Abstract
Light absorption by carotenoids is known to vary substantially with the shape or conformation of the pigment molecule induced by the molecular environment, but the role of interactions between carotenoid pigments and the proteins to which they are bound, and the resulting impact on organismal coloration, remain unclear. Here, we present a spectroscopic investigation of feathers from the brilliant red scarlet ibis (Eudocimus ruber, Threskiornithidae), the orange-red summer tanager (Piranga rubra, Cardinalidae) and the violet-purple feathers of the white-browed purpletuft (Iodopleura isabellae, Tityridae). Despite their striking differences in colour, all three of these feathers contain canthaxanthin (β,β-carotene-4,4′-dione) as their primary pigment. Reflectance and resonance Raman (rR) spectroscopy were used to investigate the induced molecular structural changes and carotenoid–protein interactions responsible for the different coloration in these plumage samples. The results demonstrate a significant variation between species in the peak frequency of the strong ethylenic vibration (ν1) peak in the rR spectra, the most significant of which is found in I. isabellae feathers and is correlated with a red-shift in canthaxanthin absorption that results in violet reflectance. Neither polarizability of the protein environment nor planarization of the molecule upon binding can entirely account for the full extent of the colour shift. Therefore, we suggest that head-to-tail molecular alignment (i.e. J-aggregation) of the protein-bound carotenoid molecules is an additional factor.
Keywords: absorption spectroscopy carotenoid, coloration, β-keratin, electronic transition, resonance Raman spectroscopy
1. Introduction
Carotenoid pigments contribute to colour of feathers and are thought to be important for communication in many bird species [1–5]. Coloration in feathers can arise from light scattering by nanostructures, or from the presence of pigments that absorb light in particular regions of the electromagnetic spectrum [2,6,7]. Nanostructures usually produce UV-to-turquoise, white or iridescent colours [6,8]. Melanins are associated with black and browns [6,7,9]. Carotenoids give rise to yellow, orange and red coloration [2]. Green colours in feathers often results from a combination of yellow pigmentation and blue structural colours [6,10,11]. All of these factors contribute to make bird feather coloration as one of the most striking displays in all of nature.
Birds do not synthesize carotenoids de novo, and therefore must ingest them along with their diet [2,12,13]. After ingestion, carotenoids are transported to various body tissues where they accumulate and carry out their biological roles [2,13]. Common carotenoids in bird diets are β-carotene, lutein, zeaxanthin and β-cryptoxanthin [13–16]. Coloration in feathers may arise directly from deposition of dietary carotenoids [2,17]. Alternatively, ingested carotenoids may be metabolically modified before deposition, for example, by ketolase activity at the β- and ε-rings [2,18,19].
It is known that carotenoids incorporated during feather development bind strongly to proteins in the structures [11,13,20]. The proteins have a heterogeneous amino acid composition that can vary even in the same feather, depending on the required physical properties [21,22]. Owing to different pigment–protein interactions, the same carotenoid composition can give rise to different colours in feathers from various species or to different coloured patches of feathers in the same species [11,20]. A similar phenomenon has been observed for the coloration of exoskeletons of crustaceans [11,20,23–27].
Resonance Raman (rR) spectroscopy provides direct, selective information regarding the structure of the electronic ground state of carotenoid molecules [28–30]. By matching the energy of incident light with the energy of the electronic absorption of a given carotenoid, selective probing of a subpopulation of molecules in a complex mixture may be achieved [28,31], thus allowing structural characterizations in situ. rR spectra of carotenoids are characterized by four distinct bands denoted ν1 through ν4, whose frequencies vary with the effective conjugation length of the π-electron double bond chain in the molecule, geometric isomeric configuration and degree of distortion [27,29,30,32]. In an analysis of yellow and red-coloured patches of the European goldfinch (Carduelis carduelis), which are pigmented by canary xanthophylls (ε,ε-carotene-3,3′-dione and 3′-hydroxy-ε,ε-caroten-3-one), Stradi et al. [11] documented variation in the energy of the strongly allowed S0 → S2 electronic transition by rR spectroscopy. This variation was due to an apparent change in the bond alternation of the conjugated polyene chain of the protein-bound carotenoids, suggesting an influence of the molecular environment on the binding of the pigments [11].
In C. carduelis, the effect of feather protein binding was to shift the colour from yellow to red—two plumage colours that are also easily produced independently by changes in carotenoid molecular structure. So, it is not clear that association with the protein can contribute to novel plumage colours. In the present study, we employed rR spectroscopy to investigate carotenoid–protein interactions responsible for the coloration of brilliant red and purple plumages of the scarlet ibis Eudocimus ruber (Threskiornithidae), summer tanager Piranga rubra (Cardinalidae) and white-browed purpletuft Iodopleura isabellae (Tityridae). All these plumage patches contain canthaxanthin (β,β-carotene-4,4′-dione) as the primary carotenoid [33–35] (figure 1). The violet colour of male I. isabellae is very rare in bird plumages, and nothing is known about its production in Iodopleura.
Figure 1.
Plumages coloration of the (a) scarlet ibis Eudocimus ruber (Threskiornithidae, image courtesy of G. Armistead/VIREO), (b) the male summer tanager Piranga rubra (Cardinalidae, image courtesy of R. Nussbaumer/VIREO) and (c) the white-browed purpletuft Iodopleura isabellae (Tityridae, photograph courtesy of Nick Athanas).
In this study, rR and absorption spectra deduced from reflectance spectra of canthaxanthin bound in situ were compared with those recorded from canthaxanthin in solution to evaluate the molecular basis for the different spectral shifts that result in distinct colours of the feathers. We predict that the spectral differences of these three birds can be attributed to novel modes of binding canthaxanthin within the keratin matrix of the feathers, and postulate the involvement of specific factors including polarizability of the molecular environment and binding-induced configurational changes (e.g. planarization of the terminal rings). It should be noted that the nature of the bond between the carotenoid pigment and the specific structural component of the feather was not explored herein, and while it is possible that the pigment could bind to lipid components of the feather, it is generally accepted that carotenoids are bound directly to keratin or to other proteins in the feather [11,20]. The characterization of the molecular factors responsible for these differences will further elucidate how birds have evolved to use variation in the molecular environment of pigment binding to tune the electronic properties of specific carotenoid molecules.
2. Material and methods
2.1. Extraction and analysis of carotenoids
Red back feathers of a male E. ruber (YPM 26570; collected March 1951), red–orange belly feathers of a male P. rubra (YPM 7362, collected June 1957; YPM 3771, no collection data) and feathers from the purple pectoral tufts of a male I. isabellae (YPM 77812, no collection data; AMNH 494716, collected in 1886) were obtained from the Yale Peabody Museum of Natural History, Yale University, New Haven, CT, USA and the American Museum of Natural History, New York, NY, USA. Specimens were chosen for the high quality of colour preservation based on visual evaluation by R.O.P.
All-trans-canthaxanthin (β,β-carotene-4,4′-dione) was obtained from Roche. Carotenoids were extracted from the feathers, as previously described by LaFountain et al. [36]. An HPLC analysis was conducted using a Waters 600E multi-solvent delivery system with an in-line Waters 2996 photodiode array detector and a Phenomenex Luna 5 µm silica column (250 × 4.6 mm). The mobile phase consisted of a linear gradient from 90 : 10 to 80 : 20 hexanes/acetone (Fisher Scientific, Pittsburgh, PA, USA) over a period of 40 min, with a flow rate of 1.5 ml min−1. The injection solvent was 14 per cent acetone and 86 per cent hexanes. The details of the mass spectrometry analysis can be found in the electronic supplementary material, figure S1.
2.2. Spectroscopy
Reflectance spectra of the feathers were measured using procedures previously described [37]. Briefly, the measurements were carried using an Ocean Optics S2000 spectrometer with an Ocean Optics DH-2000-BAL deuterium–halogen light source. Measurements were integrated for 500 ms at a distance of approximately 6 mm from the plumage with a perpendicular incident light, resulting in a approximately 3 mm2 illumination field. The probe was secured in an aluminium block to obscure ambient light. The following formula was used to calculate per cent reflectance (%R):
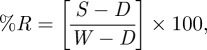 |
where S is the reflectance of the specimen, D is the reflectance of the dark standard and W is the reflectance of the white standard. The white standard was determined by measuring the reflectance of a white Spectralon tablet from Ocean Optics, while the dark standard was measured using ambient light in a darkened room [37].
Absorption spectra of all-trans-canthaxanthin in methanol, acetonitrile, tetrahydrofuran, hexane, diethylether, cyclohexane, toluene and carbon disulphide (all purchased from Sigma-Aldrich, St. Louis, MO, USA) were recorded at room temperature, using a Varian Cary E5 double-beam scanning spectrophotometer. These solvents were chosen to represent a wide range of polarizabilities. rR spectra of canthaxanthin were obtained in hexane and diethylether at room temperature, using a Jobin-Yvon U1000 rR spectrophotometer equipped with a front-illuminated, deep-depleted CCD detector (Synapse Horiba, Jobin Yvon, France). Excitation at 457.9, 488, 514.5 and 528.7 nm was provided by a Sabre argon ion laser (Coherent, Palo Alto, CA, USA) and at 568.2 nm by an Innova 90 krypton ion laser (Coherent). For in situ rR spectroscopic experiments, a confocal microscope Olympus BX41 was coupled to the spectrometer. The same slit-width (200 μm) and grating were used for both systems, thereby achieving the same spectral resolution. The excitation beam was defocused and kept at a sufficiently low power (typically less than 20 mW) to prevent degradation of the sample by the absorbed light energy. Systematic comparison of rR spectra was performed throughout the time duration of each experiment to confirm sample integrity.
2.3. Avian colour space modelling
Avian perception of colour was modelled using a tetrahedral colour space [3,37], which provides a quantitative representation of sensory experience, using the computer program TetraColorSpace v. 1. 0 for Matlab 7 [3,37] (available for free from the authors). The idealized stimulus, QI, of each single cone type was estimated by the reflectance spectrum of a plumage patch:
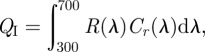 |
2.1 |
where R(λ) is the reflectance spectrum of the plumage patch, and Cr(λ) is the spectral sensitivity function of each cone type r. For each plumage colour, the idealized stimulation values of the four single cones—QI—were normalized to sum to one, yielding relative [uv/v s m l] values. The [uv/v s m l] values were converted to a colour point with spherical coordinates θ, ϕ and r, which define a colour vector pointing from the achromatic point is at the origin. Hue is the direction of the vector, given by the angles θ and ϕ, and saturation is the length of the vector, r [3,37].
We modelled E. ruber, P. rubra and I. isabellae reflectance spectra in avian tetrahedral colour space using a standard violet cone-type visual system [38], which is the ancestral condition in birds [39,40]. We further compared the colour space distribution of E. ruber, P. rubra and I. isabellae reflectance spectra to a diverse sample of avian orders and families (including data from Stoddard & Prum [3] on all non-cotinga species having plumage coloration either inferred or identified as carotenoid-based).
3. Results
Both the feather reflectance spectra (figure 2a) and the inferred absorption, spectra (figure 2b) are broad and relatively structureless, which is typical of keto-carotenoids [41]. The absorption spectra of the feathers from E. ruber and P. rubra are very similar, and have an absorption maximum (reflectance minimum) centred at 495 nm. However, the absorption spectra of E. ruber feathers are much broader than P. rubra, resulting in a substantially redder reflectance and the near elimination of the P. rubra reflectance peak in the near ultraviolet at 361 nm. The spectra of the purple feathers of I. isabellae display a maximum absorbance (minimum reflectance) shifted to longer wavelengths, peaking at approximately 570 nm. Consequently, the high-energy reflectance peak of I. isabellae is shifted well into the human visible spectrum, resulting in a blue reflectance peak at 426 nm. Combined with the long-wavelength reflectance above 600 nm (on from the far side of the 570 nm absorbance peak), the blue and red reflectance create an entirely pigmentary violet colour.
Figure 2.
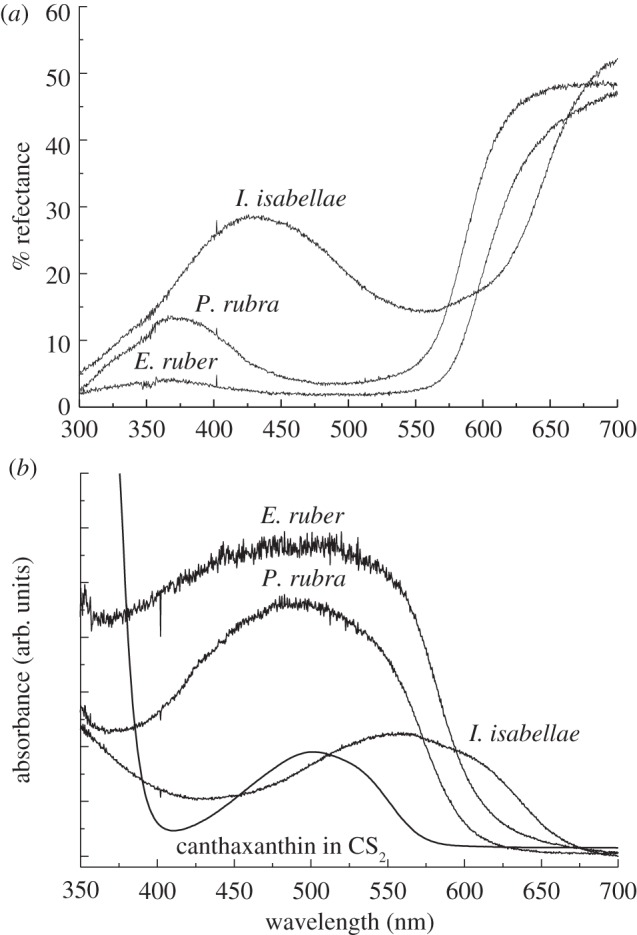
(a) Single reflectance spectra of the red back feathers of E. ruber (YPM 26570), the red–orange belly feathers of a male P. rubra (YPM 7362) and the purple breast feathers of male I. isabellae (YPM 77812); (b) Optical absorption spectra of the feathers computed from the reflectance spectra using A = −log (R), where R = reflectance.
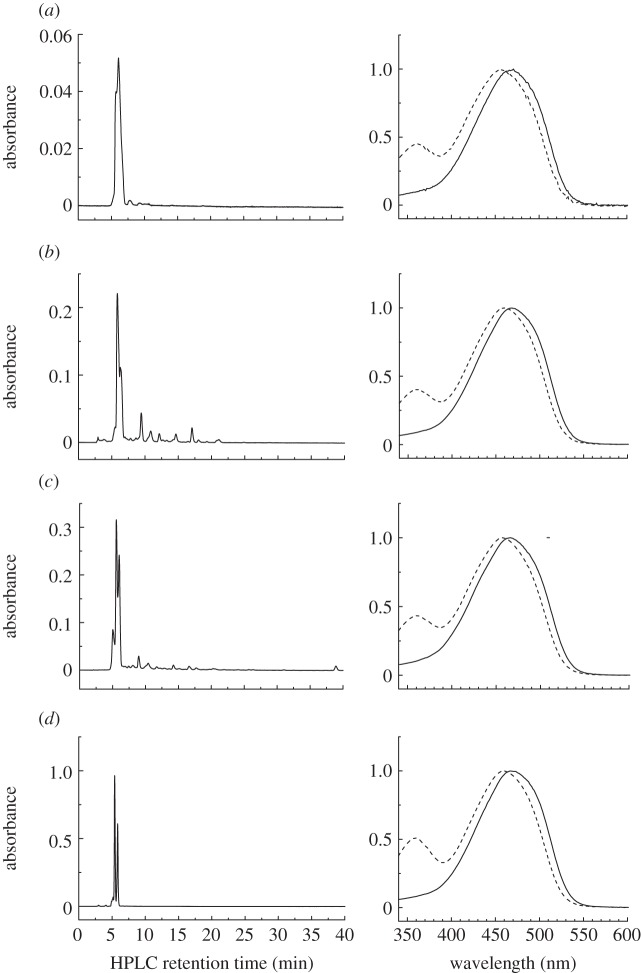
An HPLC analysis of the carotenoids extracted from feathers of E. ruber, P. rubra, and I. isabellae demonstrated the presence of a single major carotenoid in all three samples (figure 3). On the basis of its absorption spectra and retention times, the carotenoid was unambiguously identified as all-trans-canthaxanthin (β,β-carotene-4,4′-dione) (figure 3). This identification was confirmed by mass spectrometry (see electronic supplementary material, figure S1) as well as by the HPLC of a heated bona fide canthaxanthin standard run under the same conditions (figure 3d). This sample also demonstrated that the smaller peaks eluting in proximity to the major peaks are associated with cis-canthaxanthin formed during the heating and extraction process. The absorption spectrum of cis-canthaxanthin is confirmed by a slight blue-shift in the absorption spectrum relative to that of all-trans-canthaxanthin and the large cis-band appearing at approximately 360 nm (dashed traces in figure 3). These findings confirm previous reports that canthaxanthin is the primary pigment of E. ruber and P. rubra [33–35]. While canthaxanthin was found to be the dominant pigment in all three birds, E. ruber and P. rubra were also found to contain small amounts of other carotenoids. A pigment isolated from E. ruber with a retention time of 5.0 min displayed a blue-shifted absorption spectrum suggestive of a shortened conjugated chain (figure 3c). This pigment was found to have a mass of 568 m/z, which is consistent with bis-dihydrocanthaxanthin, a carotenoid previously reported in E. ruber plumage by Fox & Hopkins [33]. Piranga rubra plumage was found to contain a minor pigment that eluted at 9.7 min and had a featureless absorption spectrum consistent with a keto-carotenoid, and a mass of 580 m/z. Additional small peaks were observed in the HPLC chromatograms of these two birds; however, further analysis of these pigments was precluded owing to a lack of material. Some of the minor peaks in each chromatogram could be attributed to cis-isomers of canthaxanthin that were formed during the extraction procedure.
Figure 3.
Normal-phase HPLC chromatogram of the extracts from feathers of E. ruber, P. rubra and I. isabellae carried out as described in the text. The chromatograms were detected at 470 nm. (a) I. isabellae: solid line, 5.7 min; dashed line, 6.6 min; (b) P. rubra: solid line, 5.8 min; dashed line, 6.3 min; (c) E. ruber: solid line, 5.6 min; dashed line, 6.0 min; (d) heated canthaxanthin solid line, 5.7 min; dashed line, 6.6 min.
Saranathan et al. [42] have used small-angle X-ray scattering (SAXS) to assay the nanoscale variation in refractive index in structurally coloured and pigmented feathers. Unlike structurally coloured feathers with spongy β-keratin nanostructures [43–46], the azimuthal average of the SAXS data from I. isabellae shows no peaks for any optically relevant spatial frequencies, or q-values (see electronic supplementary material, figure S2). Rather, the azimuthal average has a slope that is closely congruent to Porod's Law (q−4), the expectation for a random heterogeneous mixture of two materials with well-defined interface [47]. In the absence of any nanostructure that could enhance the coherent scattering of blue light, the purple feathers of male I. isabellae are produced by pigmentary absorption.
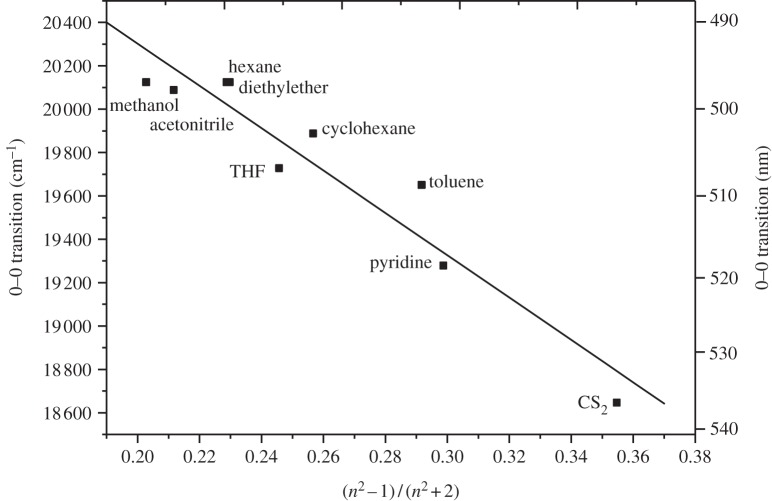
The absorption spectrum of canthaxanthin in solution exhibits only small modulations within the spectrum, and appears as a single, broad band (see the spectrum of canthaxanthin in CS2, figure 2b). This type of broad band corresponds to the strongly allowed S0 → S2 electronic transition, which gives carotenoids their characteristic visible coloration [48]. The position of the spectral origin (0–0) of the S0 → S2 transition can be determined from the longest-wavelength minimum of the second derivative of the absorption spectrum. The energy of this (0–0) band in solution depends primarily on the refractive index, n, of the solvent, and there is a small influence of the solvent dielectric constant [49]. As n increases, the polarizability, R(n), of the solvent expressed as R(n) = (n2–1)/(n2 + 2) also increases, and the position of the S0 → S2 transition shifts to longer wavelength. In the present work, we evaluated the effect of solvent polarizability on the position of the (0–0) spectral origin band of the S0 → S2 electronic transition of canthaxanthin in solution (figure 4). The spectral origin varies linearly over approximately 40 nm for a variety of solvents with a wide range of R(n) (figure 4).
Figure 4.
Position of the (0–0) bands of the S0 → S2 electronic transition of canthaxanthin as a function of polarizability in different solvents.
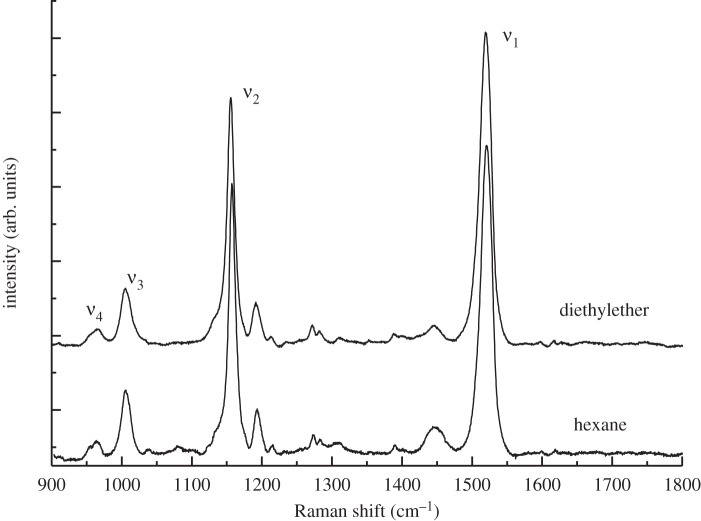
Room temperature rR spectra of canthaxanthin dissolved in hexane and diethylether contain the four main groups of bands, denoted ν1 through ν4, that are typical of carotenoid molecules (figure 5) [30,32]. The ν1 band around 1520 cm−1 arises from stretching vibrations of C═C double bonds, and its frequency depends on both the length of the π-electron conjugated chain of the carotenoid and its configuration; i.e. the extent and position of twisting along the carbon backbone of the molecule [29,50,51]. The ν2 band around 1160 cm−1 is due mainly to stretching vibrations of C–C single bonds coupled with C–H in-plane bending modes [29,32]. The rR bands in the vicinity of ν2 are also sensitive to the position of twisting along the carbon backbone and this constitutes the ‘fingerprint region’ for the assignment of carotenoid configurations [50]. The ν3 band at approximately 1010 cm−1 arises from the coupling of the in-plane rocking vibration of the methyl groups attached to the conjugated chain with the adjacent C–H in-plane bending modes [30,32]. The ν4 band around 960 cm−1 arises from C–H out-of-plane wagging motions coupled with C═C torsional modes that are out-of-plane twists of the carbon backbone [30,32]. If the π-electron conjugated system in the carotenoid is symmetric, as it is in canthaxanthin, the out-of-plane ν4 modes will not be coupled with the electronic transition when the molecule is planar. Accordingly, these bands will not be resonance-enhanced upon excitation and will exhibit very low intensity in the rR spectra. However, they will increase in intensity when distortions around C–C single bonds occur [52]. For canthaxanthin in solution (figure 5), the ν4 band exhibits two weak overlapping components between 955 and 964 cm−1. This result indicates that the average relaxed state of canthaxanthin in solution corresponds to a geometry deviating slightly from completely planar. An X-ray structure analysis of crystalline canthaxanthin supports the view that the terminal rings in the molecule are asymmetrically twisted out of the plane of the conjugated π-electron C═C chain by approximately 55° [53].
Figure 5.
Room temperature rR spectra of canthaxanthin in diethylether and hexane. Excitation wavelength, 514.5 nm. Four distinct bands denoted ν1 to ν4 are observed in the spectra whose frequencies vary as described in the text.
The rR spectra of E. ruber, P. rubra and I. isabellae were measured by rR microspectroscopy using excitation wavelengths of 457.9, 488, 514.5, 528.7 and 568.2 nm, which span the carotenoid absorption transition (figure 6). For P. rubra feathers (figure 6a), the rR spectra are not dependent on the excitation wavelength and resemble that of all-trans-canthaxanthin in solution. The ν1 band is positioned at precisely 1514.3 cm−1 for all excitation wavelengths (table 1), but its full-width at half-maximum (fwhm) is 21.4 cm−1 for excitation at 457.9 nm compared with 21.6 cm−1 at 514.5 nm, which is suggestive of a slight variation in carotenoid binding. However, the spectra indicate little out-of plane distortion upon binding canthaxanthin to the protein. From a structural point of view, the strong similarity in the spectra taken using the different excitation wavelengths are suggestive of a single pool of all-trans-canthaxanthin molecules in these feathers. Thus, little difference in canthaxanthin absorption is predicted between P. rubra and canthaxanthin in solution except for the widening of the absorbance owing to slight variation shown by the width of ν1.
Figure 6.
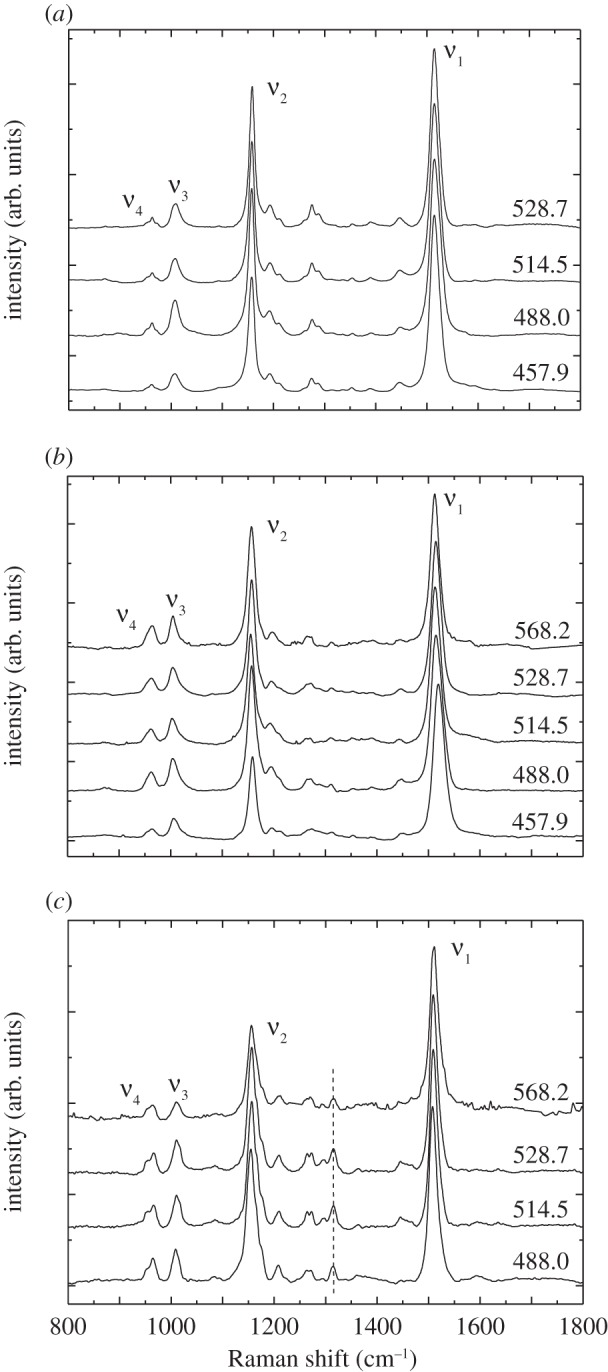
rR spectra of the feathers from: (a) P. rubra; (b) E. ruber and (c) I. isabellae. The excitation wavelengths in nm units are indicated on the right-hand side of the figure. The peak at approximately 1317 cm−1 in figure 6c is not associated with a carotenoid (dashed line).
Table 1.
Frequencies (cm−1) of rR main bands observed in the feathers from P. rubra, E. ruber and I. isabellae.
| excitation wavelength (nm) | band | P. rubra | E. ruber | I. isabellae |
|---|---|---|---|---|
| 457.9 | ν1 | 1514.3 | 1519.7 | n.a. |
| ν2 | 1157.8 | 1159.1 | n.a. | |
| ν3 | 1007.5 | 1006.4 | n.a. | |
| ν4 | 972.2; 962.2; 952 | 963.1 | n.a. | |
| 488 | ν1 | 1514.3 | 1514.8 | 1507.5 |
| ν2 | 1157.8 | 1157 | 1155; (1164.7, 1174.5) | |
| ν3 | 1007.5 | 1004.4 | 1008.7 | |
| ν4 | 972.2; 963.1; 952 | 963.1 | 965.5; 953.7 | |
| 514.5 | ν1 | 1514.3 | 1512.8 | 1508.6 |
| ν2 | 1158.2 | 1155.3 | 1156.3; (1165, 1175.7) | |
| ν3 | 1007.2 | 1003.5 | 1009.8 | |
| ν4 | 972.2; 963.1; 952 | 961.6 | 965.5; 954.9 | |
| 528.7 | ν1 | 1514.3 | 1514.1 | 1508.6 |
| ν2 | 1158.6 | 1157 | 1156.3; (1165, 1175.4) | |
| ν3 | 1008.7 | 1004.4 | 1009.8 | |
| ν4 | 972.2; 963.1; 952 | 963.1 | 965.5; 954.9 | |
| 568.2 | ν1 | n.a. | 1512.1 | 1509.6 |
| ν2 | n.a. | 1156.1 | 1156; (1165, 1176.5) | |
| ν3 | n.a. | 1004.4 | 1010.4 | |
| ν4 | n.a. | 964.6 | 964.3; 953.8 |
The rR spectra of E. ruber feathers (figure 6b) are typical of all-trans-canthaxanthin, but the frequency of the ν1 band varies between 1512.1 and 1519.7 cm−1, depending on the excitation wavelength. In addition, the fwhm of the ν1 band is 3 cm−1 larger than observed for P. rubra. The fwhm of the ν1 band is largest (approx. 28 cm−1) when the shortest wavelength (457.9 nm) excitation is used. This is suggestive of a heterogeneous population of twisted, but not fully isomerized, all-trans-canthaxanthin molecules in E. ruber feathers. These results are consistent with the reflectance and absorption spectra obtained from these feathers (figure 2), which show that the electronic absorption transition of E. ruber feathers is even broader than that observed from P. rubra. In E. ruber feathers, the overall intensity in the ν4 band region compared with that of the ν3 bands is higher than for P. rubra feathers. This is particularly true for excitation wavelengths longer than 488.0 nm and indicates distortion of the relaxed geometric state of canthaxanthin in the feathers from E. ruber.
Resonance rR spectra of I. isabellae feathers recorded using different excitation wavelengths were very similar (figure 6c), indicating the presence of a single pool all-trans-canthaxanthin molecules with some differences in the frequencies compared with the spectra taken from the other feathers and from canthaxanthin in solution. The frequency of the ν1 band, which occurs between 1507.5 and 1509.6 cm−1, is substantially lower than that observed in the feathers from the other two species and from canthaxanthin in solution (figures 5 and 6; table 1). The frequency of the ν1 band is approximately 7 cm−1 lower than the ν1 peak from P. rubra and E. ruber (at an excitation wavelength of 488 nm) and up to 12.5 cm−1 lower than the ν1 peak of canthaxanthin in solution. Given that ν1 is associated with the stretching of C═C double bonds [29,50], this result is associated with a physical extension of the effective π-electron conjugated chain within the molecular backbone of canthaxanthin in I. isabellae, which is functionally correlated with the observed red-shift in its absorbance.
Also, the structure of the ν2 band in I. isabellae is rather unusual for carotenoid molecules in organisms. Typically, the satellite bands associated with the intense band at approximately 1160 cm−1 appear as two distinct bands (see e.g. spectra of lutein in the work by Ruban et al. [54]). This is also the case for the feathers from P. rubra and E. ruber (figure 6a,b, respectively). For I. isabellae, the ν2 band at approximately 1155 cm−1 displays only one satellite band at 1209 cm−1. Purified canthaxanthin in solution also exhibits only one satellite band in that region, however at a lower frequency (approx. 1190 cm−1; figure 5). Lastly, the ν4 band in the rR spectra from I. isabellae feathers (figure 6c) has a significantly high intensity compared with that seen from canthaxanthin in solution (figure 5). This result is indicative of molecular distortion.
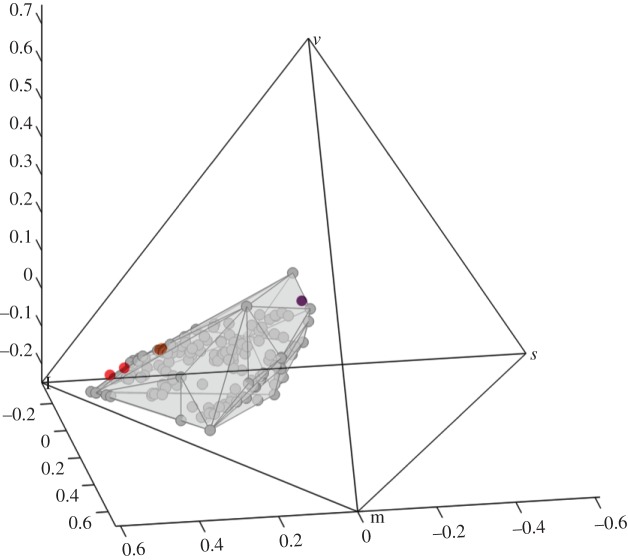
To examine further the effect of variation in carotenoid binding on the colours that birds perceive, we compared the reflectance spectra of E. ruber, P. rubra and I. isabellae to the entire gamut of avian carotenoid plumages using a tetrachromatic model of avian colour vision [3,37]. Because birds have four single cones—red, green blue and violet/ultraviolet—their colour perceptions can be modelled with a tetrahedral colour space, or simplex [3,55]. In figure 7, we show the total diversity of colour perceptions produced by carotenoid plumages of more than 50 species in 23 avian families (gray circles and polyhedron, figure 7). Using canthaxanthin pigment only, the plumage colours of E. ruber (red dots), P. rubra (orange dots) and I. isabellae (purple dot) span a huge range of the carotenoid plumage gamut. Using canthaxanthin pigments, the maximum span (the distance between E. ruber and I. isabellae) is 0.5342, compared with 0.6177, the maximum span of the entire known, avian carotenoid plumage gamut. Thus, canthaxanthin pigments in these three species achieve approximately 86 per cent of the maximum span achieved by all carotenoids. Given that the rest of these carotenoid colours set are produced by a wide diversity of molecules, these results show that the effect of carotenoid binding by feather proteins on plumage coloration perceived by birds can be at least as large as the effect on the spectra of the bound pigments induced by variation in the molecular structure itself.
Figure 7.
The plumage colours of E. ruber (red dots), P. rubra (orange dots) and I. isabellae (purple dot) modelled in a tetrahedral avian color space. The grey polyhedron represents the total diversity of colour perceptions produced by carotenoid plumages of 145 plumage patches from 52 species in 23 avian families.
4. Discussion
HPLC chromatographic analyses, chemical analysis and electronic reflectance and absorption spectroscopy carried out on differently coloured feathers of P. rubra, E. ruber and I. isabellae indicate unambiguously that their colour results from the same carotenoid pigment, canthaxanthin. We can also eliminate the possibility that the purple coloration is the result of a structural colour. Small-angle X-ray scattering (SAXS) data from Saranathan et al. [42] have shown that I. isabellae lacks any colour-producing nanostructure, and is indistinguishable from a random heterogeneous mixture of two materials with a well-defined interface (electronic supplementary material, figure S2). Thus, the colour of purple Iodopleura feathers is a distinctly pigmentary phenomenon, and is not the result of interference, or coherent scattering, of blue light wavelengths.
HPLC of extracted carotenoid molecules and the rR spectra of the feathers clearly indicate that canthaxanthin is present in these feathers in an all-trans geometric isomeric configuration. Furthermore, in all rR spectra from feathers (figure 6), the ν4 band exhibits a well-structured lineshape and little variation with the excitation wavelength, indicating that the canthaxanthin molecules in each of the feathers have a single isomeric configuration; i.e. that they are all experiencing the same the out-of-plane distortions along the π-electron conjugated chain. Such distortions are determined by the interactions experienced by the carotenoid upon binding to the protein [20]. Therefore, we can conclude that canthaxanthin in the feathers within each species is not bound in a variable fashion within the protein matrix, but is bound in a highly consistent manner in each species.
However, the binding of canthaxanthin in the different feathers of the three species of birds varies substantially in a manner that is consistent with the observed differences in absorption and feather colour. Specifically, the largest differences among the feather rR spectra were the substantial downshift of the ν1 band in the purple feathers of I. isabellae in comparison with the red feathers of P. rubra or E. ruber, or to canthaxanthin in solution (figures 5 and 6; table 1). The frequency of the ν1 band is functionally associated (i.e. correlates with the length of C═C bond conjugation) with the stretching of C═C double bonds [29,32,50]. The effective length of the C═C double bond chain of the carotenoid backbone also has a fundamental impact on the position of the allowed S0 → S2 transition, which produces the absorbance spectrum of carotenoid molecules. The effect on the absorption spectra due to differences in the length of the C═C double bond chain of carotenoid molecules is well-documented [46]. These results demonstrate that an extension of the π-electron C═C double bond chain length of a carotenoid molecule bound to feather protein can substantially affect the absorption of the pigment and the colour produced. As previously mentioned, an X-ray structure analysis of canthaxanthin has revealed that the terminal rings in the molecule are twisted out of the plane of the conjugated double bond chain by approximately 55°, which inhibits π-electron delocalization into the ring structures [53]. Thus, planarization of the terminal rings upon binding to the protein can lead to an extension of the C═C double bond chain length and produce a red-shift in the absorption of the molecule [47]. Thus, the observed increase in the C═C double bond chain length in I. isabellae predicts the red-shift in absorption and, contributes to the violet colour produced.
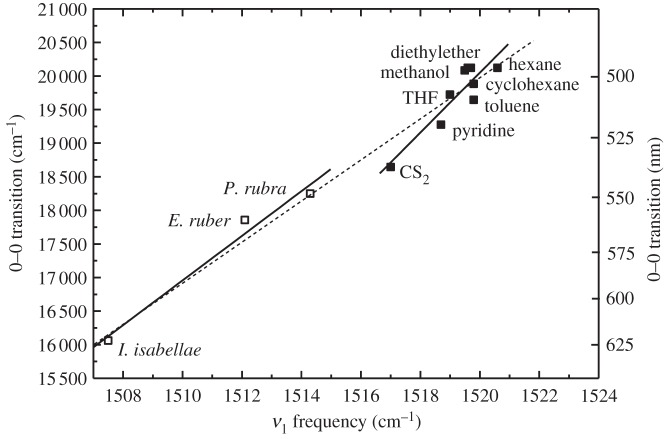
In solution, the position of the electronic absorption spectra of carotenoids depends on the polarizability of the environment (figure 4). We documented this effect by measuring the rR spectrum of canthaxanthin in many different solvents and plotting the ν1 values on the same graph with those obtained from the feathers (figure 8). The data reveal a correlation between the measured frequency of the ν1 band of canthaxanthin and the position of its electronic absorption transition, indicating that the electronic structure of the conjugated π-electron system in the ground state of canthaxanthin, which the ν1 band probes, is sensitive to solvent polarizability. If we make the same correlation between the ν1 band in feathers and the position of the lowest energy component of their absorption spectra, the slope of line associated with the canthaxanthin feathers is similar to, although slightly shallower than, the slope of the line obtained from canthaxanthin in various solvents and associated with changes in solvent polarizability (solid lines in figure 8).
Figure 8.
Correlation between the position of the (0–0) spectral origin of the S0 → S2 transition and the ν1 rR band of canthaxanthin in different solvents (solid squares) and in the feathers (open squares) of P. rubra, E. ruber and I. isabellae. All spectra were taken using 514.5 nm excitation. The solid lines represent a fit of the data to two independent linear functions. The dashed line represents a fit of the data to a single line.
For carotenoids in solution, a shallower slope of this correlation is predicted when other factors besides polarizability affect the position of the absorption bands of carotenoid molecules; e.g. differences among solvents that affect the carbon-carbon double bond/single bond order alternation [56,57]. Likewise, the difference in slope between canthaxanthin in solvents and in feathers could reflect additional properties of the binding of canthaxanthin in the feathers. However, it must be noted that it is possible to fit all the data (i.e. from both solvents and feathers) with a single line very close in slope to that determined for the feathers alone (dashed line in figure 8), although canthaxanthin in CS2 and in pyridine lie substantially off the line fitted to all the data (see the dashed line in figure 8).
The results suggest that the position of the S0 → S2 transition of canthaxanthin could be governed by the local polarizability of the carotenoid environment in the feathers. But, the likelihood of this hypothesis depends on the possibility of substantial variation in the refractive index of the protein binding site among species of birds. The refractive index, n, of the β-keratin matrix in which the carotenoid molecules are embedded is fairly high at 1.58 [58], which corresponds to a polarizability, R(n), value of 0.333, typical of many solids at room temperature. An R(n) value of 0.333 predicts the position of the S0 → S2 transition of keratin-bound canthaxanthin to be around 525 nm (figure 4), which would be blue-shifted by approximately 11 nm compared with that observed for canthaxanthin in CS2 (figure 2b). Although the exact position of the spectral origin (0–0) band of canthaxanthin in feathers is difficult to assess accurately (figure 2b), the (0–0) energies derived from the feather absorption lineshapes indicate a red-shift when compared with that observed for canthaxanthin in CS2, (figure 8) and not a blue-shift.
To obtain the observed (0–0) transition value of approximately 16 000 cm−1 for canthaxanthin in I. isabellae feathers (figure 8) based on polarizability alone would require a value of approximately 0.6 for β-keratin (figure 4), which corresponds to a refractive index of 2.3. That is an extraordinarily large, biologically impossible value that it is equivalent to many metals. Clearly, the data indicate that a change in polarizability alone cannot explain the variation in canthaxanthin absorption among feathers.
The analysis of the ν4 bands further documents that the precise configuration of canthaxanthin is different between the feathers of different species. Two parameters are important when considering the ν4 bands: the frequencies of the observed components, which depend on the position of the out-of-plane distortion in the molecule, and their intensity, which depends on the extent of the out-of plane distortion. In P. rubra feathers, the ν4 band is slightly more intense compared with that of canthaxanthin in solvents, and about one-fourth of that of the ν3 band. This indicates that the binding of canthaxanthin to the feather protein induces a small, additional distortion of the π-electron conjugated chain that is not present in organic solvents. In E. ruber, the ν4 band is nearly twice as intense as in P. rubra. Thus, carotenoid binding in E. ruber induces the same additional distortion to the molecule as in P. rubra but with a greater magnitude. Finally, in I. isabellae, the intensity of the ν4 band reaches that of the ν3 band, with two main components between 953.7 and 965.5 cm−1 (table 1). These frequencies are only slightly higher than the corresponding ν4 band frequencies observed for P. rubra and E. ruber feathers. From this, we can conclude that the binding sites provided by the three feathers induce out-of-plane distortions of canthaxanthin around carbon–carbon single bonds of varying magnitudes, and that the most substantial distortion of canthaxanthin is observed in I. isabellae feathers. It is interesting to note that the more distorted canthaxanthin becomes compared with its structure in solvents, the redder the absorption of the feathers.
Variation in the position and width of ν1 band were observed with different excitation wavelengths in each of the three species. In P. rubra, variation in the ν1 band with excitation wavelength was limited to a change in the fwhm of the peak. By contrast, in the feathers from E. ruber and I. isabellae, the ν1 band shifted noticeably in frequency with excitation wavelength. More specifically, for P. rubra feathers, where only an increase in the width of the ν1 rR band is observed, the absorption spectrum is broader when compared with that of canthaxanthin in solution. Eudocimus ruber feathers, for which ν1 frequencies varied between 1512.1 and 1519.7 cm−1 (approx. 7.5 cm−1) with excitation wavelength, exhibit an even broader absorption band (table 1). The range of ν1 frequencies observed bracket the ν1 peak of P. rubra (1514.3 cm−1), and their absorption spectrum also extends beyond both ends of that observed from P. rubra. Finally, for I. isabellae, the ν1 frequency varies between 1507.5 and 1509.6 cm−1 (approx. 2 cm−1) depending on the excitation wavelength. This result correlates with a narrower absorption spectra for I. isabellae than observed in E. ruber.
Planarization of the terminal rings of canthaxanthin will extend the π-electron conjugation farther along the carbon chain. Lengthening the extent of π-electron conjugation is known to down-shift the rR ν1 band and red-shift the absorption spectrum of carotenoids [32,59]. However, rR studies on carotenoids and polyenes having different π-electron conjugated chain lengths show that on average, there is approximately 15 nm of a red-shift in the absorption spectrum and approximately 6 cm−1 of a down-shift in the rR ν1 band position for every added double bond [29,59]. Thus, the approximately 6–7 cm−1 down-shift in the ν1 band observed in the feathers of I. isabellae compared with P. rubra (table 1) is not enough to account for all of the approximately 75 nm red-shift occurring between their absorption spectra (figure 8). Moreover, comparing the ν1 band of canthaxanthin in hexane (1520.5 cm−1) with that of the pigment in I. isabellae feathers (1508.6 cm−1), both recorded using 514.5 nm excitation, shows a difference of 11.9 cm−1. This would only amount to a difference of 11.9 cm−1 × (15 nm/6 cm−1) ≈ 30 nm between these two samples, which is clearly not enough to account for the violet purple colour of I. isabellae feathers.
An additional factor that can influence carotenoid pigment absorption is the formation of molecular aggregates. Absorption and rR spectroscopic investigations of crystalline and aggregated samples of several all-trans-carotenoids [51,60,61] described the effect of close proximity of the pigments on their spectral properties. The data reveal that carotenoids packed in a head-to-tail arrangement, called J-aggregates in the molecular exciton coupling theory [62], undergo a significant red-shift in their absorption spectra. This is because absorption into an energetically low-lying exciton state, formed when π-electron conjugated molecules are brought into close proximity, is red-shifted relative to the absorption bands associated with monomeric, unaggregated molecules. In fact, canthaxanthin undergoes a significant red-shift from 495 nm (20 202 cm−1) in solution (monomeric) to 581 nm (17 202 cm−1) in J-aggregate crystalline form [60]. Salares et al. [51] reported a 5 cm−1 downshift of the ν1 band of canthaxanthin, from 1523 cm−1 in acetone to 1518 cm−1 for the aggregated molecule in 10 per cent acetone–H2O solution. Monomeric canthaxanthin in the eight different solvents used here has ν1 values that span the 1520.5–1517 cm−1 range (using 514.5 nm excitation), and I. isabellae feathers have its ν1 band at 1508.6 cm−1 (using the same excitation λ). This corresponds to an approximately 10 cm−1 downshift, roughly twice that reported by Salares et al. [51] for carotenoid aggregates. This strongly suggests that carotenoid aggregation in the feathers is not the sole source of the spectral shift, but it appears to be an important factor, in addition to protein binding, needed to produce the violet colour of I. isabellae. It is not difficult to envision that canthaxanthin may be taken up into the keratin protein fibres of feathers in a head-to-tail manner so that it forms J-aggregates or related aggregated species, which could themselves be sensitive to the properties of the local environment provided by keratin. However, additional work will be required to confirm whether carotenoid are bound directly to β-keratin or to another protein or molecule, and whether molecular aggregates of canthaxanthin are present in the feathers of I. isabellae and other bird species as well.
5. Conclusions
These results confirm that different species of birds have evolved differences in the carotenoid binding to feather keratin proteins. Our findings support the following conclusions:
— Different canthaxanthin binding modes among the three different species examined produces changes in feather reflectance spectrum that are perceived by birds as strikingly different colours. The effect of protein binding on plumage coloration can be at least as significant on plumage colour as changes in molecular structure.
— Canthaxanthin is bound in a consistent manner by feather proteins in each of the feathers from the three species analysed, but each species induces a different degree of C═C double bond extension (ν1) and out-of-plane twists of the carbon backbone (ν4) of the bound pigment.
— Different subpopulations of all trans-canthaxanthin molecules are present in each of the feathers, which may be distinguished on the basis of their rR properties, particularly the frequency of their ν1 band. These changes do not affect the general all-trans geometric structure of canthaxanthin, but qualitatively correlate with the width of the absorption of each of the feathers.
— The degree of canthaxanthin distortion, especially the C═C double bond extension revealed through ν1, correlates well with the red-shift in the absorption spectra exhibited by canthaxanthin in each of the three feathers studied. However, the entire extent of the red-shift cannot be explained by the refractive index/polarizability of the molecular environment nor by planarization of the canthaxanthin terminal rings alone, although those are contributing factors. Very likely, linear, end-to-end molecular aggregation of the carotenoid pigments is an additional important factor.
Acknowledgements
Feather specimens were provided by the Ornithology Department of the Yale Peabody Museum of Natural History (YPM), Yale University, New Haven, CT, USA. Work in the laboratory of H.A.F was supported by the University of Connecticut Research Foundation and by the W. R. Coe Fund of Yale University. Work in the laboratory of B.R. was supported by EU program Marie Curie (FP7 Initial Training Network HARVEST), by the ERC funding agency (PHOTPROT project), by the CEA interdisciplinary program Technology for Health (MEDIASPEC project) and by the National Research Agency (ANR, Cyanoprotect Project). Richard Prum has been supported by an Ikerbasque Fellowship, and the Donostia International Physics Center, Donostia-San Sebastian, Spain. The ibis image in figure 1 is courtesy of G. Armistead/VIREO, the tanager photograph is courtesy of R. Nussbaumer/VIREO and the white-browed purpletuft photograph is courtesy of Nick Athanas. The authors thank Dr Michael Tauber for critical reading of the manuscript.
References
- 1.Blount J. D., McGraw K. J. 2008. Signal functions of carotenoid colouration. In Carotenoids volume 4: nutrition and health (eds Britton G., Liaaen-Jensen S., Pfander H.), pp. 213–232 Basel, Switzerland; Boston, MA; Berlin, Germany: Birkhäuser [Google Scholar]
- 2.McGraw K. J. 2006. Mechanics of carotenoid-based coloration. In Bird coloration volume 1: mechanisms and measurements (eds Hill G. E., McGraw K. J.), pp. 177–242 Cambridge, MA: Harvard University Press [Google Scholar]
- 3.Stoddard M. C., Prum R. O. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052 10.1093/beheco/arr088 (doi:10.1093/beheco/arr088) [DOI] [Google Scholar]
- 4.Hill G. E., McGraw K. J. (eds). 2006. Bird coloration, volume 1: mechanisms and measurements. Cambridge, MA: Harvard University Press [Google Scholar]
- 5.Hill G. E., McGraw K. J. (eds). 2006. Bird coloration, volume 2: function and evolution. Cambridge, MA: Harvard University Press [Google Scholar]
- 6.Prum R. O. 2006. Anatomy, physics, and evolution of avaian structural colors. In Bird coloration volume 1: mechanisms and measurements (eds Hill G. E., McGraw K. J.), pp. 295–353 Cambridge, MA: Harvard University Press [Google Scholar]
- 7.McGraw K. J. 2006. Mechanics of melanin-based coloration. In Bird coloration volume 1: mechanisms and measurements (eds Hill G. E., McGraw K. J.), pp. 243–294 Cambridge, MA: Harvard University Press [Google Scholar]
- 8.Prum R. O., Torres R. H., Williamson S., Dyck J. 1998. Coherent light scattering by blue feather barbs. Nature 396, 28–29 10.1038/23838 (doi:10.1038/23838) [DOI] [Google Scholar]
- 9.McGraw K. J. 2003. Melanins, metals, and mate quality. Oikos 102, 402–406 10.1034/j.1600-0579.2003.12513.x (doi:10.1034/j.1600-0579.2003.12513.x) [DOI] [Google Scholar]
- 10.Shawkey M. D., Hill G. E. 2005. Carotenoids need structural colours to shine. Biol. Lett. 1, 121–124 10.1098/rsbl.2004.0289 (doi:10.1098/rsbl.2004.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stradi R., Celentano G., Rossi E., Rovati G., Pastore M. 1995. Carotenoids in bird plumage: the carotenoid pattern in a series of Paleartic Carduelinae . Comp. Biochem. Physiol. 110B, 131–143 [Google Scholar]
- 12.Goodwin T. W. 1984. The biochemistry of carotenoids. Volume II. Animals. London, UK: Chapman Hall [Google Scholar]
- 13.Schiedt K. 1998. Absorption and metabolism of carotenoids in birds, fish and crustaceans. In Carotenoids volume 3: biosynthesis and metabolism (eds Britton G., Liaaen-Jensen S., Pfander H.), pp. 285–358 Basel, Switzerland; Boston, MA; Berlin, Germany: Birkhäuser [Google Scholar]
- 14.Brush A. H., Power D. M. 1976. House finch pigmentation—carotenoid metabolism and effect of diet. Auk 93, 725–739 [Google Scholar]
- 15.McGraw K. J., Hill G. E., Stradi R., Parker R. S. 2001. The influence of carotenoid acquisition and utilization on the maintenance of species-typical plumage pigmentation in the male American goldfinches (Carduelis tristis) and northern cardinal (Cadinalis cardinalis). Physiol. Biochem. Zool. 74, 843–852 10.1086/323797 (doi:10.1086/323797) [DOI] [PubMed] [Google Scholar]
- 16.Stradi R., Rossi E., Celentano G., Bellardi B. 1996. Carotenoids in bird plumage: the pattern in three Loxia species and in Pinicola enucleator. Comp. Biochem. Physiol. 113B, 427–432 [Google Scholar]
- 17.McGraw K. J., Hill G. E., Parker R. S. 2005. The physiological costs of being colorful: nutritional control of carotenoid utilization in the American goldfinch, Carduelis tristis. Anim. Behav. 69, 653–660 10.1016/j.anbehav.2004.05.018 (doi:10.1016/j.anbehav.2004.05.018) [DOI] [Google Scholar]
- 18.McGraw K. J., Hill G. E., Parker R. S. 2003. Carotenoid pigments in a mutant cardinal: Implications for the genetic and enzymatic control mechanisms of carotenoid metabolism in birds. Condor 105, 587–592 10.1650/7281 (doi:10.1650/7281) [DOI] [Google Scholar]
- 19.Hudon J., Brush A. 1992. Identification of carotenoid pigments in birds. In Carotenoids part a: chemistry, separation, quantitation, and antioxidation (ed. Packer L.), pp. 312–321 San Diego, CA: Academic Press [Google Scholar]
- 20.Britton G., Helliwell J. R. 2008. Carotenoid–protein interactions. In Carotenoids volume 4: nutrition and health (eds Britton G., Liaaen-Jensen S., Pfander H.), pp. 99–117 Basel–Boston–Berlin, Germany: Birkhäuser [Google Scholar]
- 21.Brush A. H. 1972. Correlation of protein electrophoretic pattern with morphology of normal and mutant feathers. Biochem. Genet. 7, 87–93 10.1007/BF00487012 (doi:10.1007/BF00487012) [DOI] [PubMed] [Google Scholar]
- 22.Brush A. H. 1986. Tissue specific protein heterogeneity in keratin structures. Biochem. Syst. Ecol. 14, 547–551 10.1016/0305-1978(86)90016-5 (doi:10.1016/0305-1978(86)90016-5) [DOI] [Google Scholar]
- 23.Weesie R. J., Jansen F. J. H. M., Merlin J. C., Lugtenburg J., Britton G., de Groot H. J. M. 1997. 13C Magic angle spinning NMR analysis and quantum chemical modeling of the bathochromic shift of astaxanthin in α-Crustacyanin, the blue carotenoprotein complex in the carapace of the lobster Homarus gammarus. Biochemistry 36, 7288–7296 10.1021/bi9631982 (doi:10.1021/bi9631982) [DOI] [PubMed] [Google Scholar]
- 24.Britton G., et al. 1997. Carotenoid blues: structural studies on carotenoproteins. Pure Appl. Chem. 69, 2075–2084 10.1351/pac199769102075 (doi:10.1351/pac199769102075) [DOI] [Google Scholar]
- 25.Cianci M., Rizkallah P. J., Olczak A., Raftery J., Chayen N. E., Zagalsky P. F., Helliwell J. R. 2002. The molecular basis of the coloration mechanism in lobster shell: β-crustacyanin at 3.2-Å resolution. Proc. Natl Acad. Sci. USA 99, 9795–9800 10.1073/pnas.152088999 (doi:10.1073/pnas.152088999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilagan R. P., Christensen R. L., Chapp T. W., Gibson G. N., Pascher T., Polivka T., Frank H. A. 2005. Femtosecond time-resolved absorption spectroscopy of astaxanthin in solution and in α-crustacyanin. J. Phys. Chem. A 109, 3120–3127 10.1021/jp0444161 (doi:10.1021/jp0444161) [DOI] [PubMed] [Google Scholar]
- 27.Veronelli M., Zerbi G., Stradi R. 1995. In situ resonance Raman spectra of carotenoids in birds feathers. J. Raman Spectrosc. 26, 683–692 10.1002/jrs.1250260815 (doi:10.1002/jrs.1250260815) [DOI] [Google Scholar]
- 28.Robert B., Horton P., Pascal A. A., Ruban A. V. 2004. Insights into the molecular dynamics of plant light-harvesting proteins in vivo. Trends Plant Sci. 9, 1360–1385 10.1016/j.tplants.2004.06.006 (doi:10.1016/j.tplants.2004.06.006) [DOI] [PubMed] [Google Scholar]
- 29.Koyama Y. 1995. Resonance Raman spectroscopy. In Carotenoids volume 1B: spectroscopy (eds Britton G., Liaaen-Jensen S., Pfander H.), pp. 135–146 Basel, Switzerland; Boston, MA; Berlin, Germany: Birkhäuser [Google Scholar]
- 30.Robert B. 1999. The electronic structure, stereochemistry and resonance Raman spectroscopy of carotenoids. In Advances in photosynthesis (eds Frank H. A., Young A. J., Britton G., Cogdell R. J.), pp. 189–201 Dordrecht, The Netherlands; Boston, MA; London, UK: Kluwer Academic Publishers. [Google Scholar]
- 31.Ruban A. V., Pascal A. A., Robert B., Horton P. 2001. Configuration and dynamics of xanthophylls in light-harvesting antennae of higher plants: spectroscopic analysis of isolated light-harvesting complex of photosystem II and thylakoid membranes. J. Biol. Chem. 276, 24 862–24 870 [DOI] [PubMed] [Google Scholar]
- 32.Robert B. 2009. Resonance Raman spectroscopy. Photosynth. Res. 101, 147–155 10.1007/s11120-009-9440-4 (doi:10.1007/s11120-009-9440-4) [DOI] [PubMed] [Google Scholar]
- 33.Fox D. L., Hopkins T. S. 1966. Carotenoid fractionation in the scarlet ibis. Comp. Biochem. Physiol. 19, 267–278 10.1016/0010-406X(66)90565-2 (doi:10.1016/0010-406X(66)90565-2) [DOI] [Google Scholar]
- 34.Fox D. L. 1962. Carotenoids of the Scarlet Ibis. Comp. Biochem. Physiol. 5, 31–43 10.1016/0010-406X(62)90139-1 (doi:10.1016/0010-406X(62)90139-1) [DOI] [PubMed] [Google Scholar]
- 35.Hudon J. 1990. Unusual carotenoid use by the western tanager (Piranga ludoviciana) and its evolutionary implications. Can. J. Zool. 69, 2311–2320 10.1139/z91-325 (doi:10.1139/z91-325) [DOI] [Google Scholar]
- 36.LaFountain A. M., Kaligotla S., Cawley S., Riedl K. M., Schwartz S. J., Frank H. A., Prum R. O. 2010. Novel methoxy-carotenoids from the burgundy-colored plumage of the pompadour cotinga Xipholena punicea. Arch. Biochem. Biophys. 504, 142–153 10.1016/j.abb.2010.08.006 (doi:10.1016/j.abb.2010.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoddard M. C., Prum R. O. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am. Nat. 171, 755–776 10.1086/587526 (doi:10.1086/587526) [DOI] [PubMed] [Google Scholar]
- 38.Endler J. A., Mielke P. W. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 10.1111/j.1095-8312.2005.00540.x (doi:10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 39.Ödeen A., Håstad O. 2003. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 20, 855–861 10.1093/molbev/msg108 (doi:10.1093/molbev/msg108) [DOI] [PubMed] [Google Scholar]
- 40.Carvalho L. S., Cowing J. A., Wilkie S. E., Bowmaker J. K., Hunt D. M. 2007. The molecular evolution of avian ultraviolet- and violet-sensitive visual pigments. Mol. Biol. Evol. 24, 1843–1852 10.1093/molbev/msm109 (doi:10.1093/molbev/msm109) [DOI] [PubMed] [Google Scholar]
- 41.Britton G., Liaaen-Jensen S., Pfander H. 2004. Carotenoids handbook. Basel, Switzerland; Boston, MA; Berlin, Germany: Birkhäuser [Google Scholar]
- 42.Saranathan V., Forster J. D., Noh H., Liew S. F., Mochrie S. G. J., Cao H., Dufresne E. R., Prum R. O. In press Structure and optical function of amorphous photonic nanostructures from avian feather barbs: a comparative small angle X-ray scattering (SAXS) analysis of 229 bird species. J. R. Soc. Interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noh H., Liew S. F., Saranathan V., Prum R. O., Mochrie S. G. J., Dufrense E. R., Cao H. 2010. Double scattering of light from biophotonic nanostructures with short-range order. Opt. Expr. 18, 11 942–11 948 10.1364/OE.18.011942 (doi:10.1364/OE.18.011942) [DOI] [PubMed] [Google Scholar]
- 44.Noh H., Liew S. F., Saranathan V., Mochrie S. G. J., Prum R. O., Dufrense E. R., Cao H. 2010. How noniridescent colors are generated by quasi-ordered structures of bird feathers. Adv. Mater. 22, 2871–2880 10.1002/adma.200903699 (doi:10.1002/adma.200903699) [DOI] [PubMed] [Google Scholar]
- 45.Glatter O., Kratky O. 1983. Small angle X-ray scattering, 2nd edn London, UK: Academic Press [Google Scholar]
- 46.Isler O. 1971. Carotenoids. Basel, Switzerland: Birkhäuser [Google Scholar]
- 47.Bartalucci G., Coppin J., Fisher S., Hall G., Helliwell J. R., Helliwell M., Liaaen J. S. 2007. Unravelling the chemical basis of the bathochromic shift in the lobster carapace; new crystal structures of unbound astaxanthin, canthaxanthin, and zeaxanthin. Acta Crystallogr. Sect. B: Struct. Sci. B63, 328–337 10.1107/S0108768106052633 (doi:10.1107/S0108768106052633) [DOI] [PubMed] [Google Scholar]
- 48.Polívka T., Frank H. A. 2010. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc. Chem. Res. 43, 1125–1134 10.1021/ar100030m (doi:10.1021/ar100030m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renge I., Sild E. 2011. Absorption shifts in carotenoids-influence of index of refraction and submolecular electric fields. J. Photochem. Photobiol. A 218, 156–161 10.1016/j.jphotochem.2010.12.015 (doi:10.1016/j.jphotochem.2010.12.015) [DOI] [Google Scholar]
- 50.Koyama Y., Kito M., Takii T., Saiki K., Tsukida K., Yamashita J. 1982. Configuration of the carotenoid in the reaction centers of photosynthetic bacteria. Comparison of the resonance Raman spectrum of the reaction centers of Rhodopseudomonas sphaeroides G1C with those of cis-trans isomers of ß-carotene. Biochim. Biophys. Acta 680, 109–118 10.1016/0005-2728(82)90001-9 (doi:10.1016/0005-2728(82)90001-9) [DOI] [Google Scholar]
- 51.Salares V. R., Young N. M., Carey P. R., Bernstein H. J. 1977. Excited state (exciton) interactions in polyene aggregates. J. Raman Spectrosc. 6, 282–288 10.1002/jrs.1250060605 (doi:10.1002/jrs.1250060605) [DOI] [Google Scholar]
- 52.Lutz M., Szponarski W., Berger G., Robert B., Neumann J.-M. 1987. The stereoisomerization of bacterial, reaction-center-bound carotenoids revisited: an electronic absorption, resonance Raman and NMR study. Biochim. Biophys. Acta 894, 423–433 10.1016/0005-2728(87)90121-6 (doi:10.1016/0005-2728(87)90121-6) [DOI] [Google Scholar]
- 53.Bart J. C. J., MacGillavry C. H. 1968. The crystal and molecular structure of canthaxanthin. Acta Crystallogr. Sect. B 24, 1587–1606 10.1107/S056774086800470X (doi:10.1107/S056774086800470X) [DOI] [PubMed] [Google Scholar]
- 54.Ruban A. V., Horton P., Robert B. 1995. Resonance Raman spectroscopy of the photosystem II light-harvesting complex of green plants: a comparison of trimeric and aggregated states. Biochem 34, 2333–2337 10.1021/bi00007a029 (doi:10.1021/bi00007a029) [DOI] [PubMed] [Google Scholar]
- 55.Cuthill I. C. 2006. Color perception. In Bird coloration, volume 1: mechanisms and measurements (eds Hill G. E., McGraw K. J.), pp. 3–40 Cambridge, MA: Harvard University Press [Google Scholar]
- 56.Araki G., Murai T. 1952. Molecular structure and absorption spectra of carotenoids. Prog. Theor. Phys. 8, 639–654 10.1143/PTP.8.639 (doi:10.1143/PTP.8.639) [DOI] [Google Scholar]
- 57.Angerhofer A., Bornhauser F., Gall A., Cogdell R. J. 1995. Optical and optically detected magnetic-resonance investigation on purple photosynthetic bacterial antenna complexes. Chem. Phys. 194, 259–274 10.1016/0301-0104(95)00022-G (doi:10.1016/0301-0104(95)00022-G) [DOI] [Google Scholar]
- 58.Brink D. J., van der Berg N. G. 2004. Structural colours from the feathers of the bird Bostrychia hagedash. J. Phys. D: Appl. Phys. 37, 813–818 10.1088/0022-3727/37/5/025 (doi:10.1088/0022-3727/37/5/025) [DOI] [Google Scholar]
- 59.Koyama Y., Fujii R. 1999. Cis-trans carotenoids in photosynthesis: configurations, excited-state properties and physiological functions. In The photochemistry of carotenoids (eds Frank H. A., Young A. J., Britton G., Cogdell R. J.), pp. 161–188 Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 60.Mori Y. 2001. Introductory studies on the growth and characterization of carotenoid solids: an approach to carotenoid solid engineering. J. Raman Spectrosc. 32, 543–550 10.1002/jrs.715 (doi:10.1002/jrs.715) [DOI] [Google Scholar]
- 61.Gruszecki W. I. 1991. Structural characterization of the aggregated forms of violaxanthin. J. Biol. Phys. 18, 99–109 10.1007/BF00395057 (doi:10.1007/BF00395057) [DOI] [Google Scholar]
- 62.Gaier K., Angerhofer A., Wolf H. C. 1991. The lowest excited electronic singlet states of all-trans β-carotene single crystals. Chem. Phys. Lett. 187, 103–109 10.1016/0009-2614(91)90492-R (doi:10.1016/0009-2614(91)90492-R) [DOI] [Google Scholar]