic potential of the endocannabinoids. Exogenous application of endocannabinoids is antinociceptive at the level of the spinal cord [79,80], and the endocannabinoid system is also tonically active during nociceptive processing. Indeed, intrathecal administration of a CB1 receptor antagonist, rimonabant, produces hyperalgesia in mice [81] and enhances C-fibre-mediated firing of WDR neurons in the dorsal horn of the spinal cord [82]. The levels of endocannabinoids in the spinal cord are also elevated in some animal models of acute and chronic pain [26,83,84]. These observations suggest that the endocannabinoids form part of an endogenous brake on the activation of nociceptive pathways, and multiple groups have investigated the effects of pharmacological manipulation of endocannabinoid catabolism. In the case of FAAH, many studies have been discussed at length across a number of reviews [18,85,86] detailing the extensive corroborative evidence that inhibition of FAAH prevents the catabolism of AEA and other NAEs, and produces CB1- and CB2-mediated analgesic effects in models of a variety of pain states. In contrast, the role(s) of 2-AG in modulating nociceptive processing, and the therapeutic potential of MAGL inhibitors have only recently been investigated.
7. 2-AG and pain processing
In the past few years, a growing number of publications concerning the role of 2-AG in pain processing have been published. Recent contributions to the field have supplied striking evidence that 2-AG may be a key molecular player in endogenous inhibition at nociceptive synapses. Nyilas et al. [10] combined electron microscopy and immunofluorescence techniques to identify the relative positions of DAGLα and CB1 on nociceptive neurons in the dorsal horn of the mouse spinal cord [10]. Both proteins are highly expressed in the superficial laminae, with dense punctate staining revealing a compartmentalized sub-cellular expression. DAGLα is localized on the intracellular surface of cell membranes in dendritic shafts and spine heads post-synaptic to C and Aδ nociceptive afferents. CB1, in contrast, is localized pre-synaptically on excitatory axon terminals corresponding to small excitatory interneurons (e.g. vertical and radial cells) and C and Aδ nociceptor boutons. This expression pattern, along with the discovery that DAGLα co-localized with mGluR5 in the peri-synaptic region, is indicative of a role for 2-AG in negative feedback at glutamatergic synapses within nociceptive pathways [87]. These data outline a role for 2-AG in the modulation of spinal nociceptive processing, which could be harnessed by preventing the catabolism of 2-AG.
Attempts to exploit this mechanism for therapeutic effect have been greatly aided by recent advances in this field, including elucidation of crystal structures for MAGL [88,89], and the development of an activity-based protein profiling technique for screening compounds [90]. These have led to the identification of several novel inhibitors of MAGL that produce cannabimimetic effects in vivo, including OMDM169 [91] and JZL184 [92]. JZL184 possesses high selectivity for MAGL over FAAH (>300-fold), HSL and other common off-target serine hydrolases and lipases, and shows a nanomolar potency in isolated mouse brain membranes [92]. Systemic administration of 8 mg kg−1 JZL184 in mice produced greater than fivefold elevation of brain levels of 2-AG, with minimal inhibition of FAAH. A higher dose of 16 mg kg−1 produced significant antinociceptive effects in both thermal and chemical models of acute pain, alleviating both cold and mechanical allodynia in the chronic constriction injury mouse model of neuropathic pain [93]. However, this dose also produced significant hypomotility and FAAH inhibition (>50%), though not elevation of AEA, complicating the interpretation of results. The potency of this compound is markedly reduced (approx. 10-fold) in rat brain membranes [92], but a small number of reports detailing the antinociceptive efficacy of local administration of JZL184 in the rat have been published. Indeed, anti-nociceptive effects of intra-plantar administration of JZL184 have been described in both phases of the formalin model of inflammatory pain [94], and also in capsaicin-induced acute pain [95], with mechanisms involving both CB1 and CB2 receptors. The effects of spinal or supra-spinal administration of JZL184 on nociceptive processing in control rats, or in models of chronic pain have yet to be reported. In the context of chronic pain states involving the activation of spinal glial cells, it is noteworthy that 2-AG signalling in microglia is thought to be terminated by ABHD12 [96], which is not sensitive to JZL184 [97].
Despite the positive indications for targeting MAGL/2-AG to produce analgesic effects, two recent publications have introduced a note of caution. Studies by Schlosburg et al. [98] and Chanda et al. [99] have reported that prolonged elevation of 2-AG levels, either by genetic deletion of MAGL or by repeated treatment with the MAGL-selective inhibitor JZL184, produced desensitization and downregulation of brain CB1 receptors. These effects were accompanied by a loss of JZL184-mediated analgesia, and cross tolerance to the antinociceptive and hypothermic effects of the CB1 receptor agonist WIN55,212,2. The development of analgesic tolerance to chronic JZL184 may be overcome, however, as repeated treatment with sub-maximal doses of JZL184 (<8 mg kg−1) produced sustained analgesic effects [100]. Although these data suggest that MAGL inhibition has therapeutic potential for the treatment of pain, the demonstration that chronic JZL184 administration precipitated withdrawal responses to rimonabant, as indicated by an increase in paw flutters [101], suggests that prolonged JZL184 treatment may be associated with physical dependence.
8. The potential impact of alternative endocannabinoid catabolism pathways on pain processing
The endocannabinoids are not only subject to metabolism by the major catabolic pathways described earlier, but also by other enzymes, resulting in the production of bioactive metabolites. In particular, AEA and 2-AG can undergo oxidative metabolism by cyclooxygenase 2 (COX-2) [102], 15-, 12- and 5-lipoxygenase (LOX) [28], and some isoforms of cytochrome P450 [29]. Levels of some of these enzymes, which may serve a role in the alternative metabolism of the endocannabinoids, are altered in pain/inflammatory states, which could impact upon the levels of endocannabinoids present under these conditions. It is well established that the expression of COX-2 is elevated both in injured tissue and in the spinal cord in models of chronic pain [103–105]. Similarly, increased spinal expression of 5-LOX and FLAP (5-LOX-activating protein), both in terms of mRNA and protein, has been reported in a model of neuropathic pain [106]. To date, the impact of chronic pain on the expression of cytochrome P450s and αβ hydrolase 6 or 12 has yet to be described.
9. Effects of novel biological metabolites of 2-AG and anandamide
The metabolism of AEA and 2-AG by COX-2 results in the generation of biologically active metabolites (prostaglandin ethanolamides and glycerol prostaglandins, respectively [107–109]). These metabolites can modulate synaptic activity with opposing effects to those of AEA and 2-AG [110]. Given that COX-2 expression is upregulated in chronic pain states, it is feasible that under these conditions, COX-2 may play a more prominent role in the catabolism of the endocannabinoids. This could occur both peripherally (at sites of injury) and centrally (at sites involved in the processing and integration of nociceptive inputs, such as the spinal cord). Levels of prostamide F2α (PMF2α) are elevated in the spinal cord of mice with knee inflammation, and spinal application of PMF2α facilitates neuronal responses. This effect is abolished in the presence of the prostamide antagonist AGN 211336 [111]. Similarly, peripheral injection of prostaglandin E2 glycerol ester (PGE2-G) induces mechanical allodynia and thermal hyperalgesia in rodents [30], and thus it appears that the catabolism of AEA and 2-AG by COX-2 to PMF2α and PGE2-G, respectively, may drive pro-nociceptive mechanisms.
To date, the potential biological consequences of LOX catabolism of the endocannabinoids on nociceptive processing have not been directly investigated either in vitro or in vivo. The demonstration that, at least in cells, 15-LOX is capable of metabolizing 2-AG to 15-HETE-G (see references in Vandevoorde & Lambert [112]), which is a ligand for the anti-inflammatory nuclear receptor PPARα [28], suggests that changes in LOX expression and metabolism of endocannabinoids via this pathway may also influence nociceptive processing. Indeed, we and others have shown that PPARα ligands can have marked inhibitory effects on inflammatory pain responses (see references in [113–116]).
Most recently, the oxidative metabolism of AEA by cytochrome P450s has been described as another enzymatic pathway, leading to the production of bioactive metabolites. 5,6-epoxyeicosatrienoic acid ethanolamide (5,6-EET-EA) generated by P450-mediated catabolism of AEA is a potent agonist at CB2 receptors [29]. Interestingly, 5,6-EET-EA is also reported to be far more stable than AEA, while having little affinity at CB1 receptors [29], although it does act as a ligand at TRPV4 (see Snider et al. [117]). Although the impact of chronic pain states on the expression of P450s (e.g. at the level of the pain-associated regions of the spinal cord or brain) remain unclear, there is evidence that 5, 6-EET-EA may modulate microglial activity in vitro [29]. Indeed, stimulation of the murine BV2 microglial cell line with IFN-γ increased the expression of CYP3A, and also enhanced the capacity of these cells to metabolize AEA into 5,6-EET-EA [29]. Given the well-documented role of microglia in the development of central sensitization in models of chronic pain, and the role of IFN-γ in mediating the exacerbation of chronic pain responses in CB2-receptor-deficient mice (see earlier), further studies of the effects of 5,6-EET-EA in vivo appear warranted.
The complexity of endocannabinoid metabolism via multiple pathways, which are dynamically altered under pathological conditions, is an important consideration when investigating the effects of drugs specifically targeting FAAH or MAGL to elevate AEA and 2-AG levels. Indeed, elevating levels of AEA appears to provide additional substrate for oxidative catabolism via COX-2, LOX and/or cytochrome P450s, depending on the level of enzyme available at the key sites involved in nociceptive processing. It remains to be determined whether FAAH inhibition is associated with an increase in the generation of 5,6-EET-EA or PGE2-G under control conditions and/or in models of chronic pain. Clearly, it is unlikely that these are the only biologically active metabolites generated by the alternative metabolism of the endocannabinoids that could impact upon nociceptive processing. Thus, further studies are required to advance our understanding of both the role of these enzymatic pathways in the generation of biologically active metabolites of the endocannabinoids and how this may influence the analgesic effects of compounds that elevate levels of endocannabinoids via the inhibition of FAAH or MAGL.
In conclusion, the analgesic effects of cannabinoid ligands mediated by CB1 receptors are well established, but limited by their side-effect profile. Recent studies of models of chronic pain states have revealed complex changes in the expression of cannabinoid receptors and levels of the endocannabinoids, in particular, at the level of the spinal cord. Dynamic changes in levels of the enzymes capable of metabolizing the endocannabinoids AEA and 2-AG, in some cases to biologically active pro-nociceptive metabolites in key areas involved in pain processing, may impact upon pain responses and the therapeutic potential of some endocannabinoid based strategies for novel analgesics.
Acknowledgements
D.R.S. and J.J.B. are funded by Arthritis Research UK Pain Centre funding (grant no. 18769). The Medical Research Council and University of Nottingham are acknowledged for S.G.W.'s studentship funding.
References
- 1.Tsou K., Brown S., Sanudo-Pena M. C., Mackie K., Walker J. M. 1998. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 10.1016/S0306-4522(97)00436-3 (doi:10.1016/S0306-4522(97)00436-3) [DOI] [PubMed] [Google Scholar]
- 2.Woolf C. J. 2011. Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl), S2–S15 10.1016/j.pain.2010.09.030 (doi:10.1016/j.pain.2010.09.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J. C., Piomelli D. 1994. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691 10.1038/372686a0 (doi:10.1038/372686a0) [DOI] [PubMed] [Google Scholar]
- 4.Leung D., Saghatelian A., Simon G. M., Cravatt B. F. 2006. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 45, 4720–4726 10.1021/bi060163l (doi:10.1021/bi060163l) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., et al. 2008. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 54, 1–7 10.1016/j.neuropharm.2007.05.020 (doi:10.1016/j.neuropharm.2007.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egertova M., Simon G. M., Cravatt B. F., Elphick M. R. 2008. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: a new perspective on N-acylethanolamines as neural signaling molecules. J. Comp. Neurol. 506, 604–615 10.1002/cne.21568 (doi:10.1002/cne.21568) [DOI] [PubMed] [Google Scholar]
- 7.Sugiura T., Kishimoto S., Oka S., Gokoh M. 2006. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 45, 405–446 10.1016/j.plipres.2006.03.003 (doi:10.1016/j.plipres.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 8.Hashimotodani Y., Ohno-Shosaku T., Maejima T., Fukami K., Kano M. 2008. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology 54, 58–67 10.1016/j.neuropharm.2007.06.002 (doi:10.1016/j.neuropharm.2007.06.002) [DOI] [PubMed] [Google Scholar]
- 9.Bisogno T., et al. 2003. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 10.1083/jcb.200305129 (doi:10.1083/jcb.200305129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyilas R., Gregg L. C., Mackie K., Watanabe M., Zimmer A., Hohmann A. G., Katona I. 2009. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur. J. Neurosci. 29, 1964–1978 10.1111/j.1460-9568.2009.06751.x (doi:10.1111/j.1460-9568.2009.06751.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravatt B. F., Giang D. K., Mayfield S. P., Boger D. L., Lerner R. A., Gilula N. B. 1996. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 10.1038/384083a0 (doi:10.1038/384083a0) [DOI] [PubMed] [Google Scholar]
- 12.Dinh T. P., Carpenter D., Leslie F. M., Freund T. F., Katona I., Sensi S. L., Kathuria S., Piomelli D. 2002. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA 99, 10 819–10 824 10.1073/pnas.152334899 (doi:10.1073/pnas.152334899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saario S. M., Savinainen J. R., Laitinen J. T., Jarvinen T., Niemi R. 2004. Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem. Pharmacol. 67, 1381–1387 10.1016/j.bcp.2003.12.003 (doi:10.1016/j.bcp.2003.12.003) [DOI] [PubMed] [Google Scholar]
- 14.Pertwee R. G. 2001. Cannabinoid receptors and pain. Prog. Neurobiol. 63, 569–611 10.1016/S0301-0082(00)00031-9 (doi:10.1016/S0301-0082(00)00031-9) [DOI] [PubMed] [Google Scholar]
- 15.Iversen L., Chapman V. 2002. Cannabinoids: a real prospect for pain relief? Curr. Opin. Pharmacol. 2, 50–55 [DOI] [PubMed] [Google Scholar]
- 16.Walker J. M., Huang S. M. 2002. Cannabinoid analgesia. Pharmacol. Ther. 95, 127–135 10.1016/S0163-7258(02)00252-8 (doi:10.1016/S0163-7258(02)00252-8) [DOI] [PubMed] [Google Scholar]
- 17.Hohmann A. G., Suplita R. L., 2nd 2006. Endocannabinoid mechanisms of pain modulation. AAPS J. 8, E693–E708 10.1208/aapsj080479 (doi:10.1208/aapsj080479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagar D. R., Gaw A. G., Okine B. N., Woodhams S. G., Wong A., Kendall D. A., Chapman V. 2009. Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol. Pain 5, 59. 10.1186/1744-8069-5-59 (doi:10.1186/1744-8069-5-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guindon J., Hohmann A. G. 2009. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 8, 403–421 10.2174/187152709789824660 (doi:10.2174/187152709789824660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon S. B., Bennett D. H., Bevan S. 2005. Inflammatory mediators and modulators of pain In Textbook of pain (eds McMahon S. B., Kolzenberg M.). Philadelphia, PA: Churchill Livingstone [Google Scholar]
- 21.Woolf C. J., Salter M. W. 2005. Plasticity and pain: role of the dorsal horn In Wall and Melzack's textbook of pain (eds McMahon S. B., Kolzenberg M.). Philadelphia, PA: Churchill Livingstone [Google Scholar]
- 22.Gosselin R. D., Suter M. R., Ji R. R., Decosterd I. 2010. Glial cells and chronic pain. Neuroscientist 16, 519–531 10.1177/1073858409360822 (doi:10.1177/1073858409360822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan E. D., Watkins L. R. 2009. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 10.1038/nrn2533 (doi:10.1038/nrn2533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pernia-Andrade A. J., et al. 2009. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325, 760–764 10.1126/science.1171870 (doi:10.1126/science.1171870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salio C., Doly S., Fischer J., Franzoni M. F., Conrath M. 2002. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci. Lett. 329, 13–16 10.1016/S0304-3940(02)00549-9 (doi:10.1016/S0304-3940(02)00549-9) [DOI] [PubMed] [Google Scholar]
- 26.Sagar D. R., et al. 2010. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 62, 3666–3676 10.1002/art.27698 (doi:10.1002/art.27698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhaveri M. D., Richardson D., Kendall D. A., Barrett D. A., Chapman V. 2006. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J. Neurosci. 26, 13 318–13 327 10.1523/JNEUROSCI.3326-06.2006 (doi:10.1523/JNEUROSCI.3326-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak K. R., Gupta R. A., Moody J. S., Ji C., Boeglin W. E., DuBois R. N., Brash A. R., Marnett L. J. 2002. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. J. Biol. Chem. 277, 23 278–23 286 10.1074/jbc.M201084200 (doi:10.1074/jbc.M201084200) [DOI] [PubMed] [Google Scholar]
- 29.Snider N. T., Nast J. A., Tesmer L. A., Hollenberg P. F. 2009. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol. Pharmacol. 75, 965–972 10.1124/mol.108.053439 (doi:10.1124/mol.108.053439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu S. S., Bradshaw H. B., Chen J. S., Tan B., Walker J. M. 2008. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br. J. Pharmacol. 153, 1538–1549 10.1038/bjp.2008.33 (doi:10.1038/bjp.2008.33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegyi Z., Kis G., Hollo K., Ledent C., Antal M. 2009. Neuronal and glial localization of the cannabinoid-1 receptor in the superficial spinal dorsal horn of the rodent spinal cord. Eur. J. Neurosci. 30, 251–262 10.1111/j.1460-9568.2009.06816.x (doi:10.1111/j.1460-9568.2009.06816.x) [DOI] [PubMed] [Google Scholar]
- 32.Romero-Sandoval A., Nutile-McMenemy N., DeLeo J. A. 2008. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 108, 722–734 10.1097/ALN.0b013e318167af74 (doi:10.1097/ALN.0b013e318167af74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wotherspoon G., Fox A., McIntyre P., Colley S., Bevan S., Winter J. 2005. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience 135, 235–245 10.1016/j.neuroscience.2005.06.009 (doi:10.1016/j.neuroscience.2005.06.009) [DOI] [PubMed] [Google Scholar]
- 34.Racz I., et al. 2008. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 28, 12 125–12 135 10.1523/JNEUROSCI.3400-08.2008 (doi:10.1523/JNEUROSCI.3400-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahn E. J., Zvonok A. M., Thakur G. A., Khanolkar A. D., Makriyannis A., Hohmann A. G. 2008. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J. Pharmacol. Exp. Ther. 327, 584–591 10.1124/jpet.108.141994 (doi:10.1124/jpet.108.141994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahn E. J., Makriyannis A., Hohmann A. G. 2007. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 152, 765–777 10.1038/sj.bjp.0707333 (doi:10.1038/sj.bjp.0707333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagar D. R., Kelly S., Millns P. J., O'Shaughnessey C. T., Kendall D. A., Chapman V. 2005. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur. J. Neurosci. 22, 371–379 10.1111/j.1460-9568.2005.04206.x (doi:10.1111/j.1460-9568.2005.04206.x) [DOI] [PubMed] [Google Scholar]
- 38.Elmes S. J., Jhaveri M. D., Smart D., Kendall D. A., Chapman V. 2004. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur. J. Neurosci. 20, 2311–2320 10.1111/j.1460-9568.2004.03690.x (doi:10.1111/j.1460-9568.2004.03690.x) [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim M. M., et al. 2003. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl Acad. Sci. USA 100, 10 529–10 533 10.1073/pnas.1834309100 (doi:10.1073/pnas.1834309100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valenzano K. J., et al. 2005. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 48, 658–672 10.1016/j.neuropharm.2004.12.008 (doi:10.1016/j.neuropharm.2004.12.008) [DOI] [PubMed] [Google Scholar]
- 41.Whiteside G. T., et al. 2005. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur. J. Pharmacol. 528, 65–72 10.1016/j.ejphar.2005.10.043 (doi:10.1016/j.ejphar.2005.10.043) [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto W., Mikami T., Iwamura H. 2008. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. Eur. J. Pharmacol. 583, 56–61 10.1016/j.ejphar.2008.01.010 (doi:10.1016/j.ejphar.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 43.Luongo L., et al. 2010. 1-(2′,4′-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol. Dis. 37, 177–185 10.1016/j.nbd.2009.09.021 (doi:10.1016/j.nbd.2009.09.021) [DOI] [PubMed] [Google Scholar]
- 44.Brownjohn P. W., Ashton J. C. 2012. Spinal cannabinoid CB2 receptors as a target for neuropathic pain: an investigation using chronic constriction injury. Neuroscience 203, 180–193 10.1016/j.neuroscience.2011.12.028 (doi:10.1016/j.neuroscience.2011.12.028) [DOI] [PubMed] [Google Scholar]
- 45.Paszcuk A. F., Dutra R. C., da Silva K. A., Quintao N. L., Campos M. M., Calixto J. B. 2011. Cannabinoid agonists inhibit neuropathic pain induced by brachial plexus avulsion in mice by affecting glial cells and MAP kinases. PLoS ONE 6, e24034. 10.1371/journal.pone.0024034 (doi:10.1371/journal.pone.0024034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerrero A. V., Quang P., Dekker N., Jordan R. C., Schmidt B. L. 2008. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci. Lett. 433, 77–81 10.1016/j.neulet.2007.12.053 (doi:10.1016/j.neulet.2007.12.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozano-Ondoua A. N., Wright C., Vardanyan A., King T., Largent-Milnes T. M., Nelson M., Jimenez-Andrade J. M., Mantyh P. W., Vanderah T. W. 2010. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 86, 646–653 10.1016/j.lfs.2010.02.014 (doi:10.1016/j.lfs.2010.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curto-Reyes V., Llames S., Hidalgo A., Menéndez L., Baamonde A. 2010. Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain. Br. J. Pharmacol. 160, 561–573 10.1111/j.1476-5381.2009.00629.x (doi:10.1111/j.1476-5381.2009.00629.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu X., Mei F., Liu Y., Zhang R., Zhang J., Ma Z. 2011. Intrathecal administration of the cannabinoid 2 receptor agonist JWH015 can attenuate cancer pain and decrease mRNA expression of the 2B subunit of N-methyl-d-aspartic acid. Anesth. Analg. 113, 405–411 10.1213/ANE.0b013e31821d1062 (doi:10.1213/ANE.0b013e31821d1062) [DOI] [PubMed] [Google Scholar]
- 50.Potenzieri C., Harding-Rose C., Simone D. A. 2008. The cannabinoid receptor agonist, WIN 55, 212-2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 1215, 69–75 10.1016/j.brainres.2008.03.063 (doi:10.1016/j.brainres.2008.03.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui J. H., Kim W. M., Lee H. G., Kim Y. O., Kim C. M., Yoon M. H. 2011. Antinociceptive effect of intrathecal cannabinoid receptor agonist WIN 55,212-2 in a rat bone tumor pain model. Neurosci. Lett. 493, 67–71 10.1016/j.neulet.2010.12.052 (doi:10.1016/j.neulet.2010.12.052) [DOI] [PubMed] [Google Scholar]
- 52.Schuelert N., Zhang C., Mogg A. J., Broad L. M., Hepburn D. L., Nisenbaum E. S., Johnson M. P., McDougall J. J. 2010. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthritis Cartilage 18, 1536–1543 10.1016/j.joca.2010.09.005 (doi:10.1016/j.joca.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 53.Yao B. B., et al. 2008. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br. J. Pharmacol. 153, 390–401 10.1038/sj.bjp.0707568 (doi:10.1038/sj.bjp.0707568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox M. L., Haller V. L., Welch S. P. 2007. The antinociceptive effect of delta9-tetrahydrocannabinol in the arthritic rat involves the CB(2) cannabinoid receptor. Eur. J. Pharmacol. 570, 50–56 10.1016/j.ejphar.2007.05.024 (doi:10.1016/j.ejphar.2007.05.024) [DOI] [PubMed] [Google Scholar]
- 55.Bridges D., Ahmad K., Rice A. S. 2001. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 133, 586–594 10.1038/sj.bjp.0704110 (doi:10.1038/sj.bjp.0704110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim G., Sung B., Ji R. R., Mao J. 2003. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain 105, 275–283 10.1016/S0304-3959(03)00242-2 (doi:10.1016/S0304-3959(03)00242-2) [DOI] [PubMed] [Google Scholar]
- 57.Palazzo E., Luongo L., Bellini G., Guida F., Marabese I., Boccella S., Rossi F., Maione S., de Novellis V. 2012. Changes in cannabinoid receptor subtype 1 activity and interaction with metabotropic glutamate subtype 5 receptors in the periaqueductal gray-rostral ventromedial medulla pathway in a rodent neuropathic pain model. CNS Neurol. Disord. Drug Targets 11, 148–161 [DOI] [PubMed] [Google Scholar]
- 58.Heinricher M. M., Tavares I., Leith J. L., Lumb B. M. 2009. Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev. 60, 214–225 10.1016/j.brainresrev.2008.12.009 (doi:10.1016/j.brainresrev.2008.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter G. T., Flanagan A. M., Earleywine M., Abrams D. I., Aggarwal S. K., Grinspoon L. 2011. Cannabis in palliative medicine: improving care and reducing opioid-related morbidity. Am. J. Hosp. Palliat. Med. 28, 297–303 10.1177/1049909111402318 (doi:10.1177/1049909111402318) [DOI] [PubMed] [Google Scholar]
- 60.Furuse S., Kawamata T., Yamamoto J., Niiyama Y., Omote K., Watanabe M., Namiki A. 2009. Reduction of bone cancer pain by activation of spinal cannabinoid receptor 1 and its expression in the superficial dorsal horn of the spinal cord in a murine model of bone cancer pain. Anesthesiology 111, 173–186 10.1097/ALN.0b013e3181a51e0d (doi:10.1097/ALN.0b013e3181a51e0d) [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim M. M., et al. 2005. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl Acad. Sci. USA 102, 3093–3098 10.1073/pnas.0409888102 (doi:10.1073/pnas.0409888102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galiegue S., et al. 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61 10.1111/j.1432-1033.1995.tb20780.x (doi:10.1111/j.1432-1033.1995.tb20780.x) [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Hoffert C., Vu H. K., Groblewski T., Ahmad S., O'Donnell D. 2003. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 17, 2750–2754 10.1046/j.1460-9568.2003.02704.x (doi:10.1046/j.1460-9568.2003.02704.x) [DOI] [PubMed] [Google Scholar]
- 64.Zhang F., Hong S., Stone V., Smith P. J. W. 2007. Expression of cannabinoid CB1 receptors in models of diabetic neuropathy. J. Pharmacol. Exp. Ther. 323, 508–515 10.1124/jpet.107.128272 (doi:10.1124/jpet.107.128272) [DOI] [PubMed] [Google Scholar]
- 65.Windebank A. J., Grisold W. 2008. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 13, 27–46 10.1111/j.1529-8027.2008.00156.x (doi:10.1111/j.1529-8027.2008.00156.x) [DOI] [PubMed] [Google Scholar]
- 66.Burgos E., Gomez-Nicola D., Pascual D., Martin M. I., Nieto-Sampedro M., Goicoechea C. 2012. Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur. J. Pharmacol. 682, 62–72 10.1016/j.ejphar.2012.02.008 (doi:10.1016/j.ejphar.2012.02.008) [DOI] [PubMed] [Google Scholar]
- 67.Pascual D., Goicoechea C., Suardiaz M., Martin M. I. 2005. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 118, 23–34 10.1016/j.pain.2005.07.008 (doi:10.1016/j.pain.2005.07.008) [DOI] [PubMed] [Google Scholar]
- 68.Doğrul A., Gül H., Yıldız O., Bilgin F., Güzeldemir M. E. 2004. Cannabinoids blocks tactile allodynia in diabetic mice without attenuation of its antinociceptive effect. Neurosci. Lett. 368, 82–86 10.1016/j.neulet.2004.06.060 (doi:10.1016/j.neulet.2004.06.060) [DOI] [PubMed] [Google Scholar]
- 69.Ulugol A., Karadag H. C., Ipci Y., Tamer M., Dokmeci I. 2004. The effect of WIN 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci. Lett. 371, 167–170 10.1016/j.neulet.2004.08.061 (doi:10.1016/j.neulet.2004.08.061) [DOI] [PubMed] [Google Scholar]
- 70.Hald A., et al. 2008. Differential effects of repeated low dose treatment with the cannabinoid agonist WIN 55,212-2 in experimental models of bone cancer pain and neuropathic pain. Pharmacol. Biochem. Behav. 91, 38–46 10.1016/j.pbb.2008.04.021 (doi:10.1016/j.pbb.2008.04.021) [DOI] [PubMed] [Google Scholar]
- 71.Racz I., et al. 2008. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 28, 12 136–12 145 10.1523/JNEUROSCI.3402-08.2008 (doi:10.1523/JNEUROSCI.3402-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walter L., Franklin A., Witting A., Wade C., Xie Y., Kunos G., Mackie K., Stella N. 2003. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 23, 1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romero-Sandoval E. A., Horvath R., Landry R. P., DeLeo J. A. 2009. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol. Pain 5, 25. 10.1186/1744-8069-5-25 (doi:10.1186/1744-8069-5-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuelert N., McDougall J. J. 2008. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum. 58, 145–153 10.1002/art.23156 (doi:10.1002/art.23156) [DOI] [PubMed] [Google Scholar]
- 75.McDougall J. J., Yu V., Thomson J. 2008. In vivo effects of CB2 receptor-selective cannabinoids on the vasculature of normal and arthritic rat knee joints. Br. J. Pharmacol. 153, 358–366 10.1038/sj.bjp.0707565 (doi:10.1038/sj.bjp.0707565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao B. B., et al. 2009. Characterization of a cannabinoid CB2 receptor-selective agonist, A-836339 [2,2,3,3-tetramethyl-cyclopropanecarboxylic acid [3-(2-methoxy-ethyl)-4,5-dimethyl-3H-thiazol-(2Z)-ylidene]-amide], using in vitro pharmacological assays, in vivo pain models, and pharmacological magnetic resonance imaging. J. Pharmacol. Exp. Ther. 328, 141–151 10.1124/jpet.108.145011 (doi:10.1124/jpet.108.145011) [DOI] [PubMed] [Google Scholar]
- 77.Richardson D., et al. 2008. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteo- and rheumatoid arthritis. Arthritis Res. Ther. 10, R43. 10.1186/ar2401 (doi:10.1186/ar2401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curto-Reyes V., Boto T., Hidalgo A., Menendez L., Baamonde A. 2011. Antinociceptive effects induced through the stimulation of spinal cannabinoid type 2 receptors in chronically inflamed mice. Eur. J. Pharmacol. 668, 184–189 10.1016/j.ejphar.2011.06.057 (doi:10.1016/j.ejphar.2011.06.057) [DOI] [PubMed] [Google Scholar]
- 79.Starowicz K., Makuch W., Osikowicz M., Piscitelli F., Petrosino S., Di Marzo V., Przewlocka B. 2011. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology 62, 1746–1755 10.1016/j.neuropharm.2011.11.021 (doi:10.1016/j.neuropharm.2011.11.021) [DOI] [PubMed] [Google Scholar]
- 80.Smith F. L., Fujimori K., Lowe J., Welch S. P. 1998. Characterization of delta9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol. Biochem. Behav. 60, 183–191 10.1016/S0091-3057(97)00583-2 (doi:10.1016/S0091-3057(97)00583-2) [DOI] [PubMed] [Google Scholar]
- 81.Richardson J. D., Aanonsen L., Hargreaves K. M. 1997. SR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur. J. Pharmacol. 319, R3–R4 10.1016/S0014-2999(96)00952-1 (doi:10.1016/S0014-2999(96)00952-1) [DOI] [PubMed] [Google Scholar]
- 82.Chapman V. 1999. The cannabinoid CB1 receptor antagonist, SR141716A, selectively facilitates nociceptive responses of dorsal horn neurones in the rat. Br. J. Pharmacol. 127, 1765–1767 10.1038/sj.bjp.0702758 (doi:10.1038/sj.bjp.0702758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sagar D. R., Jhaveri M. D., Richardson D., Gray R. A., de Lago E., Fernandez-Ruiz J., Barrett D. A., Kendall D. A., Chapman V. 2010. Endocannabinoid regulation of spinal nociceptive processing in a model of neuropathic pain. Eur. J. Neurosci. 31, 1414–1422 10.1111/j.1460-9568.2010.07162.x (doi:10.1111/j.1460-9568.2010.07162.x) [DOI] [PubMed] [Google Scholar]
- 84.Okine B. N., et al. 2012. Lack of effect of chronic pre-treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: evidence for plastic changes in the endocannabinoid system. Br. J. Pharmacol. 10 10.1111/j.1476-5381.2012.02028.x (doi:10.1111/j.1476-5381.2012.02028.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schlosburg J. E., Kinsey S. G., Lichtman A. H. 2009. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 11, 39–44 10.1208/s12248-008-9075-y (doi:10.1208/s12248-008-9075-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn K., McKinney M. K., Cravatt B. F. 2008. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 108, 1687–1707 10.1021/cr0782067 (doi:10.1021/cr0782067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katona I., Freund T. F. 2008. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14, 923–930 10.1038/nm.f.1869 (doi:10.1038/nm.f.1869) [DOI] [PubMed] [Google Scholar]
- 88.Bertrand T., et al. 2010. Structural basis for human monoglyceride lipase inhibition. J. Mol. Biol. 396, 663–673 10.1016/j.jmb.2009.11.060 (doi:10.1016/j.jmb.2009.11.060) [DOI] [PubMed] [Google Scholar]
- 89.Labar G., Bauvois C., Borel F., Ferrer J. L., Wouters J., Lambert D. M. 2009. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem 11, 218–227 10.1002/cbic.200900621 (doi:10.1002/cbic.200900621) [DOI] [PubMed] [Google Scholar]
- 90.Blankman J. L., Simon G. M., Cravatt B. F. 2007. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14, 1347–1356 10.1016/j.chembiol.2007.11.006 (doi:10.1016/j.chembiol.2007.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bisogno T., Ortar G., Petrosino S., Morera E., Palazzo E., Nalli M., Maione S., Di Marzo V. 2009. Development of a potent inhibitor of 2-arachidonoylglycerol hydrolysis with antinociceptive activity in vivo. Biochim. Biophys. Acta 1791, 53–60 10.1016/j.bbalip.2008.10.007 (doi:10.1016/j.bbalip.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 92.Long J. Z., et al. 2009. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 5, 37–44 10.1038/nchembio.129 (doi:10.1038/nchembio.129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kinsey S. G., Long J. Z., O'Neal S. T., Abdullah R. A., Poklis J. L., Boger D. L., Cravatt B. F., Lichtman A. H. 2009. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J. Pharmacol. Exp. Ther. 330, 902–910 10.1124/jpet.109.155465 (doi:10.1124/jpet.109.155465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guindon J., Guijarro A., Piomelli D., Hohmann A. G. 2011. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br. J. Pharmacol. 163, 1464–1478 10.1111/j.1476-5381.2010.01192.x (doi:10.1111/j.1476-5381.2010.01192.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spradley J. M., Guindon J., Hohmann A. G. 2010. Inhibitors of monoacylglycerol lipase, fatty-acid amide hydrolase and endocannabinoid transport differentially suppress capsaicin-induced behavioral sensitization through peripheral endocannabinoid mechanisms. Pharmacol. Res. 62, 249–258 10.1016/j.phrs.2010.03.007 (doi:10.1016/j.phrs.2010.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fiskerstrand T., et al. 2010. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 87, 410–417 10.1016/j.ajhg.2010.08.002 (doi:10.1016/j.ajhg.2010.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Long J. Z., Nomura D. K., Cravatt B. F. 2009. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem. Biol. 16, 744–753 10.1016/j.chembiol.2009.05.009 (doi:10.1016/j.chembiol.2009.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schlosburg J. E., et al. 2010. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 13, 1113–1119 10.1038/nn.2616 (doi:10.1038/nn.2616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chanda P. K., et al. 2010. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol. Pharmacol. 78, 996–1003 10.1124/mol.110.068304 (doi:10.1124/mol.110.068304) [DOI] [PubMed] [Google Scholar]
- 100.Ghosh S., Wise L. E., Chen Y., Gujjar R., Mahadevan A., Cravatt B. F., Lichtman A. H. 2012. The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci. 10 10.1016/j.lfs.2012.06.020 (doi:10.1016/j.lfs.2012.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lichtman A. H., Sheikh S. M., Loh H. H., Martin B. R. 2001. Opioid and cannabinoid modulation of precipitated withdrawal in Δ9-tetrahydrocannabinol and morphine-dependent mice. J. Pharmacol. Exp. Ther. 298, 1007–1014 [PubMed] [Google Scholar]
- 102.Fowler C. J. 2007. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br. J. Pharmacol. 152, 594–601 10.1038/sj.bjp.0707379 (doi:10.1038/sj.bjp.0707379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guay J., Bateman K., Gordon R., Mancini J., Riendeau D. 2004. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J. Biol. Chem. 279, 24 866–24 872 10.1074/jbc.M403106200 (doi:10.1074/jbc.M403106200) [DOI] [PubMed] [Google Scholar]
- 104.Hay C., de Belleroche J. 1997. Carrageenan-induced hyperalgesia is associated with increased cyclo-oxygenase-2 expression in spinal cord. Neuroreport 8, 1249–1251 10.1097/00001756-199703240-00038 (doi:10.1097/00001756-199703240-00038) [DOI] [PubMed] [Google Scholar]
- 105.Samad T. A., Moore K. A., Sapirstein A., Billet S., Allchorne A., Poole S., Bonventre J. V., Woolf C. J. 2001. Interleukin-1beta-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410, 471–475 10.1038/35068566 (doi:10.1038/35068566) [DOI] [PubMed] [Google Scholar]
- 106.Okubo M., Yamanaka H., Kobayashi K., Noguchi K. 2009. Leukotriene synthases and the receptors induced by peripheral nerve injury in the spinal cord contribute to the generation of neuropathic pain. Glia 58, 599–610 10.1002/glia.20948 (doi:10.1002/glia.20948) [DOI] [PubMed] [Google Scholar]
- 107.Kozak K. R., Crews B. C., Morrow J. D., Wang L. H., Ma Y. H., Weinander R., Jakobsson P. J., Marnett L. J. 2002. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 277, 44 877–44 885 10.1074/jbc.M206788200 (doi:10.1074/jbc.M206788200) [DOI] [PubMed] [Google Scholar]
- 108.Kozak K. R., Crews B. C., Ray J. L., Tai H. H., Morrow J. D., Marnett L. J. 2001. Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and in vivo. J. Biol. Chem. 276, 36 993–36 998 10.1074/jbc.M105854200 (doi:10.1074/jbc.M105854200) [DOI] [PubMed] [Google Scholar]
- 109.Yu M., Ives D., Ramesha C. S. 1997. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J. Biol. Chem. 272, 21 181–21 186 10.1074/jbc.272.34.21181 (doi:10.1074/jbc.272.34.21181) [DOI] [PubMed] [Google Scholar]
- 110.Sang N., Zhang J., Chen C. 2006. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. J. Physiol. 572, 735–745 10.1113/jphysiol.2006.105569 (doi:10.1113/jphysiol.2006.105569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gatta L., Piscitelli F., Giordano C., Boccella S., Lichtman A., Maione S., Di Marzo V. 2012. Discovery of prostamide F2α and its role in inflammatory pain and dorsal horn nociceptive neuron hyperexcitability. PLoS ONE 7, e31111. 10.1371/journal.pone.0031111 (doi:10.1371/journal.pone.0031111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vandevoorde S., Lambert D. M. 2007. The multiple pathways of endocannabinoid metabolism: a zoom out. Chem. Biodivers. 4, 1858–1881 10.1002/cbdv.200790156 (doi:10.1002/cbdv.200790156) [DOI] [PubMed] [Google Scholar]
- 113.Re G., Barbero R., Miolo A., Di Marzo V. 2007. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: potential use in companion animals. Vet. J. 173, 21–30 10.1016/j.tvjl.2005.10.003 (doi:10.1016/j.tvjl.2005.10.003) [DOI] [PubMed] [Google Scholar]
- 114.Jhaveri M. D., et al. 2008. Inhibition of fatty acid amide hydrolase and cyclooxygenase-2 increases levels of endocannabinoid related molecules and produces analgesia via peroxisome proliferator-activated receptor-alpha in a model of inflammatory pain. Neuropharmacology 55, 85–93 10.1016/j.neuropharm.2008.04.018 (doi:10.1016/j.neuropharm.2008.04.018) [DOI] [PubMed] [Google Scholar]
- 115.Costa B., Comelli F., Bettoni I., Colleoni M., Giagnoni G. 2008. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 139, 541–550 10.1016/j.pain.2008.06.003 (doi:10.1016/j.pain.2008.06.003) [DOI] [PubMed] [Google Scholar]
- 116.Sagar D. R., Kendall D. A., Chapman V. 2008. Inhibition of fatty acid amide hydrolase produces PPAR-alpha-mediated analgesia in a rat model of inflammatory pain. Br. J. Pharmacol. 155, 1297–1306 10.1038/bjp.2008.335 (doi:10.1038/bjp.2008.335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Snider N. T., Walker V. J., Hollenberg P. F. 2010. Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications. Pharmacol. Rev. 62, 136–154 10.1124/pr.109.001081 (doi:10.1124/pr.109.001081) [DOI] [PMC free article] [PubMed] [Google Scholar]