Abstract
A better understanding of the inherent properties of vascular tissue to adapt to its mechanical environment is crucial to improve the predictability of biomechanical simulations. Fibrillar collagen in the vascular wall plays a central role in tissue adaptation owing to its relatively short lifetime. Pathological alterations of collagen turnover may fail to result in homeostasis and could be responsible for abdominal aortic aneurysm (AAA) growth at later stages of the disease. For this reason our previously reported multiscale constitutive framework (Martufi, G. & Gasser, T. C. 2011 J. Biomech. 44, 2544–2550 (doi:10.1016/j.jbiomech.2011.07.015)) has been enriched by a collagen turnover model. Specifically, the framework's collagen fibril level allowed a sound integration of vascular wall biology, and the impact of collagen turnover on the macroscopic properties of AAAs was studied. To this end, model parameters were taken from the literature and/or estimated from clinical follow-up data of AAAs (on average 50.7 mm-large). Likewise, the in vivo stretch of the AAA wall was set, such that 10 per cent of collagen fibres were engaged. Results showed that the stretch spectrum, at which collagen fibrils are deposed, is the most influential parameter, i.e. it determines whether the vascular geometry grows, shrinks or remains stable over time. Most importantly, collagen turnover also had a remarkable impact on the macroscopic stress field. It avoided high stress gradients across the vessel wall, thus predicted a physiologically reasonable stress field. Although the constitutive model could be successfully calibrated to match the growth of small AAAs, a rigorous validation against experimental data is crucial to further explore the model's descriptive and predictive capabilities.
Keywords: remodelling, aneurysm, growth, rupture, constitutive modelling, vascular tissue
1. Introduction
Abdominal aortic aneurysms (AAAs) are defined as local and permanent dilatations of the infrarenal aorta that are frequently observed in the elderly male population. AAAs can grow into shapes that are prone to rupture, which are lethal in up to 90 per cent [1] of all cases. A rupture risk assessment is central to the management of AAA patients, and accurate rupture risk assessments could reduce the related mortality rate without unnecessarily increasing the rate of AAA repair interventions. Growth rate and wall strength of AAAs are thought to be linked [2–5], such that a reliable growth predictor would reinforce an AAA rupture risk assessment, i.e. give a better-graded indication for elective repair.
Describing the growth of aneurysms is an active field of research, and models for abdominal aortic [6–9] and cerebral [10–14] aneurysms have been reported. Although these models are able to predict some clinical observations, they still require significant development to be launched clinically, i.e. to predict patient–individual aneurysm rupture in a clinical setting.
AAAs are the result of irreversible pathological remodelling of the extracellular matrix (ECM) [15]. ECMs provide an essential mechanical environment to which vascular tissue is continuously exposed, and mainly contain elastin, collagen and proteoglycans (PGs) [16]. While elastin is a stable protein having a half-life of tens of years [17], collagen is normally in a continual state of deposition and degradation [18]. Specifically, vascular collagen has a normal half-life of 60–70 days [19], which decreases by up to 10-fold in the case of disease and injury [20]. Moreover, elastin decreases significantly [21] and fragments [22] through the aneurysmatic expansion of the aorta.
Collagen fibrils are regarded as one basic building block of ECMs and give mechanical strength, stiffness and toughness to the vasculature. Collagen fibrils are locally secreted by fibroblasts and assembled into organized suprafibrillar structures that, to a large extent, determine the macroscopic mechanical behaviour [23]. Consequently, understanding the development of hierarchical collagen structures is crucial to understanding the mechanical properties of the vascular wall.
It is well accepted that the collagen structure develops under the action of mechanical loading and eventually leads to mechanically optimized structures. Physiological maintenance of these structures relies on a delicate (coupled) balance between continual degradation and synthesis of collagen. The maintenance of fibrillar collagen is realized by fibroblast cells, i.e. by synthesis of new collagen and degradation by metalloproteinases (MMPs) of existing collagen [24]. Mature fibroblasts perceive changes in the mechanical strains/stresses and adjust their expression and synthesis of collagen molecules in order to account for the changes in their micro-mechanical environment. While an alteration of collagen turnover is essential in response to injury [24–28], a malfunction of collagen turnover could fail to result in homeostasis and determine AAAs disease [29]. Specifically, at later stages of aneurysm disease, collagen synthesis is insufficient to counteract higher mechanical wall stress [29]. Consequently, the structural integrity of the wall is not ensured, such that wall strength decreases and the risk for AAA rupture increases proportionally.
The present work introduces a novel growth model for AAAs, where their expansion is a direct consequence of the pathological remodelling of fibrillar collagen. Collagen fibrils are thought to be assembled into collagen fibres that, in turn, define the tissue's macroscopic properties [30–33]. This multi-scale view allowed us to integrate vascular wall biology within the constitutive framework and led to robust and fast finite element (FE) implementation. The model was calibrated to the growth of AAAs that were followed-up with computer tomography–angiography (CT–A).
In contrast to earlier models [6,7,9,11–14], the present description does not constrain the collagen fibres to develop along certain orientations, and the predicted collagen organizations were in good agreement with our histological study of the AAA wall [34].
2. Methods
2.1. Passive constitutive response
The wall of later stage aneurysms shows a negligible amount [21] of fragmented [22] elastin, as well as a severe decrease in smooth muscle cells [35]. Consequently, the AAA wall was regarded as a fibrous collagenous tissue, where collagen fibres reinforced an otherwise isotropic matrix material. At low strains, collagen fibres are (mechanically) inactive, and the non-collagenous (matrix) material determines the vascular wall's properties. The matrix material was described by the (classical) isotropic neoHookean strain energy function ψnH = c(I1 − 3), where c > 0 was a stress-like material parameter, i.e. twice the referential shear stiffness, and I1 = J−2/3FTF : I denoted a strain invariant [36].
An orientation density function ρ(N) = ρ(−N) defined the alignment of the collagen fibres with respect to their referential orientations N (with |N| = 1) [37]. For simplicity, the passive wall response was modelled with an isotropic collagen orientation density (ρ(N) = ρ0), which adjusted over time according to the local stretch field (see §2.2).
Collagen fibres were assembled from bundles of collagen fibrils with different undulations, following a virtually linear stress–strain law [33]. The classic ‘shear-lag’ theory [38,39] was adopted, and shear forces were thought to be distributed all along the length of the collagen fibril in order to support inter-fibrilar load transition. Specifically, small PGs such as decorin bind non-covalently but specifically to the collagen fibril at about 60 nm intervals [40]. The PG-based cross-linking was supported by numerous experimental studies showing that PGs play a direct role in inter-fibril load sharing [40–46], and this has also been verified through theoretical investigations [47–49]. However, it was also noted that the biomechanical role of PGs is discussed controversially, and some data indicate minimal, if any, PG contribution to tensile properties of the tissue [47,50,51].
The undulation of collagen fibrils, within a collagen fibre, was determined by a triangular probability density function (PDF). Specifically, the triangular PDF defined the relative amount of engaged collagen fibrils in response to the stretch λ that was applied to the collagen fibre [33]. Consequently, the limits λmin and λmax of the triangular PDF denoted fibre stretches, at which the first and last collagen fibrils engaged, i.e. straightened out. Assuming an incompressible collagen fibre, these assumptions yielded piece-wise analytical expressions for the collagen fibre's Cauchy stress [33]
 |
2.1 |
with Δλ = λmax−λmin and  and k denoting the stiffness of the collagen fibril proteoglycan-complexes. Finally, an affine deformation between the continuum and the collagen fibre was considered, i.e. λ = |FN|.
and k denoting the stiffness of the collagen fibril proteoglycan-complexes. Finally, an affine deformation between the continuum and the collagen fibre was considered, i.e. λ = |FN|.
2.2. Collagen turnover model
Collagen turnover in the vascular wall is accomplished by fibroblast cells that are spread throughout the collagen network. Apart from continuously producing and degrading collagen fibrils, fibroblasts are thought to sense the state of stress or strain and even pre-stretch collagen fibrils prior to deposition. Specifically, a biomechanical mode for collagen turnover has to fulfil three tasks:
— Detail what quantity stimulates collagen turnover, i.e. define the sensing mechanism.
— Quantify how the stimulus is translated into degradation and production of collagen.
— Detail how matured collagen is removed and how newly formed collagen is integrated into the existing collagen structure.
2.2.1. Sensing mechanism
We considered fibroblasts being connected to collagen fibrils, which in turn allowed them to sense the collagen fibre stretch λ. At a certain λ, those fibroblast cells that are connected to active collagen fibrils will experience a mechanical stimulus, whereas those that are connected to undulated fibrils cannot feel any stretch (figure 1a). Consequently, a stretch-based model [6,7,12–14] that allowed a gradual sensing of the collagen fibre stretch was considered.
Figure 1.

The hierarchical structure of a collagen fibre built-up of statistically distributed collagen fibrils that are interlinked by proteoglycan (PG) bridges and populated by fibroblast cells. (a) Homeostatic condition at 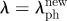 that leads to
that leads to  (b) Supra-physiological stretched condition at
(b) Supra-physiological stretched condition at  that leads to
that leads to  .
.
In a normal vessel wall, only about 6 or 7 per cent of collagen fibrils are engaged at physiological deformation [52,53]. Unfortunately, such data do not exist for the AAA wall and, motivated by the higher stress, we assumed that 10 per cent of collagen fibrils were engaged in the AAA wall. On the basis of triangular PDF, the density of engaged collagen fibrils was related to the distribution parameters according to  for λmin ≤ λ ≤ Δλ/2. Consequently, the physiological stretch could be expressed through
for λmin ≤ λ ≤ Δλ/2. Consequently, the physiological stretch could be expressed through  with
with  denoting the density of engaged collagen fibrils at the physiological deformation, i.e. 10 per cent. Similarly, we introduced the homeostatic collagen fibre stretch
denoting the density of engaged collagen fibrils at the physiological deformation, i.e. 10 per cent. Similarly, we introduced the homeostatic collagen fibre stretch
| 2.2 |
In order to reach this stretch, the newly formed collagen fibrils had to be deposited with 
 and
and  Here,
Here,  and
and  denote the lower undulation limit, the higher undulation limit and the width of the triangular PDF that defines the collagen that was deposited. At homeostasis and physiological loading, the collagen stretch will tend to
denote the lower undulation limit, the higher undulation limit and the width of the triangular PDF that defines the collagen that was deposited. At homeostasis and physiological loading, the collagen stretch will tend to  and a suitable optimality condition reads
and a suitable optimality condition reads
 |
2.3 |
where ζ(N) serves as the mechanical stimulus. For ζ > 1, the existing collagen is stretched higher than the newly formed collagen, such that in total, more collagen is required to reach homeostasis. In that case, collagen turnover needs to be amplified in order to increase the total collagen density in the tissue. Similarly, for ζ < 1, collagen turnover needs to slow down to reach homeostasis through a net loss of collagen. It is emphasized that ζ depends on the orientation through the collagen orientation vector N, and similar collagen-stretch-based stimuli have been proposed earlier in the literature [6,7,12–14]. In contrast, other stimuli such as stress [8–11] and the ratio between systolic and diastolic stretches [54] have also been suggested.
2.2.2. Collagen degradation and production rates
It was assumed that collagen degraded isotropically according to
| 2.4 |
where η defines the time-scale of the degradation process and ρ is the total collagen density. Equation (2.4) is independent from the orientation N and the local strain, such that degrading collagen is purely time-based; i.e. as soon as collagen matures, it is removed.
The proposed collagen production was based on the idea that fibroblasts will produce collagen in an effort to maintain a homeostatic environment. Consequently, for a particular direction N, the production of collagen fibrils was related to the stimulus ζ(N) and reads
| 2.5 |
Here,  denotes the maximum collagen production rate, which aimed at modelling the limited capability of fibroblasts to produce collagen fibrils [55]. According to equation (2.5), the collagen production along the referential direction N is proportional to the mechanical stimulus ζ(N), but it cannot exceed the maximum rate
denotes the maximum collagen production rate, which aimed at modelling the limited capability of fibroblasts to produce collagen fibrils [55]. According to equation (2.5), the collagen production along the referential direction N is proportional to the mechanical stimulus ζ(N), but it cannot exceed the maximum rate  According to equations (2.4) and (2.5), the net production of collagen vanishes at homeostatic conditions
According to equations (2.4) and (2.5), the net production of collagen vanishes at homeostatic conditions  , i.e.
, i.e.  Likewise, the model agrees with the observation that mechanical stretching of collagen fibrils protect them from enzymatic degradation by MMPs [56].
Likewise, the model agrees with the observation that mechanical stretching of collagen fibrils protect them from enzymatic degradation by MMPs [56].
2.2.3. Collagen fibril disintegration and integration
Collagen fibrils disintegrate from the existing collagen structure without changing their undulation characteristics; i.e. the collagen density ρ is reduced, but the limits λmin and λmax of the triangular PDF that determine the fibril undulation spectrum remain unchanged. In contrast, newly formed collagen fibrils are integrated at a certain distribution of pre-stretches. The idea of integrating pre-stretched collagen has been used in the past [57] and is supported by the observation that fibroblasts have the ability to contract the surrounding ECM [17]. Likewise, fibroblasts are observed to direct the formation of groups of short collagen fibrils that appear to connect fibroblast cells [58].
The deformation of a newly formed collagen fibril is demonstrated in figure 2, where we defined a local intermediate configuration Ωst i at which the individual ith collagen fibril was straight but did not carry any load, i.e. it was stress-free. The individual ith collagen fibril was thought to be integrated (cross-linked) in the current (deformed) configuration Ω at a certain stretch  that was measured relative to its hypothetical intermediate configuration Ωst i. Consequently, the straightening stretch
that was measured relative to its hypothetical intermediate configuration Ωst i. Consequently, the straightening stretch  was required to straighten this collagen fibril relative to the reference (undeformed) configuration Ω0, where λ = |FN| denotes the total macroscopic stretch in direction of the fibril. Similar to the passive model, collagen fibrils were considered in a statistical sense, and the undulation of the newly formed collagen fibrils followed a triangular PDF having the limits
was required to straighten this collagen fibril relative to the reference (undeformed) configuration Ω0, where λ = |FN| denotes the total macroscopic stretch in direction of the fibril. Similar to the passive model, collagen fibrils were considered in a statistical sense, and the undulation of the newly formed collagen fibrils followed a triangular PDF having the limits  and
and  Finally, and as demonstrated later, adding the newly formed collagen to the already existing one, can be regarded as a continuous update of the PDF that represents the collagen fibril distribution.
Finally, and as demonstrated later, adding the newly formed collagen to the already existing one, can be regarded as a continuous update of the PDF that represents the collagen fibril distribution.
Figure 2.

Finite strain kinematics of the collagenous tissue. The ith newly formed collagen fibril maps from the reference configuration Ω0 through its intermediate configuration Ωst i into the current configuration Ω.
2.2.4. Updating of the collagen fibril distribution
The cumulative collagen distribution X was defined by adding the newly formed collagen distribution  to the already existing collagen distribution
to the already existing collagen distribution  . For convenience, the mean E(X) = (λmax + λmin)/2 and variance var(X) = (λmax − λmin)2/24 of the triangular PDF were introduced, such that the cumulative triangular distribution was given by
. For convenience, the mean E(X) = (λmax + λmin)/2 and variance var(X) = (λmax − λmin)2/24 of the triangular PDF were introduced, such that the cumulative triangular distribution was given by
 |
2.6 |
Here, the weighted mean and the weighted variance decomposition were applied, and  denotes the relative amount of newly formed collagen. In addition, the abbreviations
denotes the relative amount of newly formed collagen. In addition, the abbreviations
and
 |
were used.
Consequently, the distribution X was defined through equation (2.6) as soon as  and
and  and dξ+ of the newly formed collagen were given. In vivo collagen fibrils are deposited on top of the cyclically stretched vascular wall, such that differently oriented collagen fibrils might have different
and dξ+ of the newly formed collagen were given. In vivo collagen fibrils are deposited on top of the cyclically stretched vascular wall, such that differently oriented collagen fibrils might have different  and
and  Unfortunately, no related experimental data are available, and we simply used orientation-independent undulation limits throughout this study.
Unfortunately, no related experimental data are available, and we simply used orientation-independent undulation limits throughout this study.
Solving the set of equations (2.6) for  and
and  of the cumulative distribution of collagen fibrils gives
of the cumulative distribution of collagen fibrils gives
| 2.7 |
with 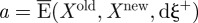 and
and
 |
Finally, it is noted that equation (2.7) only held for small differences between  and
and  , such that collagen turnover that was close only to the homeostatic condition could be described within this statistical framework.
, such that collagen turnover that was close only to the homeostatic condition could be described within this statistical framework.
2.3. Numerical implementation
The proposed multiscale model for vascular tissue was implemented in FE software (FEAP v. 8.2, University of California at Berkeley, CA, USA) at the Gauss point level of a Q1P0 mixed Finite Element [59] formulation. To this end, a quasi-incompressible implementation was followed, and the volumetric energy contribution ψvol = κ(J − 1)2 was added to the constitutive description. The volumetric stiffness κ was adjusted at each time step such that the volume ratio J → 1 with J = det F. Consequently, the macroscopic Cauchy stress was additively decomposed and reads
| 2.8 |
where σvol is the volumetric stress contribution, and  and
and  are contributions that arose from the matrix material and the collagen reinforcement, respectively. The superimposed bar indicates isochoric stress quantities, and σvol and
are contributions that arose from the matrix material and the collagen reinforcement, respectively. The superimposed bar indicates isochoric stress quantities, and σvol and  are detailed elsewhere [60]. The macroscopic stress
are detailed elsewhere [60]. The macroscopic stress 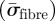 that arose from the collagen fibres was computed by integration over all fibres, i.e. over all directions. This contribution is detailed in §2.3.1.
that arose from the collagen fibres was computed by integration over all fibres, i.e. over all directions. This contribution is detailed in §2.3.1.
2.3.1. Microfibre model
Following the pioneering work of Lanir [30], the macroscopic stress contribution from the collagen fibres was computed by integration over the solid angle ω, i.e. by a spherical integration over the unit sphere, and reads
| 2.9 |
This integral was numerically approximated by a spherical design [31,61], i.e.
 where lint denotes the total number of integration points. Details regarding the numerical implementations are given in table 1. It is noted that the applied two-scale simulation required considerable memory demands owing to storing the microstructural data (λmin, λmax and ρ) at each Gauss point.
where lint denotes the total number of integration points. Details regarding the numerical implementations are given in table 1. It is noted that the applied two-scale simulation required considerable memory demands owing to storing the microstructural data (λmin, λmax and ρ) at each Gauss point.
Table 1.
Numerical implementation of the collagen-related contributions of the multiscale vascular tissue model. The Cauchy stress  and the spatial stiffness
and the spatial stiffness  at the Gauss point of a finite element were computed. The microstructure was represented by the collagen orientation density ρ and the undulation limits λmin and λmax, which were stored in the history vector.
at the Gauss point of a finite element were computed. The microstructure was represented by the collagen orientation density ρ and the undulation limits λmin and λmax, which were stored in the history vector.
 |
3. Results
In this section, the application of the introduced constitutive framework is demonstrated. Specifically, the interplay between collagen structure and wall stress, as well as the applicability to investigate realistic (clinically relevant) problems is shown. Passive constitutive properties were taken from the literature, and turnover properties were estimated from clinical follow-up data of 30 small AAAs, i.e. having maximum diameters smaller than 55 mm. To this end, the expansion of the aneurysms between two consecutive CT–A scans was measured, and patient-specific mean arterial pressures (MAPs = 1/3 systolic + 2/3 diastolic blood pressure) were computed from the patient charts. The data analysis revealed a maximum outer diameter of 50.7 (s.d. 5.05) mm, MAP of 103 (s.d. 10.44) mmHg (13.73 (s.d. 1.39) kPa) and an average growth rate of 2.9 (s.d. 5.6) mm yr−1. Structural FE simulations (A4research, VASCOPS GmbH, Austria) of these cases showed a circumferential strain and Cauchy stress in the aneurysmatic sac of 6 (s.d. 1.13) per cent and 106 (s.d. 48.76) kPa, respectively.
3.1. Parameter estimation
The material parameters c,k and the structural parameters λmin, λmax defines the passive (time-independent) constitutive response. Previously reported parameters [33] that capture the mean population properties of the AAA wall [62], were used in the present study. Similarly, the width of the triangular PDF that defined the newly formed collagen was estimated from [33,34] and set to Δλnew = 0.24.
The turnover parameter η that defined the time scale for homeostatic collagen turnover was set to two months, i.e. the half-life of vascular collagen [19]. In contrast, no applicable data to estimate the turnover parameters  and
and  are reported in the literature, and these parameters were estimated from the response of a numerical relaxation test and the expansion of an AAA tube model.
are reported in the literature, and these parameters were estimated from the response of a numerical relaxation test and the expansion of an AAA tube model.
3.2. Relaxation test
Because the spectrum of the collagen deposition stretch was assumed to be constant,  was also constant, and the mechanical stimulus through equation (2.3) for a relaxation test, i.e. where the macroscopic stretch λ is fixed, is time-independent. Without constraining the collagen production rate (i.e.
was also constant, and the mechanical stimulus through equation (2.3) for a relaxation test, i.e. where the macroscopic stretch λ is fixed, is time-independent. Without constraining the collagen production rate (i.e.  ), collagen would be constantly produced, such that the stress increases continuously until infinity. Consequently, we used such a relaxation test to define the limit of the collagen production rate
), collagen would be constantly produced, such that the stress increases continuously until infinity. Consequently, we used such a relaxation test to define the limit of the collagen production rate  [63]. To this end, a strain of 6 per cent was applied and a steady-state target stress of 106 kPa was reached with
[63]. To this end, a strain of 6 per cent was applied and a steady-state target stress of 106 kPa was reached with  Here, sr stands for steradian and denotes the unit of the solid angle. The undulation parameters
Here, sr stands for steradian and denotes the unit of the solid angle. The undulation parameters  and
and  were used, where §3.3 motivates this choice.
were used, where §3.3 motivates this choice.
3.3. Abdominal aortic aneurysm tube model
The AAA tube model was loaded with a pressure of 13.73 kPa, and had an outer diameter and wall thickness of 50.7 mm and 2 mm, respectively. Symmetry conditions were applied and 300 hexahedral finite elements represented a quarter of the model (figure 5). Bending effects from non-homogeneous stress across the wall were captured by five finite elements across the thickness. The limit of the collagen production was fixed at  By setting the undulation of the newly formed collagen to
By setting the undulation of the newly formed collagen to  and
and  the tube model showed homeostasis, i.e. its diameter neither grew nor shrank over time (see dashed line in figure 3). Here, homeostasis is understood as a purely hypothetical condition, because the AAA is known to grow, i.e. to continuously change its tissue constitution over time.
the tube model showed homeostasis, i.e. its diameter neither grew nor shrank over time (see dashed line in figure 3). Here, homeostasis is understood as a purely hypothetical condition, because the AAA is known to grow, i.e. to continuously change its tissue constitution over time.
Figure 5.

Maximum principal Cauchy stress computed with the abdominal aortic aneurysm (AAA) tube model. (a) Prediction that neglected collagen turnover (passive model). (b) Prediction that accounted for collagen turnover and used parameters (


 ) leading to homeostasis. (c) Prediction that accounted for collagen turnover and used parameters
) leading to homeostasis. (c) Prediction that accounted for collagen turnover and used parameters 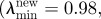

 ) that matched the growth of small AAAs.
) that matched the growth of small AAAs.
Figure 3.

Evolution of the abdominal aortic aneurysm (AAA) tube model over time with respect to different undulations of the newly formed collagen fibrils. The maximum collagen production rate was set to  The width of the triangular probability density function (PDF) was fixed at Δλnew = 0.24, while the lower undulation limit
The width of the triangular probability density function (PDF) was fixed at Δλnew = 0.24, while the lower undulation limit  was changed.
was changed.
In addition to the homeostatic solution, the implications of a step-change in  were investigated (figure 3). Increasing the undulation of collagen fibrils, i.e. decreasing their pre-stretch at deposition, triggered continuous growth while the AAA tube model shrank when depositing the newly formed collagen fibrils at a lower undulation. Finally, the AAA tube model matched the average expansion of small AAAs for
were investigated (figure 3). Increasing the undulation of collagen fibrils, i.e. decreasing their pre-stretch at deposition, triggered continuous growth while the AAA tube model shrank when depositing the newly formed collagen fibrils at a lower undulation. Finally, the AAA tube model matched the average expansion of small AAAs for  and
and  i.e. the diameter increased by 2.9 mm yr−1 (see the black solid line in figures 3 and 4).
i.e. the diameter increased by 2.9 mm yr−1 (see the black solid line in figures 3 and 4).
Figure 4.

Calibration of the abdominal aortic aneurysm (AAA) tube model (thick line) with respect to clinical follow-up data (thin lines) of small AAAs.
Owing to its highly nonlinear character, the passive constitutive model predicted a strong stress gradient across the wall thickness (figure 5a). Such a stress gradient is considered to be non-physiological and, among other constitutive refinements, residual strains in the load-free configuration are able to smooth the stress across the wall thickness. Activating collagen turnover  and
and  ) on the top of the passive formulation redistributed the stress in the collagen fibres. Consequently, at homeostatic conditions, the stress across the wall was almost uniform (figure 5b). Depositing newly formed collagen at a higher undulation (
) on the top of the passive formulation redistributed the stress in the collagen fibres. Consequently, at homeostatic conditions, the stress across the wall was almost uniform (figure 5b). Depositing newly formed collagen at a higher undulation ( and
and  ) caused not only continuous growth of the tube but also failed to reach homeostasis, leading to a weak stress gradient across the wall (figure 5c).
) caused not only continuous growth of the tube but also failed to reach homeostasis, leading to a weak stress gradient across the wall (figure 5c).
3.4. Organ-level analysis
3.4.1. Modelling assumptions
The aneurysm geometry between the renal arteries and 1.5 cm distal the aortic bifurcation was segmented from routinely taken CT–A data (A4research). In order to provide an artefact-insensitive and user-independent reconstruction, deformable (active) contour models were applied [64]. A single finite element across the wall thickness was used, and in total, 6209 hexahedral elements represented the AAA. A constant wall thickness of 2 mm was prescribed, and the influence of the intra-luminal thrombus was disregarded. The model was loaded by 100 mmHg (13.33 kPa) MAP and fixed at the bottom and top slices (nodal degrees of freedom were locked). Finally, no contact with surrounding organs that could have influenced the expansion of the aneurysm was considered.
3.4.2. Problem-solving and numerical predictions
Passive response. The stress field without collagen turnover, i.e. with the passive constitutive description, was computed first. To this end, the pressure loading was incremented and the quasi-static solution was computed using a direct solver (FEAP v. 8.2, University of California at Berkeley). We neither observed numerical instabilities nor required any kind of numerical regularization. In line with the results from alternative passive AAA wall models [65], the macroscopic stress was complex distributed over the wall and showed regions of stress concentrations (figure 6a).
Figure 6.

Stress and shape development of a patient-specific abdominal aortic aneurysm (AAA) model. (a–c) Maximum principal Cauchy stress in a patient-specific AAA model. (a) Maximum principal Cauchy stress prediction that neglected collagen turnover (passive model). (b) Maximum principal Cauchy stress prediction that accounted for collagen turnover and used parameters 

 ) leading to homeostasis (dashed line). (c) Maximum principal Cauchy stress prediction after one year collagen turnover with parameters (
) leading to homeostasis (dashed line). (c) Maximum principal Cauchy stress prediction after one year collagen turnover with parameters (

 ) that matched the growth of small AAAs. (d) AAA shape difference between the homeostatic solution (b) (dashed line) and the prediction after 1-year growth (solid line; c).
) that matched the growth of small AAAs. (d) AAA shape difference between the homeostatic solution (b) (dashed line) and the prediction after 1-year growth (solid line; c).
Homeostatic conditions. On top of the passive prediction, collagen turnover was activated (

 ), such that the AAA geometry remained stable over time. This homogenized the wall stress distribution (compare figure 6a,b) and shifted the initially isotropic collagen distribution to a locally orthotropic distribution (figure 7a,b). Consequently, collagen adapted [66] until the optimal microstructure configuration was reached and the macroscopic stress converged to the homeostatic solution.
), such that the AAA geometry remained stable over time. This homogenized the wall stress distribution (compare figure 6a,b) and shifted the initially isotropic collagen distribution to a locally orthotropic distribution (figure 7a,b). Consequently, collagen adapted [66] until the optimal microstructure configuration was reached and the macroscopic stress converged to the homeostatic solution.
Figure 7.
Numerically predicted collagen distribution in the abdominal aortic aneurysm (AAA) wall. The view is perpendicular to the wall and taken at the site of peak wall stress (PWS). (a) Isotropic distribution used by the mode that neglected collagen turnover (passive model). (b) Predicted distribution at homeostasis. (c) Predicted distribution after 10 years' growth.
Abdominal aortic aneurysm growth. On top of the homeostatic prediction, collagen undulation parameters were changed (
 ), such that the AAA enlarged over time (figure 6). While the macroscopic stress did not change considerably (figure 6c), the collagen distribution in the wall was altered with respect to its initial configuration (figure 7). Finally, figure 6d illustrates the AAA shape development for 1-year growth.
), such that the AAA enlarged over time (figure 6). While the macroscopic stress did not change considerably (figure 6c), the collagen distribution in the wall was altered with respect to its initial configuration (figure 7). Finally, figure 6d illustrates the AAA shape development for 1-year growth.
To carry higher wall stress, more collagen was required, such that collagen turnover (production  and degradation
and degradation  ) rates peaked at the site of peak wall stress. Owing to the fibroblast's limited capability to produce collagen, the collagen production was no longer able to compensate for the increasing wall stress. Consequently, AAA growth was amplified and the risk for rupture increased, i.e. wall stress increased considerably over time (figure 8).
) rates peaked at the site of peak wall stress. Owing to the fibroblast's limited capability to produce collagen, the collagen production was no longer able to compensate for the increasing wall stress. Consequently, AAA growth was amplified and the risk for rupture increased, i.e. wall stress increased considerably over time (figure 8).
Figure 8.

Development of the maximum principal Cauchy stress in the abdominal aortic aneurysm (AAA) wall after 2, 5 and 10 years. Simulation used parameters (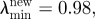

 ) that matched the growth of small AAAs over a period of about one year.
) that matched the growth of small AAAs over a period of about one year.
4. Discussion
Biological tissues respond to their mechanical environment and predictions based on passive constitutive models, i.e. suppressing tissue remodelling and growth can only cover a limited time period. Vascular tissue develops at its in vivo loading state, which induces residual strains in its (hypothetical) load-free configuration, i.e. in the setting that typically serves as a reference for computations. Predicting realistic physiological stress states with passive constitutive models requires residual strains in the load-free configuration, which, for complex geometries, are unfortunately unknown. Consequently, the key for improving biomechanical models is to understand the tissue's inherent property to adapt to mechanical environments, and in that respect, computational simulations can be potentially helpful. Specifically, multiscale models allow considering biological process at the microscale that, in turn, define the development of the vascular wall's macroscopic mechanical properties.
The present work enriched our previously reported multiscale constitutive model [33] by considering collagen turnover. Specifically, the model's collagen fibril level provided an interface to integrate the vascular wall biology and to study the impact of collagen turnover on the macroscopic properties of AAAs. The applied concept aimed at reflecting the mechanics of later stage AAAs, i.e. where the impact of elastin and smooth muscle cells can be neglected. However, such a modelling approach does not reflect aneurysm formation, where the interaction between elastin, collagen and smooth muscle cells should be particularly considered [9,11,67]. That explains also the manifold overestimation of growth of the non-aneurysmal regions as it was predicted by our model.
Most importantly, the results indicated a remarkable impact of collagen turnover: it avoided high stress gradients across the vessel that is thought to be non-physiological. Likewise, the deposition of collagen fibrils at certain stretch defined homeostatic conditions and vascular geometry remained stable over time. In contrast, depositing collagen fibrils at a higher undulation limit triggered continuous growth, where the expansion rate of small AAAs was used for parameter identification.
Finally, setting an upper boundary for the collagen production rate (constraint collagen turnover) destabilized the equilibrium of collagen turnover and amplified growth. The observation that collagen cross-linking increased in the aneurysmatic aortic wall justified limiting the collagen production. Specifically, increased collagen cross-linking suggested that at a certain stage the synthesis of collagen was reduced, while existing collagen continued to be cross-linked [68,69]. By limiting the collagen production in our model, the mechanical stimulus was deactivated and the collagen synthesis could no longer support a net increase of collagen. Considering the rapidity of collagen remodelling, this circumstance could quickly lead to aneurysm rupture [29]. Note that the final common pathway of aneurysm progression involves proteolytic activity, and collagen degradation is a prerequisite for aneurysm rupture [70].
The undulation limits  and
and  of the newly formed collagen as well as the maximum collagen production rate
of the newly formed collagen as well as the maximum collagen production rate  were key parameters in our model. The experimental identification of these parameters was not straightforward and, when only considering macroscopic observations, not unique either. Specifically, increasing the undulation limits and lowering the maximum collagen production both led to an increased AAA growth rate. The definition of experimental protocols that would allow the unique identification of these parameters remains to be addressed in future studies.
were key parameters in our model. The experimental identification of these parameters was not straightforward and, when only considering macroscopic observations, not unique either. Specifically, increasing the undulation limits and lowering the maximum collagen production both led to an increased AAA growth rate. The definition of experimental protocols that would allow the unique identification of these parameters remains to be addressed in future studies.
Collagen undulation is a microstructural parameter that is directly related to the load transmission from the ECM to fibroblast cells. Consequently, we proposed that alteration of the distribution of collagen undulation is the main contributor to control AAA expansion rate. Through the histomechanical collagen fibre model, the distribution of collagen undulation is directly related to the stress–stretch property of the collagen fibre. Consequently, our mechanical stimulus could be formulated as the strain-based equation (2.3), i.e. very similar to earlier models [6,7,12–14]. With the help of the histomechanical relation equation (2.1), the mechanical stimulus could also be formulated in stress space that leads to a stress-based stimulus, which has also been suggested in the literature [8–11].
One key assumption in our model (similar to model [14]) was linking the collagen turnover rate to the amount of existing collagen, i.e. through the collagen density ρ in the rate equations. This assumption implied that the fibroblast–collagen ratio remained constant, and, to the authors' knowledge, no sound experimental evidence for that has been reported in the literature. Similarly, we assumed a constant pre-stretch of newly formed collagen without sound experimental evidence.
Apart from the macroscopic consequences of collagen turnover, it shifted the initially isotropic collagen orientation density into a locally orthotropic distribution. This corresponds well with our histological study of the AAA wall [34].
Most interestingly, AAAs do not always enlarge. Sometimes, they might remain stable or even shrink over time [71]. While the present collagen turnover model is basically able to predict shrinking, i.e. when placing the newly formed collagen fibrils at lower undulation limits, no portion of the investigated AAA in this study shrank over time.
Although collagen turns over on top of a pulsating vascular wall, our collagen turnover model considered average rather than pulsating mechanical quantities. Specifically, the differences in strain pulsations in circumferential and axial directions may influence the undulation characteristics of collagen fibrils, i.e. collagen fibrils aligned with circumferential directions and axial directions may differ. This difference has also been found by fitting the passive model to biaxial AAA data [34]. Consequently, relating the width of the undulation spectrum (i.e. the parameter Δλnew) of collagen fibrils to the physiological strain pulsation might improve the present model.
Certainly, a change in collagen density will affect the thickness of the vessel wall. To the authors' best knowledge, no related experimental data have been reported in the literature and, for simplicity, the incompressibility assumption was used in the present study. However, for larger growth, this assumption leads to an unrealistically thin wall. It is also noted that approaches that predict the wall thickness based on the homeostasis assumption have been reported in the literature [72]. Although an intra-luminal thrombus is known to be an important solid structure [73] that increases the predictability of AAA models [74], it was disregarded in the present study. Besides the intra-luminal thrombus's direct solid mechanical impact, it must also be seen as a dynamic structure that develops in time. Consequently, a clinically relevant AAA growth model should also account for the flow [75] of blood through the aneurysm and its coagulation process [76] to thrombus formation. Similarly, possible contact with surrounding organs was not considered through our AAA model. Naturally, the present growth model could be improved by considering the intra-luminal thrombus and possible contact of the AAA, especially with the spine. Finally, although the model was able to successfully capture the enlargement of small AAAs, a rigorous validation against experimental data would be crucial to evaluate its descriptive and predictive capabilities.
Acknowledgements
The retrospective use of data from human subjects was approved by the local ethics committee.
We gratefully acknowledge the support given to this work by the Young Faculty grant no. 2006-7568 that was provided by the Swedish Research Council, VINNOVA, the Swedish Foundation for Strategic Research and the EC Seventh Framework Programme: ‘Fighting Aneurysmal Diseases (FAD-200647)’.
References
- 1.Upchurch G. R., Jr, Schaub T. A. 2006. Abdominal aortic aneurysm. Am. Fam. Physician 73, 1198–1204 [PubMed] [Google Scholar]
- 2.Wilson K. A., Lindholt J. S., Hoskins P. R., Heickendorff L., Vammen S., Bradbury A. W. 2001. The relationship between abdominal aortic aneurysm distensibility and serum markers of elastin and collagen metabolism. Eur. J. Vasc. Endovasc. Surg. 21, 175–178 10.1053/ejvs.2001.1303 (doi:10.1053/ejvs.2001.1303) [DOI] [PubMed] [Google Scholar]
- 3.Petersen E., Wagberg F., Angquist K. A. 2002. Proteolysis of the abdominal aortic aneurysm wall and the association with rupture. Eur. J. Vasc. Endovasc. Surg. 23, 153–157 10.1053/ejvs.2001.1572 (doi:10.1053/ejvs.2001.1572) [DOI] [PubMed] [Google Scholar]
- 4.Wilson K. A., Lee A. J., Lee A. J., Hoskins P. R., Fowkes F. G., Ruckley C. V., Bradbury A. W. 2003. The relationship between aortic wall distensibility and rupture of infrarenal abdominal aortic aneurysm. J. Vasc. Surg. 37, 112–117 10.1067/mva.2003.40 (doi:10.1067/mva.2003.40) [DOI] [PubMed] [Google Scholar]
- 5.DiMartino E. S., Bohra A., VandeGeest J. P., Gupta N. Y., Makaroun M. S., Vorp D. A. 2006. Biomechanical properties of ruptured versus electively repaired abdominal aortic aneurysm wall tissue. J. Vasc. Surg. 43, 570–576 10.1016/j.jvs.2005.10.072 (doi:10.1016/j.jvs.2005.10.072) [DOI] [PubMed] [Google Scholar]
- 6.Watton P. N., Heil M., Hill N. A. 2004. A mathematical model for the growth of the abdominal aortic aneurysm. Biomech. Model. Mechanobiol. 3, 98–113 10.1007/s10237-004-0052-9 (doi:10.1007/s10237-004-0052-9) [DOI] [PubMed] [Google Scholar]
- 7.Watton P. N., Hill N. A. 2009. Evolving mechanical properties of a model of abdominal aortic aneurysm. Biomech. Model. Mechanobiol. 8, 25–42 10.1007/s10237-007-0115-9 (doi:10.1007/s10237-007-0115-9) [DOI] [PubMed] [Google Scholar]
- 8.Volokha K. Y., Vorp D. A. 2008. A model of growth and rupture of abdominal aortic aneurysm. J. Biomech. 41, 1015–1021 10.1016/j.jbiomech.2007.12.014 (doi:10.1016/j.jbiomech.2007.12.014) [DOI] [PubMed] [Google Scholar]
- 9.Zeinali-Davarani S., Baek S. 2012. Medical image-based simulation of abdominal aortic aneurysm growth. Mech. Res. Commun 42, 107–117 10.1016/j.mechrescom.2012.01.008 (doi:10.1016/j.mechrescom.2012.01.008) [DOI] [Google Scholar]
- 10.Humphrey J. D., Canham P. B. 2000. Structure, mechanical properties,and mechanics of intracranial saccular aneurysms. J. Elasticity 61, 49–81 10.1023/A:1010989418250 (doi:10.1023/A:1010989418250) [DOI] [Google Scholar]
- 11.Baek S., Rajagopal K. R., Humphrey J. D. 2006. A theoretical model of enlarging intracranial fusiform aneurysms. J. Biomech. Eng. 128, 142–149 10.1115/1.2132374 (doi:10.1115/1.2132374) [DOI] [PubMed] [Google Scholar]
- 12.Kroon M., Holzapfel G. A. 2007. A model for saccular cerebral aneurysm growth by collagen fibre remodelling. J. Theor. Biol. 247, 775–787 10.1016/j.jtbi.2007.03.009 (doi:10.1016/j.jtbi.2007.03.009) [DOI] [PubMed] [Google Scholar]
- 13.Kroon M., Holzapfel G. A. 2009. A theoretical model for fibroblast-controlled growth of saccular cerebral aneurysms. J. Theor. Biol. 257, 73–83 10.1016/j.jtbi.2008.10.021 (doi:10.1016/j.jtbi.2008.10.021) [DOI] [PubMed] [Google Scholar]
- 14.Watton P. N., Selimovic A., Raberger N. B., Huang P., Holzapfel G. A., Ventikos Y. 2011. Modelling evolution and the evolving mechanical environment of saccular cerebral aneurysms. Biomech. Model. Mechanobiol. 10, 109–132 10.1007/s10237-010-0221-y (doi:10.1007/s10237-010-0221-y) [DOI] [PubMed] [Google Scholar]
- 15.Davies M. J. 1998. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation 98, 193–195 10.1161/01.CIR.98.3.193 (doi:10.1161/01.CIR.98.3.193) [DOI] [PubMed] [Google Scholar]
- 16.Carey D. 1991. Control of growth and differentiation of vascular cells by extracellular matrix proteins. Ann. Rev. Physiol. 53, 161–177 10.1146/annurev.ph.53.030191.001113 (doi:10.1146/annurev.ph.53.030191.001113) [DOI] [PubMed] [Google Scholar]
- 17.Alberts B., Bray D., Lewis J., Raff M., Roberts K., Watson J. D. 1994. Molecular biology of the cell, 4th edn Ypsilanti, MI: Garland Publishing [Google Scholar]
- 18.Humphrey J. D. 1999. Remodelling of a collagenous tissue at fixed lengths. J. Biomech. Eng. 121, 591–597 10.1115/1.2800858 (doi:10.1115/1.2800858) [DOI] [PubMed] [Google Scholar]
- 19.Nissen R., Cardinale G. J., Udenfriend S. 1978. Increased turnover of arterial collagen in hypertensive rats. Proc. Natl Acad. Sci. USA 75, 451–453 10.1073/pnas.75.1.451 (doi:10.1073/pnas.75.1.451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bashey R. I., Cox R., McCann J., Jimenez S. A. 1989. Changes in collagen biosynthesis, types, and mechanics of aorta in hypertensive rats. J. Lab. Clin. Med. 113, 604–611 [PubMed] [Google Scholar]
- 21.Rizzo R. J., McCarthy W. J., Dixit S. N., Lilly M. P., Shively V. P., Flinn W. R., Yao J. S. T. 2011. Collagen types and matrix protein content in human abdominal aortic aneurysms. J. Vasc. Surg. 10, 365–373 [DOI] [PubMed] [Google Scholar]
- 22.Kazi M., Thyberg J., Religa P., Roy J., Eriksson P., Hedin U., Swedenborg J. 2003. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J. Vasc. Surg. 38, 1283–1292 10.1016/S0741-5214(03)00791-2 (doi:10.1016/S0741-5214(03)00791-2) [DOI] [PubMed] [Google Scholar]
- 23.Wess T. J. 2008. Collagen fibrillar structure and hierarchies. In Collagen structure and mechanics (ed. Fratzl P.), pp. 49–80 New York, NY: Springer [Google Scholar]
- 24.Gupta V., Grande-Allen K. J. 2006. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc. Res. 72, 375–383 10.1016/j.cardiores.2006.08.017 (doi:10.1016/j.cardiores.2006.08.017) [DOI] [PubMed] [Google Scholar]
- 25.Barnes M. J. 1985. Collagens in atherosclerosis. Coll. Relat. Res. 5, 65–97 10.1016/S0174-173X(85)80048-0 (doi:10.1016/S0174-173X(85)80048-0) [DOI] [PubMed] [Google Scholar]
- 26.Bishop J. E., Lindahl G. 1999. Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc. Res. 42, 27–44 10.1016/S0008-6363(99)00021-8 (doi:10.1016/S0008-6363(99)00021-8) [DOI] [PubMed] [Google Scholar]
- 27.Sluijter J. P. G., Smeets M. B., Velema E., Pasterkamp G., de Kleijn D. P. V. 2004. Increased collagen turnover is only partly associated with collagen fiber deposition in the arterial response to injury. Cardiovasc. Res. 61, 186–195 10.1016/j.cardiores.2003.09.028 (doi:10.1016/j.cardiores.2003.09.028) [DOI] [PubMed] [Google Scholar]
- 28.Strauss B. H. 1996. In vivo collagen turnover following experimental balloon angioplasty injury and the role of matrix metalloproteinases. Circ. Res. 79, 541–550 10.1161/01.RES.79.3.541 (doi:10.1161/01.RES.79.3.541) [DOI] [PubMed] [Google Scholar]
- 29.Choke E., Cockerill G., Wilson W. R., Sayed S., Dawson J., Loftus I., Thompson M. M. 2005. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 30, 227–244 10.1016/j.ejvs.2005.03.009 (doi:10.1016/j.ejvs.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 30.Lanir Y. 1983. Constitutive equations for fibrous connective tissues. J. Biomech. 16, 1–12 10.1016/0021-9290(83)90041-6 (doi:10.1016/0021-9290(83)90041-6) [DOI] [PubMed] [Google Scholar]
- 31.Federico S., Gasser T. C. 2010. Nonlinear elasticity of biological tissues with statistical fiber orientation. J. R. Soc. Interface 7, 955–966 10.1098/rsif.2009.0502 (doi:10.1098/rsif.2009.0502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasser T. C. 2011. An irreversible constitutive model for fibrous soft biological tissue: a 3D microfiber approach with demonstrative application to abdominal aortic aneurysms. Acta Biomater. 7, 2457–2466 10.1016/j.actbio.2011.02.015 (doi:10.1016/j.actbio.2011.02.015) [DOI] [PubMed] [Google Scholar]
- 33.Martufi G., Gasser T. C. 2011. A constitutive model for vascular tissue that integrates fibril, fiber and continuum levels with application to the isotropic and passive properties of the infrarenal aorta. J. Biomech. 44, 2544–2550 10.1016/j.jbiomech.2011.07.015 (doi:10.1016/j.jbiomech.2011.07.015) [DOI] [PubMed] [Google Scholar]
- 34.Gasser T. C., Gallinetti S., Xing X., Forsell C., Swedenborg J., Roy J. 2012. Spatial orientation of collagen fibers in the abdominal aortic aneurysm's wall and its relation to wall mechanics. Acta Biomater. 8, 3091–3103 10.1016/j.actbio.2012.04.044 (doi:10.1016/j.actbio.2012.04.044) [DOI] [PubMed] [Google Scholar]
- 35.López-Candales A., Holmes D. R., Liao S., Scott M. J., Wickline S. A., Thompson R. W. 1997. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathol. 150, 993–1007 [PMC free article] [PubMed] [Google Scholar]
- 36.Ogden R. W. 1997. Non-linear elastic deformations. New York, NY: Dover [Google Scholar]
- 37.Gasser T. C., Ogden R. W., Holzapfel G. A. 2006. Hyperelastic modelling of arterial layers with distributed collagen fiber orientations. J. R. Soc. Interface 3, 15–35 10.1098/rsif.2005.0073 (doi:10.1098/rsif.2005.0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox H. L. 1952. The elasticity and strength of paper and other fibrous materials. Br. J. Appl. Phys. 3, 72–79 10.1088/0508-3443/3/3/302 (doi:10.1088/0508-3443/3/3/302) [DOI] [Google Scholar]
- 39.Kelly A. 1970. Interface effects and the work of fracture of a fibrous composite. Proc. R. Lond. A 319, 95–116 10.1098/rspa.1970.0168 (doi:10.1098/rspa.1970.0168) [DOI] [Google Scholar]
- 40.Scott J. E. 2003. Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage, etc. a sliding proteoglycan-filament model. J. Physiol. 553, 335–343 10.1113/jphysiol.2003.050179 (doi:10.1113/jphysiol.2003.050179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao J., Vesely I. 2007. Skewness angle of interfibrillar proteoglycans increases with applied load on mitral valve chordae tendineae. J. Biomech. 40, 390–398 10.1016/j.jbiomech.2005.12.011 (doi:10.1016/j.jbiomech.2005.12.011) [DOI] [PubMed] [Google Scholar]
- 42.Liao J., Vesely I. 2003. A structural basis for the size-related mechanical properties of mitral valve chordae tendineae. J. Biomech. 36, 1125–1133 10.1016/S0021-9290(03)00109-X (doi:10.1016/S0021-9290(03)00109-X) [DOI] [PubMed] [Google Scholar]
- 43.Robinson P. S., Huang T. F., Kazam E., Iozzo R. V., Birk D. E., Soslowsky L. J. 2005. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J. Biomech. Eng. 127, 181–185 10.1115/1.1835363 (doi:10.1115/1.1835363) [DOI] [PubMed] [Google Scholar]
- 44.Sasaki N., Odajima S. 1996. Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. J. Biomech. 29, 1131–1136 10.1016/0021-9290(96)00024-3 (doi:10.1016/0021-9290(96)00024-3) [DOI] [PubMed] [Google Scholar]
- 45.Scott J. E. 1990. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J. Anat. 169, 23–35 [PMC free article] [PubMed] [Google Scholar]
- 46.Cribb A. M., Scott J. E. 1995. Tendon response to tensile stress: an ultrastructural investigation of collagen : proteoglycan interactions in stressed tendon. J. Anat. 187(Pt 2), 423–428 [PMC free article] [PubMed] [Google Scholar]
- 47.Fessel G., Snedeker J. G. 2011. Equivalent stiffness after glycosaminoglycan depletion in tendon: an ultra-structural finite element model and corresponding experiments. J. Theor. Biol. 268, 77–83 10.1016/j.jtbi.2010.10.007 (doi:10.1016/j.jtbi.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 48.Redaelli A., Vesentini S., Soncini M., Vena P., Mantero S., Montevecchi F. M. 2003. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons: a computational study from molecular to microstructural level. J. Biomech. 36, 1555–1569 10.1016/S0021-9290(03)00133-7 (doi:10.1016/S0021-9290(03)00133-7) [DOI] [PubMed] [Google Scholar]
- 49.Vesentini S., Redaelli A., Montevecchi F. M. 2005. Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J. Biomech. 38, 433–443 10.1016/j.jbiomech.2004.04.032 (doi:10.1016/j.jbiomech.2004.04.032) [DOI] [PubMed] [Google Scholar]
- 50.Rigozzi S., Muller R., Snedeker J. G. 2009. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J. Biomech. 42, 1547–1552 10.1016/j.jbiomech.2009.03.031 (doi:10.1016/j.jbiomech.2009.03.031) [DOI] [PubMed] [Google Scholar]
- 51.Rigozzi S., Muller R., Snedeker J. G. 2010. Collagen fibril morphology and mechanical properties of the Achilles tendon in two inbred mouse strains. J. Anat. 216, 724–731 10.1111/j.1469-7580.2010.01225.x (doi:10.1111/j.1469-7580.2010.01225.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armentano R. L., Levenson J., Barra J. G., Fischer E. I., Breitbart G. J., Pichel R. H., Simon A. 1991. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am. J. Physiol. 260, H1870–H1877 [DOI] [PubMed] [Google Scholar]
- 53.Greenwald S. E., Moore J. E., Jr, Rachev A., Kane T. P., Meister J. J. 1997. Experimental investigation of the distribution of residual strains in the artery wall. J. Biomech. Eng. 119, 438–444 10.1115/1.2798291 (doi:10.1115/1.2798291) [DOI] [PubMed] [Google Scholar]
- 54.Kim J., Baek S. 2011. Circumferential variations of mechanical behavior of the porcine thoracic aorta during the inflation test. J. Biomech. 44, 1941–1947 10.1016/j.jbiomech.2011.04.022 (doi:10.1016/j.jbiomech.2011.04.022) [DOI] [PubMed] [Google Scholar]
- 55.Li Q., Muragaki Y., Hatamura I., Ueno H., Ooshima A. 1998b. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J. Vasc. Res. 35, 93–103 10.1159/000025570 (doi:10.1159/000025570) [DOI] [PubMed] [Google Scholar]
- 56.Flynn B. P., Bhole A. P., Saeidi N., Liles M., Dimarzio C. A., Ruberti J. W. 2010. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS ONE 5, e12337. 10.1371/journal.pone.0012337 (doi:10.1371/journal.pone.0012337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphrey J. D., Rajagopal K. R. 2002. A constrained mixture model for growth and remodeling of soft tissues. Math. Model. Methods Appl. Sci. 12, 407–430 10.1142/S0218202502001714 (doi:10.1142/S0218202502001714) [DOI] [Google Scholar]
- 58.McBridge D. J. 1984. Hind limb extensor tendon development in the chick: a light and transmission electron microscopic study. M.S. Thesis in Physiology, Rutgers University, New Jersey [Google Scholar]
- 59.Simo J. C., Taylor R. L. 1991. Quasi-incompressible finite elasticity in principal stretches. Continuum basis and numerical algorithms. Comput. Methods Appl. Mech. Eng. 85, 273–310 10.1016/0045-7825(91)90100-K (doi:10.1016/0045-7825(91)90100-K) [DOI] [Google Scholar]
- 60.Miehe C. 1994. Aspects of the formulation and finite element implementation of large strain isotropic elasticity. Int. J. Numer. Methods Eng. 37, 1981–2004 10.1002/nme.1620371202 (doi:10.1002/nme.1620371202) [DOI] [Google Scholar]
- 61.Hardin R. H., Sloane N. J. A. 1996. McLaren's improved snub cube and other new spherical designs in three dimensions. Discrete Comput. Geom. 15, 429–441 10.1007/BF02711518 (doi:10.1007/BF02711518) [DOI] [Google Scholar]
- 62.Vande Geest J. P., Sacks M. S., Vorp D. A. 2006. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J. Biomech. 39, 1324–1334 10.1016/j.jbiomech.2005.03.003 (doi:10.1016/j.jbiomech.2005.03.003) [DOI] [PubMed] [Google Scholar]
- 63.Martufi G., Gasser T. C. 2012. Histo-mechanical modeling of the wall of abdominal aorta aneurysms. In Proc. MATHMOD 2012 7th Vienna Int. Conf. on Mathematical Modelling, Vienna, Austria, February 15–17, 2012 [Google Scholar]
- 64.Auer M., Gasser T. C. 2010. Reconstruction and finite element mesh generation of abdominal aortic aneurysms from computerized tomography angiography data with minimal user interactions. IEEE Trans. Med. Imaging 29, 1022–1028 10.1109/TMI.2009.2039579 (doi:10.1109/TMI.2009.2039579) [DOI] [PubMed] [Google Scholar]
- 65.Raghavan M. L., Vorp D. A. 2000. Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J. Biomech. 33, 475–482 10.1016/S0021-9290(99)00201-8 (doi:10.1016/S0021-9290(99)00201-8) [DOI] [PubMed] [Google Scholar]
- 66.Humphrey J. D. 2002. Cardiovascular solid mechanics. Cells, tissues, and organs. New York, NY: Springer [Google Scholar]
- 67.Wilson J. S., Baek S., Humphrey J. D. 2012. Importance of initial aortic properties on the evolving regional anisotropy, stiffness and wall thickness of human abdominal aortic aneurysms. J. R. Soc. Interface. 10.1098/rsif.2012.0097 (doi:10.1098/rsif.2012.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindeman J. H. N., et al. 2010. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc. Natl Acad. Sci. USA 107, 862–865 10.1073/pnas.0910312107 (doi:10.1073/pnas.0910312107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carmo M., Colombo L., Bruno A., Corsi F. R. M., Roncoroni L., Cuttin M. S., Radice F., Mussinib E., Settembrini P. G. 2002. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 23, 543–549 10.1053/ejvs.2002.1620 (doi:10.1053/ejvs.2002.1620) [DOI] [PubMed] [Google Scholar]
- 70.Dobrin P. B., Baker W. H., Gley W. C. 1984. Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch. Surg. 119, 405–409 10.1001/archsurg.1984.01390160041009 (doi:10.1001/archsurg.1984.01390160041009) [DOI] [PubMed] [Google Scholar]
- 71.Brady A. R., Thompson S. G., Fowkes F. G. R., Greenhalgh R. M., Powell J. T. 2004. Abdominal aortic aneurysm expansion risk factors and time intervals for surveillance. Circulation 100, 16–21 10.1161/01.CIR.0000133279.07468.9F (doi:10.1161/01.CIR.0000133279.07468.9F) [DOI] [PubMed] [Google Scholar]
- 72.Zeinali-Davarani S., Raguin L. G., Baek S. 2011. An inverse optimization approach toward testing different hypotheses of vascular homeostasis using image-based models. Int. J. Struct. Changes Solids 3, 33–45 [Google Scholar]
- 73.Gasser T. C., Görgülü G., Folkesson M., Swedenborg J. 2008. Failure properties of intra-luminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J. Vasc. Surg. 48, 179–188 10.1016/j.jvs.2008.01.036 (doi:10.1016/j.jvs.2008.01.036) [DOI] [PubMed] [Google Scholar]
- 74.Gasser T. C., Auer M., Labruto F., Swedenborg J., Roy J. 2010. Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur. J. Vasc. Endovasc. Surg. 40, 176–185 10.1016/j.ejvs.2010.04.003 (doi:10.1016/j.ejvs.2010.04.003) [DOI] [PubMed] [Google Scholar]
- 75.Biasetti J., Hussain F., Gasser T. C. 2011. Blood flow and coherent vortices in the normal and aneurysmatic aortas. A fluid dynamical approach to intra-luminal Thrombus formation. J. R. Soc. Interface 8, 1449–1461 10.1098/rsif.2011.0041 (doi:10.1098/rsif.2011.0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biasetti J., Spazzini P. G., Gasser T. C. 2012. An integrated fluid-chemical model toward modeling the formation of intra-luminal thrombus in abdominal aortic aneurysms. Front. Physiol. 3, 266. 10.3389/fphys.2012.00266 (doi:10.3389/fphys.2012.00266) [DOI] [PMC free article] [PubMed] [Google Scholar]



