Abstract
Background
EMR for early neoplastic Barrett’s esophagus is gaining favor over esophagectomy. Esophageal stricture development has been reported as a common complication of EMR, photodynamic therapy, and combination endoscopic therapy.
Objective
To determine clinical and procedural predictors of symptomatic stricture formation after EMR.
Design
Retrospective analysis.
Setting
Tertiary-care referral university hospital.
Patients
Data were retrospectively reviewed on 73 patients at our institution who underwent EMR monotherapy for Barrett’s esophagus with high-grade dysplasia or intramucosal cancer since January 2006.
Intervention
EMR.
Main Outcome Measurements
Symptomatic esophageal stricture formation.
Results
Symptomatic esophageal stricture formation was noted in 24.7% of patients undergoing EMR. Stricture formation on univariate analysis was associated with percentage of circumference of esophageal lumen resected, total pieces resected, number of EMR sessions, and tobacco use. A threshold effect was found at 50% of esophageal circumference resected (66.7% vs 27.2% developed strictures above and below the threshold, respectively; P = .004). A 25-pack-year or greater history of tobacco use had a threshold effect on esophageal stricture formation (77.8% vs 7.2% developed strictures above and below the threshold, respectively; P =.02). In multivariate analysis, resection of >50% of the circumference was strongly associated with stricture formation (odds ratio [OR] 4.17; 95% confidence interval [CI], 1.27–13.7). A 25-pack-year or greater history of tobacco use also trended toward stricture formation (OR 3.33; 95% CI, 0.929–12.1).
Limitations
Retrospective design, sample size.
Conclusion
Resection of at least 50% of the esophageal mucosal circumference is strongly associated with stricture formation. Patients with strong histories of tobacco use also may be more likely to develop esophageal strictures following EMR.
Barrett’s esophagus (BE) is an intestinal metaplasia of the distal aspect of the esophagus.1 The recognition of BE as a premalignant lesion of the esophagus that progresses through various stages of dysplasia to esophageal adenocarcinoma has prompted multiple specialty society guidelines regarding screening, surveillance, and therapy of this lesion.2,3 Traditionally, esophagectomy has been regarded as the standard intervention when high-grade dysplasia (HGD) or intramucosal cancer was detected on surveillance endoscopy. In an effort to deliver comparable effectiveness of esophagectomy for cancer control, while avoiding the substantial mortality and morbidity of the surgery, researchers have studied and proposed multiple endoscopic therapies. Endoscopic therapies have focused on the use of photodynamic therapy (PDT), thermal therapy, radiofrequency ablation, EMR, and combinations of these. Both PDT and radiofrequency ablation have been demonstrated in prospective, randomized trials to decrease the risk of esophageal adenocarcinoma in BE.4–6 Recently, complete EMR of BE has been shown to be an effective treatment modality as well.7,8
Esophageal strictures are a common complication of EMR. Symptomatic stricture formation has been reported with EMR, PDT, and combination therapy, ranging from 13% to 50% of patients.7–10 Predictors of stricture development have been examined in patients treated with PDT and include multiple sessions of PDT, the presence of intramucosal cancer, and the length of BE.9 We aimed to estimate the effects of clinical and procedural factors on the development of esophageal strictures after mono-therapy with EMR for Barrett’s neoplasia. We hypothesized a priori that the extent of mucosa resected would be associated with stricture formation.
METHODS
Data extraction
Records of patients who underwent EMR without other adjunctive therapies such as PDT, radiofrequency ablation, or argon plasma coagulation for BE with HGD or intramucosal cancer at the University of Michigan Health System were reviewed from January 2006 until February 2010. This retrospective study was approved by the University of Michigan Institutional Review Board.
Sources of data included endoscopy reports with photographic documentation, pathology reports, and clinic notes. All data were extracted by a single investigator (J.L.) not involved in direct care of the patients.
Diagnosis of BE
Diagnosis of BE with HGD or intramucosal cancer was established by using the standard biopsy protocol of 4-quadrant biopsy every 1 to 2 centimeters of Barrett’s mucosa. All biopsy specimens were reviewed by an expert GI pathologist at our institution. Any biopsy specimens obtained at an outside facility were reviewed by our GI pathologists to confirm diagnosis before EMR. Total lengths of BE and circumferential lengths were documented by using the Prague (CM) system.11 Before EMR, all patients with confirmed or suspected cancer underwent an EUS excluding nodal involvement or submucosal cancer.
EMR
EMR was carried out in all but one patient by using the Duette banding system (Cook Medical, Bloomington, Ind) over an Olympus (Center Valley, Pa) single-channel therapeutic upper endoscope. A single patient’s procedure was performed by using the Olympus cap system. All resections were performed by advanced, fellowship-trained, experienced endoscopists (C.P., B.J.E., R.K.). The submucosa was injected to create a lift and to facilitate overlapping resection specimens by using a mixture of 1:60,000 dilution epinephrine in saline solution with a few drops of methylene blue to make the solution a medium-blue, translucent color. A band was then applied to create a pseudopolyp, followed by electrocautery hexagonal snare removal. When multiple resections were done, the specimens were removed contiguously so that the resection pieces overlapped. Post-EMR recommendations included proton pump inhibitor therapy twice daily, sucralfate suspension 1 g by mouth 4 times daily for 2 weeks, clear liquid diet for 2 days, and avoidance of nonsteroidal anti-inflammatory drugs (NSAIDs) and antiplatelet agents for 2 weeks after the procedure, unless patients were at high risk for a vascular event, in which case resumption of antiplatelet medications was allowed earlier. The cumulative proportion of circumference resected was summed retrospectively based on endoscopic reports, and photographic examples of resections of 25%, 50%, and 75% of the esophageal lumen are demonstrated in Figures 1 through 3. The minimum time between index EMR and subsequent therapeutic sessions was 4 weeks, and the maximum was 3 months.
Figure 1.
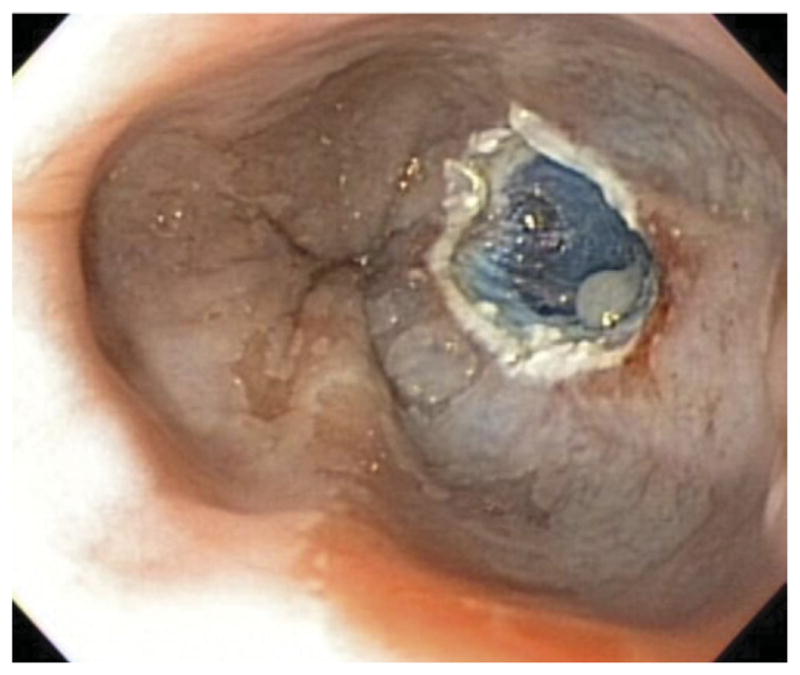
Endoscopic resection of 25% of the esophageal lumen.
Figure 3.

Endoscopic resection of 75% of the esophageal lumen.
Definition of esophageal stricture
After EMR, symptomatic esophageal stricture was defined as an endoscopically identified narrowing or stenosis producing any patient complaints of dysphagia requiring dilation therapy. An asymptomatic esophageal stricture was defined as evidence of endoscopic narrowing after EMR without reported symptoms of dysphagia. In the absence of symptoms of dysphagia, these were not dilated at the time of endoscopy. In general, dilation was attempted with at least a 13-mm wire-guided bougie dilator with discretion to size of the dilator determined by the endoscopist at the time of endoscopy.
Follow-up and surveillance
All patients had at least one surveillance endoscopy at our institution after EMR. Patients who were scheduled for radiofrequency ablation, PDT, or argon plasma coagulation at a later date were included in this review, and data were collected up to the point at which they underwent this adjunctive therapy.
Data analysis
All data analysis was performed by using Stata 10.0 (Stata Corp, College Station, Tex). Univariate analysis was performed on both clinical and procedural factors comparing patients who formed symptomatic strictures with patients who were asymptomatic (some of whom had asymptomatic strictures) by chi-square or Fisher exact tests for smaller sample sizes for categorical variables as appropriate. Continuous variables were assessed by using the t test. Analyses also were performed comparing all participants who formed strictures (including symptomatic and asymptomatic) with those who did not form strictures. Clinical factors examined included age, sex, body mass index, length of BE, tobacco use, alcohol use, NSAID use, proton pump inhibitor use/dosing, esophageal stricture before EMR, Charlson Comorbidity Index,12 and the presence of hiatal hernia, erosive esophagitis, or distinct nodules at the time of index endoscopy. Procedural factors included degree of circumference resected, number of total pieces resected, and total number of EMR sessions.
Significant variables noted on univariate analysis were examined in a multivariate logistic regression model. Statistical significance was defined as a P value of .05 on both univariate and multivariate analysis. It is recognized that there were several statistical tests of hypotheses performed on outcome data arising from individual patients. The P values for the exploratory univariate statistical tests as well as the univariate analysis of data on the subgroup that underwent only a single therapeutic session are not corrected for multiple testing because their role was to suggest candidate variables for further study. The subsequent multivariate logistic regression analysis was performed without defining it as a primary or secondary outcome. It is noted that because of the observational, retrospective nature of the study, the results should be taken as descriptive.
RESULTS
Baseline patient characteristics
Seventy-three patients with BE underwent a total of 102 sessions of EMR. Twenty-two patients (30%) underwent multiple sessions, with 16 undergoing 2 sessions, 5 undergoing 3 sessions, and a single patient undergoing 4 sessions.
Characteristics noted before EMR are summarized in Table 1. Patients were typically older, male, overweight tobacco smokers with substantial comorbidities. Circumferential and maximal lengths of BE were noted in all patients. The median circumferential length was 1 cm, and the maximal length was 2 cm. Nodular mucosa or a distinct nodule was noted in 76.7% of patients undergoing EMR.
TABLE 1.
Baseline characteristics of patients undergoing EMR
| Characteristic | |
|---|---|
| Age, median (interquartile range), y | 71 (64–77) |
| Male sex | 76.8% |
| BMI, median (interquartile range), kg/m2 | 28.5 (25.4–33.8) |
| Maximal length of Barrett’s esophagus, median (interquartile range), cm | 2 (1–4) |
| Length of circumferential Barrett’s esophagus, median (interquartile range), cm | 1 (0–3) |
| Diagnosis of HGD before EMR | 68.5% |
| Diagnosis of intramucosal cancer before EMR | 31.5% |
| Presence of distinct nodule | 76.7% |
| Charlson comorbidity index, median (interquartile range) | 5 (3–7) |
| History of esophageal stricture | 11% |
| Alcohol use, median (interquartile range), drinks/wk | 1 (0–7) |
| Tobacco use history, median (interquartile range), pack-years | 25 (0–41) |
| Ever smoked | 73.9% |
BMI, Body mass index; HGD, high-grade dyslplasia.
Stricture rate
The overall incidence of esophageal strictures after EMR was 39.7% (29/73). Symptomatic strictures requiring intervention in the form of wire-guided bougie or balloon dilation developed in 24.7% (18/73) of patients. All patients reported improvement in symptoms of dysphagia after dilation. In patients with dysphagia, 94.4% required only a single dilation to alleviate symptoms. One patient required 2 sessions of dilation. There were no reported complications of stricture dilation. A single patient required admission to the hospital for overnight observation for hematemesis after EMR. This patient did not require transfusion, and therapeutic endoscopy for treatment of a bleeding lesion was not necessary. There were no immediate or delayed perforations noted in our population.
Univariate analysis of factors associated with stricture formation
In univariate analysis of patients undergoing EMR for neoplastic BE, factors associated with symptomatic stricture formation were assessed. Those who formed symptomatic strictures were compared with those who did not form symptomatic strictures (the latter group includes patients with asymptomatic strictures). Symptomatic strictures were associated with greater total circumference resected, total number of pieces resected, number of EMR sessions, and smoking history (Table 2). In addition, symptomatic stricture formation tended to have longer length of circumferential BE and total length of BE. Length of resection, although not uniformly reported, did not appear to effect symptomatic stricture formation. There was no difference in proton pump inhibitor use between the two populations, regardless of a once or twice daily dosing frequency. NSAID use tended to be more frequent in the patients who developed symptomatic strictures, although this value did not reach statistical significance. Only two patients in this study were noted to have erosive esophagitis, and both developed symptomatic strictures. Endoscopist procedure volume or experience did not differ in stricture rate on univariate analysis.
TABLE 2.
Univariate predictors of symptomatic stricture formation following EMR
| Factor | Symptomatic stricture formation, n = 18 | No symptomatic stricture, n = 55 | P value |
|---|---|---|---|
| Age, median (interquartile range), y | 72 (61–80) | 71 (53–83) | .75 |
| Male sex | 78.8% | 76.4% | 1.0 |
| BMI, median (interquartile range), kg/m2 | 29.1 (25.8–35.3) | 28.2 (21.7–43.2) | .67 |
| Length of BE, median (interquartile range), cm | 2.5 (1–4) | 2 (0.5–8) | .39 |
| Length of circumferential BE, median (interquartile range), cm | 2 (0–4) | 0.5 (0–7) | .24 |
| PPI use | 88.9% | 81.8% | .98 |
| NSAID use | 66.7% | 45.5% | .07 |
| Presence of hiatal hernia | 38.9% | 27.3% | .35 |
| Presence of intramucosal cancer | 38.9% | 29.1% | .44 |
| Presence of nodule | 77.8% | 76.3% | 1.0 |
| Percentage of circumference resected, median (interquartile range) | 60% (50–100) | 50% (25–80) | .002 |
| Percentage of patients with >50% circumference resected | 66.7% | 27.2% | .004 |
| Total pieces resected, median (interquartile range) | 6 (4–10) | 4 (1–9) | .02 |
| No. of sessions, median (interquartile range) | 2 (1–4) | 1 (1–3) | .005 |
| Prior history of esophageal stricture | 11.1% | 10.9% | 1.0 |
| Charlson Comorbidity Index, median (interquartile range) | 4.5 (5–8) | 5 (1–9) | .24 |
| Tobacco use history, median (interquartile range), pack-years | 39 (0–50) | 20 (0–60) | .02 |
| Percentage of patients with 25 pack-years or greater history of tobacco use | 77.8% | 7.2% | .015 |
| Ever smoked | 77.8% | 72.7% | .77 |
| Alcohol use, median (interquartile range), drinks/wk | 1 (0–5) | 1 (0–7) | .56 |
BMI, Body mass index; BE, Barrett’s esophagus; PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug.
With respect to degree of circumference resected, a threshold effect was noted in those who formed symptomatic strictures as evidenced by Figure 4. Risk of symptomatic stricture formation began to increase with >50% of the esophageal lumen circumference resected; 66.7% of patients who had at least 50% of the circumference resected developed a symptomatic stricture versus 27.2% of those who had <50% resected; P = .004.
Figure 4.
Effect of percentage of esophageal circumference resected on symptomatic esophageal stricture formation.
A similar threshold effect was noted for extent of tobacco use history stratified by the median value of our total population—25 pack-years of tobacco use. As evidenced by Figure 5, each assessed interval of >25 pack-years of tobacco use resulted in the majority of patients developing a symptomatic esophageal stricture, significantly more so than individuals who had <25 pack-years of tobacco use.
Figure 5.

Effect of tobacco pack-year exposure on esophageal stricture.
Comparing all endoscopically identified patients who formed strictures (both symptomatic and asymptomatic) with those who did not form strictures, significant values included degree of circumference resected, total pieces resected, number of sessions, and proportion of patients with >50% of the esophageal circumference resected.
On subgroup analysis of patients who underwent a single therapeutic session of EMR, those who developed a symptomatic stricture had a median circumference resected of 58% compared with 50% in those who did not develop a stricture; P = .01.
Multivariate logistic regression
Multivariate logistic regression was then carried out on the significant factors identified on univariate analysis (P < .05) (Table 3). Total sessions and total number of pieces resected were omitted because they were found to be co-linear with degree of circumference resected, and we believed that circumference resected would be more likely to be causally related to stricture formation than the other two parameters. Circumference resected and tobacco use were treated as dichotomous variables because of their threshold effects. In the multivariate model shown in Table 3, resection of >50% of the esophageal lumen was associated with symptomatic stricture formation (OR 4.17; 95% CI, 1.27–13.7). Tobacco use of 25 pack-years or greater demonstrated a strong trend toward symptomatic stricture formation but did not reach statistical significance (OR 3.33; 95% CI, 0.929–12.1, respectively).
TABLE 3.
Multivariate logistic regression for symptomatic strictures
| Factor | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| >50% esophageal circumference resected | 5.33 | 1.70–16.8 | 4.17 | 1.27–13.7 |
|
| ||||
| 25 pack-year or greater tobacco use | 4.52 | 1.32–15.5 | 3.33 | 0.929–12.1 |
OR, Odds ratio; CI, confidence interval.
DISCUSSION
EMR is an endoscopic technique gaining favor for removing BE with HGD and intramucosal cancer for individuals not thought to be candidates for esophagectomy because of medical comorbidities or, in some circumstances, when the patient prefers to avoid an operation. This technique has been used as a form of monotherapy often resulting in complete BE eradication as well as an adjunctive procedure to ablative therapies. EMR in combination with PDT recently has been compared with esophagectomy in a retrospective review of treatment of HGD in BE, and it demonstrated comparable mortality at 5-year follow-up.13 Additionally, several large cohort studies have demonstrated efficacy in eradication of neoplasia and intestinal metaplasia.7,8
Our study demonstrated that the percentage of circumference of dysplastic Barrett’s epithelium resected predicted the development of symptomatic post-EMR esophageal strictures. Other procedural factors including number of EMR sessions and total number of pieces resected conferred a risk of stricture formation on univariate analysis and were found to be co-linear with degree of circumference resected. We believe that the circumference resected is more likely to be causally related to stricture formation than these other procedural factors, but the current data cannot prove that contention. We found a threshold effect of circumference resected at 50%. Below this threshold, symptomatic stricture formation was less common.
In addition to procedural factors, we assessed possible clinical factors that could predict post-EMR stricture formation. We found no evidence for association with stricture formation with most clinical factors including age or length of BE with the presence of intramucosal cancer, the presence of a nodule or nodular mucosa, and history of esophageal stricture. However, tobacco use was associated with stricture formation, with a threshold effect identified at 25 pack-years. This finding lost statistical significance after adjustment for circumference resected, likely because of the relatively small sample size, albeit this is the largest study to date we are aware of on this topic. We are not aware of prior studies exploring tobacco use as a risk factor for esophageal stricture formation after endoscopic therapy, and tobacco use has not been described in the literature as a risk factor for benign esophageal strictures. Tobacco use, however, has been associated with the development of a stricturing phenotype early in the course of Crohn’s disease.14–15 Unfortunately, given the retrospective nature of this study, we do not have accurate data on whether patients were still smoking at the time of their EMRs, and if so, in what quantity and frequency. Future studies may confirm this finding. In the meantime, it would be worthwhile to counsel patients undergoing EMR to discontinue tobacco use, because it would have additional unrelated health benefits.
Although EMR is a technically challenging endoscopic procedure, it offers benefit over esophagectomy in terms of complications. Recently, the rate of major complications (anastomotic leak, chylous leak, and venous thrombo-embolism) from esophagectomy was reported at 13%, with minor complications (pneumonia, atrial fibrillation, wound infection, stricture, and pneumothorax) accounting for 63%.13 Within that analysis, anastomotic stricture requiring dilation therapy was seen in 47% of patients. Common complications of endoscopic therapy include bleeding, reported as high as 3% of cases,13 and esophageal strictures seen in 13% to 50% of patients.7–10,12,13 We noted no significant bleeding requiring transfusion or admission in our patient population, and the stricture rate was comparable to what is reported in the literature. Furthermore, these strictures seen after EMR are often easily managed with standard dilation techniques; in our population, 94.4% of patients required only a single dilation for resultant improvement in symptoms of dysphagia. This is contrary to what has been previously reported with PDT strictures, which often are more challenging to manage endoscopically.10,16 A review of the individual who required two dilation sessions demonstrated that 50% of the esophageal lumen was resected in two pieces; however, this individual did have a significant tobacco-use history consisting of 50 pack-years and had only mild symptoms at the time of his second dilation. Although our data suggest that the total circumference resected is predictive of stricture development, many endoscopists favor resecting up to 50% of the esophageal lumen in one session. Our subgroup analysis of those undergoing a single resection shows that this may be a reasonable consideration at least in the short term because the median circumference resected in those who formed strictures was greater than this threshold. It should be noted, however, that those individuals who required multiple sessions for complete resection did not require interval dilation between therapeutic sessions. Strictures in all of these individuals did not develop until completion of resection of dysplastic tissue. Ultimately, it is most important to obtain an adequate resection with clear margins, which often results in greater than 50% of the esophageal lumen being resected. Although these strictures have been reported to be very difficult to manage and can lead to significant morbidity, in our population these strictures were almost uniformly managed with a single dilation at the time of surveillance. This may be in part due to technical factors unique to our institutional practice and post-EMR care including routine sucralfate, clear liquid diet, and high-dose proton pump inhibitor use. We anticipate a further prospective study focused on specific technical interventions and the development and difficulty of management of EMR strictures. It should be pointed out that all of these procedures were performed at a tertiary-care center by expert endoscopists, and although these results are reassuring, this procedure should not be attempted by an inexperienced gastroenterologist without adequate case volume.
Because stricture development has been reported as the most common complication of endoscopic therapy for neoplastic BE, predictors of stricture formation have been examined in patients undergoing PDT.10 Within that analysis, patients undergoing EMR were not more likely to develop a PDT stricture. However, another study did demonstrate an association for stricture formation in patients who had undergone EMR before PDT.16 Stricture formation appears at least in part to relate to the inflammatory process that develops after the creation of iatrogenic mucosal injury. Animal models have demonstrated lack of epithelization and a chronic, active inflammatory infiltrate that appears to have both polymorphonuclear cells and disorganized fibrotic collagen deposition.17 We postulate that tobacco exposure may cause further hindrance of the healing response to mucosal injury. This finding, however, could be partially confounded by a greater degree of circumference involved with dysplasia in heavy smokers, resulting in greater circumference of resection.
Our aim was to identify both clinical and procedural predictors of esophageal stricture formation in patients undergoing EMR without planned thermal ablative therapy. A prior report focused on procedural predictors of stricture formation for using EMR for resection of superficial esophageal lesions.18 Resection of greater than 75% of the esophageal lumen was associated with stricture formation within that cohort. The vast majority of patients in that study had an underlying diagnosis of squamous cell carcinoma, and clinical predictors were not reported. Our study assessed patients undergoing EMR for BE with HGD or intramucosal cancer only.
Our study had some significant limitations. The observational design introduces the possibility of unmeasured confounders within both our patient population and our intervention. Additionally, there is potential for under-ascertainment of strictures or misclassification of potential exposures extracted from the medical records of these patients. The relatively small number of symptomatic patients who formed strictures also limits the precision of effect estimates. With recognition of these limitations, the current study did have a significant number of strengths. Our population represents the largest cohort to date of patients with BE and HGD or intramucosal cancer that has been studied to assess the risk of stricture formation after EMR. Additionally, by assessing only EMR without adjunctive endoscopic therapy, the current study avoids potential confusion as to the cause of the stricture.
In summary, we have demonstrated that resecting greater than 50% of the esophageal lumen and heavy tobacco use are associated with the risk of symptomatic post-EMR stricture formation. Fortunately, these strictures were routinely easily treated, often with a single dilation. Further study is necessary to assess the importance of tobacco exposure on development of esophageal stricture formation after EMR.
Figure 2.
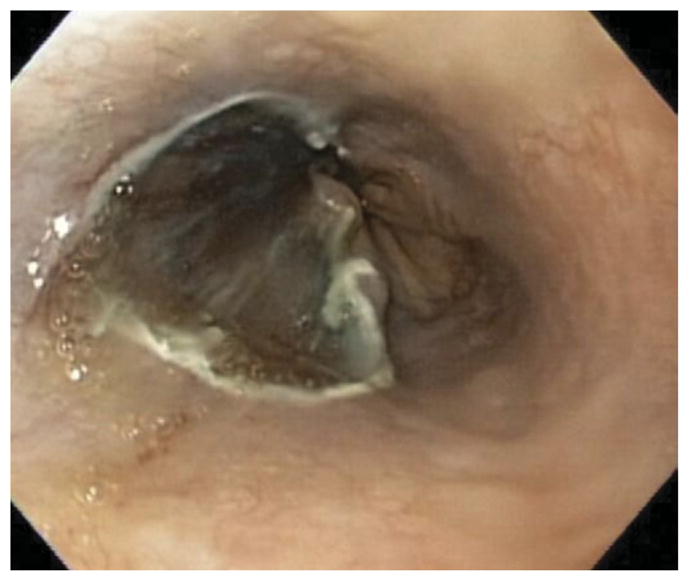
Endoscopic resection of 50% of the esophageal lumen.
Take-home Message.
Multivariate analysis showed a strong association for symptomatic esophageal stricture formation after endoscopic mucosal resection if more than 50% of the esophageal lumen was resected.
Unlike strictures associated with photodynamic therapy, strictures associated with EMR were easily treated with endoscopic dilation therapy, with the vast majority of patients requiring only a single dilation.
Abbreviations
- BE
Barrett’s esophagus
- CI
confidence interval
- HGD
high-grade dysplasia
- NSAID
nonsteroidal anti-inflammatory drug
- OR
odds ratio
- PDT
photodynamic therapy
Footnotes
DISCLOSURE: All authors disclosed no financial relationships relevant to this publication.
References
- 1.Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med. 2002;346:836–42. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 2.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy for Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Overholt B, Lightdale C, Wang K, et al. Photodynamic therapy with porfimer sodium for the ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded randomized phase III trial. Gastrointest Endosc. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 5.Pouw RE, Gondrie JJ, Sondermeijer CM, et al. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;10:1627–36. doi: 10.1007/s11605-008-0629-1. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 7.Chennat J, Konda VJA, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684–92. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 8.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicenter randomized trial. Gut Epub. 2010 Jun 4; doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy PA, Tsung TW, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815–23. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yachimski P, Puricelli WP, Nishioka NS. Patient predictors of esophageal stricture development after photodynamic therapy. Clin Gastro Hep. 2008;6:302–8. doi: 10.1016/j.cgh.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–9. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M, Szratowski TP, Peterson J, et al. Validation of combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 13.Prasad GA, Wang KK, Buttar NS, et al. Long term survival following endoscopic and surgical treatment of high grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226–33. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pico MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and structuring behaviors in the first 8 years of Crohn’s disease. Am J Gastroenterol. 2003;98:363–8. doi: 10.1111/j.1572-0241.2003.07240.x. [DOI] [PubMed] [Google Scholar]
- 15.Louis E, Michel V, Hugot JP, et al. Early development of structuring or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003;52:552–7. doi: 10.1136/gut.52.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad GA, Wang KK, Buttar NS, et al. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2007;65:60–6. doi: 10.1016/j.gie.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Nieponice A, McGrath K, Quereshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289–96. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165–9. doi: 10.1067/mge.2003.73. [DOI] [PubMed] [Google Scholar]



