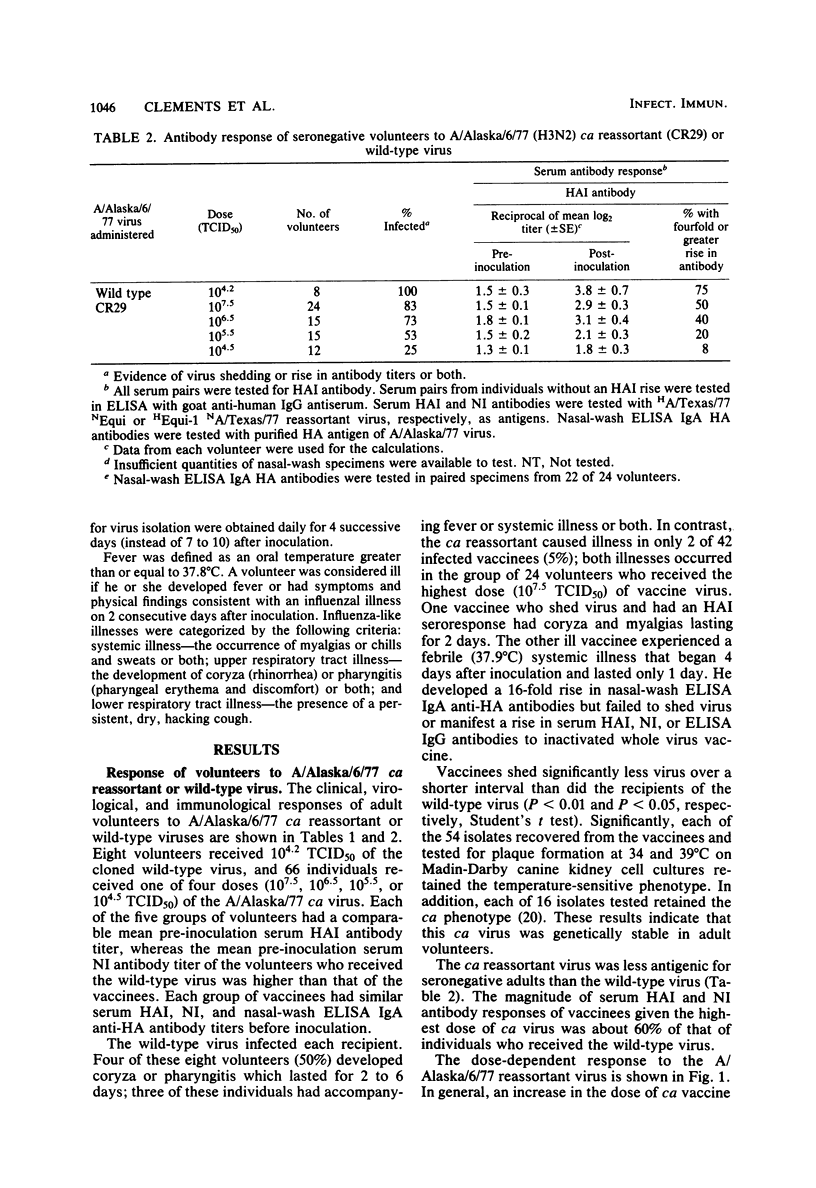
Abstract
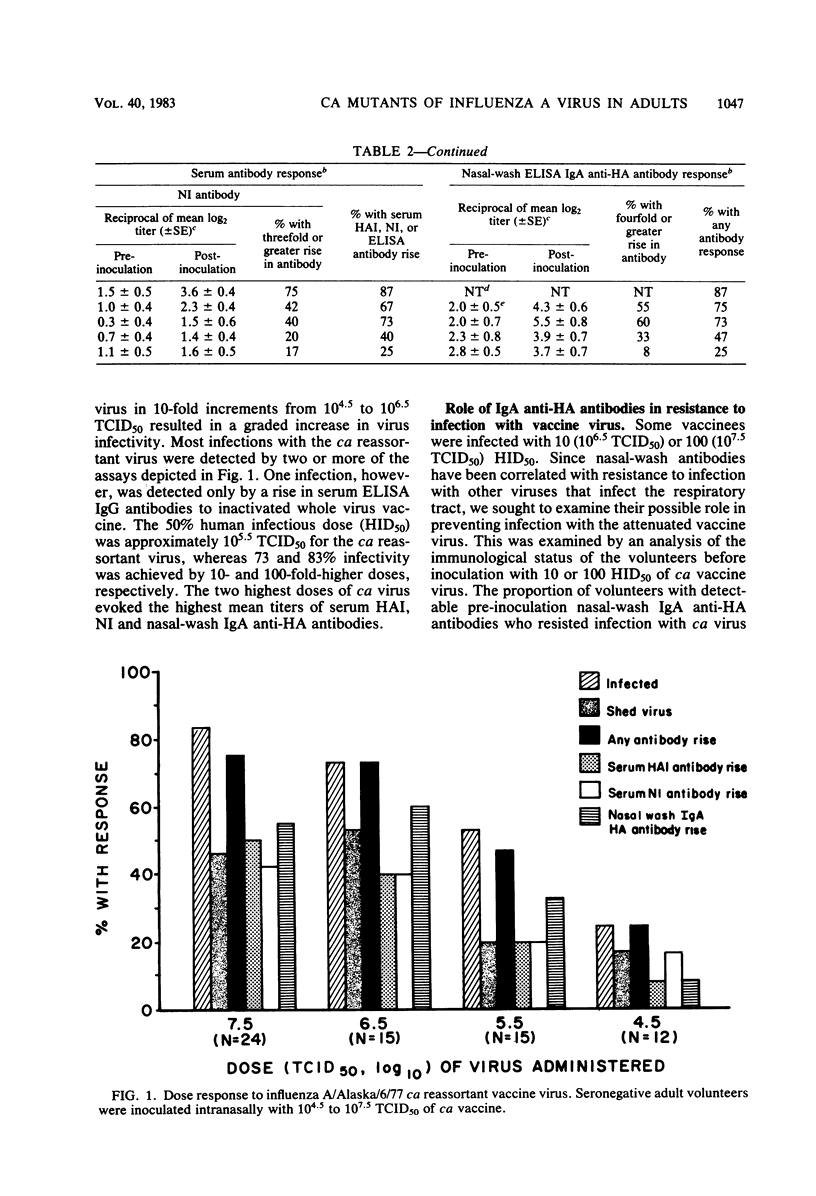
An attenuated influenza A candidate vaccine virus, derived from the A/Ann Arbor/6/60 (H2N2) cold-adapted (ca) donor virus and the A/Alaska/6/77 (H3N2) wild-type virus, was evaluated in adult seronegative volunteers (serum hemagglutination-inhibiting antibody titer, less than or equal to 1:8) for level of attenuation, infectivity, antigenicity, and genetic stability. Four groups with similar preinoculation mean titers of serum and nasal wash antibodies were inoculated intranasally with 10(4.5), 10(5.5), 10(6.5), or 10(7.5) 50% tissue culture infectious doses (TCID50) of the ca reassortant virus, and eight other seronegative adult volunteers received the wild-type virus. Only 2 of 66 vaccinees developed fever or mild and brief systemic or upper respiratory tract illness or both. Both volunteers with vaccine-related reactions received the highest dose (10(7.5) TCID50) of ca virus, which indicates that the vaccine retains some mild reactogenicity at a high dosage. In contrast, four of eight volunteers infected with the wild-type virus became ill. Each of the 54 isolates tested retained the temperature-sensitive phenotype of the vaccine virus. Thus, the ca reassortant was genetically stable and attenuated at 10(4.5) to 10(7.5) TCID50 for seronegative adults. The 50% human infective dose of ca virus was approximately 10(5.3) TCID50. Ten and one hundred 50% human infectious doses infected 73 and 83% of vaccinees, respectively, and approximately 75% developed an immunological response at these doses. The failure of the vaccine virus to infect some volunteers was correlated with the presence of pre-inoculation nasal wash immunoglobulin A hemagglutinin antibody.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber W. H., Small P. A., Jr Local and systemic immunity to influenza infections in ferrets. Infect Immun. 1978 Jul;21(1):221–228. doi: 10.1128/iai.21.1.221-228.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock R. M. Joseph E. Smadel memorial lecture. Strategy for development of respiratory and gastrointestinal tract viral vaccines in the 1980s. J Infect Dis. 1981 Mar;143(3):364–374. doi: 10.1093/infdis/143.3.364. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Maassab H. F., Kendal A. P. Comparative studies of wild-type and cold-mutant (temperature-sensitive) influenza viruses: nonrandom reassortment of genes during preparation of live virus vaccine candidates by recombination at 25 degrees between recent H3N2 and H1N1 epidemic strains and cold-adapted A/An Arbor/6/60. Virology. 1979 Aug;97(1):190–194. doi: 10.1016/0042-6822(79)90386-6. [DOI] [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V., Maassab H. F., Minuse E., Clark L. C., Abrams G. D., Mitchell J. R. Pilot studies on recombinant cold-adapted live type A and B influenza virus vaccines. J Infect Dis. 1977 Jul;136(1):17–25. doi: 10.1093/infdis/136.1.17. [DOI] [PubMed] [Google Scholar]
- Hoskins T. W., Davies J. R., Smith A. J., Miller C. L., Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979 Jan 6;1(8106):33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- Hrabar A., Vodopija I., André F. E., Mitchell J. R., Maassab H. F., Hennessy A. V., Davenport F. M. A placebo-controlled dose-response study of the reactogenicity and immunogenicity of a cold-adapted recombinant A/Victoria/3/75 (H3N2) live influenza virus candidate vaccine in healthy volunteers. Dev Biol Stand. 1977 Jun 1;39:53–60. [PubMed] [Google Scholar]
- KNIGHT V. THE USE OF VOLUNTEERS IN MEDICAL VIROLOGY. Prog Med Virol. 1964;6:1–26. [PubMed] [Google Scholar]
- Kendal A. P., Cox N. J., Murphy B. R., Spring S. B., Maassab H. F. Comparative studies of wild-type and 'cold-mutant' (temperature sensitive) influenza viruses: geneology of the matrix (M) and non-structural (NS) proteins in recombinant cold-adapted H3N2 viruses. J Gen Virol. 1977 Oct;37(1):145–159. doi: 10.1099/0022-1317-37-1-145. [DOI] [PubMed] [Google Scholar]
- Lazar A., Okabe N., Wright P. F. Humoral and cellular immune responses of seronegative children vaccinated with a cold-adapted influenza A/HK/123/77 (H1N1) recombinant virus. Infect Immun. 1980 Mar;27(3):862–866. doi: 10.1128/iai.27.3.862-866.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassab H. F. Adaptation and growth characteristics of influenza virus at 25 degrees c. Nature. 1967 Feb 11;213(5076):612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- Maassab H. F., Cox N. J., Murphy B. R., Kendal A. P. Biological, genetic and biochemical characterization of a cold-adapted recombinant A/Victoria/3/75 virus and its evaluation in volunteers. Dev Biol Stand. 1977 Jun 1;39:25–31. [PubMed] [Google Scholar]
- Maassab H. F., Francis T., Jr, Davenport F. M., Hennessy A. V., Minuse E., Anderson G. Laboratory and clinical characteristics of attenuated strains of influenza virus. Bull World Health Organ. 1969;41(3):589–594. [PMC free article] [PubMed] [Google Scholar]
- Meyer H. M., Jr, Hopps H. E., Parkman P. D., Ennis F. A. Review of existing vaccines for influenza. Am J Clin Pathol. 1978 Jul;70(1 Suppl):146–152. [PubMed] [Google Scholar]
- Mills J., 5th, Van Kirk J. E., Wright P. F., Chanock R. M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971 Jul;107(1):123–130. [PubMed] [Google Scholar]
- Mortiz A. J., Kunz C., Hofman H., Liehl E., Reeve P., Maassab H. F. Studies with a cold-recombinant A/Victoria/3/75 (H3N2) virus. II. Evaluation in adult volunteers. J Infect Dis. 1980 Dec;142(6):857–860. doi: 10.1093/infdis/142.6.857. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chanock R. M., Clements M. L., Anthony W. C., Sear A. J., Cisneros L. A., Rennels M. B., Miller E. H., Black R. E., Levine M. M. Evaluation of A/Alaska/6/77 (H3N2) cold-adapted recombinant viruses derived from A/Ann Arbor/6/60 cold-adapted donor virus in adult seronegative volunteers. Infect Immun. 1981 May;32(2):693–697. doi: 10.1128/iai.32.2.693-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Chanock R. M., Douglas R. G., Betts R. F., Waterman D. H., Holley H. P., Jr, Hoover D. L., Suwanagool S., Nalin D. R., Levine M. M. Temperature-sensitive mutants of influenza A virus: evaluation of the Alaska/77-ts-1A2 temperature-sensitive recombinant virus in seronegative adult volunteers. Arch Virol. 1980;65(2):169–173. doi: 10.1007/BF01317328. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Holley H. P., Jr, Berquist E. J., Levine M. M., Spring S. B., Maassab H. F., Kendal A. P., Chanock R. M. Cold-adapted variants of influenza A virus: evaluation in adult seronegative volunteers of A/Scotland/840/74 and A/Victoria/3/75 cold-adapted recombinants derived from the cold-adapted A/Ann Arbor/6/60 strain. Infect Immun. 1979 Feb;23(2):253–259. doi: 10.1128/iai.23.2.253-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Tierney E. L., Barbour B. A., Yolken R. H., Alling D. W., Holley H. P., Jr, Mayner R. E., Chanock R. M. Use of the enzyme-linked immunosorbent assay to detect serum antibody responses of volunteers who received attenuated influenza A virus vaccines. Infect Immun. 1980 Aug;29(2):342–347. doi: 10.1128/iai.29.2.342-347.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Wood F. T., Massicot J. G., Chanock R. M. Temperature-sensitive mutants of influenza A virus. Transfer of the two ts-1A2 ts lesions present in the Udorn/72-ts-1A2 donor virus to the influenza A/Alaska/6/77 (H3N2) wild type virus. Arch Virol. 1980;65(2):175–186. doi: 10.1007/BF01317329. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Poliovirus antibody response in serum and nasal secretions following intranasal inoculation with inactivated poliovaccine. J Immunol. 1969 Jan;102(1):15–23. [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knopf H. L., Wenzel R. P., Kapikian A. Z., Chanock R. M. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969 Dec;90(6):519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- Puck J. M., Glezen W. P., Frank A. L., Six H. R. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis. 1980 Dec;142(6):844–849. doi: 10.1093/infdis/142.6.844. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Cogliano R. C., Shands J. W., Jr, Small P. A., Jr Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect Immun. 1979 Sep;25(3):992–997. doi: 10.1128/iai.25.3.992-997.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve P., Gerendas B., Moritz A., Liehl E., Kunz C., Hofmann H., Maassab H. F. Studies in man with cold-recombinant influenza virus (H1N1) live vaccines. J Med Virol. 1980;6(1):75–83. doi: 10.1002/jmv.1890060110. [DOI] [PubMed] [Google Scholar]
- Small P. A., Jr, Waldman R. H., Bruno J. C., Gifford G. E. Influenza infection in ferrets: role of serum antibody in protection and recovery. Infect Immun. 1976 Feb;13(2):417–424. doi: 10.1128/iai.13.2.417-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Pollard R. Vaccination against influenza: a five-year study in the Post Office. J Hyg (Lond) 1979 Aug;83(1):157–170. doi: 10.1017/s0022172400025936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Maassab H. F., Kendal A. P., Murphy B. R., Chanock R. M. Cold-adapted variants of influenza virus A. I. Comparison of the genetic properties of ts mutants and five cold-adapted variants of influenza virus A. Virology. 1977 Mar;77(1):337–343. doi: 10.1016/0042-6822(77)90430-5. [DOI] [PubMed] [Google Scholar]
- Stuart-Harris C. From the National Institute of Allergy and Infectious Diseases. The present status of live influenza virus vaccine. J Infect Dis. 1980 Nov;142(5):784–793. doi: 10.1093/infdis/142.5.784. [DOI] [PubMed] [Google Scholar]
- Tremonti L. P., Lin J. S., Jackson G. G. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J Immunol. 1968 Sep;101(3):572–577. [PubMed] [Google Scholar]
- Wright P. F., Okabe N., McKee K. T., Jr, Maassab H. F., Karzon D. T. Cold-adapted recombinant influenza A virus vaccines in seronegative young children. J Infect Dis. 1982 Jul;146(1):71–79. doi: 10.1093/infdis/146.1.71. [DOI] [PubMed] [Google Scholar]
- van Voorthuizen F., Jens D., Saes F. Characterization and clinical evaluation of live influenza A vaccine prepared from a recombinant of the A/USSR/92/77 (H1N1) and the cold-adapted A/Ann Arbor/6/60 (H2N2) strains. Antiviral Res. 1981 Jun;1(2):107–122. doi: 10.1016/0166-3542(81)90037-1. [DOI] [PubMed] [Google Scholar]



