Abstract
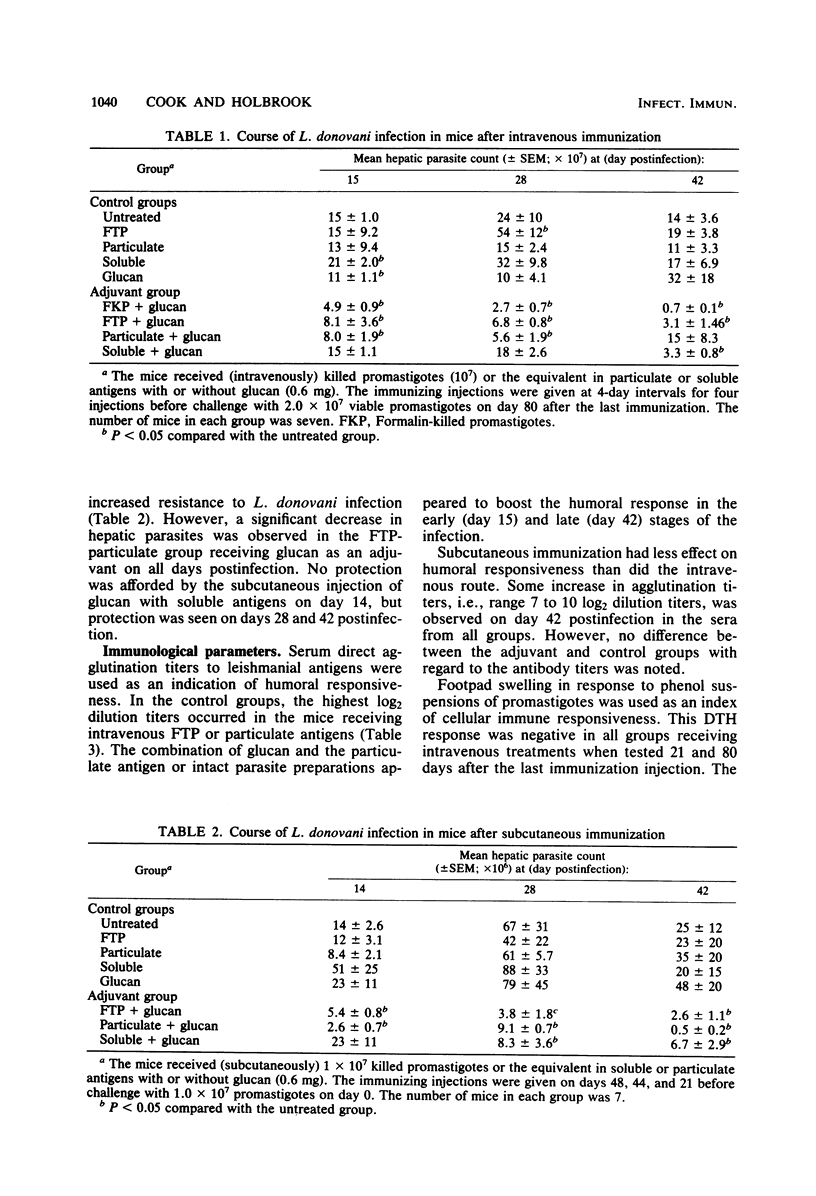
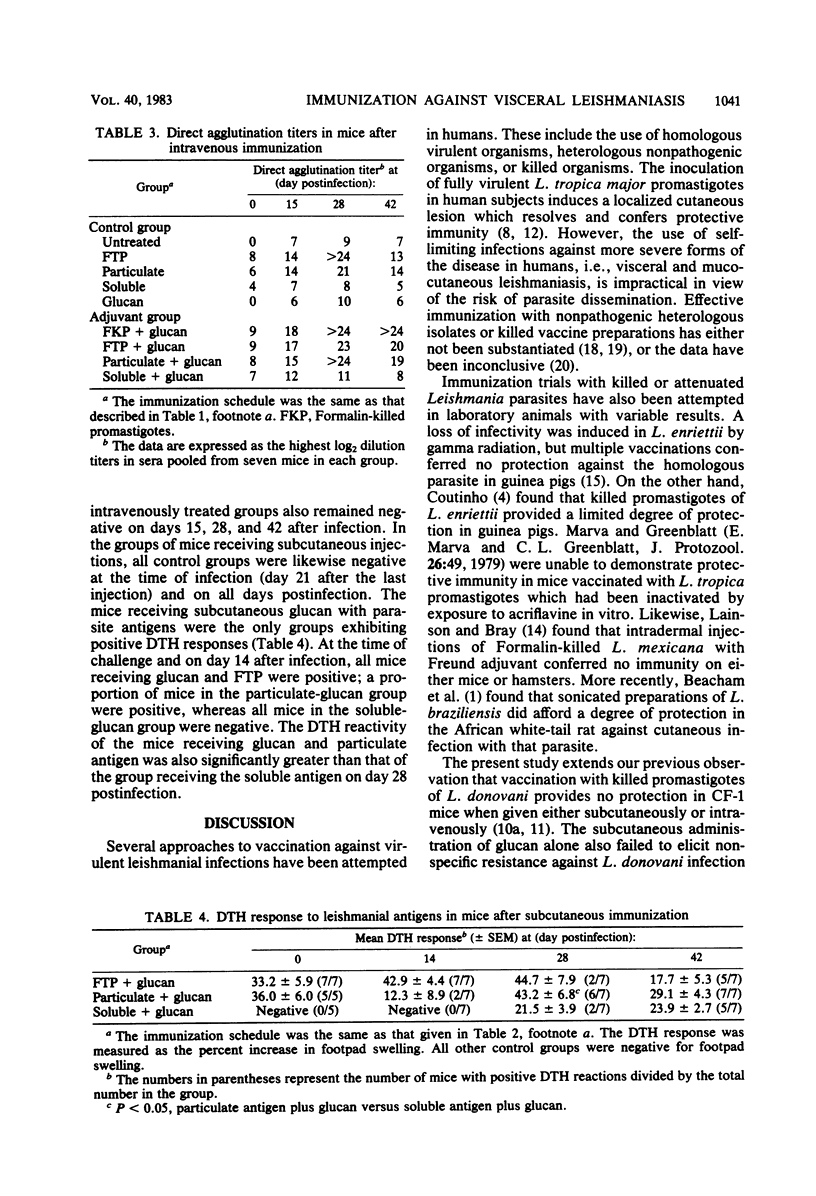
The protective efficacy of glucan as an adjuvant with killed promastigotes of Leishmania donovani was compared with that of soluble or particulate fractions of the parasite. When these vaccine preparations were injected either intravenously or subcutaneously in CF-1 mice, glucan potentiated resistance against L. donovani infections as reflected by significant reductions in hepatic amastigote counts relative to infected control mice. The leishmanial antigens alone afforded no protection. Serum direct agglutination titers to leishmanial antigens were highest in all groups given the vaccine intravenously, whereas the delayed-type hypersensitivity response to the antigen was positive only in groups immunized subcutaneously with glucan as an adjuvant. Some index of protection and immune response against visceral infection with the parasite was seen in groups vaccinated with glucan and soluble antigens. However, the protection afforded by glucan and particulate antigens of L. donovani more closely paralleled the resistance of mice treated with glucan and unfractionated killed promastigotes. Further antigenic analysis of particulate fractions of L. donovani may optimize effective immunization when used with appropriate adjuvants, e.g., glucan.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beacham B. E., Romito R., Kay H. D. Vaccination of the African white-tailed rat, Mystromys albacaudatus, with sonicated Leishmania braziliensis panamensis promastigotes. Am J Trop Med Hyg. 1982 Mar;31(2):252–258. doi: 10.4269/ajtmh.1982.31.252. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Holbrook T. W., Dougherty W. J. Protective effect of glucan against visceral leishmaniasis in hamsters. Infect Immun. 1982 Sep;37(3):1261–1269. doi: 10.1128/iai.37.3.1261-1269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. A., Holbrook T. W., Parker B. W. Visceral leishmaniasis in mice: protective effect of glucan. J Reticuloendothel Soc. 1980 Jun;27(6):567–573. [PubMed] [Google Scholar]
- Di Luzio N. R., Williams D. L., McNamee R. B., Edwards B. F., Kitahama A. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int J Cancer. 1979 Dec 15;24(6):773–779. doi: 10.1002/ijc.2910240613. [DOI] [PubMed] [Google Scholar]
- Feit C., Tewari R. P. Immunogenicity of Ribosomal Preparations from Yeast Cells of Histoplasma capsulatum. Infect Immun. 1974 Nov;10(5):1091–1097. doi: 10.1128/iai.10.5.1091-1097.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt C. L. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Holbrook T. W., Cook J. A. Immunization of mice against Leishmania donovani by subcutaneous injections of dead promastigotes. Am J Trop Med Hyg. 1983 Jan;32(1):51–53. doi: 10.4269/ajtmh.1983.32.51. [DOI] [PubMed] [Google Scholar]
- Holbrook T. W., Cook J. A., Parker B. W. Immunization against Leishmania donovani: glucan as an adjuvant with killed promastigotes. Am J Trop Med Hyg. 1981 Jul;30(4):762–768. doi: 10.4269/ajtmh.1981.30.762. [DOI] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufman Z., Egoz N., Greenblatt C. L., Handman E., Montilio B., Even-Paz Z. Observations on immunization against cutaneous leishmaniasis in Israel. Isr J Med Sci. 1978 Feb;14(2):218–222. [PubMed] [Google Scholar]
- Lemma A., Cole L. Leishmania enriettii: radiation effects and evaluation of radioattenuated organisms for vaccination. Exp Parasitol. 1974 Feb;35(1):161–169. doi: 10.1016/0014-4894(74)90019-8. [DOI] [PubMed] [Google Scholar]
- Leon L. L., Leon W., Chaves L., Costa S. C., Cruz M. Q., Brascher H. M., Lima A. O. Immunization of mice with Trypanosoma cruzi polyribosomes. Infect Immun. 1980 Jan;27(1):38–43. doi: 10.1128/iai.27.1.38-43.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- MANSON-BAHR P. E., SOUTHGATE B. A. RECENT RESEARCH ON KALA AZAR IN EAST AFRICA. J Trop Med Hyg. 1964 Apr;67:79–84. [PubMed] [Google Scholar]
- Mayrink W., da Costa C. A., Magalhães P. A., Melo M. N., Dias M., Lima A. O., Michalick M. S., Williams P. A field trial of a vaccine against American dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73(4):385–387. doi: 10.1016/0035-9203(79)90159-7. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Immunogenicity of a ribosomal antigen of Leishmania enriettii. Trans R Soc Trop Med Hyg. 1971;65(1):18–19. [PubMed] [Google Scholar]
- Stauber L. A. Characterization of strains of Leishmania donovani. Exp Parasitol. 1966 Feb;18(1):1–11. doi: 10.1016/0014-4894(66)90002-6. [DOI] [PubMed] [Google Scholar]
- Vattuone N. H., Yanovsky J. F. Trypanosoma cruzi: agglutination activity of enzyme-treated epimastigotes. Exp Parasitol. 1971 Dec;30(3):349–355. doi: 10.1016/0014-4894(71)90098-1. [DOI] [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. The effect of metabolic inhibitors and hydroxylamine on the immune response in mice to mycobacterial ribonucleic acid vaccines. J Immunol. 1974 Jan;112(1):271–284. [PubMed] [Google Scholar]



