Abstract
This study determined the effects of exogenous hyaluronic acid (HA) on the biomechanical and biochemical properties of self-assembled bovine chondrocytes, and investigated biophysical and genetic mechanisms underlying these effects. The effects of HA commencement time, concentration, application duration and molecular weight were examined using histology, biomechanics and biochemistry. Additionally, the effects of HA application on sulphated glycosaminoglycan (GAG) retention were assessed. To investigate the influence of HA on gene expression, microarray analysis was conducted. HA treatment of developing neocartilage increased compressive stiffness onefold and increased sulphated GAG content by 35 per cent. These effects were dependent on HA molecular weight, concentration and application commencement time. Additionally, applying HA increased sulphated GAG retention within self-assembled neotissue. HA administration also upregulated 503 genes, including multiple genes associated with TGF-β1 signalling. Increased sulphated GAG retention indicated that HA could enhance compressive stiffness by increasing the osmotic pressure that negatively charged GAGs create. The gene expression data demonstrate that HA treatment differentially regulates genes related to TGF-β1 signalling, revealing a potential mechanism for altering matrix composition. These results illustrate the potential use of HA to improve cartilage regeneration efforts and better understand cartilage development.
Keywords: tissue engineering, glycosaminoglycan, biomechanics, gene expression
1. Introduction
Articular cartilage lines the articulating surface of bones and provides a low-friction, load-bearing surface. Developing an effective in vitro method for cartilage formation could provide a platform technology for investigating novel regeneration strategies, studying cartilage pathogenesis and examining cartilage development. However, numerous problems, including chondrocyte de-differentiation [1] and difficulty in reproducing the cartilage matrix [2–5] have hindered the development of effective in vitro cartilage growth models.
Recent efforts have focused on developing in vitro neocartilage that recapitulates the biochemical composition and biomechanical properties of native tissue. The primary matrix constituents of cartilage are glycosaminoglycans (GAGs) and collagen, which confer tensile and compressive integrity to the tissue. Aggrecan, the major proteoglycan of cartilage, binds negatively charged GAGs and subsequently increases the fixed charge density of the tissue, increasing resistance to compressive loading [6]. Collagen contributes primarily to tensile mechanics; both the amount and organization of collagen in articular cartilage correlate with tensile properties [7,8]. Because the extracellular matrix (ECM) contributes extensively to cartilage biomechanics, modulating the composition, organization and interactions between matrix molecules is a central goal of in vitro cartilage development.
Our laboratory has pioneered a novel self-assembly approach for scaffoldless cartilage formation [9,10] based on high-density chondrocyte culture. Self-assembly avoids many of the problems associated with biomaterial scaffolds such as decreased retention of phenotype [1], limited cell–cell communication [11,12] and potential toxicity of degradation byproducts [13]. Self-assembly also mimics cartilage development [14], providing a platform for investigating in vitro cartilage formation. Surprisingly, a recent study showed that self-assembled constructs lacked hyaluronic acid (HA) [14], a high molecular weight polysaccharide present in the ECM that acts a scaffold for proteoglycans [15], suggesting that it may be necessary to exogenously add HA to reproduce native cartilage formation.
HA interacts with chondrocytes primarily via the CD44 receptor. CD44 has been shown to play a wide variety of roles, including pericellular matrix assembly [16], intracellular signalling [17] and matrix remodelling [18]. Although the potential developmental role of the CD44 receptor has not been elucidated, CD44 expression has been shown to increase during chondrogenic condensation [19]. The mechanisms underlying CD44 signalling in chondrocytes are not well-understood and may be the result of co-receptor activity. For instance, CD44 has been shown to interact with tyrosine kinase receptors [20], TGF-β1 receptors [21] and c-Src kinase receptors [22]. Additionally, the size of HA influences CD44 signalling, potentially due to receptor clustering [23]. To understand the interaction between chondrocytes and HA, these complex signalling pathways need to be considered.
HA is a major component of embryonic mesenchymal tissue and has been shown to promote chondrogenesis. HA levels appear to be higher during cell development and migration, but lower after chondrogenic differentiation [24]. Additionally, low HA concentrations inhibit cartilage formation in chick limb buds [25]. These results have been supported by in vitro studies demonstrating that HA mediates important events in morphogenesis, including cell movement and proliferation [26]. HA has also been shown to promote chondrodifferentiation. For instance, supplementing media with HA has been demonstrated to increase collagen II and GAG production in mesenchymal stem cells [27]. The contribution of HA to cartilage development suggests that HA could be a powerful agent for promoting in vitro chondrogenesis.
Applying HA to chondrocyte cultures has been shown to increase biosynthesis in various experimental models. For instance, HA application to monolayer cultures has been observed to increase proteoglycan synthesis in equine articular cartilage [28], rabbit chondrocytes [29,30] and bovine articular cartilage [31]. Furthermore, exogenous HA administration has been shown to promote the expression of adhesion-related molecules, such as integrin receptors, paxillin, focal adhesion kinase and mitogen-activated protein kinase, suggesting that HA application could modulate chondrocyte–matrix interactions [32]. These studies show that HA has a significant signalling role that could influence the functional properties of neotissue, but there have not yet been studies on how exogenous HA treatment influences biomechanical properties.
In this paper, we present a series of studies evaluating the effects of exogenous HA application on the de novo formation of self-assembled articular cartilage and potential mechanisms underlying these effects, which have implications for both developmental biology and regenerative medicine. The influence of HA application time, concentration and molecular weight on the biochemical and biomechanical properties of neocartilage were investigated. Our hypotheses were that exogenous HA treatment would (i) increase sulphated GAG content, (ii) enhance compressive stiffness, (iii) differentially regulate functionally relevant genetic pathways, and (iv) increase GAG retention. To interrogate these hypotheses, HA was administered to neotissue in three sequential phases to examine the effects of HA commencement time (phase I), HA concentration and duration (phase II) and HA molecular weight (phase III). The most promising treatment from each phase, based on biomechanical and biochemical properties, was carried forward to the subsequent phase.
2. Material and methods
2.1. Chondrocyte isolation and culture
Bovine chondrocytes were isolated and cultured as described previously [10]. Chondrocytes were isolated from the immature bovine patellofemoral surface, and then digested in 0.2 per cent type II collagenase (Worthington) with 3 per cent FBS for 18 h. Chondrocytes were frozen at −80°C for 24 h, and then transferred to liquid nitrogen for long-term storage. To form constructs, chondrocytes were thawed and seeded in 5 mm cylindrical agarose moulds at a density of 5.5 million chondrocytes in 100 μl of chondrogenic media. Chondrocytes were resuspended in media supplemented with HA prior to construct formation for groups receiving HA at seeding. After 4 h, 400 μl of media was added and 500 μl of media was changed for subsequent days. Chondrogenic media was formulated by supplementing Dulbecco's modified eagle medium (DMEM) with 4.5 mg ml–1 glucose and l-glutamine (Biowhittaker), 100 nM dexamethasone (Sigma), 1% PSF (Lonza), 1% ITS+ (BD Scientific), 50 µg ml–1 ascorbate-2-phosphate, 40 μg ml–1 l-proline and 100 µg ml–1 sodium pyruvate (Sigma). HA was applied in three sequential phases: phase I (effects of HA commencement time), phase II (effects of HA concentration and duration), and phase III (effects of HA molecular weight). For HA treatment in phases I and II, HA with an average molecular mass of 1.6 × 106 Da (Sigma 53 747) was dissolved in chondrogenic media [10] at a concentration of 1 mg ml–1 by stirring at 4°C for 48 h and applied daily during construct feeding. For phase III, HA polymers with average molecular masses of 5.1 × 103, 1.2 × 104, 3.1 × 104, 1.7 × 106 and 2.7 × 106 Da (Lifecore Biosciences) were employed. Both Sigma and Lifecore HA were derived from bacteria.
2.2. Construct processing
All experiments except for gene expression analyses were four weeks in duration. At four weeks, samples were prepared for histology, quantitative biochemistry and mechanical testing. A 3 mm diameter punch was removed from the construct's centre for creep indentation testing. The outer region was divided to create samples for biochemical assays and tensile testing.
2.3. Histology and immunohistochemistry
At four weeks, samples were cryoembedded and frozen at −20°C for at least 24 h prior to cryosectioning at 14 μm. Some of these sections were fixed in formalin and stained with Safranin-O/fast green and Picrosirius Red to show GAG and collagen distributions, respectively. Other sections were fixed in 4°C acetone and processed for collagen II as described previously [33] or for HA IHC. Peroxidase activity was quenched, and each sample was blocked with goat serum for collagen type II or rabbit serum for HA (Vectastain ABC kit). Sections were then incubated for 1 h with rabbit anti-collagen II (Cedar Lane, CL50211AP) or sheep anti-HA (Abcam, ab53842). Antibodies were diluted in the ratio 1 : 100 and 1 : 300 for HA and collagen II, respectively. Secondary antibodies (Vectastain ABC kit) were applied for 30 min followed by incubation with the Vectastain ABC reagent and 5 min of 3′,3′-diaminobenzidine (DAB) staining.
2.4. Quantitative biochemistry
Samples were frozen at −20°C for at least 24 h, and then lyophilized for 48 h to obtain dry weights. Subsequently, each sample was digested using pepsin and elastase as described previously [33]. Sulphated GAG content was assayed using the Blyscan Glycosaminoglycan Assay kit (Accurate Chemical and Scientific Corporation). Total collagen content was determined using a chloramine-T hydroxyproline assay [34]. To assess sulphated GAG retention, constructs were incubated in PBS at 37°C and removed at 1, 2, 4, 6 or 8 h and then assayed for GAG content. All collagen and GAG contents were expressed as percentages of tissue wet weight.
2.5. Creep indentation testing
The aggregate modulus of each sample was determined using a creep indentation apparatus [35]. A tare load of 0.2 g followed by a test load of 0.7 g was applied via a 0.8 mm porous indentation tip. Material properties were determined using a semi-analytical, semi-numeric biphasic model [36,37].
2.6. Tensile testing
Samples were cut into dog-bone shapes and glued to paper tabs for gripping [38]. Prior to positioning the samples on the paper, pictures were taken and then analysed using ImageJ to determine the width and gauge length. A micrometer was used to determine sample thicknesses. Tensile tests were conducted at a strain rate of 1 per cent of the gauge length per second on a materials testing system (Instron Model 5565). Young's modulus was calculated as the slope of the linear region of the stress–strain curve; the maximum stress was reported as ultimate tensile strength (UTS).
2.7. RNA isolation and microarray hybridization
For gene expression experiments, neocartilage was treated with 1 mg ml–1, 1.6 × 106 Da HA beginning at seeding. Gene expression was analysed at 4 days based on prior data showing elevated levels of surface receptors on day 4 [14]. RNA was isolated at 4 days (n = 3) for control constructs and HA-treated constructs (4 h following the application of HA) using a Qiagen RNeasy Lipid Tissue Mini Kit. To assess RNA purity and concentration, RNA was analysed using a nanodrop. An Agilent bioanalyser was employed to assess the quality of the RNA. Bovine microarrays were acquired from Agilent. 400 ng of total RNA were processed according to Agilent's one colour gene expression protocol. The arrays were scanned on Agilent's high-resolution C scanner and analysed using Agilent's Feature Extraction Software v. 10.5.
2.8. Microarray data analysis
Current annotations for the microarray probes were obtained from Agilent technologies (https://earray.chem.agilent.com/earray). The Entrez Gene IDs from the Agilent annotation were cross-referenced with HomoloGene build 65 (ftp://ftp.ncbi.nih.gov/pub/HomoloGene/build65/) to find the gene names for the corresponding human orthologues. Changes in gene expression were assessed between control and HA treatment based on the normalized intensities of each gene. Based on n = 3 arrays for each treatment, the Benjamini–Hochberg p-value correction was used to generate adjusted p-values. Genes with adjusted values of p < 0.01 were considered to be differentially expressed. Differentially expressed genes were entered into the Database for Annotation, Visualization and Integrated Discovery to determine enriched Kyoto Encyclopedia of Genes and Genomes pathways [39]. Enriched pathways were defined as p < 0.005 and a minimal five genes appearing among the differentially expressed genes.
2.9. Real-time PCR
Primers for GAPDH, COL2 and COL1 were synthesized as described previously [40]; other primers, including c-Myc (cat. no. Bt03229599_m1), E2F4 (cat. no. Bt03247677_g1) and protein phosphatase (cat. no. Bt03229328_m1) were purchased from Invitrogen. Reverse transcription was performed by incubating 500 ng of RNA with SuperScript III (Invitrogen) as recommended by the manufacturer. Real-time PCR was done with a Rotor-gene 3000 real-time PCR machine (Corbett Research). SYBR Green mastermix was used with primers at a concentration of 1 μM. A 10 min denaturing step was employed, followed by 45 cycles of 95°C (15 s) and 60°C (60 s). The threshold cycle (CT) for each gene of interest (GOI) was compared with the housekeeping gene GAPDH. Relative gene expressions were computed as 2-ΔΔCT, where ΔΔCT = (CT,GOI – CT,GAPDH)HA – (CT,GOI – CT,GAPDH)control.
2.10. Statistical analysis
Biochemistry and biomechanics samples (n = 6) were analysed using a one-factor ANOVA in phases I and III. For assessing phase II and sulphated GAG retention, a two-factor ANOVA was employed. When warranted, Tukey's post hoc testing was performed to determine statistically significant differences. All microarray and PCR analysis was conducted as described earlier based on n = 3. Significance was defined as p < 0.05. All data are presented as means ± standard deviation.
3. Results
3.1. Hyaluronic acid-treated neotissue resembles native cartilage phenotype
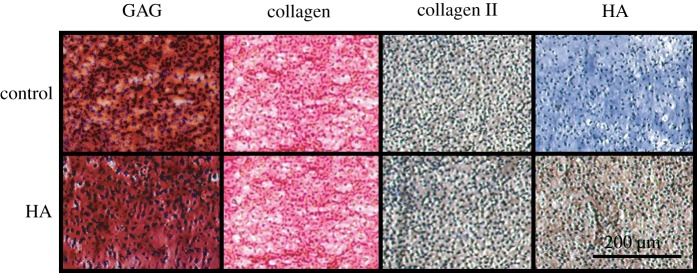
In all experiments in this paper (except gene expression analysis), self-assembled neocartilage was cultured for four weeks. The diameter of HA-treated constructs was not statistically different from the control value of 5.8 ± 0.2 mm. Wet weight was statistically greater for HA treatment, with values of 14 ± 2 and 20 ± 1 mg for control and HA-treated groups, respectively. Histological analysis (figure 1) showed that neocartilage produced collagen and GAG uniformly. Immunohistochemistry indicated that both control and treated samples stained positive for collagen II, while only HA-treated constructs stained positive for HA. Additionally, HA-treated neotissue produced matrix with a uniform distribution of GAGs, total collagen, collagen II and HA.
Figure 1.
Photomicrographs of histology staining for glycosaminoglycans (GAGs), collagen and immunohistochemistry for collagen II and hyaluronic acid (HA). Sections were prepared after four weeks of culture. All images are shown at 10× magnification. Scale bar, 200 µm.
3.2. Hyaluronic acid increases sulphated glycosaminoglycan content and compressive stiffness when applied at seeding
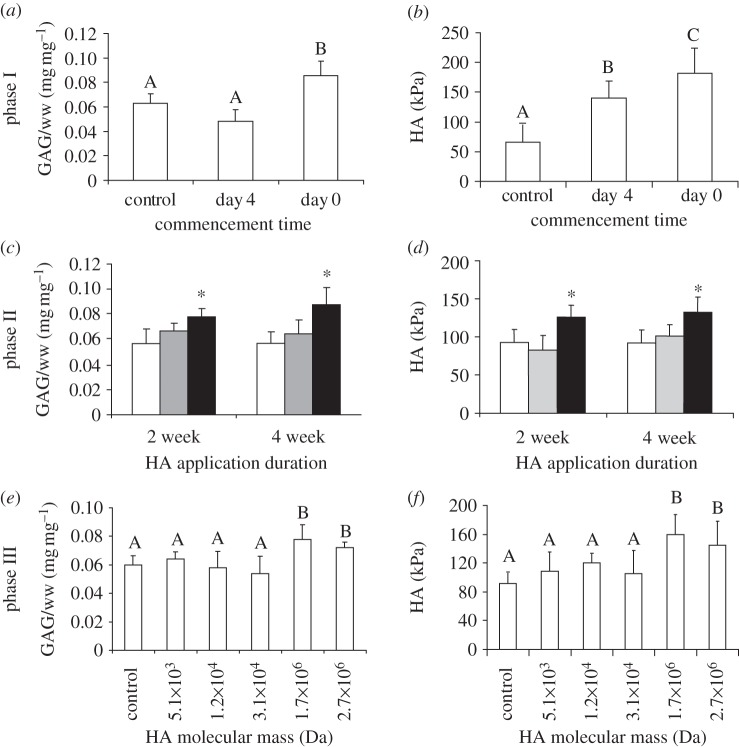
In phase I, the effects of application commencement time were studied. Neotissue was treated with 1 mg ml–1 HA at seeding (day 0) or at day 4, with subsequent continual HA exposure through media changes. To assess the effects of HA application on biochemical composition, collagen and sulphated GAG were quantified. Treated neotissue did not exhibit statistically different collagen contents normalized to neocartilage wet weight (col/ww) from the control value of 9.5 ± 1.8% (mg mg–1). However, sulphated GAG/ww was statistically higher for the day 0 group, which had a sulphated GAG/ww of 8.5 ± 1.1% (mg mg–1) when compared with the control value of 6.5 ± 0.7% (mg mg–1) at four weeks (figure 2a). The effects of HA on construct biomechanical properties were quantified by measuring Young's modulus (EY), UTS and aggregate modulus (a measure of compressive stiffness). For tensile properties, no group was statistically different from the control group, which had a UTS of 370 ± 124 kPa and EY of 641 ± 296 kPa. The aggregate modulus was statistically higher for constructs treated with HA: the group treated at seeding reached 181 ± 40 kPa while the control group was 65 ± 26 kPa (figure 2b).
Figure 2.
Biomechanical and biochemical properties. Phase I: (a) day 0 HA application significantly increased the aggregate modulus. (b) Application of HA at day 0 resulted in a significantly higher GAG content. Phase II: (c) 1 mg ml–1 HA administration enhanced compressive stiffness compared with 0.1 mg ml–1 (grey bars) and control (white bars), whereas 5 mg ml–1 HA treatment disrupted construct formation and precluded neocartilage culture. (d) Application duration did not have a significant effect on GAG content, though HA application at 1 mg ml–1 (black bars) significantly increased GAG content. Phase III: (e) high molecular weight HA also enhanced the aggregate moduli of constructs. (f) HA with higher molecule weights (1.7 × 106 Da and 2.7 × 106 Da) resulted in higher GAG content. Data are presented as means with standard deviation error bars, n = 6 per bar. Distinct letters above the bars indicate significant differences (p < 0.05). For parts (c,d), the asterisk denotes statistical significance.
3.3. Intermediate hyaluronic acid concentration enhances compressive stiffness
In phase II, we carried forward the application of HA at seeding and then performed a full-factorial examination of the treatment duration (weeks 1–2 and weeks 1–4) and HA concentration (0.1, 1 and 5 mg ml–1). Neocartilage was again cultured for a total of four weeks. Constructs treated with 5 mg ml–1 HA did not self-assemble. Sulphated GAG results (figure 2c) showed differences only for the 1 mg ml–1 group; no statistical differences were observed for the 0.1 mg ml–1 groups. HA application duration did not significantly change the sulphated GAG content. For the 1 mg ml–1 HA treatment, sulphated GAG content reached 8.3 ± 0.7% (mg mg–1), which was 38 per cent greater than the control value. Collagen contents for treated groups were not statistically different than the control value of 8.9 ± 0.4% (mg mg–1). Biomechanical properties of neotissue treated with 1 mg ml–1 paralleled phase I results. Treating with HA at 1 mg ml–1 increased the aggregate modulus by approximately 40 per cent (figure 2d); however, the 0.1 mg ml–1 group did not exhibit statistically higher compressive stiffness. Additionally, the HA application duration did not significantly alter the compressive stiffness. Neither the 0.1 mg ml–1 group nor the 1 mg ml–1 group had tensile properties that statistically differed from control values of 748 ± 78 and 362 ± 94 kPa for the EY and UTS, respectively. Thus, the effects of HA treatment depended on concentration, but did not depend on whether application duration was two weeks or four weeks. Based on these results, two-week HA treatment was carried forward to phase III.
3.4. Hyaluronic acid molecular weight influences biomechanical and biochemical properties
To examine the influence of HA size, we assessed the effects of five different molecular masses applied during the first two weeks of a four-week culture: 5.1 × 103, 1.2 × 104, 3.1 × 104, 1.7 × 106 and 2.7 × 106 Da, all at concentrations of 1 mg ml–1. Treatment commenced at seeding and was continuous for the first two weeks of culture based on phase II results. Constructs were assessed after four weeks of culture. Neocartilage had statistically higher sulphated GAG content (figure 2e) when treated with 1.7 or 2.7 × 106 Da HA. Both the 1.7 × 106 and 2.7 × 106 groups showed increased compressive stiffness with values of 163 ± 26 and 136 ± 30 kPa, respectively (figure 2f). Hence, only higher molecular mass HA (1.7 or 2.7 × 106 Da HA) had the ability to enhance the biochemical and biomechanical properties of neocartilage.
3.5. Hyaluronic acid promotes sulphated glycosaminoglycan retention
After four weeks of culture, sulphated GAG retention was assessed by incubating control and HA-treated constructs in PBS for 1, 2, 4, 6 or 8 h and quantifying the sulphated GAG content of each construct (figure 3). HA-treated neotissue had 1.6 × 106 Da HA applied at 1 mg ml–1 from seeding for the first two weeks of culture. Analysis of sulphated GAG using a two-way ANOVA showed that both HA administration and duration exhibited statistical differences. Duration statistically altered sulphated GAG content, with incubation times of greater than 2 h resulting in decreased GAG content. By 8 h, sulphated GAG content for control constructs had decreased to 3.7 per cent of the initial amount. For instance, after 8 h of incubation in PBS total sulphated GAG content of HA-treated neocartilage was three times higher than that of the control (0.15 ± 0.10 and 0.05 ± 0.03 mg per construct, respectively).
Figure 3.
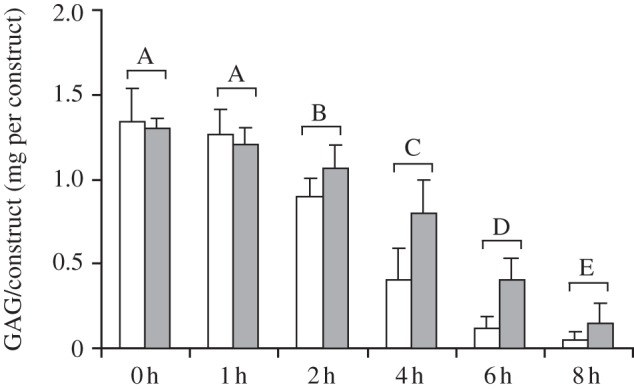
GAG retention of control constructs (white bars) and constructs treated with 1 mg ml–1 HA (grey bars) for two weeks. To assess GAG retention, total GAG content (mg per construct) was quantified after various incubation periods in PBS following four weeks of culture. HA administration increased GAG retention for HA-treated constructs based on a two-way ANOVA of the factors duration and HA treatment. Data are presented as means with standard deviation error bars, n = 6 per bar. Distinct letters above the bars indicate significant differences (p < 0.05).
3.6. Neocartilage properties are on par with native tissue
To quantitatively compare the properties of neocartilage to native tissue, we employed a functionality index (FI) by giving equal weight to the sulphated GAG content (G), collagen content (C), compressive stiffness (EC) and tensile stiffness (ET) (see equation (3.1)). The subscripts ‘nat’ and ‘sac’ denote native and self-assembled construct values, respectively.
 |
3.1 |
The FI is structured such that a score of 1 represents a construct with properties equivalent to those of native immature bovine cartilage. Treating with 1 mg ml–1, 1.6 × 106 Da HA beginning at seeding for a total of two weeks resulted in an FI of 0.85, producing a considerable improvement over the control FI of 0.56.
3.7. Microarray and PCR analyses show that hyaluronic acid enriches functional gene pathways
To investigate possible genetic mechanisms underlying the changes in functional properties, microarray analysis comparing the gene expression profiles of control constructs and HA-treated constructs was performed. Results showed that HA application differentially regulated 794 genes: 503 genes were upregulated and 291 genes were downregulated. HA treatment downregulated collagen I expression and upregulated several genes relating to chondrocyte phenotype [41], including collagen II, collagen XI and chondroadherin. HA synthases were not differentially regulated, exhibiting fold changes of 1.27 (p = 0.73), 1.08 (p = 0.45), 1.15 (p = 0.83) for HAS1, HAS2 and HAS3, respectively. Additionally, CD44 was not differentially expressed due to HA administration (1.21, p = 0.71). As summarized in table 1, differentially expressed genes only enriched pathways relating to TGF-β1 signalling (nine genes, p = 0.003) and ECM–receptor interactions (11 genes, p = 0.0001).
Table 1.
Differentially expressed genes of enriched pathways. HA treatment enriched only pathways relating to TGF-β signalling and ECM–receptor interactions. Fold changes represent the gene expression of neotissue at 4 days treated with HA (1 mg ml–1, 1.6 × 106 Da HA) relative to control values.
| gene | fold change | regulation |
|---|---|---|
| TGF-β pathway targets | ||
| activin | 6.82 | up |
| inhibitor of DNA binding 1 | 8.57 | down |
| inhibitor of DNA binding 2 | 4.67 | up |
| c-Myc transcription factor | 5.34 | up |
| E2F4 transcription factor | 4.78 | up |
| protein phosphatase | 3.47 | up |
| thrombospondin 1a | 4.92 | up |
| thrombospondin 2a | 8.22 | up |
| thrombospondin 3a | 4.26 | up |
| thrombospondin 4a | 6.82 | up |
| ECM–receptor pathway targets | ||
| chondroadherin | 13.42 | up |
| collagen, type I, α1 | 4.92 | down |
| collagen, type II, α1 | 4.47 | up |
| collagen, type III, α1 | 3.42 | up |
| collagen, type XI, α2 | 5.01 | up |
| integrin-α7 | 4.07 | up |
| syndecan 4 | 5.52 | up |
aTargets that were differentially expressed in both the TGF-β signalling and ECM–receptor interactions pathways.
Quantitative real-time PCR was used to validate microarray results. Gene expression values for collagen II, collagen I, c-Myc, E2F4 and protein phosphatase were quantified (figure 4). HA treatment increased the expression of collagen II, c-Myc, E2F4 and protein phosphatase and downregulated collagen I expression. These results paralleled the fold changes observed with microarray results.
Figure 4.
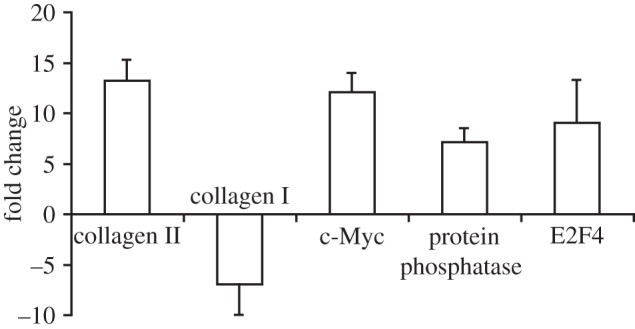
PCR results for neocartilage treated with 1 mg ml–1 HA. All reported values are gene expression values of HA neocartilage normalized to control expression levels computed using the 2-ΔΔCT method. Data are presented as means with standard deviation error bars, n = 3 per bar.
4. Discussion
This study has shown that exogenous HA application improves the biochemical and biomechanical properties of neocartilage and also enriches pathways relating to TGF-β1 signalling and ECM–receptor signalling. Through several phases, an HA administration regimen was identified that enhances the biochemical and biomechanical properties of developing neocartilage. Subsequent study revealed potential mechanisms to explain the observed results. The experiments of this study confirmed the hypotheses that exogenous HA treatment (i) increases sulphated GAG content, (ii) enhances compressive stiffness, (iii) differentially regulates functionally relevant genetic pathways, and (iv) increases sulphated GAG retention. This is the first study to show that HA can be applied exogenously to improve the biochemical and biomechanical properties of tissue engineered cartilage. This work also demonstrates potential biophysical and genetic mechanisms that could produce these enhancements.
Diffusion limitations could be underlying the observed dependence on commencement time but not application duration. Other studies have documented increased diffusion limitations during cartilage construct culture. For instance, 3 × 103 Da dextran showed decreased diffusion as constructs were cultured; the authors attributed this reduced diffusion to increased matrix deposition [42]. Time-dependent diffusion limitations could decrease HA penetration at later time points and explain why HA application at seeding had a larger effect than when it was applied at day 4. The lack of dependence on application duration implies that the HA effects are maximized at the earlier stages of self-assembly, which could be a result of diffusion limitations present at later stages of culture. Previous diffusion studies [42,43] and the effects observed in this study suggest that HA must be applied in the early stages of neocartilage formation to enhance construct properties.
This study also showed that higher molecular weight HA can increase compressive stiffness by 75 per cent and increase sulphated GAG content by 30 per cent, which builds upon other studies that have shown the importance of HA molecular weight. In general, larger molecular weight HA is considered to be chondroinductive [44], whereas smaller fragments, such as HA hexamers, are generally associated with inflammatory events and disruption of pericellular matrix formation [16]. HA molecular weight could influence signalling because HA size alters how many CD44 receptors it can bind, which in turn changes the signalling events related to CD44 receptor clustering [23]. These results suggest that HA molecular weight has a profound impact on its biological function, and imply that modulating chain length may be a powerful tool for altering the effects of HA applied in vitro. In particular, this study suggests that only higher molecular weight HA increases the sulphated GAG content and compressive stiffness of self-assembled neocartilage.
It is important to distinguish endogenous and exogenous HA deposition, which has been shown to influence biomechanical properties [45]. The absence of HA observed in this study for control constructs (figure 1) and in published work using self-assembled neocartilage [14] indicates a lack of endogenous HA production. Additionally, microarray data demonstrate that HA synthases were not differentially expressed following HA treatment, suggesting that the HA present in treatment groups was not endogenously produced. Based on the presence of HA in the treated groups and the lack of upregulated HA synthases, it appears that the observed HA is exogenous. However, post-translation modification of HA synthases [46] could also alter HA deposition and merits further investigation.
Results regarding the retention of sulphated GAGs suggest that the effects of HA are also mediated by a biophysical mechanism. Because negatively charged GAGs create a large fixed charge density in the matrix, enhanced sulphated GAG retention could increase osmotic pressure and subsequently increase resistance to compressive loading [15]. Thus, increasing GAG retention could increase the osmotic pressure of neocartilage and subsequently enhance its compressive properties. Exogenous HA has been shown to suppress proteoglycan release from chondrocyte cultures [47,48] and reduce in vivo GAG release from canine joints [49]. Interactions between HA and other matrix molecules increase the viscosity of the matrix [50], which could decrease GAG release. Because increased GAG retention could increase compressive stiffness, this presents an exciting potential biophysical mechanism underlying the enhanced mechanical properties.
In addition to biophysical effects, microarray and PCR results suggest that HA application has a molecular signalling role. The ECM–receptor pathway included the upregulation of several collagen molecules (collagen II, III and XI) and surface receptors (syndecan-4 and integrin-α7), suggesting that HA could modulate chondrocyte interactions with the surrounding ECM. Because HA upregulated chondrogenic genes, such as collagen II and downregulated collagen I, it could be a potent chondrogenic agent for cartilage formation. Furthermore, the observed upregulation of chondroadherin, which increases extracellular signal-regulated kinase signalling [51], could create secondary signalling effects. Increased expression of syndecan-4, which binds to TGF-β1 via its heparan sulphate chains [52], also suggests that TGF-β1 and HA could potentially be applied simultaneously to produce synergistic effects. These findings illustrate that HA administration increased the expression of key surface molecules such as syndecan-4 and integrin-α7 and matrix constituents, including collagen II and collagen XI, which collectively could influence chondrocyte–matrix interactions.
In addition to the differential expression of matrix molecules, the TGF-β1 signalling pathway was also differentially regulated. TGF-β1 signalling is mediated by Smads, which are intracellular signalling proteins that transduce TGF-β1 signals to the nucleus. HA treatment upregulated the expression of transcription factors, including c-Myc and E2F4, both of which have been shown to be highly expressed during cartilage development [53,54]. Although there is not a well-established mechanism regarding CD44 signalling in chondrocytes [55], data from this study indicate that HA may primarily signal using the TGF-β1 pathway in self-assembled neocartilage (figure 5). This supports previous work showing that HA activates the TGF-β1 receptor and subsequently induces Smad protein phosphorylation [21]. Based on our previous investigations of direct TGF-β1 treatment [56,57], signalling via the TGF-β1 pathway could upregulate matrix synthesis, and thus improve biochemical and biomechanical properties.
Figure 5.
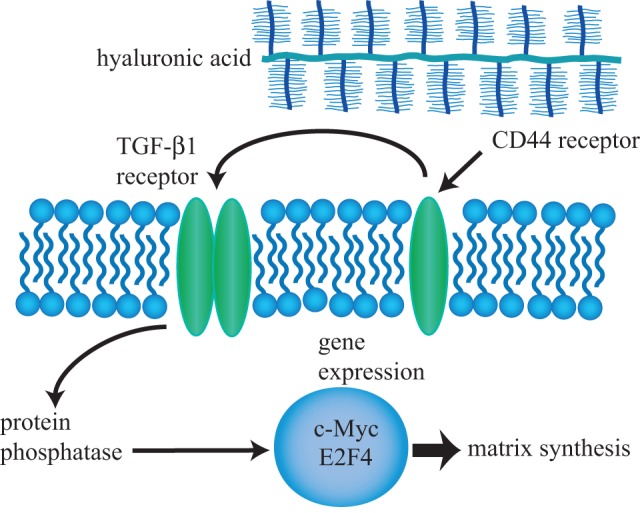
Potential HA signalling pathway mediated by CD44 and TGF-β1 receptors. Microarray and PCR analysis showed that several TGF-β1 intermediates, including the transcription factors c-Myc and E2F4, are upregulated following HA treatment. HA-induced signalling may be mediated by HA binding to CD44, which is known to bind to and activate the TGF-β1 receptor. (Online version in colour.)
The observed changes in gene expression could be mediated by the surface receptor CD44, which has been shown to bind the cytoplasmic domain of the TGF-β1 receptor and influence signalling [21]. However, conflicting results exist that show CD44 as a positive [21] and negative [58] regulator of the TGF-β1 signalling pathway. Interestingly, while anti-CD44 antibodies completely inhibit HA binding to chondrocytes, HA-mediated gene expression is not completely blocked [59]. This result shows that factors in addition to CD44 signalling play a role in HA-induced gene expression changes. Because these studies were conducted using other cell types, further work will be required to elucidate CD44 signalling in chondrocytes. Signalling via TGF-β1 intermediates could supplement the biophysical effects to enhance the functional properties of cartilage neotissue.
In summary, this study has shown that exogenous HA could act via both biophysical and genetic mechanisms to enhance the biomechanical and biochemical properties of self-assembled neocartilage. Although exogenous HA application has been studied in monolayer chondrocyte culture, this study has been the first to illustrate how HA can be applied exogenously to influence functional-relevant genetic pathways, and also improve biochemical and biomechanical properties during in vitro cartilage formation. Future studies could block CD44 to further investigate how HA influences intracellular signalling, apply HA in conjunction with other agents such as TGF-β1 to synergistically improve construct functionality, and probe the biophysical mechanism of HA-induced GAG retention.
Acknowledgments
This work was supported by NIH R01 AR053286.
References
- 1.Grande D. A., Halberstadt C., Naughton G., Schwartz R., Manji R. 1997. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. 34, 211–220 (doi:10.1002/(SICI)1097-4636(199702)34:2<211::AID-JBM10>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 2.Riesle J., Hollander A. P., Langer R., Freed L. E., Vunjak-Novakovic G. 1998. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J. Cell Biochem. 71, 313–327 (doi:10.1002/(SICI)1097-4644(19981201)71:3<313::AID-JCB1>3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- 3.Ng K. W., Saliman J. D., Lin E. Y., Statman L. Y., Kugler L. E., Lo S. B., Ateshian G. A., Hung C. T. 2007. Culture duration modulates collagen hydrolysate-induced tissue remodeling in chondrocyte-seeded agarose hydrogels. Ann. Biomed. Eng. 35, 1914–1923 10.1007/s10439-007-9373-z (doi:10.1007/s10439-007-9373-z) [DOI] [PubMed] [Google Scholar]
- 4.Wong M., Siegrist M., Gaschen V., Park Y., Graber W., Studer D. 2002. Collagen fibrillogenesis by chondrocytes in alginate. Tissue Eng. 8, 979–987 10.1089/107632702320934074 (doi:10.1089/107632702320934074) [DOI] [PubMed] [Google Scholar]
- 5.Kafienah W., Cheung F. L., Sims T., Martin I., Miot S., Von Ruhland C., Roughley P. J., Hollander A. P. 2008. Lumican inhibits collagen deposition in tissue engineered cartilage. Matrix Biol. 27, 526–534 10.1016/j.matbio.2008.04.002 (doi:10.1016/j.matbio.2008.04.002) [DOI] [PubMed] [Google Scholar]
- 6.Urban J. P., Maroudas A., Bayliss M. T., Dillon J. 1979. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology 16, 447–464 [DOI] [PubMed] [Google Scholar]
- 7.Kempson G. E., Muir H., Pollard C., Tuke M. 1973. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim. Biophys. Acta 297, 456–472 10.1016/0304-4165(73)90093-7 (doi:10.1016/0304-4165(73)90093-7) [DOI] [PubMed] [Google Scholar]
- 8.Woo S. L., Akeson W. H., Jemmott G. F. 1976. Measurements of nonhomogeneous, directional mechanical properties of articular cartilage in tension. J. Biomech. 9, 785–791 10.1016/0021-9290(76)90186-X (doi:10.1016/0021-9290(76)90186-X) [DOI] [PubMed] [Google Scholar]
- 9.Hoben G. M., Hu J. C., James R. A., Athanasiou K. A. 2007. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng. 13, 939–946 10.1089/ten.2006.0116 (doi:10.1089/ten.2006.0116) [DOI] [PubMed] [Google Scholar]
- 10.Hu J. C., Athanasiou K. A. 2006. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 12, 969–979 10.1089/ten.2006.12.969 (doi:10.1089/ten.2006.12.969) [DOI] [PubMed] [Google Scholar]
- 11.Elder S. H., Sanders S. W., McCulley W. R., Marr M. L., Shim J. W., Hasty K. A. 2006. Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. J. Orthop. Res. 24, 740–747 10.1002/jor.20086 (doi:10.1002/jor.20086) [DOI] [PubMed] [Google Scholar]
- 12.Bryant S. J., Chowdhury T. T., Lee D. A., Bader D. L., Anseth K. S. 2004. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng. 32, 407–417 10.1023/B:ABME.0000017535.00602.ca (doi:10.1023/B:ABME.0000017535.00602.ca) [DOI] [PubMed] [Google Scholar]
- 13.Anderson J. M. 2001. Biological responses to materials. Annu. Rev. Mater. Res. 31, 81–110 10.1146/annurev.matsci.31.1.81 (doi:10.1146/annurev.matsci.31.1.81) [DOI] [Google Scholar]
- 14.Ofek G., Revell C. M., Hu J. C., Allison D. D., Grande-Allen K. J., Athanasiou K. A. 2008. Matrix development in self-assembly of articular cartilage. PLoS ONE 3, e2795. 10.1371/journal.pone.0002795 (doi:10.1371/journal.pone.0002795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudson C. B., Knudson W. 2001. Cartilage proteoglycans. Semin. Cell Dev. Biol. 12, 69–78 10.1006/scdb.2000.0243 (doi:10.1006/scdb.2000.0243) [DOI] [PubMed] [Google Scholar]
- 16.Knudson C. B. 1993. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J. Cell Biol. 120, 825–834 10.1083/jcb.120.3.825 (doi:10.1083/jcb.120.3.825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turley E. A. 1989. Hyaluronic acid stimulates protein kinase activity in intact cells and in an isolated protein complex. J. Biol. Chem. 264, 8951–8955 [PubMed] [Google Scholar]
- 18.Hua Q., Knudson C. B., Knudson W. 1993. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J. Cell. Sci. 106, 365–375 [DOI] [PubMed] [Google Scholar]
- 19.Rousche K. T., Knudson C. B. 2002. Temporal expression of CD44 during embryonic chick limb development and modulation of its expression with retinoic acid. Matrix Biol. 21, 53–62 10.1016/S0945-053X(01)00189-5 (doi:10.1016/S0945-053X(01)00189-5) [DOI] [PubMed] [Google Scholar]
- 20.Wobus M., Rangwala R., Sheyn I., Hennigan R., Coila B., Lower E. E., Yassin R. S., Sherman L. S. 2002. CD44 associates with EGFR and erbB2 in metastasizing mammary carcinoma cells. Appl. Immunohistochem. Mol. Morphol. 10, 34–39 10.1097/00022744-200203000-00006 (doi:10.1097/00022744-200203000-00006) [DOI] [PubMed] [Google Scholar]
- 21.Bourguignon L. Y., Singleton P. A., Zhu H., Zhou B. 2002. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J. Biol. Chem. 277, 39703–39712 10.1074/jbc.M204320200 (doi:10.1074/jbc.M204320200) [DOI] [PubMed] [Google Scholar]
- 22.Bourguignon L. Y., Zhu H., Shao L., Chen Y. W. 2001. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J. Biol. Chem. 276, 7327–7336 10.1074/jbc.M006498200 (doi:10.1074/jbc.M006498200) [DOI] [PubMed] [Google Scholar]
- 23.Ohno S., Im H. J., Knudson C. B., Knudson W. 2006. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFκB and p38 MAP kinase in articular chondrocytes. J. Biol. Chem. 281, 17952–17960 10.1074/jbc.M602750200 (doi:10.1074/jbc.M602750200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kujawa M. J., Carrino D. A., Caplan A. I. 1986. Substrate-bonded hyaluronic acid exhibits a size-dependent stimulation of chondrogenic differentiation of stage 24 limb mesenchymal cells in culture. Dev. Biol. 114, 519–528 10.1016/0012-1606(86)90215-0 (doi:10.1016/0012-1606(86)90215-0) [DOI] [PubMed] [Google Scholar]
- 25.Toole B. P., Jackson G., Gross J. 1972. Hyaluronate in morphogenesis: inhibition of chondrogenesis in vitro. Proc. Natl. Acad. Sci. USA 69, 1384–1386 10.1073/pnas.69.6.1384 (doi:10.1073/pnas.69.6.1384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toole B. P. 1997. Hyaluronan in morphogenesis. J. Intern. Med. 242, 35–40 10.1046/j.1365-2796.1997.00171.x (doi:10.1046/j.1365-2796.1997.00171.x) [DOI] [PubMed] [Google Scholar]
- 27.Schwartz Z., Griffon D. J., Fredericks L. P., Lee H. B., Weng H. Y. 2011. Hyaluronic acid and chondrogenesis of murine bone marrow mesenchymal stem cells in chitosan sponges. Am. J. Vet. Res. 72, 42–50 10.2460/ajvr.72.1.42 (doi:10.2460/ajvr.72.1.42) [DOI] [PubMed] [Google Scholar]
- 28.Frean S. P., Abraham L. A., Lees P. 1999. In vitro stimulation of equine articular cartilage proteoglycan synthesis by hyaluronan and carprofen. Res. Vet. Sci. 67, 183–190 10.1053/rvsc.1999.0328 (doi:10.1053/rvsc.1999.0328) [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki K., Ochi M., Uchio Y., Adachi N., Matsusaki M. 1999. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J. Cell. Physiol. 179, 142–148 (doi:10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi T., Yamada H., Shimmei M. 1996. Effect of high molecular weight hyaluronan on cartilage degeneration in a rabbit model of osteoarthritis. Osteoarthritis Cartilage 4, 99–110 10.1016/S1063-4584(05)80319-X (doi:10.1016/S1063-4584(05)80319-X) [DOI] [PubMed] [Google Scholar]
- 31.Fukuda K., Dan H., Takayama M., Kumano F., Saitoh M., Tanaka S. 1996. Hyaluronic acid increases proteoglycan synthesis in bovine articular cartilage in the presence of interleukin-1. J. Pharmacol. Exp. Ther. 277, 1672–1675 [PubMed] [Google Scholar]
- 32.Takeuchi R., et al. 2006. Effects of vibration and hyaluronic acid on activation of three-dimensional cultured chondrocytes. Arthritis Rheum. 54, 1897–1905 10.1002/art.21895 (doi:10.1002/art.21895) [DOI] [PubMed] [Google Scholar]
- 33.Natoli R. M., Responte D. J., Lu B. Y., Athanasiou K. A. 2009. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J. Orthop. Res. 27, 949–956 10.1002/jor.20821 (doi:10.1002/jor.20821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almarza A. J., Athanasiou K. A. 2004. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 10, 1787–1795 10.1089/ten.2004.10.1787 (doi:10.1089/ten.2004.10.1787) [DOI] [PubMed] [Google Scholar]
- 35.Athanasiou K. A., Agarwal A., Dzida F. J. 1994. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J. Orthop. Res. 12, 340–349 10.1002/jor.1100120306 (doi:10.1002/jor.1100120306) [DOI] [PubMed] [Google Scholar]
- 36.Mow V. C., Gibbs M. C., Lai W. M., Zhu W. B., Athanasiou K. A. 1989. Biphasic indentation of articular cartilage. II. A numerical algorithm and an experimental study. J. Biomech. 22, 853–861 10.1016/0021-9290(89)90069-9 (doi:10.1016/0021-9290(89)90069-9) [DOI] [PubMed] [Google Scholar]
- 37.Athanasiou K. A., Niederauer G. G., Schenck R. C., Jr 1995. Biomechanical topography of human ankle cartilage. Ann. Biomed. Eng. 23, 697–704 10.1007/BF02584467 (doi:10.1007/BF02584467) [DOI] [PubMed] [Google Scholar]
- 38.Aufderheide A. C., Athanasiou K. A. 2007. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 13, 2195–2205 10.1089/ten.2006.0291 (doi:10.1089/ten.2006.0291) [DOI] [PubMed] [Google Scholar]
- 39.Huang D., Sherman B. T., Lempicki R. A. 2009. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 4, 44–57 10.1038/nprot.2008.211 (doi:10.1038/nprot.2008.211) [DOI] [PubMed] [Google Scholar]
- 40.Darling E. M., Athanasiou K. A. 2005. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23, 425–432 10.1016/j.orthres.2004.08.008 (doi:10.1016/j.orthres.2004.08.008) [DOI] [PubMed] [Google Scholar]
- 41.Mansson B., Wenglen C., Morgelin M., Saxne T., Heinegard D. 2001. Association of chondroadherin with collagen type II. J. Biol. Chem. 276, 32883–32888 10.1074/jbc.M101680200 (doi:10.1074/jbc.M101680200) [DOI] [PubMed] [Google Scholar]
- 42.Leddy H. A., Awad H. A., Guilak F. 2004. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J. Biomed. Mater. Res. B Appl. Biomater. 70, 397–406 10.1002/jbm.b.30053 (doi:10.1002/jbm.b.30053) [DOI] [PubMed] [Google Scholar]
- 43.Maroudas A. 1970. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 10, 365–379 10.1016/S0006-3495(70)86307-X (doi:10.1016/S0006-3495(70)86307-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caplan A. I. 2000. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 6, 1–8 10.1089/107632700320838 (doi:10.1089/107632700320838) [DOI] [PubMed] [Google Scholar]
- 45.Allison D. D., Braun K. R., Wight T. N., Grande-Allen K. J. 2009. Differential effects of exogenous and endogenous hyaluronan on contraction and strength of collagen gels. Acta Biomater. 5, 1019–1026 10.1016/j.actbio.2008.11.013 (doi:10.1016/j.actbio.2008.11.013) [DOI] [PubMed] [Google Scholar]
- 46.Tammi R. H., Passi A. G., Rilla K., Karousou E., Vigetti D., Makkonen K., Tammi M. I. 2011. Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 278, 1419–1428 10.1111/j.1742-4658.2011.08070.x (doi:10.1111/j.1742-4658.2011.08070.x) [DOI] [PubMed] [Google Scholar]
- 47.Shimazu A., Jikko A., Iwamoto M., Koike T., Yan W., Okada Y., Shinmei M., Nakamura S., Kato Y. 1993. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum. 36, 247–253 10.1002/art.1780360217 (doi:10.1002/art.1780360217) [DOI] [PubMed] [Google Scholar]
- 48.Morris E. A., Wilcon S., Treadwell B. V. 1992. Inhibition of interleukin 1-mediated proteoglycan degradation in bovine articular cartilage explants by addition of sodium hyaluronate. Am. J. Vet. Res. 53, 1977–1982 [PubMed] [Google Scholar]
- 49.Abatangelo G., Botti P., Del Bue M., Gei G., Samson J. C., Cortivo R., De Galateo A., Martelli M. 1989. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. I. Biochemical results. Clin. Orthop. Relat. Res. 241, 278–285 [PubMed] [Google Scholar]
- 50.Kato Y., Mukudai Y., Okimura A., Shimazu A., Nakamura S. 1995. Effects of hyaluronic acid on the release of cartilage matrix proteoglycan and fibronectin from the cell matrix layer of chondrocyte cultures: interactions between hyaluronic acid and chondroitin sulfate glycosaminoglycan. J. Rheumatol. Suppl. 43, 158–159 [PubMed] [Google Scholar]
- 51.Haglund L., Tillgren V., Addis L., Wenglen C., Recklies A., Heinegard D. 2011. Identification and characterization of the integrin α2β1 binding motif in chondroadherin mediating cell attachment. J. Biol. Chem. 286, 3925–3934 10.1074/jbc.M110.161141 (doi:10.1074/jbc.M110.161141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishiguro K., et al. 2001. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J. Biol. Chem. 276, 47483–47488 10.1074/jbc.M106268200 (doi:10.1074/jbc.M106268200) [DOI] [PubMed] [Google Scholar]
- 53.Dagnino L., Fry C. J., Bartley S. M., Farnham P., Gallie B. L., Phillips R. A. 1997. Expression patterns of the E2F family of transcription factors during mouse nervous system development. Mech. Dev. 66, 13–25 10.1016/S0925-4773(97)00083-X (doi:10.1016/S0925-4773(97)00083-X) [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Toury R., Hauchecorne M., Balmain N. 1997. Expression and subcellular localization of the Myc superfamily proteins: c-Myc, Max, Mad1 and Mxi1 in the epiphyseal plate cartilage chondrocytes of growing rats. Cell Mol. Biol. (Noisy-le-grand) 43, 175–188 [PubMed] [Google Scholar]
- 55.Knudson W., Loeser R. F. 2002. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol. Life Sci. 59, 36–44 10.1007/s00018-002-8403-0 (doi:10.1007/s00018-002-8403-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elder B. D., Athanasiou K. A. 2009. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage 17, 114–123 10.1016/j.joca.2008.05.006 (doi:10.1016/j.joca.2008.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elder B. D., Athanasiou K. A. 2008. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE 3, e2341. 10.1371/journal.pone.0002341 (doi:10.1371/journal.pone.0002341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito T., Williams J. D., Fraser D. J., Phillips A. O. 2004. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. J. Biol. Chem. 279, 25 326–25 332 10.1074/jbc.M403135200 (doi:10.1074/jbc.M403135200) [DOI] [PubMed] [Google Scholar]
- 59.McKee C. M., Penno M. B., Cowman M., Burdick M. D., Strieter R. M., Bao C., Noble P. W. 1996. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Invest. 98, 2403–2413 10.1172/JCI119054 (doi:10.1172/JCI119054) [DOI] [PMC free article] [PubMed] [Google Scholar]