Abstract
Background
The efficacy of screening and surveillance EGD for esophageal adenocarcinoma (EAC) is controversial.
Objective
To examine the effect of an EGD before the diagnosis of EAC on survival after the diagnosis of cancer among patients with gastroesophageal reflux (GER).
Design
A retrospective, controlled cohort study.
Subjects
The national administrative databases of the Veterans Affairs were accessed, and patients diagnosed with EAC, from 1995 through 2003, who had a prior diagnosis consistent with GER were identified. Electronic medical records were then abstracted. Cases were subjects who had an EGD performed between 1 and 5 years before the diagnosis of EAC; controls were those subjects without a prior EGD.
Results
A total of 155 subjects with EAC and GER were identified. Cases with a history of an EGD at least 1 year before a diagnosis of EAC (n =25) were diagnosed at earlier stages than those without a prior EGD (P =.02) but did not experience a significant improvement in survival (adjusted hazard ratio 0.93 [95% CI, 0.58–1.50]). Cases who had been enrolled in surveillance programs that adhered to published guidelines trended toward improved survival, but long-term survival reverted toward the rate found without any surveillance.
Conclusions
A prior EGD was associated with an improved stage at the diagnosis of EAC but did not alter long-term survival. In the absence of prospective, randomized, controlled trials, the benefit of screening and surveillance to decrease mortality from EAC cannot be confirmed.
The incidence of esophageal adenocarcinoma (EAC) is rising faster than that of any other cancer in the United States and many other westernized nations.1,2 Barrett’s esophagus (BE) is the accepted precursor of EAC,3–5 and gastroesophageal reflux (GER) increases the risk of both BE and EAC.3,4 It is commonly accepted that GER leads to the development of BE in some individuals, and BE then progresses to low-grade and high-grade dysplasia before developing into invasive cancer in a subset of patients.4 A number of retrospective studies suggested that a prior EGD among patients with EAC is associated with an earlier stage of cancer at the time of initial diagnosis and improved survival.6–11 Therefore, multiple gastroenterology societies recommend screening patients with GER symptoms for BE and EAC, and repeated surveillance of patients who have BE.4,12–14 Because of the limitations of the previous studies, including potential lead-time bias, length-time bias, and selection bias, the efficacy of screening and surveillance remains controversial and is not uniformly recommended.15–17
To warrant the expense of endoscopic surveillance in the population of patients with GER, a strategy of screening and surveillance ought to be clearly effective in improving outcomes from EAC. Our primary hypothesis was that, among patients with GER and EAC, a history of a screening EGD would be associated with a more favorable stage at the time of diagnosis, an increased likelihood of surgical resection, and improved long-term survival after the diagnosis of cancer compared with patients with GER and EAC but with no prior EGD. We also hypothesized that adherence to published guidelines for the interval of surveillance would be associated with improved outcomes from EAC among patients with GER and documented BE.
PATIENTS AND METHODS
Databases
Subjects were retrospectively identified within the United States Department of Veterans Affairs (VA) National Patient Care Datasets (NPCD). The NPCD is a computerized administrative database that includes all inpatient admissions at any VA hospital throughout the country since 1970 and all outpatient encounters within the VA system since 1990.
Identification of subjects
Veterans were initially identified who were diagnosed with adenocarcinoma of the distal third of the esophagus or of the gastric cardia (International Classification of Diseases [ICD] 151.0) from 1995 through 2003, and who had gastroesophageal reflux (GER) diagnosed (ICDs 530.10–530.12, 530.81, or 787.1) before the diagnosis of cancer. Potential subjects were excluded if they did not have at least one admission or outpatient encounter in each of the 5 years before the cancer diagnosis. The electronic medical records of each potential subject were accessed via remote Internet access upon approval of the institutional review board of the VA Ann Arbor Healthcare System. The histology and location of the cancer were abstracted based on pathology, surgery, and endoscopy reports. The subjects without EAC (such as gastric cardia adenocarcinoma, which shares the same ICD code as EAC) were excluded based on the review of the electronic medical records. Subjects with dysplasia but no evidence of EAC were excluded. Electronic medical records were also abstracted for the date of pathologic diagnosis, stage at diagnosis, and date of death.
Comorbidities and upper endoscopies
Comorbid diagnoses during the 1 year before the diagnosis of cancer were collected from the NPCD.18–20 Diagnostic EGDs that were performed between 5 years and 1 year before the diagnosis of cancer were identified by using the NPCD (Current Procedural Terminology codes: 43200, 43202, 43221, 43222, 43234, 43235, 43239; or ICD-9 procedural codes: 42.23, 42.24, 44.13, 45.13, 45.16). In the primary analysis, cases were defined as eligible subjects who had an EGD between 1 and 5 years before the diagnosis of EAC, and controls were eligible subjects without a prior EGD in that time frame. If available, all prior endoscopic and histologic findings were abstracted from the electronic medical records. Subjects with BE were assessed as nonadherent with the 2002 American College of Gastroenterology (ACG) guidelines for surveillance of BE if they had a prior EGD, but they were overdue for surveillance at the time of cancer diagnosis based on the most recent histologic findings.4
RESULTS
Subject population with EAC
A total of 311 subjects were identified with GER and a billing diagnosis consistent with EAC and who had been active in the NPCD for each of the 5 years before the diagnostic code of cancer. On review of the electronic medical records, subjects were excluded if their cancer type was other than EAC (133), if they had BE but no documented evidence of cancer (5), if there were insufficient electronic medical records to validate a cancer diagnosis (16), or if the EAC was diagnosed before 1995 (2).
The resulting cohort contained 155 subjects with GER and EAC; 99% were men, 84% were white, 5% were His-panic, 3% were African American, and 8% were of unknown race or ethnicity. At the time of diagnosis, 15% were stage I, 37% stage II, 22% stage III, and 26% stage IV. Fifty-one percent of these subjects underwent surgical resection. As expected, the stage at diagnosis predicted survival (P < .0001). Five-year stage-specific survival (I, 42%; II, 12%; III, 6%; IV, 3%) was at least as good as for male patients with esophageal cancer who were enrolled in the Surveillance Epidemiology and End Results registry (localized, 29%; regional, 13%; distant, 2%).21 In the survival analysis, when using the Cox proportional hazard model, advancing age (hazard ratio [HR] 1.02 for each year [95% CI, 1.00–1.04]) and increasing number of comorbidities (HR 1.19 for each [95% CI, 1.05–1.36]) were also significant risk factors for death. Subjects who were undergoing surgical resection had improved survival (HR 0.45 [95% CI, 0.32–0.63]) compared with those not undergoing resection.
Influence of prior EGD on outcomes from EAC
Of the 155 subjects with GER who developed EAC, there were 25 patients (16%) who had undergone EGD between 1 and 5 years before the diagnosis of cancer (Fig. 1). Compared with the 130 controls with no history of a prior EGD, subjects with a prior EGD were older (72.0 vs 68.3 years, P =.08) and had fewer comorbidities (Charlson comorbidity score among those with a prior EGD and no prior EGD: 0, 48% and 29%; 1, 40% and 37%; 2 or more, 12% and 34%, respectively; P =.03). Subjects with a prior EGD were diagnosed at an earlier stage than those without a prior EGD (Fig. 2) and were more likely to undergo surgical resection (64% vs 48%, P =.16). However, there was no difference in long-term survival after the diagnosis of cancer among subjects compared with controls (HR 0.82 [95% CI, 0.52–1.29]) (Fig. 3, Table 1). Adjusting for age, comorbidities, and year of diagnosis yielded similar results (HR 0.93 [95% CI, 0.58–1.50]). Almost all deaths in both groups were because of EAC (82% in subjects with a prior EGD vs 92% in controls without a prior EGD, P =.23).
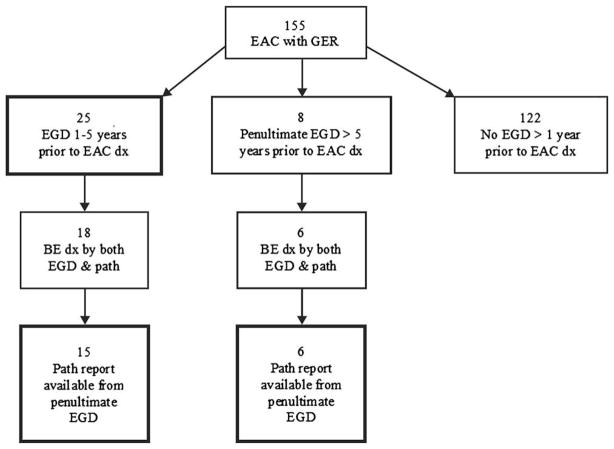
Figure 1.
Flow chart of endoscopic and histologic findings of prior EGDs. Twenty-five subjects had an EGD performed between 1 and 5 years before the diagnosis of EAC. These represent the cases in the primary analysis of the effect of a prior EGD on outcomes from EAC. Fifteen of these subjects had been diagnosed with BE, were confirmed by histology, and the pathology reports from their penultimate EGDs before the diagnosis of EAC were available. In 6 additional subjects with BE, the penultimate EGD occurred more than 5 years before the diagnosis of cancer. The analysis of adherence to guidelines included the 15 plus 6 subjects (21 total). dx, Diagnosis.
Figure 2.
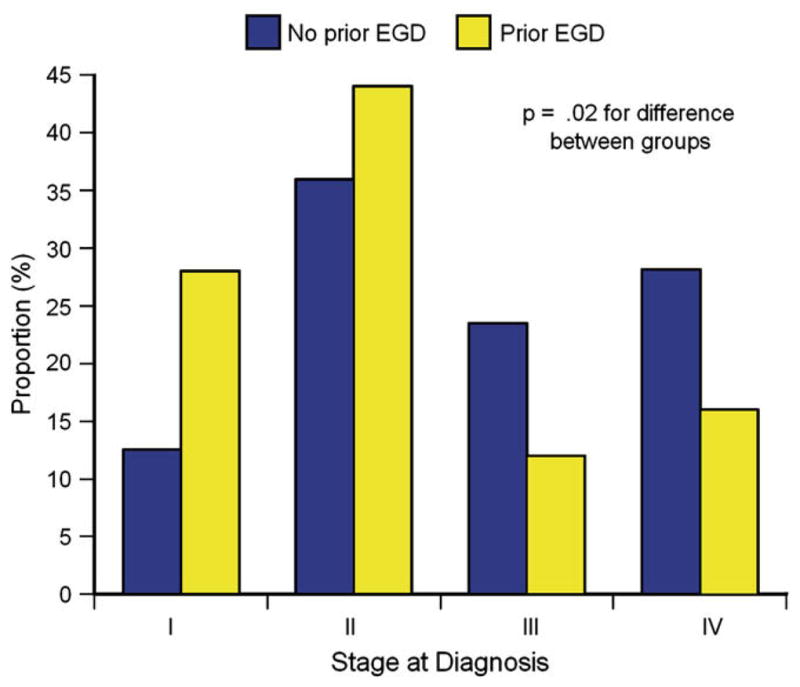
Stage at the time of diagnosis of cancer was more favorable in subjects who had undergone a prior EGD.
Figure 3.
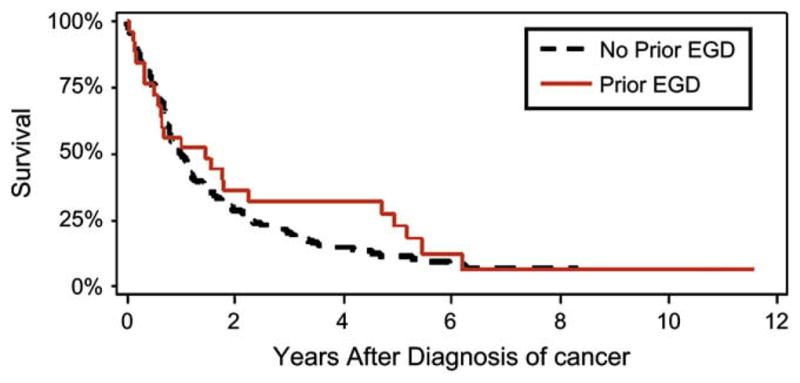
Kaplan-Meier survival analysis after diagnosis of EAC. There was no difference in survival between subjects who had received a prior EGD and controls who had not received a prior EGD. Vital status was determined, on average, 6.5 years after the diagnosis of EAC among subjects with no prior EGD (range 4.1–11.9 years), and 7.6 years among those with a prior EGD (range 4.1–11.8 years, P =.04 compared with cases).
TABLE 1.
Cox proportional HRs for the association between predictors and mortality
| Model* | Covariates | HR (95% CI) |
|---|---|---|
| A | Age (1-y increments)† | 1.02 (1.00–1.04) |
| B | Charlson comorbidity score | 1.19 (1.05–1.36) |
| C | Year of cancer diagnosis (1-y increments)† | 1.08 (0.98–1.19) |
| D | Surgical resection | 0.45 (0.32–0.63) |
| E | EGD 1–5 y before EAC dx | 0.82 (0.52–1.29) |
| F | Adherence with ACG guidelines | 0.46 (0.21–0.98) |
| G | EGD 1–5 y before EAC dx | 0.93 (0.58–1.50) |
| Age (1-y increments)† | 1.02 (1.00–1.03) | |
| Charlson comorbidity score | 1.17 (1.02–1.33) | |
| Year of cancer diagnosis (1-y increments)† | 1.07 (0.97– 1.18) | |
| H | Adherence with ACG surveillance guidelines | 0.52 (0.24–1.12) |
| Age (1-y increments)† | 1.01 (0.99–1.01) | |
| Charlson comorbidity score | 1.17 (1.03–1.34) | |
| Year of cancer diagnosis (1-y increments)† | 1.08 (1.04–1.21) |
dx, Diagnosis.
Each model (A–H) only included the covariates for which results are displayed (1–4 covariates in each model); for instance, model H included adherence, age, Charlson comorbidity score, and year of cancer diagnosis.
The HR for a continuous predictor is estimated for a specified difference in that exposure (indicated in parentheses after the predictor).
Diagnosis of BE before EAC
The electronic medical records were reviewed for each subject to determine whether BE had been diagnosed before the diagnosis of cancer (Fig. 1). Only 1 subject had cancer and BE diagnosed simultaneously by an initial screening EGD that was clearly documented to have been performed for screening purposes for longstanding GER, with no changes in symptoms or dysphagia. Of the 25 subjects with an EGD between 1 and 5 years before the diagnosis of cancer, 18 had an EGD and pathology reports both confirming the diagnosis of BE (Fig. 1), and, in 15 of those subjects, the pathology report from the penultimate EGD before the diagnosis of cancer was available for review. In an additional 8 subjects, the penultimate EGD occurred more than 5 years before the diagnosis of cancer, and the pathology report was available and confirmed the diagnosis of BE in 6. Thus, 21 subjects were confirmed to have a prior diagnosis of BE and also had pathology reports available from the penultimate EGD. These 21 subjects were used to examine the influence of adherence with guidelines of surveillance.
Adherence with guidelines of surveillance for BE
The 21 subjects with an EGD more than 1 year before the diagnosis of EAC were classified as adherent or nonadherent with the ACG guidelines based on the presence and severity of dysplasia on the penultimate EGD, and the interval between the penultimate EGD and the diagnosis of EAC (Fig. 4).4 The ACG guidelines recommend performing a surveillance EGD at 3-month intervals for high-grade dysplasia, 12 months for low-grade dysplasia, and 3 years for BE without dysplasia. Eleven subjects were considered nonadherent, because the interval was longer than recommended, and 10 were considered adherent. Sixty-five percent of all these subjects had no evidence of dysplasia on their first EGD, and only 40% of those adherent with surveillance had high-grade dysplasia found at any time before the diagnosis of EAC.
Figure 4.
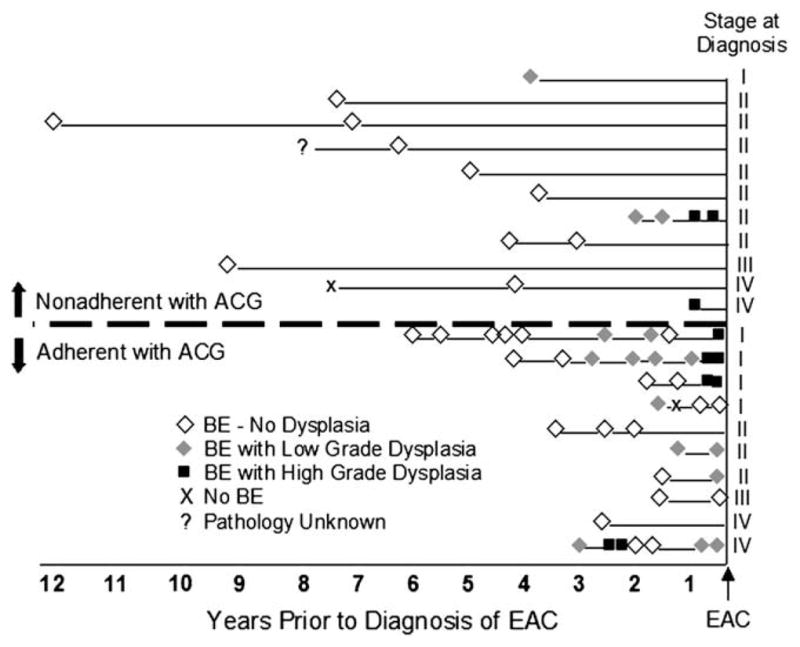
History of endoscopic surveillance of subjects with BE. The intersection at the right end reflects the date of diagnosis of cancer. Histologic findings from EGDs performed at various times before the diagnosis of cancer are reflected on timelines for each subject. The stage of cancer at the time of diagnosis is recorded for each subject at the end of the timeline. Subjects are stratified by adherence to the published guidelines of the ACG.
Influence of intensive surveillance of BE on outcomes
Adherence with guidelines was associated with a diagnosis at earlier stages (Table 2), but there was no difference in the likelihood of undergoing surgical resection (odds ratio [OR] 0.68 [95% CI, 0.17–2.7], adjusted for age and comorbidities). Subjects who had been adherent with the ACG guidelines appeared to be associated with better survival compared with those with no prior EGD, but this result lost statistical significance after adjustment for potential confounders (Table 1). Although long-term survival appeared better among cases adherent with ACG guidelines, it was still poor (19% at 5.4 years [95% CI, 0%–49%]) and appeared to approach the survival observed in the controls with no prior EGD (10% at 5.2 years [95% CI, 4%–15%]). Among subjects who did not undergo surgical resection, adherence with ACG guidelines still appeared to be associated with improved survival (HR 0.42 [95% CI, 0.16–1.09], when adjusting for age, comorbidities, and year of diagnosis).
TABLE 2.
Comparisons of EAC subjects adherent with surveillance versus EAC subjects without a prior EGD
| Factor | Adherent to surveillance (n Z 10) | No prior EGD (n Z 122) | P value |
|---|---|---|---|
| Mean (SD) | 69.5 ± 9.0 | 68.2 ± 9.8 | .69 |
| age (y) | |||
| Stage | .09 | ||
| I | 40% | 12% | |
| II | 30% | 35% | |
| III | 10% | 24% | |
| IV | 20% | 29% | |
| Cause of death: cancer | 71% | 91% | .15 |
DISCUSSION
We performed a retrospective cohort study by using the national administrative databases and the electronic medical records of the VA to evaluate the effect of a prior EGD on outcomes after the diagnosis of EAC. Although a prior EGD was associated with a more favorable cancer stage at the time of diagnosis, there was no overall effect on survival after a diagnosis of cancer. This is likely because of the poor survival from even locally staged EAC.
Our results differed from a previously published account that also used the VA NPCD but that did not use a manual medical record review; that study found that patients with GER who had died and who had had either EAC or gastric cardia cancer were less likely to have undergone a prior EGD than living GER controls.11 The investigators of that study expected that any survival benefit from EGD ought to be because of early diagnosis of cancer or dysplasia that led to an esophagectomy, but they found that none of the controls were diagnosed with nonfatal cancer or underwent an esophagectomy, which led them to raise the possibility that their results may have been because of unmeasured confounders or healthy volunteer bias. Although we cannot know with certainty why we found different results from their study, we think it is most likely because of the choice of controls. All of our cases and controls had cancer and, therefore, were likely more similar across many unmeasured confounders (such as severity of reflux, diet, smoking, and health-seeking behaviors), but they used control subjects who did not later develop cancer and may have differed in important respects from the subjects in unmeasured factors (eg, increased proportion with functional etiology of GER symptoms, more frequent visits to doctors, better diet, less smoking, more exercise) that may also be associated with the likelihood of requesting or being referred for an EGD. Still, these effects might not fully explain the differing results from our study. A prospective, randomized, controlled study of screening and surveillance is needed to address the efficacy of such a strategy.
By using the electronic medical records, which contain the endoscopic and histologic findings from prior EGDs, we were also able to stratify the results based on the quality of surveillance, as defined by adherence with the recommended interval between EGDs. Although this secondary analysis was limited by a small sample size, these results confirmed that subjects who underwent surveillance tended to be diagnosed at more favorable stages. Although subjects who had undergone surveillance at the recommended intervals trended toward improved survival, this advantage attenuated over the long-term toward the survival found among control subjects without any surveillance. This pattern suggests the presence of an underlying lead-time bias (increasing the proportion of life with known cancer, without actually extending the duration of life) rather than a true causal effect. This possibility is corroborated by the fact that adherence with guidelines was associated with a trend toward improved short-term survival, despite similar rates of surgical resection as controls who did not undergo a prior EGD. This pattern could also be explained by competing risk of death from causes other than esophageal cancer in this elderly population. However, we are unaware of any rational biological explanation for the finding that adherence was associated with better survival among subjects who did not undergo surgical resection. Another possible explanation for these findings is length-time bias (surveillance preferentially identifying slower-growing, less-fatal cancers rather than altering the duration of life).
Limitations of the current study, as well as of previous studies, include the possibility of ascertainment bias (controls may have received EGDs outside of the VA), and selection bias (healthier or more adherent patients requesting and being referred for EGD). We found that subjects who had undergone a prior EGD indeed had fewer comorbidities than those who did not, but they were also slightly older; we adjusted for these factors in the survival analyses, but there may have been differences in other important unmeasured factors related to selection effects. We minimized the chances of ascertainment bias by limiting subjects to those who were continuously active within the VA system and by using 2 methods for ascertaining EGDs (manual electronic chart review plus billing data that would likely capture most EGDs performed outside the VA, because the VA would usually be billed for eligible expenses by civilian facilities for services that a local VA is not capable of providing). Although ascertainment bias could explain a negative finding, it would not explain the equally poor survival among subjects and controls if a prior EGD was in fact effective. If subjects whom we had classified as not having received prior EGDs had them performed at outside hospitals and these prior EGDs actually improved survival from EAC, then one would expect better survival than we observed in both groups: those classified as having prior EGDs in the VA and those classified as not having prior EGDs in the VA.
In addition, our results might not be generalizable to patients without GER who are found to have BE (because all of our subjects had GER), patients with GER and with rarer encounters with health care facilities (because we excluded patients without an encounter every year) or to the general population (because our subjects all received care in the VA medical system). However, the stage-specific survivals of our subjects were at least as good as among patients enrolled in the national Surveillance Epidemiology and End Results cancer registry. Potential confounders that we were not able to control for were the number of biopsy specimens obtained or the quality of endoscopic or pathologic interpretation. There may have been additional unmeasured confounders. Also, the study may have been underpowered to detect a survival advantage for subjects who were adherent with surveillance guidelines; however, even if such an advantage existed, the absolute survival still appeared to be poor (19% survival at 5.4 years). Although there may have been biases toward the null in this study, and the study may be limited by sample size, the effectiveness of surveillance ought to be clearly evident to justify the expense of endoscopic surveillance for the large population with GER. In the absence of such clear evidence, prospective, randomized, controlled studies of surveillance are required to guide policy.
Our study contained a number of favorable features, including a national population-based design rather than a single-referral center experience, robust classification of tumor type and ascertainment of outcome, long follow-up, and access to medical records, including prior EGDs and pathology reports. In contrast to the prior studies on this topic, we focused our examination on subjects with documented invasive cancer and excluded subjects who had dysplasia. In addition, we limited our study population to subjects who had a diagnosis of GER before the diagnosis of their cancer: the group of people who are recommended for screening by published guidelines.4,12,22 There is no standardized care across the VA about whether to perform screening or surveillance, or in what time intervals. Furthermore, the updated ACG guidelines were published toward the end of the time period studied.4 These facts allowed for substantial variation among practice patterns and, therefore, the ability to compare different practices.
In conclusion, although surveillance for EAC is associated with more favorable stage cancer at the time of diagnosis, we did not find a significant improvement in long-term survival after the diagnosis of cancer. Surveillance with short intervals may be associated with slightly improved survival, but this is likely mostly because of lead-time and length-time effects, rather than a true causative effect. Randomized, controlled trials are needed to evaluate surveillance for EAC, but significant hurdles remain to be able to develop an effective strategy for reducing mortality from EAC.
Capsule Summary.
What is already known on this topic
Screening and surveillance EGD for esophageal adenocarcinoma (EAC) are not uniformly recommended because of limitations in earlier studies, including potential lead-time bias, length-time bias, and selection bias.
What this study adds to our knowledge
In a retrospective review of 155 subjects with EAC and gastroesophageal reflux, those with a history of EGD at least 1 year before a diagnosis of EAC were identified at earlier stages than those without a prior EGD, but no significant improvement in survival was seen.
Acknowledgments
We thank Brenda Vibbart for her assistance in creating graphic art.
Abbreviations
- ACG
American College of Gastroenterology
- BE
Barrett’s esophagus
- EAC
esophageal adenocarcinoma
- GER
gastroesophageal reflux
- HR
hazard ratio
- ICD
International Classification of Diseases
- NPCD
National Patient Care Datasets
- OR
odds ratio
- VA
United States Department of Veterans Affairs
Footnotes
DISCLOSURE
The authors report that there are no disclosures relevant to this publication. J. H. Rubenstein is the Damon Runyon-Gordon Family Clinical Investigator, supported in part by the Damon Runyon Cancer Research Foundation (CI-36-07), and by the NIDDK 1K23DK079291-01. J. M. Inadomi is funded through the NCI (R01 CA106773).
References
- 1.El-Serag HB, Mason AC, Petersen N, et al. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368–72. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–55. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888–95. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 5.Portale G, Peters JH, Hagen JA, et al. Comparison of the clinical and histological characteristics and survival of distal esophageal-gastroesophageal junction adenocarcinoma in patients with and without Barrett mucosa. Arch Surg. 2005;140:570–4. doi: 10.1001/archsurg.140.6.570. [DOI] [PubMed] [Google Scholar]
- 6.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 7.Peters JH, Clark GW, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg. 1994;108:813–21. [PubMed] [Google Scholar]
- 8.van Sandick JW, van Lanschot JJ, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216–22. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streitz JM, Jr, Andrews CW, Jr, Ellis FH., Jr Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg. 1993;105:383–7. [PubMed] [Google Scholar]
- 10.Cooper GS, Yuan Z, Chak A, et al. Association of prediagnosis endoscopy with stage and survival in adenocarcinoma of the esophagus and gastric cardia. Cancer. 2002;95:32–8. doi: 10.1002/cncr.10646. [DOI] [PubMed] [Google Scholar]
- 11.Kearney DJ, Crump C, Maynard C, et al. A case-control study of endoscopy and mortality from adenocarcinoma of the esophagus or gastric cardia in persons with GERD. Gastrointest Endosc. 2003;57:823–9. doi: 10.1016/s0016-5107(03)70015-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang KK, Wongkeesong M, Buttar NS, et al. American Gastroenterological Association medical position statement: role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology. 2005;128:1468–70. doi: 10.1053/j.gastro.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 13.The role of endoscopy in the surveillance of premalignant conditions of the upper gastrointestinal tract. Gastrointest Endosc. 1998;48:663–8. doi: 10.1016/s0016-5107(98)70055-0. [DOI] [PubMed] [Google Scholar]
- 14.Boyer J, Robaszkiewicz M. Guidelines of the French Society of Digestive Endoscopy: monitoring of Barrett’s esophagus. The Council of the French Society of Digestive Endoscopy Endoscopy. 2000;32:498–9. doi: 10.1055/s-2000-9007. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Watson A, Heading RC, Shepherd NA. [Accessed March 21, 2008];Guidelines for the diagnosis and management of Barrett’s columnar-lined oesophagus. Available at: http://www.bsg.org.uk/bsgdisp1.php?id=3f4a76385e42599499e9&h=1&sh=1&i=1&b=1&m=00023.
- 17.Armstrong D, Marshall JK, Chiba N, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults: update 2004. Can J Gastroenterol. 2005;19:15–35. doi: 10.1155/2005/836030. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. [Accessed March 21, 2008];SEER-Medicare: calculation of comorbidity weights. Available at: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–90. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. [Accessed March 21, 2008];Fast stats: esophagus cancer. 2006 Available at: http://seer.cancer.gov/faststats/sites.php?site=Esophagus%20Cancer&stat=Survival. [Google Scholar]
- 22.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]