Abstract
Background
There are limited data on the phenotypic differences between patients with hereditary hemochromatosis (HH) and other forms of iron overload.
Aims
To describe and compare patients suspected of having iron overload disease.
Methods
Patients were evaluated at a university iron overload clinic over a 5-year period. Biochemical and clinical profiles of patients with HH and non-HH causes of suspected iron overload were retrospectively compared.
Results
A total of 270 patients were evaluated during the enrollment period, and 137 (51%) were diagnosed with HH. The most common reasons for referral were elevated serum iron markers (155 patients), followed by positive family history (40 patients), and known HH (75 patients). In patients without HH referred for suspected iron overload, the most common diagnoses were nonalcoholic fatty liver disease (NAFLD) (24%), chronic hepatitis C infection (14%), and alcohol related liver disease (9%). Of the patients with HH, 108 were C282Y homozygotes, 20 were compound heterozygotes (C282Y/H63D), and nine had neither mutation. The following clinical characteristics were significantly different (p < 0.05) between patients with HH and all other referred patients: arthralgia (42 vs. 16%) and decreased libido (11 vs. 4%). There was a nonsignificant trend towards increased fatigue (44 vs. 33%), diabetes (10 vs. 6%), impotence (8 vs. 4%), and hypothyroidism (10 vs. 6%) in the HH group.
Conclusions
(1) A large proportion of patients referred for suspected iron overload have diagnoses other than HH. (2) NAFLD, chronic hepatitis C, and chronic alcohol use were the most common alternative diagnoses. (3) Arthralgia and fatigue are the most common symptoms among patients with HH.
Keywords: Hemochromatosis, Iron overload, Liver disease
Background
Acquired causes of iron overload such as chronic viral hepatitis, thalassemia, and alcoholic liver disease are more common than hereditary hemochromatosis (HH); however, HH is the best described inherited disorder of iron metabolism. Aberrant regulation of iron in this inherited disorder leads to increased absorption and may cause widespread end-organ damage. The classic HH phenotype includes abnormal biochemical markers and features such as arthropathy involving the second and third metacarpophalangeal joint, skin hyperpigmentation, diabetes, liver fibrosis, cirrhosis, and hepatocellular carcinoma. The majority of Caucasian patients with the HH phenotype carry the homozygous C282Y mutation in the HFE gene; up to 80% of C282Y homozygotes develop abnormal iron indices, but there is a poor correlation with significant disease and many subjects remain clinically silent [1].
Different estimates of disease expression have been reported, ranging from as few as 2% of C282Y homozygotes to 28% because of variable penetrance, expressivity, and definition of disease related to iron overload [2, 3]. The initial symptoms of excess body iron are vague and may include lethargy, abdominal pain, and loss of libido, but are often not considered iron-overload-related disease because they are nonspecific [4]. Therefore, early diagnosis of hemochromatosis requires a high degree of clinical suspicion and appropriate use of screening and confirmatory tests by practicing clinicians.
Although serum ferritin (SF) is an acute phase reactant, it can also be a reliable indicator of total body iron stores and prompt diagnostic evaluation. It is used in conjunction with fasting transferrin-iron saturation (TS) to diagnose HFE-associated HH [5]. Unlike TS, elevated SF is associated with numerous secondary iron overload syndromes and has been considered to be the single, best screening tool for assessment of risk for end-organ damage related to iron overload [6]. Among patients with HH, SF levels greater than 1,000 μg/l are more likely to be associated with fatigue and other symptoms attributed to iron accumulation and are also linked to an increased risk of advanced hepatic fibrosis [7].
There are limited data about the clinical features and phenotypic characteristics of patients referred for evaluation of iron overload. It has been proposed that liver disorders such as chronic hepatitis C, nonalcoholic fatty liver disease, and liver disease related to alcohol may also result in elevated serum iron markers, but there are limited data describing the frequency with which these disorders might lead to clinical suspicion of iron overload. Furthermore, there are few reports in the literature comparing the clinical presentation of patients with HH to these other diseases with regard to symptoms or signs related to iron overload.
Methods
The records of patients referred to a dedicated iron overload clinic (IOC) at an academic teaching hospital were retrospectively reviewed over a 5-year period. The IOC was created as a referral center for patients with known or suspected iron overload based on abnormal serum iron markers. A detailed routine clinical intake form including review of systems was completed by all patients as part of the medical record. Records were also reviewed for physical examination findings, liver biopsy, and laboratory results. Gender, age, reason for referral, HFE genotypes, hepatic iron index (HII), possible disease related characteristics, final diagnoses, and laboratory values, i.e., transferrin saturation (TS) and serum ferritin (SF), were collected and compared between groups classified by diagnosis. Patients were stratified into two groups based on final diagnosis: HH and non-HH. A diagnosis of HH was made on the basis of HFE genotype or a hepatic iron index greater than 1.9. For comparing biochemical profiles, the non-HH group was further stratified into chronic hepatitis C and nonalcoholic fatty liver disease (NAFLD). Diabetes was based on a history of fasting hyperglycemia (glucose > 122 mg/dl) or glucose intolerance controlled by diet, oral hypoglycemic agents, or insulin.
Mean SF levels, TS levels, and hepatic iron indices (HII) were compared using Student’s t-test. A Chi-square test was used to compare proportions. Statistics were analyzed using Analyse-It (Analyse-it Software, Ltd). The study was approved by the Institutional Review Board at the University of Washington.
Results
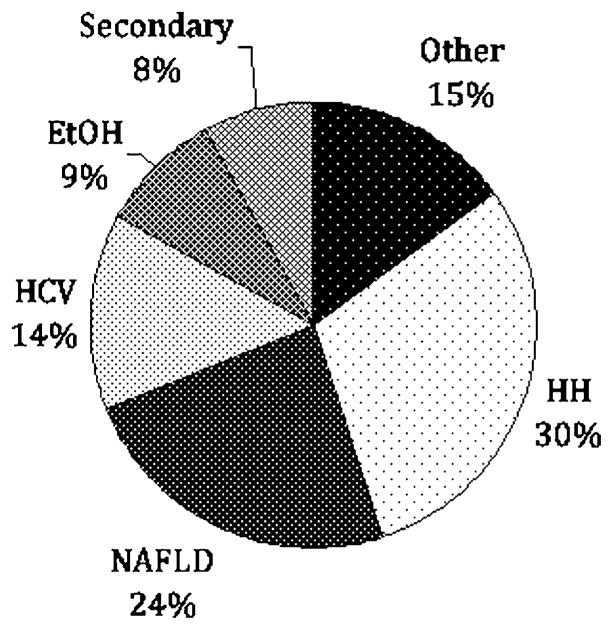
A total of 270 patients were evaluated during the study period. Reasons for referral included elevated serum iron markers in 155 patients (57%), known history of HH in 75 patients (28%), and family history of HH in 40 (15%) (Fig. 1). Among those patients referred with a family history of hemochromatosis, 24 of 40 (60%) were found to have HH based on the criteria noted above. The remaining 16 patients either tested negative for the characteristic HFE mutations (12 patients) or had normal iron studies on initial evaluation (four patients). Among the 155 patients referred because of elevated iron studies, 46 were found to have HH based on genetic testing (43 patients) or elevated hepatic iron index (three patients). For the remaining 109 patients, the most common diagnoses were NAFLD in 37 patients (24%), hepatitis C in 22 patients (14%), and alcohol related liver disease in 14 patients (9%) (Fig. 2). Final diagnoses for all patients included in this study are shown in Fig. 3.
Fig. 1.
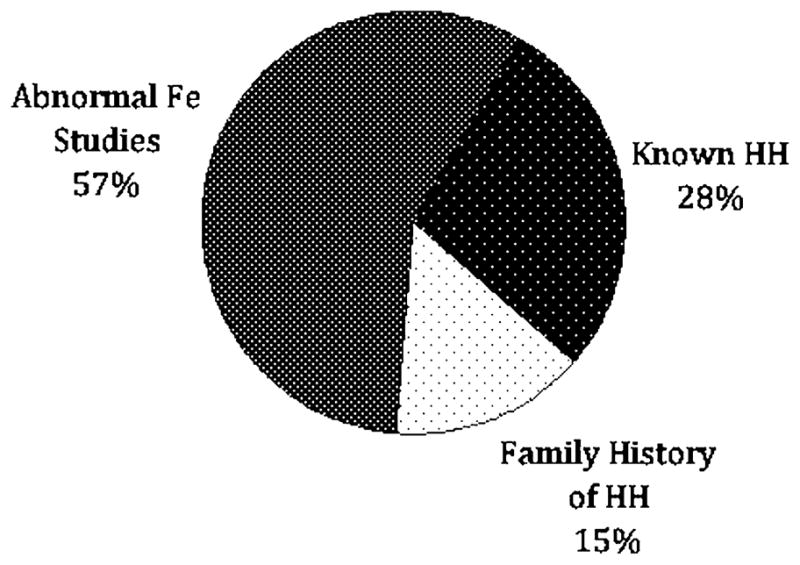
Reasons for patient referral (n = 270). Fe iron, HH hereditary hemochromatosis
Fig. 2.
Diagnoses in patients (n = 155) referred for elevated iron studies. HH hereditary hemochromatosis, NAFLD nonalcoholic fatty liver disease, HCV hepatitis C, EtOH alcoholic liver disease, Secondary secondary iron overload (hemolytic anemia, G6PD, sideroblastic anemia, thalassemia), Other autoimmune hepatitis, osteoarthritis, hepatitis B, hyperferritinemia cataract syndrome, Wilson’s disease, unknown (three patients), no disease (eight patients)
Fig. 3.
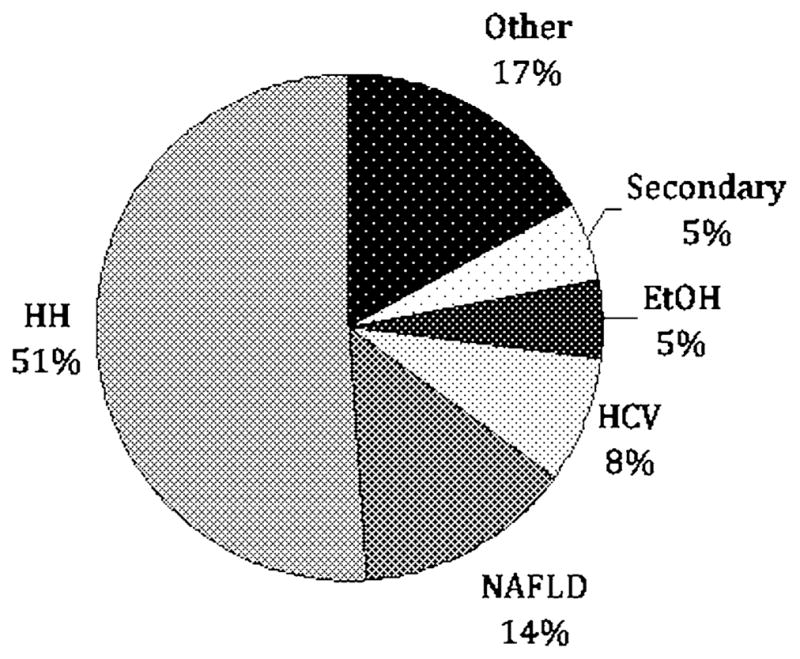
Final diagnoses of patients (n = 270) referred to an IOC. HH hereditary hemochromatosis, NAFLD nonalcoholic fatty liver disease, HCV hepatitis C, EtOH alcoholic liver disease, Secondary secondary iron overload (hemolytic anemia, G6PD, sideroblastic anemia, thalassemia), Other autoimmune hepatitis, osteoarthritis, hepatitis B, hyperferritinemia cataract syndrome, Wilson’s disease, unknown (three patients), no disease (24 patients)
The mean SF level for patients with HH was 726 μg/l and was significantly different than those with hepatitis C (648 μg/l) and NAFLD (610 μg/l) (Table 1). The mean TS level for patients with HH was 73% and was significantly different than those with hepatitis C (55%) and NAFLD (39%). The mean hepatic iron index for patients with HH was 3.0 compared to 0.8 for hepatitis C patients, and 0.6 for NAFLD patients. HH patients who did not have the homozygous C282Y mutation were more likely to have steatosis or chronic hepatitis on liver histology compared to C282Y homozygotes, although we were unable to perform statistical comparisons because of the small number of subjects with non-HFE HH.
Table 1.
Comparison of biochemical profiles between HH and non-HH patients
| Mean values | HH (n = 137) | HCV (n = 22) | NAFLD (n = 37) |
|---|---|---|---|
| Ferritin (μg/l) | 726 | 648 (p < .05) | 610 (p < .05) |
| Transferrin saturation (%) | 73 | 55 (p < .05) | 39 (p < .05) |
| Hepatic iron index | 3.0 | 0.8 (p < .05) | 0.6 (p < .05) |
HH hereditary hemochromatosis, HCV hepatitis C, NAFLD nonalcoholic fatty liver disease
HH was diagnosed in 137 of 270 patients (51%) either by HFE gene testing (132 patients) or elevated hepatic iron index on liver biopsy (five patients). These 137 patients included 78 men and 64 women with average ages of 45 and 47 years, respectively. For the HH patients, 108 (79%) were homozygous for the C282Y mutation, 20 (15%) were compound heterozygotes for C282Y and H63D, four (3%) were H63D homozygotes, and five (4%) were wild type for both the C282Y and H63D mutations. Of the 137 patients with HH, 86 (63%) underwent phlebotomy treatment. The remaining HH patients did not have excessive iron stores to warrant phlebotomy.
The clinical symptoms and physical exam findings of all patients were assessed at presentation (Fig. 4). Among patients with HH and non-HH causes of suspected iron overload, fatigue was the most common symptom. For patients with HH compared to those with other diagnoses, there was a significant difference in both arthralgia (42 vs. 16%) and decreased libido (11 vs. 4%). There was a nonsignificant trend in the HH group towards unexplained fatigue (44 vs. 33%), diabetes (10 vs. 6%), impotence (8 vs. 4%), and hypothyroidism (10 vs. 6%). There was no difference in abdominal pain (12 vs. 11%), infertility (2 vs. 1%), bronzing (3 vs. 2%), low testosterone levels (2 vs. 2%), or congestive heart failure (4 vs. 3%).
Fig. 4.
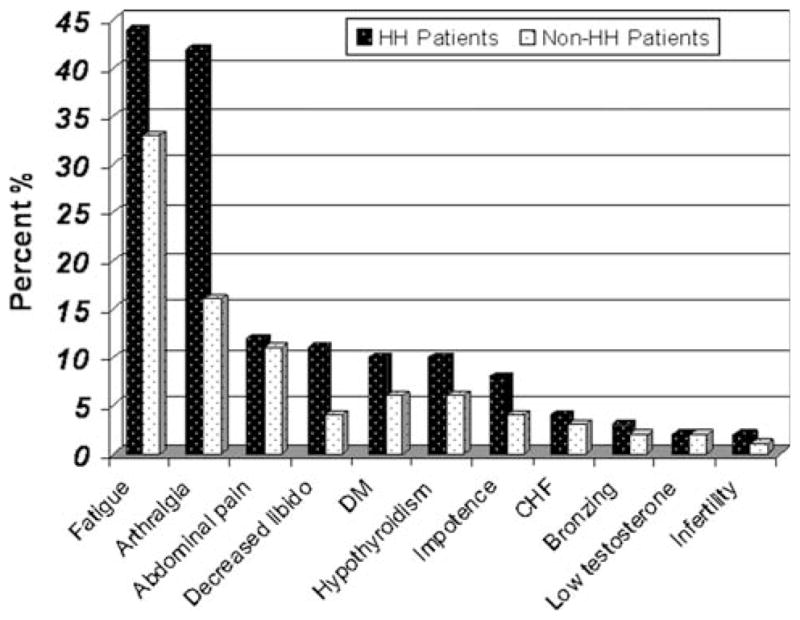
HH hereditary hemochromatosis, DM diabetes mellitus, CHF congestive heart failure
Discussion
Since the discovery of the HFE gene, a number of subsequent studies have examined the relationship between genotype and phenotype in individual cohorts as well as in cross-sectional population-based samples [8]. The current study is unique in that we describe a large series of patients ascertained by referral to a dedicated academic clinic focused on the management of iron overload disorders. As such, our cohort has identified that after HH, chronic hepatitis C, alcoholic and nonalcoholic fatty liver disease are the most common causes of elevated serum iron markers that lead to referral for suspected iron overload. Approximately half (51%) of all our patients had a final diagnosis of HH but of the patients referred because of abnormal iron studies, 70% had alternative diagnoses. NAFLD/NASH was the second most common diagnosis after HH and is now recognized to be an important cause of hyperferritinemia [9, 10].
We have also identified several clinical features that were more common among patients with HH compared to those with other reasons for elevated serum iron markers. Similar to previous reports, we also identified a high prevalence of arthralgia and fatigue among our HH patients, even when compared patients with other diagnoses. Furthermore, a high proportion of patients referred for suspected hemochromatosis had other reasons for elevated serum iron indices. Our study reinforces the importance of confirmatory tests such as HFE gene testing to establish the diagnosis of HH and the importance of liver biopsy to identify these other causes of elevated serum TS and ferritin among patients who do not carry the characteristic mutations diagnostic of HH. Our study is also unique in describing the symptoms frequently reported among patients referred for suspected iron overload and in examining the differences in the prevalence of symptoms attributable to iron overload among patients with and without HH.
HH subjects incurred a higher frequency of numerous signs and symptoms similar to their biochemical profiles, which were significantly different compared to other liver diseases. Fatigue, arthralgia, abdominal pain, and low libido have been reported to be the earliest and most common symptoms of HH and were also the most common in our cohort. We also showed that 79% of HH patients were homozygous for C282Y, which is consistent with other studies citing 70–100% of patients were positive for the two mutant alleles [11, 12].
We recognize that the current study has several limitations. Our sample included a small number of HH patients without HFE mutations and thus lacked sufficient power to adequately determine differences between HFE and non-HFE HH groups. Other possible limitations include the retrospective study design and lack of comprehensive information on all subjects. We did not collect and compare data on serum liver biochemical tests.
In summary, this study found that arthralgia and fatigue are the most common symptoms among patients with HH referred for clinical evaluation and that a large proportion of patients referred for further evaluation of elevated serum iron markers have alternative diagnoses, the majority of which can be diagnosed by liver biopsy. We suggest that clinicians be alert for other causes of elevated serum TS and SF among patients who lack the C282Y homozygous mutation.
Acknowledgments
This study was supported in part by NIH grant DK02957 to Kris V. Kowdley, MD.
Footnotes
Authors’ contributions J.B.D. contributed to the design, provided intellectual content, created the figures and table, and drafted the manuscript. M.A.M., J.E.M., and D.W. contributed equally by collecting and analyzing the data. K.V.K. designed the study, revised the manuscript, and provided important intellectual content. All authors read and approved the final manuscript.
Competing Interests The authors declare that they have no competing interests.
Contributor Information
John B. Dever, Email: deverjohn@hotmail.com, Digestive Disease Institute, Liver Center of Excellence, Virginia Mason Medical Center, 1201 9th Ave, Seattle, WA, USA.
Mark A. Mallory, Email: markma89@yahoo.com, University of Washington School of Medicine, Seattle, WA, USA.
Julie E. Mallory, Email: julesmallory@gmail.com, Williams College, Williamstown, MA, USA.
Dorothy Wallace, Email: Dorothy.Wallace@vmmc.org, Digestive Disease Institute, Liver Center of Excellence, Virginia Mason Medical Center, 1201 9th Ave, Seattle, WA, USA.
Kris V. Kowdley, Email: kris.kowdley@vmmc.org, kkowdley@u.washington.edu, Digestive Disease Institute, Liver Center of Excellence, Virginia Mason Medical Center, 1201 9th Ave, Seattle, WA, USA. University of Washington School of Medicine, Seattle, WA, USA.
References
- 1.Gurrin LC, Osborne NJ, Constantine CC, et al. The natural history of serum iron indices for HFE C282Y homozygosity associated with hereditary hemochromatosis. Gastroenterology. 2008;135:1945–1952. doi: 10.1053/j.gastro.2008.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E, Felitti JVJ, Koziol JA, et al. Penetrance of the 845G → A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359:211–218. doi: 10.1016/S0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- 3.Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 4.Yen AW, Fancher TL, Bowlus CL. Revisiting hereditary hemochromatosis: current concepts and progress. Am J Med. 2006;119:391–399. doi: 10.1016/j.amjmed.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Alexander J, Kowdley KV. HFE-associated hereditary hemochromatosis. Genet Med. 2009;11:307–313. doi: 10.1097/GIM.0b013e31819d30f2. [DOI] [PubMed] [Google Scholar]
- 6.Waalen J, Felitti VJ, Gelbart T, et al. Screening for hemochromatosis by measuring ferritin levels: a more effective approach. Blood. 2008;111:3373–3376. doi: 10.1182/blood-2007-07-102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison ED, Brandhagen DJ, Phatak PD, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med. 2003;138:627–633. doi: 10.7326/0003-4819-138-8-200304150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Koziol JA, Ho NJ, Felitti VJ, et al. Reference centiles for serum ferritin and percentage of transferrin saturation, with application to mutations of the HFE gene. Clin Chemo. 2001;47:1804–1810. [PubMed] [Google Scholar]
- 9.Wong K, Adams PC. The diversity of liver diseases among outpatient referrals for an elevated serum ferritin. Can J Gastroenterol. 2006;20:467–470. doi: 10.1155/2006/357340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licata A, Nebbia ME, Cabibbo G, et al. Hyperferritinemia is a risk factor for steatosis in chronic liver disease. World J Gastroenterol. 2009;15:2132–2138. doi: 10.3748/wjg.15.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 12.Jouanolle AM, Fergelot P, Gandon G, et al. A candidate gene for hemochromatosis: frequency of the C282Y and H63D mutations. Hum Genet. 1997;100:544–547. doi: 10.1007/s004390050549. [DOI] [PubMed] [Google Scholar]