Abstract
In bacteria, the production of exopolysaccharides—polysaccharides secreted by the cells into their growth medium—is integral to the formation of aggregates and biofilms. These exopolysaccharides often form part of a matrix that holds the cells together. Investigating the bacterium Sinorhizobium meliloti, we found that a mutant that overproduces the exopolysaccharide succinoglycan showed enhanced aggregation, resulting in phase separation of the cultures. However, the aggregates did not appear to be covered in polysaccharides. Succinoglycan purified from cultures was applied to different concentrations of cells, and observation of the phase behaviour showed that the limiting polymer concentration for aggregation and phase separation to occur decreased with increasing cell concentration, suggesting a ‘crowding mechanism’ was occurring. We suggest that, as found in colloidal dispersions, the presence of a non-adsorbing polymer in the form of the exopolysaccharide succinoglycan drives aggregation of S. meliloti by depletion attraction. This force leads to self-organization of the bacteria into small clusters of laterally aligned cells, and, furthermore, leads to aggregates clustering into biofilm-like structures on a surface.
Keywords: bacterial aggregation, depletion attraction, Sinorhizobium meliloti, exopolysaccharide, biofilm
1. Introduction
In the laboratory, pure cultures of bacteria are often grown as dispersed single cells. However, bacteria can also form aggregates. During wastewater treatment, for example, bacteria form multi-species aggregates that break down organic waste. Bacteria also colonize surfaces, forming microcolonies and aggregative structures known as biofilms. Integral to aggregation is the production of exopolysaccharides by the bacteria; for example, observations of bacterial aggregates from wastewater treatment by electron microscopy showed the presence of extracellular fibrils that were later confirmed to be polysaccharides [1–6], and, more recently, studies of Agrobacterium species [7–10], Rhizobium leguminosarum [11–13], Xanthomonas campestris [14] and Sinorhizobium meliloti [15,16] have shown that mutants affected in exopolysaccharide production have altered aggregation and biofilm formation.
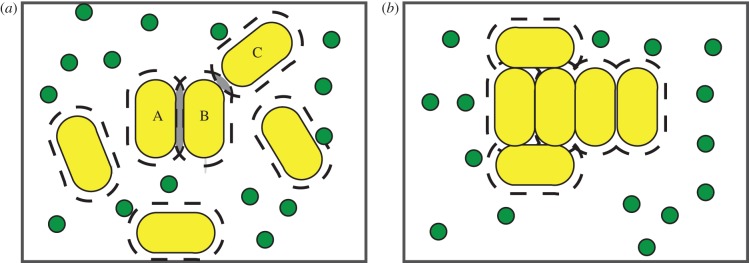
The principles of soft condensed matter physics can be applied to understanding how bacterial aggregates form; cultures of non-motile bacteria are effectively colloidal dispersions, and many mechanisms that underlie the stability of colloidal dispersions will be relevant to bacterial cultures [17]. Polymers can contribute to the destabilization of colloidal dispersions via two generic mechanisms: polymer bridging and depletion attraction. Polymer bridging occurs when polymers adsorb to multiple colloidal particles and draw them together. The presence of an extracellular matrix composed of polysaccharides around many bacterial aggregates suggests that polymer bridging often occurs [18]. On the other hand, depletion attraction relies on non-adsorbing polymers, which give rise to an entropic force because clustering of the colloidal particles increases the total entropy of the system by increasing the entropy of the polymers. This is illustrated in figure 1. In such a mixture, the centre of a polymer coil (represented as small circles in figure 1) cannot approach the surface of a particle (larger spherocylinders in figure 1) by a distance less than the size of its own radius (as measured, for example, by its radius of gyration, rg): doing so would restrict the configurational freedom of the polymer coils and result in loss of entropy. Each particle can therefore be considered as surrounded by a ‘depletion zone’ (delineated by dashed lines in figure 1). As two nearby particles come closer and increase the region of overlapping depletion zones, the volume of space available to the polymer molecules increases, thus increasing the entropy of the latter, leading to a state of lower free energy. In cultures of bacteria producing exopolysaccharides, which are differentiated from other polysaccharides such as capsular polysaccharides by the fact that they are secreted into the medium rather than being attached to the outer membrane [19,20], this mechanism will operate.
Figure 1.
(a,b) Schematic of how depletion attraction operates in a culture of bacteria producing exopolysaccharide. Bacteria, large spherocylinders; exopolysaccharide, small spheres. Dashed lines around the bacteria are zones of depletion or excluded volumes and represent the volume that is not accessible to the centre of mass of a given polymer after it has been secreted into the medium. As bacteria come closer together, these excluded volumes overlap (as shown in grey shaded zones; (a)). When bacteria aggregate, this volume then becomes available to the succinoglycan, increasing its entropy and maximizing the entropy of the system. As shown in (a), a greater volume is made available to the succinoglycan if cells align laterally (cells A and B) rather than end-on (cells B and C), so lateral alignment of cells would be expected to be favoured if a crowding mechanism is occurring. (Online version in colour.)
Here, we investigate aggregation in cultures of the bacterium Sinorhizobium meliloti. Under most conditions, the laboratory strain of S. meliloti, Rm1021, produces the exopolysaccharide succinoglycan, which is a polymer of an octasaccharide that consists of one galactose and seven glucose molecules with acetyl, succinyl and pyruvyl modifications [21]. Sinorhizobium meliloti can also produce a second exopolysaccharide, EPSII or galactoglucan, but only in strains that have a functional expR gene; Rm1021 does not produce EPSII under most conditions as it carries an insertion sequence in the expR gene, which is therefore not functional. The succinoglycan biosynthetic pathway has been extremely well characterized from a set of Rm1021 mutants. We have used these mutants to investigate how altering succinoglycan biosynthesis affects aggregation in these cells, and have found that overproduction of succinoglycan can lead to aggregation by depletion attraction.
2. Material and methods
2.1. Bacterial strains and culture media
The S. meliloti and Escherichia coli strains used in this study are listed in tables 1 and 2, respectively. Sinorhizobium meliloti strains were grown routinely at 30°C in LBMC (Luria–Bertani broth supplemented with 2.5 mM CaCl2.2H2O and MgSO4.7H2O). Initially, 5 ml cultures were grown in test tubes in LBMC for 24 h (with the addition of antibiotic relevant for that strain), after inoculating a single colony from an LBMC agar plate. This culture was then sub-inoculated into fresh LBMC at OD600 0.1, followed by incubation to the required growth phase (late-exponential phase approx. 15 h). For large-scale cultures (greater than 100 ml), 5 ml of culture of that strain was grown from a single colony in LBMC (with antibiotics) for 24 h. This was then sub-inoculated into 100 ml fresh LBMC followed by incubation for approximately 9 h to exponential phase. This culture was then sub-inoculated into the required amount of fresh LBMC at OD600 0.1 and grown to the required growth phase. Escherichia coli cultures were grown with shaking (200 r.p.m.) at 37°C. Initially, 5 ml cultures were grown in the presence of the relevant antibiotic from a single colony isolated from an LB agar plate overnight. The culture was then sub-inoculated into fresh LB at OD600 0.1 and grown to the required growth phase.
Table 1.
Sinorhizobium meliloti strains used in this study. R denotes antibiotic resistance.
| strain | description | reference |
|---|---|---|
| Rm1021 | spontaneous SU47 mutant, SmR | [22] |
| Rm7210 | Rm1021 exoY:: Tn5, SmR, NmR | [23,24] |
| Rm7096 | Rm1021 exoS::Tn5, SmR, NmR | [25] |
| Rm8396 | Rm1021 exoS::Tn5-233, SmR, NmR, GmR, SpR | [25] |
| Rm7095 | Rm1021 exoR::Tn5, SmR, NmR | [25] |
| Rm7094 | Rm1021 exoB::Tn5, SmR, NmR | [26] |
| Rm8341 | Rm1021 exoZ::Tn5, SmR, NmR | [27] |
| Rm7154 | Rm1021 exoH::Tn5, SmR, NmR | [28] |
| Rm7445 | Rm1021 exoK::Tn5, SmR, NmR | [29] |
| Rm7013 | Rm1021 exsH::Tn5, SmR, NmR | [29] |
| Rm8826 | Rm1021 exoK::Tn5-233 exsH::Tn5, SmR, NmR, GmR, SpR | [29] |
| Rm ΔfliF | Rm1021 ΔfliF, SmR | G. P. Ferguson |
| RmGD01 | Rm1021 exoS::Tn5-233, exoY::Tn5, SmR, NmR, GmR, SpR | this study |
Table 2.
Escherichia coli strains used in this study. R denotes antibiotic resistance.
For growth of S. meliloti in nitrogen-limited M9 [25,32], 5 ml cultures were grown in LBMC for 24 h, the cells were pelleted by centrifugation (8000g) and resuspended in nitrogen-limited M9. This suspension was inoculated into fresh nitrogen-limited M9 at OD600 0.5, followed by incubation to the required growth phase.
Antibiotics were used at the following concentrations: streptomycin 500 μg ml–1, neomycin 200 μg ml–1, gentamicin 50 μg ml−1, spectinomycin 100 μg ml–1 and kanamycin 50 μg ml−1.
For S. meliloti transductions, the phage φM12 was used [33]; for E. coli, phage P1 was used [34].
2.2. Sedimentation screen
The different strains were assessed for aggregation by monitoring of sedimentation. The bacterial strains were grown to the required growth phase in the medium indicated in the figure legends. One millilitre of the culture was then transferred to a cuvette, which was covered with Parafilm and incubated for 24 h at 30°C without shaking. Images were acquired using a digital camera (Nikon). Percentage aggregation was measured as previously described [16,35]. Briefly, OD600 of the upper phase was measured when the culture was added to the cuvette and again after 24 h. Percentage aggregation was calculated using
 |
Sedimentation was also screened for cultures in the presence of exoS mutant supernatant. A 10 ml late-exponential phase exoS mutant culture was centrifuged at 8000g for 5 min. The resulting supernatant was removed and filter sterilized through a 0.2 μm pore filter. Strains of S. meliloti to be tested in the presence of the exoS supernatant were grown to late-exponential phase, 1 ml was then centrifuged at 13 000g for 2 min and the pelleted cells were dispersed in the exoS supernatant. As a control, each strain was also centrifuged at 13 000g and then resuspended in its own supernatant. The sedimentation screen was then carried out as detailed above.
The aggregation of Rm1021 was also tested in the presence of the exoS supernatant after the cells had been heat-treated. Late-exponential phase cultures of the parent strain Rm1021 and the exoS mutant were heat-treated by incubating the cultures at 60°C for 3 h. Cell viability was assessed by counting colony-forming units (CFU) per millilitre before and after heat treatment. A sedimentation screen was then performed.
2.3. Microscopy
For phase contrast microscopy, cultures of the strains required for observation were grown to the required growth phase and 5 μl was spotted onto glass slides, with a coverslip. Coverslips were sealed with clear nail varnish. Phase contrast microscopy images were taken with a ×100 oil immersion phase contact lens. All phase contrast microscopy was carried out on a Carl Zeiss Axioscope microscope and Axiocam using Metamorph software.
Confocal microscopy was carried out with an inverted microscope (Nikon TE300) coupled to a BioRad radiance 2100 scanning system using a ×60 water immersion lens. Images were acquired by scanning the samples with optimal settings for green fluorescent protein (GFP) (488 nm excitation argon laser line and 505 nm long-pass emission) and using Lasersharp v. 2000 software. Images were subsequently processed using ImageJ v. 1.40 g software [36].
Cultures of S. meliloti strains expressing GFP from the pHC60 [37] plasmid were grown to late-exponential phase and transferred into chambered coverglass slides containing a borosilicate glass base 1 μm thick (Lab-Tek Nunc; no. 155411) and incubated without shaking at 30°C for the time indicated. To reduce evaporation from the chambers, incubation was carried out in humidified Petri dishes.
2.4. Quantification of succinoglycan production by the anthrone–sulphuric acid assay
Succinoglycan was purified from culture supernatants by precipitation by cetrimide (hexadecyltrimethylammonium bromide) and quantified using the anthrone–sulphuric acid assay [38]. Three 10 ml S. meliloti cultures for each strain were grown to the required phase. The cultures were then centrifuged for 1 h at 13 000g. The supernatant was removed and 0.3 volumes of 1 per cent cetrimide was added to precipitate the succinoglycan. The precipitate was pelleted by centrifugation at 13 000g for 1 h and re-dissolved in 1 ml of 10 per cent NaCl. This was then diluted 1 : 10 in a total volume of 2 ml in a test tube and assayed by the anthrone–sulphuric acid assay. Acetone (0.5 ml) was carefully layered on the top of the sample. Sulphuric acid (5 ml) was then added to the test tube, which was mixed slowly until a floc of anthrone appeared. The samples were then vortexed briefly and placed on ice for 5 min. Absorbance was measured at 620 nm. A standard curve was generated from glucose ranging from 0 to 40 μg ml–1. Succinoglycan production was normalized to OD600 of the culture.
2.5. Isolation and purification of succinoglycan
Isolation of succinoglycan was adapted from a previous method [39]. A 500 ml culture of the exoS mutant was grown to late-exponential phase in M9. This was centrifuged at 13 000g for 1 h. The supernatant was then treated by ultrafiltration with an ultrafiltration filter disc with a molecular weight cut-off of 300 kDa (Millipore). Overnight proteinase K treatment at 37°C was followed by ultradialysis with at least 1 l of de-ionized water. The resulting solution was then lyophilized and stored at −20°C. Solutions were made in phosphate-buffered saline (PBS) or to the required concentration from the dried material. Purity of the sample was assessed by UV spectrophotometry and the Bradford assay; the purified succinoglycan contained no DNA and less than 1 per cent protein, consistent with previous work [39].
2.6. Effect of cell concentration and succinoglycan concentration on phase separation
The S. meliloti exoY mutant was grown to late-exponential phase. The cultures were then centrifuged at 13 000g for 30 min in pre-weighed sterile centrifuge flasks. The supernatant was removed and the flask weighed to give the weight of the pellet. PBS was then added to give a final concentration of 25 per cent (w/v) of cells, and the cells were resuspended by gentle pipetting. In cuvettes, cells, polymer (succinoglycan or xanthan) and PBS were added in required volumes to give final concentrations of 2.5–20 per cent of cells and 0.05 per cent succinoglycan or 0.035 per cent xanthan in a total volume of 1 ml per cuvette. For each concentration of cells used, a control with no added polymer was also made. The cuvettes were then sealed with Parafilm and incubated without shaking for 24 h at 30°C. The degree of aggregation and rapid sedimentation was assessed by visual observation of the cuvettes after 24 h of static incubation. To find the limiting polymer concentration, the cuvettes were diluted by first mixing the contents of the cuvette, then removing 100 μl and adding 100 μl of fresh PBS. After each dilution, the cuvettes were incubated without shaking for 24 h before visual observation to determine the amount of polymer at each cell concentration below which no rapid sedimentation was observed during this time.
3. Results
3.1. The Rm1021 exoS mutant exhibits enhanced aggregation
In a bacterial culture, autoaggregation of cells leads to enhanced sedimentation and phase separation of the cultures, which results in the cells collecting at the bottom of the culture vessel, leaving a clear supernatant [14,16]. We used this phenotype to screen S. meliloti Rm1021 exopolysaccharide biosynthesis mutants for altered aggregation.
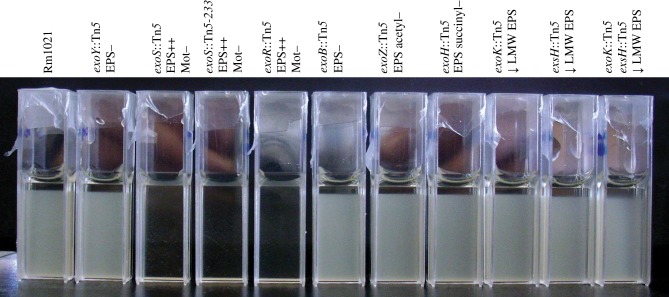
Strains were grown in LBMC (LB supplemented with 2.5 mM CaCl2.2H2O and MgSO4.7H2O) to late-exponential phase, transferred to cuvettes and incubated for 24 h without shaking (figure 2). Most of the strains, including the parent strain Rm1021, remained more or less homogeneous, showing only the first signs of sedimentation after a day in the form of a small layer of clear liquid right at the top of each sample. In all of these samples, the clear layer measured between 2 and 3 mm after 24 h. Modelling a typical cell as a 0.7 × 1.7 μm ellipsoid (average dimensions taken from optical micrographs), we predict the sedimentation speed of a single cell to be 33 nm s–1, or 2.8 mm d−1 (see appendix A for details). Our observations are therefore consistent with the presence of unaggregated cells.
Figure 2.
Screening of exopolysaccharide mutants for differences in rate of sedimentation. Rm1021 and the indicated succinoglycan biosynthesis mutants were grown to late-exponential phase in LBMC, transferred into cuvettes and incubated without shaking for 24 h. For each mutant strain, the gene location of the transposon is indicated, along with the relevant phenotype relative to Rm1021, which is motile and can make succinoglycan: EPS, succinoglycan; Mot, motility; +/−, gain or loss of that phenotype. ExoK and ExsH are extracellular glycanases; mutants have a decrease (down arrow) of low-molecular-weight (LMW) succinoglycan. Repeated experiments gave the same result. (Online version in colour.)
In contrast, exoS and exoR mutants were both found to sediment at much higher rates: all the cells had sedimented to the bottom of the cuvettes after 24 h, leaving a clear supernatant. We take this as indicative of autoaggregation, which gives rise to cell clusters with significantly enhanced sedimentation speed.
3.2. Enhanced aggregation in the exoS mutant is due to succinoglycan
The exoS mutant is a gain-of-function mutant that overproduces succinoglycan and also has lost motility owing to downregulation of flagella synthesis. The exoR mutant is a loss-of-function mutant that has exactly the same phenotype [25,40,41]. Because of the similarities in phenotype, and because the exoR mutant was less well understood at the start of this study compared with the exoS mutant, we chose to focus on the exoS mutant.
We hypothesized that overproduction of succinoglycan was the cause of the exoS phenotype. However, it was unclear as to why the parent strain Rm1021 and the mutant strains capable of producing succinoglycan did not appear to differ from the strains incapable of producing succinoglycan (exoY and exoB mutants), which all remained as stable, turbid cultures (figure 2). Therefore, to test whether succinoglycan overproduction was the cause of the exoS mutant phenotype, an exoSexoY double mutant was constructed. This mutant cannot produce succinoglycan owing to loss of ExoY, but still shows any other effects that may result from the exoS gain-of-function mutation. A ΔfliF mutant, which lacks flagella and is immotile (data not shown), was used as a control to investigate whether loss of flagella alone could result in aggregation (see table 3 for a summary of these strains).
Table 3.
Relevant phenotypes and proteins affected in S. meliloti strains used in this study.
| strain/mutant | phenotype | proteins affected in mutants |
|---|---|---|
| Rm1021 | parent; motile/can make succinoglycan | |
| exoY | motile/cannot make succinoglycan | ExoY: galactosyl transferase |
| exoS | non-motile/overproduces succinoglycan | ExoS: sensor histidine kinase of a two-component regulator |
| exoSexoY | non-motile/cannot make succinoglycan | ExoS and ExoY |
| ΔfliF | non-motile/parent levels of succinoglycan | FliF: encodes MS ring at base of flagella |
| exoZ | motile/makes non-acetylated succinoglycan | ExoZ: acetyl transferase |
| exoH | motile/makes non-succinylated succinoglycan | ExoH: succinyl transferase |
| exoSexoZ | non-motile/overproduces non-acetylated succinoglycan | ExoS and ExoZ |
| exoSexoH | non-motile/overproduces non-succinylated succinoglycan | ExoS and ExoH |
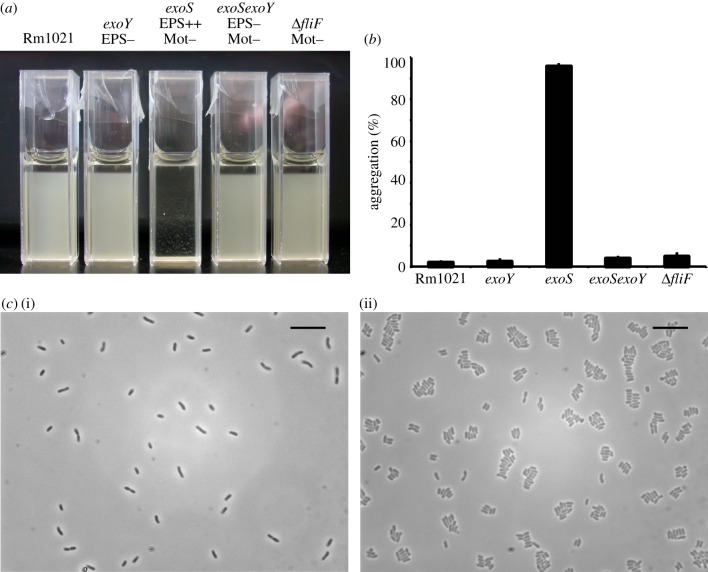
We measured the concentration of succinoglycan in cultures grown to late-exponential phase in LBMC. As has been found previously, the exoS mutant overproduced succinoglycan when compared with the parent [25], as well as the ΔfliF mutant, and strains carrying an exoY mutation, which cannot make succinoglycan [23,42] (Rm1021, 0.07±0.15; exoY, 0.15±0.22; exoS, 50.78±16.54; exoSexoY, 0±0.11; ΔfliF 0.31±0.06 μg glucose equivalents ml–1 culture ± s.d., n = 3). A comparison of the sedimentation rate and aggregation of these strains showed that the exoSexoY double mutant and the ΔfliF mutant sediment at the same rate as Rm1021, whereas the exoS mutant has enhanced sedimentation and aggregation, which was confirmed by microscopy (figure 3a–c). This suggests that in the exoS mutant the overproduction of succinoglycan leads to aggregation.
Figure 3.
The exoS mutant has enhanced aggregation and lateral stacking of cells. (a) Sedimentation screen of Rm1021 and the indicated mutants. Strains were grown to late-exponential phase in LBMC, transferred into cuvettes and incubated without shaking for 24 h. (b) Percentage of aggregation as quantified from the change in OD600 over 24 h from the experiment performed as in (a). Data are means ± s.d., n = 3. (c) Phase contrast microscopy images of Rm1021 (i) and the exoS mutant (ii). The exoY, exoSexoY and ΔfliF mutants all looked the same as Rm1021. Strains were grown to late-exponential phase, 1 μl of culture was placed on a microscope slide and, after sealing on a coverslip, the slide was immediately imaged. The exoS mutant forms small aggregates, where the cells are aligned laterally. (c) Scale bars, 10 μm. (Online version in colour.)
As succinoglycan is an exopolysaccharide, it is secreted into the medium rather than remaining attached to the cells and can therefore be washed away from the cells through centrifugation. As such, the supernatant from an exoS culture will contain succinoglycan. To confirm whether enhanced sedimentation in the exoS mutant was due to overproduction of succinoglycan, cultures of all the strains were centrifuged, and the resulting pellet of cells was re-dispersed in the supernatant from a centrifuged exoS culture. Dispersion in the exoS supernatant resulted in rapid sedimentation of all the strains (figure 4a).
Figure 4.
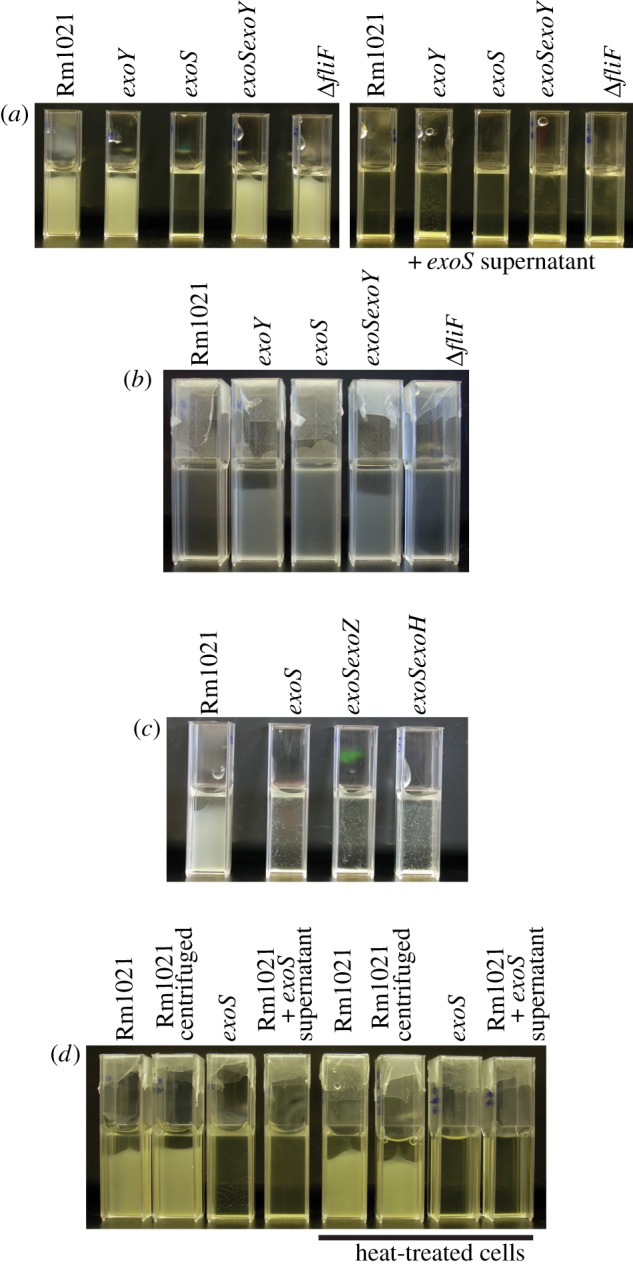
Addition of the exoS supernatant to S. meliloti results in aggregation. (a) Left: cells of the indicated strains were grown to late-exponential phase, transferred to cuvettes and incubated without shaking for 24 h. Right: cultures of the indicated strains dispersed in exoS supernatant. An exoS culture was grown to late-exponential phase, centrifuged and the supernatant was filter sterilized to remove any remaining cells. The indicated strains were grown to late-exponential phase, and centrifuged to pellet the cells. The pelleted cells were then dispersed in the filter-sterilized exoS supernatant, transferred to cuvettes and incubated without shaking for 24 h. (b) The indicated strains were grown in nitrogen-free M9. After 30 h, cells were transferred to cuvettes and left without shaking for 24 h. (c) The indicated strains were grown to late-exponential phase, transferred to cuvettes and incubated without shaking for 24 h. (d) Rm1021 and the exoS mutant were grown to late-exponential phase. Heat-treated cultures were incubated at 65°C for 2 h before preparation. Rm1021 centrifuged, Rm1021 that were centrifuged and the pellet then re-dispersed without changing the supernatant. Rm1021 cells dispersed in exoS supernatant were prepared as in (a). All experiments were performed at least three times with the same result. (Online version in colour.)
To upregulate succinoglycan biosynthesis in the parent strain without mutation, we next grew the strains in a minimal M9 medium lacking nitrogen. This high-carbon, low-nitrogen medium maximizes exopolysaccharide production, and has been used to isolate succinoglycan from S. meliloti cultures [19,25]. Under these conditions, Rm1021 did indeed produce more succinoglycan (Rm1021, 35.83±5.84; exoY, 0.24±1.04; exoS, 139.69±18.38; exoSexoY, 0.84±1.03; ΔfliF 40.50±3.72 μg glucose equivalents ml−1 culture±s.d., n = 3). After culturing the strains in this medium, cells were transferred to cuvettes and incubated without shaking for 24 h (figure 4b). The Rm1021 strain had enhanced sedimentation, compared with the exoY and exoSexoY mutants, which cannot produce succinoglycan. The ΔfliF mutant, which produces succinoglycan at a similar level to Rm1021, also had enhanced sedimentation. Interestingly, we observed little sedimentation of the exoS mutant in this minimal medium. So much succinoglycan was produced that the medium became highly viscous, as observed during pipetting, and this viscosity seemed to effectively prevent sedimentation during our observation window.
To test whether the chemical structure and molecular weight of succinoglycan affected phase separation, we made an exoSexoZ and an exoSexoH double mutant (table 3). The exoZ mutation results in strains that produce succinoglycan with no acetyl group [27], and the exoH mutation results in production of succinoglycan with no succinyl group [28]. The alteration in the chemical structure of the polymers also alters the molecular weight of the polymers produced, owing to altered susceptibility of the polymer to cleavage by extracellular glycancases [43]. Compared with Rm1021, the exoZ mutant produces more low-molecular-weight succinoglycan, whereas the exoH mutant produces succinoglycan that has a higher molecular weight, and a higher degree of polymerization [43]. As with the exoS single mutant, both mutants had enhanced phase separation when compared with the parent strain (figure 4c).
3.3. exoS mutant aggregates do not have an extracellular matrix and are laterally aligned
Microscopic observation of exoS cultures in LBMC showed that the cells were forming aggregates, unlike the parent strain where the cells were dispersed singly throughout the culture (figure 3c). Phase contrast microscopy of bacterial aggregates often shows cells encased in an extracellular matrix [14,44]. Surprisingly, however, we found that no extracellular material was visible around the exoS aggregates. Notably, the cells also had an ordered arrangement, with cells within each aggregate showing a predominantly side-by-side, or lateral, alignment.
As the aggregates did not seem to be encased in succinoglycan, it seemed possible that the presence of the polysaccharide could act as a signal and cause the cells to adapt their cell surface, resulting in aggregation. As a preliminary test of this, we heat-treated Rm1021 cells to kill them (as assessed by the CFU assay; before heat treatment, 7.33×109 ± 1.1×107 cells per ml; after heat treatment, less than 100 cells per ml), before pelleting the cells and adding the exoS supernatant. This again resulted in enhanced sedimentation and aggregation, with the cells forming aggregates of laterally aligned cells, suggesting that the enhanced aggregation caused by the presence of succinoglycan was due to a physico-chemical mechanism (figure 4d).
3.4. Succinoglycan causes aggregation of S. meliloti through a crowding mechanism
It therefore seemed that succinoglycan, rather than encasing the cells and sticking them together (polymer bridging), could be inducing aggregation through depletion attraction.
The ordered arrangement of cells within the exoS aggregates provides support for the depletion attraction mechanism. The alignment of the cells side by side (figure 3c) maximizes the overlap of the depletion zones between the rod-shaped cells (figure 1), giving rise to the strongest depletion attraction among all possible mutual orientations of two neighbouring cells. Alternatively, side-by-side aggregation maximizes the volume available to the succinoglycan. Moreover, we found that the process shown in figure 3 was easily reversible, consistent with a depletion mechanism. Shaking of the sedimented cuvettes re-dispersed the cells, which, if left undisturbed, would again sediment. This is consistent with there being no permanent ‘sticking’ between the cells; aggregates formed by bridging tend to be permanent.
To understand the depletion-induced aggregation process further, we need to be more precise about the phenomenon that until now we have called ‘enhanced sedimentation’. The strength of the depletion attraction in a mixture of polymers and particles increases with the polymer concentration. At low polymer concentration, small aggregates are formed that are transient: they form, break apart owing to thermal agitation (Brownian motion) and then new clusters re-form. However, at high-enough polymer concentration, depletion causes thermodynamic ‘phase separation’: a homogeneous sample separates into two coexisting phases with different compositions; a lower (because denser) phase with a higher concentration of particles, and an upper phase with a lower concentration of particles [45]. One way of viewing this is to say that the lower phase is the result of the formation of a macroscopically large cluster. At low overall particle concentration, essentially all the particles separate out into the concentrated phase, coexisting with a dilute phase with almost no particles. Such phase separation is the cause of the rapid sedimentation we observe in the exoS mutant (figures 2 and 3a). At any single overall particle concentration, the concentration of polymers needed to cause phase separation is sharply defined, as this is a thermodynamic phase transition point (much like the way the melting and boiling points of a molecular substance are sharply defined).
We studied the phase behaviour of different concentrations of exoY mutant upon the addition of succinoglycan purified from the supernatant of an exoS mutant culture by ultrafiltration and proteinase K treatment. exoY mutant cells were harvested in late-exponential phase and diluted to concentrations ranging from 2.5 to 20 per cent (w/v) in PBS. Various amounts of succinoglycan were added and the samples were left to incubate for 24 h without shaking. The observed aggregation and phase behaviour are summarized in figure 5b. In this phase diagram, each symbol represents a sample of given composition, and shows whether the sample remained homogeneous (or, equivalently, single-phased), or whether it has separated out into coexisting upper and lower phases with low and high concentrations of bacteria after incubating for 24 h. The visual appearance of samples with various cell concentrations at the highest succinoglycan concentration investigated (0.05%) are shown in figure 5a side by side with corresponding samples with no succinoglycan for comparison.
Figure 5.
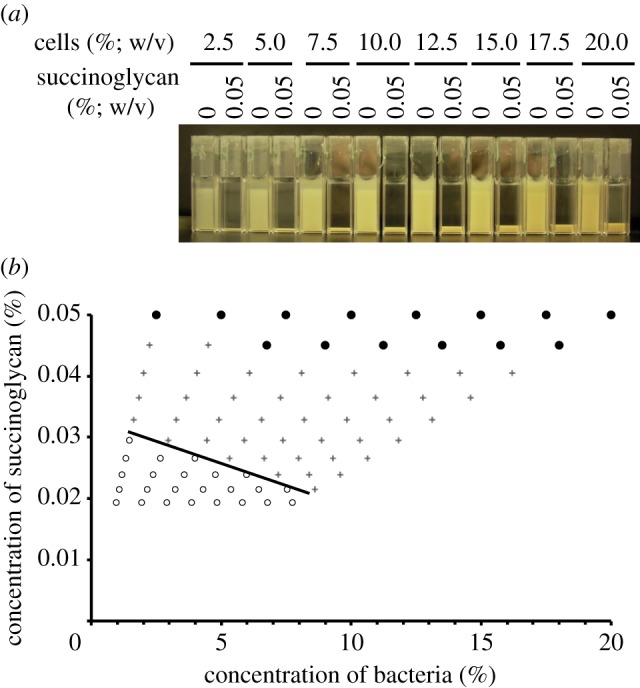
Application of succinoglycan to the Rm1021 exoY mutant leads to aggregation, which is stronger under crowded conditions. (a) Cultures of the exoY mutant were grown to late-exponential phase and then diluted to a range of concentrations with the addition of 0.05% (w/v) succinoglycan where indicated. exoY cultures with no succinoglycan were used as controls. Cultures were incubated for 24 h without shaking. (b) Cultures as shown in (a) were successively diluted. After each dilution, cultures containing succinoglycan were visually scored for extent of aggregation and compared with control cuvettes, after 24 h without shaking. Top row indicates results for cuvettes containing 0.05% succinoglycan as shown in (a). Filled circles indicate cultures that are aggregating and leave a clear upper phase; plus symbols indicate cultures where a sediment is still visible but the upper phase is now turbid; open circles indicate cultures that are now equal turbidity to the relevant control. Line is drawn as a guide to the eye. n = 3. (Online version in colour.)
The negative slope of the boundary separating samples that remained homogeneous from samples that have undergone significant sedimentation in figure 5b is significant: less succinoglycan is needed to cause autoaggregation in a sample with a higher concentration of cells, consistent with a crowding mechanism.
3.5. Application of xanthan to S. meliloti cultures and application of succinoglycan to E. coli cultures cause aggregation consistent with a crowding mechanism
Xanthan is a bacterial exopolysaccharide, and is structurally similar to succinoglycan, as both carry acidic groups and acetyl modifications. Thus, the addition of xanthan should bring about similar results to the addition of succinoglycan.
Experiments were performed as above, whereby various concentrations of S. meliloti exoY were incubated in PBS with xanthan (figure 6). Xanthan led to reversible autoaggregation (figure 6a). Again, at a higher concentration of cells, less xanthan is needed for autoaggregation to be observed (figure 6b).
Figure 6.
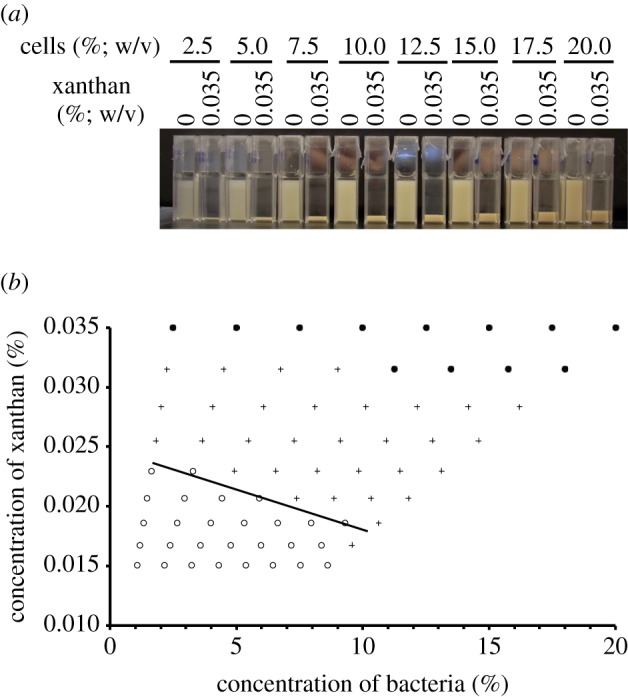
Application of xanthan also results in aggregation consistent with a crowding mechanism. (a) Cultures of the exoY mutant were grown to late-exponential phase and then diluted to a range of concentrations with the addition of 0.035% (w/v) xanthan where indicated. exoY cultures with no xanthan were used as controls. Cultures were incubated for 24 h without shaking. (b) Cultures as shown in (a) were successively diluted. After each dilution, cultures containing xanthan were visually scored for extent of aggregation and compared with control cuvettes, after 24 h without shaking. Top row indicates results for cuvettes as shown in (a). Filled circles indicate cultures that are aggregating and leave a clear upper phase; plus symbols indicate cultures where a sediment is still visible but the upper phase is now turbid; open circles indicate cultures that are now equal turbidity to the relevant control. Line is drawn as a guide to the eye. n = 3. (Online version in colour.)
To test whether the effect of succinoglycan was unique to S. meliloti, we applied purified succinoglycan to E. coli MG1655 cultures. Interestingly, the effect of succinoglycan on phase separation of the cultures was dependent on the growth phase of the MG1655 cultures; phase separation of late-exponential phase cultures was more variable than had been seen with S. meliloti, with the upper phase of the cultures often remaining more turbid (figure 7a). Escherichia coli cultures grown to stationary phase did, however, phase separate in the presence of succinoglycan (figure 7a). A large number of changes may occur to bacteria in the transition from exponential to stationary phase. Given the results we had seen with the S. meliloti exoS mutant, we focused on the presence of flagella. We constructed an MG1655 fliF mutant, and found that, unlike the parent strain MG1655, when the E. coli MG1655 fliF mutant was grown to late-exponential phase, the cultures did phase separate in the presence of succinoglycan (figure 7a). Phase diagrams of both stationary phase E. coli MG1655 and the E. coli MG1655 fliF mutant showed that the limiting polymer concentration for phase separation decreased with increasing cell concentration, again consistent with depletion attraction (figure 7b).
Figure 7.
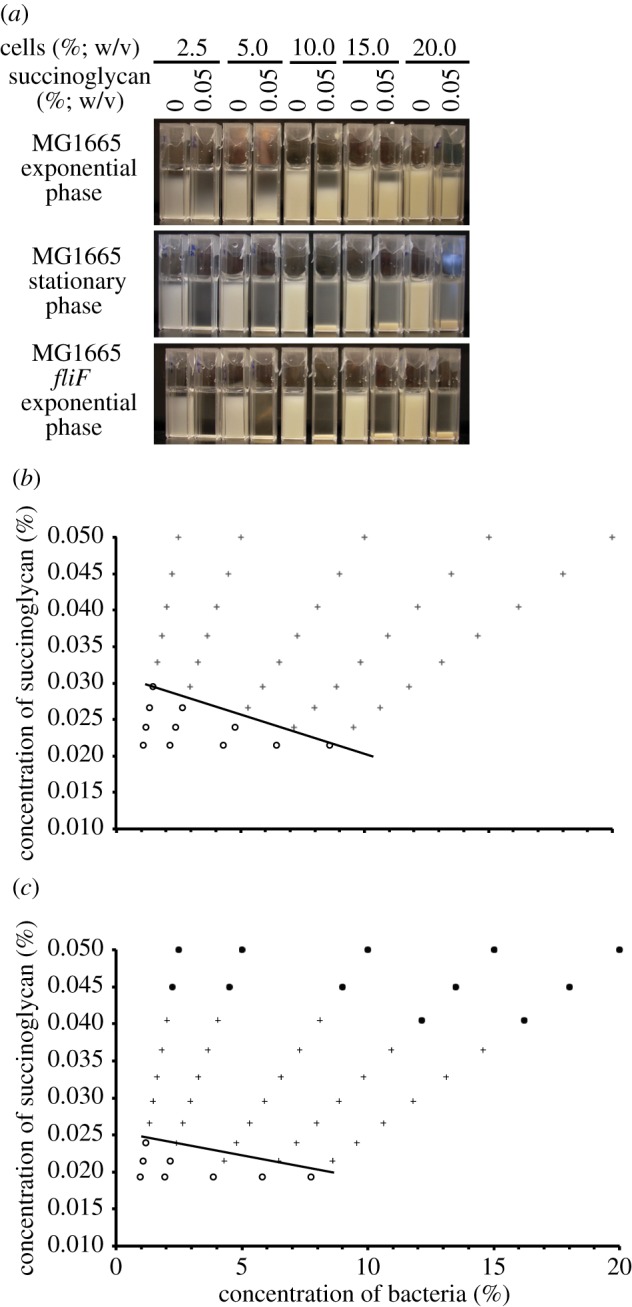
Addition of succinoglycan to E. coli results in aggregation consistent with a crowding mechanism. (a) Cultures of E. coli MG1655 or a MG1655 fliF mutant were grown to late-exponential phase or stationary phase as indicated and then diluted to a range of concentrations with the addition of 0.05% (w/v) succinoglycan where indicated. Cultures with no succinoglycan were used as controls. Cultures were incubated for 24 h without shaking. (b,c) Escherichia coli MG1655 stationary phase cultures (b) and MG1655 fliF mutant late exponential phase cultures (c) were prepared as shown in (a) and successively diluted. After each dilution, cultures containing succinoglycan were visually scored for extent of aggregation and compared with control cuvettes, after 24 h without shaking. Top row indicates results for cuvettes as shown in (a). Filled circles indicate cultures that are aggregating and leave a clear upper phase; plus symbols indicate cultures where a sediment is still visible but the upper phase is now turbid; open circles indicate cultures that are now equal turbidity to the relevant control. Lines are guides to the eye. (Online version in colour.)
3.6. The sediment formed by the exoS aggregates has a biofilm-like structure
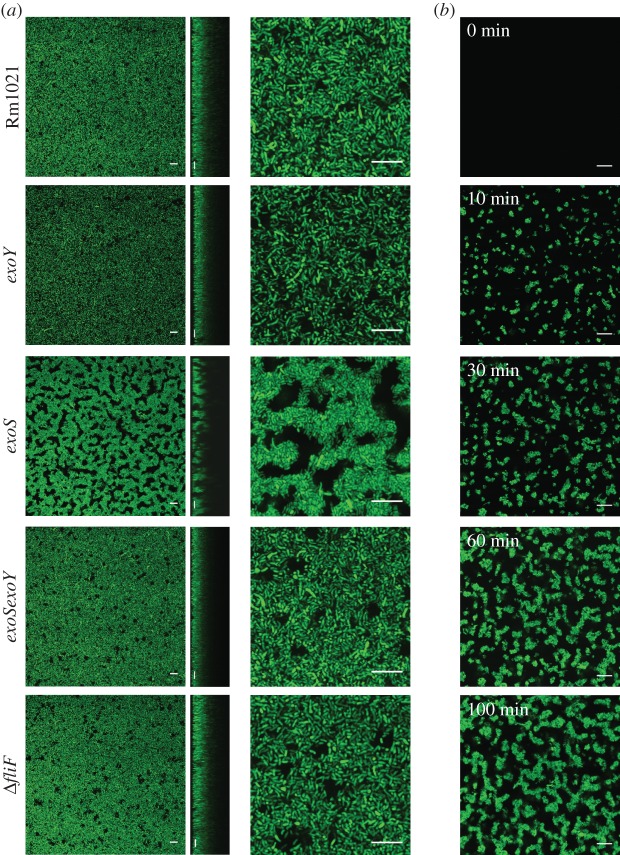
Confocal microscopy has been extensively used to image bacterial biofilms on surfaces. In studies on rhizobial biofilms, many have used chambered coverslides, which consist of a coverslip with culture chambers mounted on top, that are imaged using an inverted confocal microscope [11,46,47]. We used the same arrangement in our work as it allowed the visualization of the sediment that formed as bacteria aggregate and sediment.
Rm1021 and the exoY, exoS, exoSexoY and ΔfliF mutants, all expressing GFP, were grown to late-exponential phase, transferred to chambered coverslides, incubated without shaking for 24 h and then imaged. The sediment formed by the exoS mutant was significantly more inhomogeneous than that formed by the other strains (figure 8a). The aggregated cells were separated by empty spaces similar to the ‘water channels’ that have been described in biofilms for other species. There is a predominance of lateral (side-by-side) aggregation within the structure, as had been observed by phase contrast microscopy of individual aggregates from the exoS culture (figures 3b and 8). These inhomogeneities are similar to those described for the biofilms of other species. In contrast, Rm1021 and the exoY, exoSexoY and ΔfliF mutants all formed a flat lawn of cells with little apparent structure. Moreover, the cells in these sediments were observed to undergo Brownian motion (small random displacements), whereas cells in the exoS sediment were more firmly fixed to each other and the surface.
Figure 8.
The sediment formed by aggregation of the exoS mutant has a biofilm-like structure. (a) The indicated strains, expressing GFP, were grown to late-exponential phase in LBMC, transferred to chambered coverslides, incubated without shaking for 24 h and then imaged by confocal microscopy. As the coverslip forms the base of the incubation chamber, this allows imaging of the sediment that forms through aggregation. Left and right, x–y images; middle, x–z. (b) Cultures of the exoS mutant were grown to late-exponential phase in LBMC and then transferred to chambered coverslides on a confocal microscope. Images were taken from the base of the chamber at the indicated times. (a,b) Scale bars, 10 μm. (Online version in colour.)
To observe the formation of the biofilm-like structure over time, late-exponential phase cultures of the exoS mutant were transferred to the chambered coverslides and then imaged immediately (figure 8b). Small aggregates started to form on the coverslip almost immediately. Sedimentation and accumulation of these small aggregates at the base of the chamber eventually lead to a mature biofilm-like structure as seen in figure 8a.
4. Discussion
In this study, we have shown that, when S. meliloti overproduces succinoglycan, the cells can aggregate in the bulk and also form biofilm-like structures on surfaces by depletion attraction. This mechanism does not require the formation of a sticky extracellular matrix, but is instead the result of the crowding effects originating from the volume exclusion between the cells and the polysaccharide. We found that, as the concentration of S. meliloti cells in a culture is increased, less succinoglycan is required to induce aggregation and rapid sedimentation of the sample. This rules out bridging owing to stickiness as the causative mechanism. In the bridging process, a larger number of cells require a correspondingly larger number of polymer bridges. In contrast, in depletion, the polymer molecules (succinoglycan) push out the cells by ‘crowding’; thus, at higher cell concentration, less polymer is needed for phase separation, as observed here (figure 5b) and in model systems in which the operation of the depletion mechanism is not in doubt (for review, see [45]).
For completeness, we note the presence of cells in our culture that are still in the process of dividing, and as such have not fully separated (see figure 3c). For the purposes of depletion physics, these ‘twins’ behave as cells that are twice as long, giving a bimodal length distribution. It is known that the physics of colloid-rod mixtures with bimodal length distributions, where the longer component is less than approximately three times the length of the shorter component, is not qualitatively different from that of a system in which the length distribution is monomodal [48].
Depletion attraction may be a widespread phenomenon in bacteria producing exopolysaccharides. Exopolysaccharides are secreted into the medium [19,20]; therefore, in a laboratory or industrial culture or in a natural confined environment, a high concentration of non-adsorbing polymers can accumulate. Furthermore, in the majority of bacterial species, the outer cell surface carries a net negative charge, militating against electrostatic attraction with the majority of exopolysaccharides, which are anionic polymers [19]. Under these circumstances, depletion attraction between the cells is inevitable. We have previously demonstrated this to be the case using synthetic polymers: the addition of high enough concentrations of sodium polystyrene sulphonate (an anionic polyelectrolyte) to cultures of E. coli indeed brought about depletion-driven autoaggregation and rapid sedimentation [49,50]. In the present work, we have also shown that addition of a different bacterial exopolysaccharide, xanthan, to S. meliloti cultures results in similar phenomenology that is consistent with depletion, lending further support to the hypothesis that depletion attraction may be ubiquitous in bacterial cultures.
As part of this mechanism, the cells form an ordered structure of laterally aligned cells, which enhances the depletion attraction. The ability of depletion attraction to provide a mechanism for self-organization has been shown to have a role in the organization of organelles in eukaryotes [51] and the stacking of thylakoid membranes in the chloroplasts of plant cells [52]. However, we found that, as well as forming aggregates in the bulk, the exoS mutant can form structures similar to the biofilms described in other species, suggesting that depletion attraction can also result in the organization of cells into biofilm-like structures. We used chambered coverslides, whereby the base of the culture chamber forms a coverslip, to visualize biofilm formation, as this allowed us to visualize aggregates as they sedimented. Chambered coverslides have also been used in studies of R. leguminosarum [11], S. meliloti [46] and X. campestris [47] biofilms. We found that the exoS mutant forms an inhomogeneous structure, consisting of interlinked aggregates separated by gaps that are characteristic of the ‘water channels’ described in many biofilms. The speed with which aggregates appeared on the base of the chamber suggests that depletion attraction may also drive the interaction between cells and a surface as well as between surfaces of cells, as these initial aggregates are unlikely to be due to sedimentation. We calculate that a single cell sediments only approximately 20 μm in 10 min (appendix A). Instead, initial aggregates are probably the consequence of the depletion attraction between cells and the flat surface of the bottom of our observation chamber. The depletion mechanism giving rise to an effective attraction between two cells also operates between a cell and a flat surface (for the colloidal analogue, see [53]). This attraction leads to a layer of cells at the surface with enhanced density. The usual depletion mechanism between cells still operates within this layer, and can give rise to aggregation within the surface layer of cells [54]. This is probably the mechanism for the formation of the initial surface inhomogeneities observed in figure 8b. Sedimentation of aggregates formed in the bulk then contributes to the later build up of the biofilm-like structures formed by the exoS mutant as shown in figure 8a. We are now carrying out further experiments to test this hypothesis.
The role of flagella in this process remains unclear. When exoS supernatant or succinoglycan was added to Rm1021 cultures, cells could be observed swimming between aggregates (data not shown), suggesting that motile cells may be able to overcome the depletion attraction and leave the aggregates. Sinorhizobium meliloti possesses up to five peritrichous flagella, and a study of S. meliloti L5–30 suggested that up to 40 per cent of cells did not possess flagella in exponential phase, with the number increasing to over 60 per cent by stationary phase [55]. It is interesting that the ExoS protein positively controls succinoglycan biosynthesis while negatively regulating flagella biosynthesis. It may be that the aggregation of cells by production of succinoglycan and depletion attraction requires downregulation of flagella. This hypothesis is supported by the data from adding succinoglycan to E. coli MG1655 cultures. In this case, E. coli in late-exponential phase separated in the presence of succinoglycan, but with a turbid upper phase still present, whereas in S. meliloti there was a clear upper phase. However, adding succinoglycan to stationary phase cultures of E. coli MG1655 gave phase separation more consistent with what is observed with S. meliloti. Flagella are known to be downregulated in the transition to stationary phase [56]. Consistent with this, we found that an E. coli MG1655 mutant that lacks flagella phase separated in the presence of succinoglycan in late-exponential phase, suggesting that flagella may have a prominent role in depletion-driven bacterial aggregation, just as has been suggested for biofilm formation generally. Indeed, very recently, experiments, theory and simulation show that the presence of flagella-driven motility weakens the depletion interaction, so that more polymers are needed to cause phase separation; the flagella themselves may also serve as ‘steric stabilization’, hindering the formation of large clusters [57]. The reasons for the difference in behaviour of late-exponential phase S. meliloti and E. coli in the presence of succinoglycan are still being investigated. It is possible that S. meliloti cultures possess a greater proportion of non-flagellated cells that provide nuclei on which further aggregation can occur.
Many species of bacteria live in confined environments (water droplets, marine detritus, inside host cells, etc.) as part of their life cycles. If these species secrete exopolysaccharides, then the depletion mechanism will inevitably come into operation as polymers accumulate. Future studies will address the role of this potentially ubiquitous phenotype of depletion-induced aggregation in bacterial biology.
Acknowledgements
We thank Sarah Spragg for assistance in making the exoSexoY mutant, Sebastian Beck for help with exopolysaccharide isolation and helpful discussions and Graham C. Walker for providing S. meliloti strains. G.D. was funded by an EPSRC studentship, and W.C.K.P. was supported by EPSRC grant EP/D071070/1. Conceived and designed the experiments: G.D., G.P.F., C.E.F., W.C.K.P. Performed the experiments: G.D. Analysed the data: G.D., C.E.F, W.C.K.P. Contributed reagents/materials/analysis tools: G.P.F., C.E.F., W.C.K.P. Wrote the paper: G.D., W.C.K.P.
APPENDIX A. Single-cell sedimentation speed
An ellipsoid of semi-minor and semi-major axes a and b, respectively, translating in a liquid of viscosity η at speed u along its major axis experiences a drag force f given by fD = ξu, where the ‘friction coefficient’ ξ is given by [58]
We model individual S. meliloti cells as ellipsoids with a = 0.35 μm and b = 0.85 μm, so that the denominator in ξ is very close to unity. We therefore use the approximation ξ ≈ 4πηb. We expect an ellipsoid to sediment with its long axis parallel to gravity. In the steady state, the drag force is balanced by the gravitational force on the cell, which is given by fG = ΔρVg, where g = 9.8 m s−2 is the acceleration due to gravity, V = 4πa2b/3 is the volume of the cell modelled as an ellipsoid and Δρ ≈ 80 kg m−3 [59] is the density difference between the cell and the buffer. Equating fD and fG, we obtain the sedimentation speed
 |
which works out to be 33 nm s−1 using parameters already quoted.
References
- 1.Deinema M. H., Zevenhuizen L. P. 1971. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch. Mikrobiol. 78, 42–51 10.1007/BF00409087 (doi:10.1007/BF00409087) [DOI] [PubMed] [Google Scholar]
- 2.Friedman B. A., Dugan P. R. 1968. Identification of Zoogloea species and the relationship to zoogloeal matrix and floc formation. J. Bacteriol. 95, 1903–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman B. A., Dugan P. R., Pfister R. M., Remsen C. C. 1969. Structure of exocellular polymers and their relationship to bacterial flocculation. J. Bacteriol. 98, 1328–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinney R. E. 1953. Staining bacterial polysaccharides. J. Bacteriol. 66, 453–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney R. E., Weichlein R. G. 1953. Isolation of floc-producing bacteria from activated sludge. Appl. Microbiol. 1, 259–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavoni J. L., Tenney M. W., Echelberger W. F., Jr 1972. Bacterial exocellular polymers and biological flocculation. J. Water Pollut. Control Fed. 44, 414–429 [PubMed] [Google Scholar]
- 7.Matthysse A. G. 1987. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J. Bacteriol. 169, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthysse A. G. 1983. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J. Bacteriol. 154, 906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthysse A., Marry M., Krall L., Kaye M., Ramey B., Fuqua C., White A. 2005. The effect of cellulose overproduction on binding and biofilm formation on roots by Agrobacterium tumefaciens. Mol. Plant Microbe Interact. 18, 1002–1010 10.1094/MPMI-18-1002 (doi:10.1094/MPMI-18-1002) [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson A. D., Ramey-Hartung B., Day T. W., Merritt P. M., Fuqua C. 2010. Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiology (UK) 156, 2670–2681 10.1099/mic.0.039032-0 (doi:10.1099/mic.0.039032-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo D. M., Williams A., Edwards A., Posadas D. M., Finnie C., Dankert M., Downie J. A., Zorreguieta A. 2006. Proteins exported via the PrsD–PrsE type I secretion system and the acidic exopolysaccharide are involved in biofilm formation by Rhizobium leguminosarum. J. Bacteriol. 188, 4474–4486 10.1128/JB.00246-06 (doi:10.1128/JB.00246-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smit G., Kijne J. W., Lugtenberg B. J. 1986. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J. Bacteriol. 168, 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smit G., Kijne J. W., Lugtenberg B. J. 1987. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J. Bacteriol. 169, 4294–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dow J. M., Crossman L., Findlay K., He Y.-Q., Feng J.-X., Tang J.-L. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc. Natl Acad. Sci. USA 100, 10 995–11 000 10.1073/pnas.1833360100 (doi:10.1073/pnas.1833360100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujishige N. A., Kapadia N. N., De Hoff P. L., Hirsch A. M. 2006. Investigations of Rhizobium biofilm formation. FEMS Microbiol. Ecol. 56, 195–206 10.1111/j.1574-6941.2005.00044.x (doi:10.1111/j.1574-6941.2005.00044.x) [DOI] [PubMed] [Google Scholar]
- 16.Sorroche F. G., Rinaudi L. V., Zorreguieta A., Giordano W. 2010. EPS II-dependent autoaggregation of Sinorhizobium meliloti planktonic cells. Curr. Microbiol. 61, 465–470 10.1007/s00284-010-9639-9 (doi:10.1007/s00284-010-9639-9) [DOI] [PubMed] [Google Scholar]
- 17.Wilking J. N., Angelini T. E., Seminara A., Brenner M. P., Weitz D. A. 2011. Biofilms as complex fluids. MRS Bull. 36, 385–391 10.1557/mrs.2011.71 (doi:10.1557/mrs.2011.71) [DOI] [Google Scholar]
- 18.Harris R. H., Mitchell R. 1973. The role of polymers in microbial aggregation. Annu. Rev. Microbiol. 27, 27–50 10.1146/annurev.mi.27.100173.000331 (doi:10.1146/annurev.mi.27.100173.000331) [DOI] [PubMed] [Google Scholar]
- 19.Sutherland I. W. 1990. Biotechnology of microbial exopolysaccharides. Cambridge: Cambridge University Press [Google Scholar]
- 20.Whitfield C. 1988. Bacterial extracellular polysaccharides. Can. J. Microbiol. 34, 415–420 10.1139/m88-073 (doi:10.1139/m88-073) [DOI] [PubMed] [Google Scholar]
- 21.Åman P., McNeil M., Franzén L.-E., Darvill A. G., Albersheim P. 1981. Structural elucidation, using h.p.l.c.-m.s. and g.l.c.-m.s., of the acidic polysaccharide secreted by Rhizobium meliloti strain 1021. Carbohydr. Res. 95, 263–282 10.1016/S0008-6215(00)85582-2 (doi:10.1016/S0008-6215(00)85582-2) [DOI] [Google Scholar]
- 22.Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149, 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuber T. L., Walker G. C. 1993. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74, 269–280 10.1016/0092-8674(93)90418-P (doi:10.1016/0092-8674(93)90418-P) [DOI] [PubMed] [Google Scholar]
- 24.Zhan H. J., Leigh J. A. 1990. Two genes that regulate exopolysaccharide production in Rhizobium meliloti. J. Bacteriol. 172, 5254–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty D., Leigh J. A., Glazebrook J., Walker G. C. 1988. Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J. Bacteriol. 170, 4249–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buendia A. M., Enenkel B., Köplin R., Niehaus K., Arnold W., Pühler A. 1991. The Rhizobium meliloti exoZl exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose 4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar viciae strain TOM. Mol. Microbiol. 5, 1519–1530 10.1111/j.1365-2958.1991.tb00799.x (doi:10.1111/j.1365-2958.1991.tb00799.x) [DOI] [PubMed] [Google Scholar]
- 27.Reuber T. L., Walker G. C. 1993. The acetyl substituent of succinoglycan is not necessary for alfalfa nodule invasion by Rhizobium meliloti Rm1021. J. Bacteriol. 175, 3653–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh J. A., Reed J. W., Hanks J. F., Hirsch A. M., Walker G. C. 1987. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 51, 579–587 10.1016/0092-8674(87)90127-9 (doi:10.1016/0092-8674(87)90127-9) [DOI] [PubMed] [Google Scholar]
- 29.York G. M., Walker G. C. 1997. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol. Microbiol. 25, 117–134 10.1046/j.1365-2958.1997.4481804.x (doi:10.1046/j.1365-2958.1997.4481804.x) [DOI] [PubMed] [Google Scholar]
- 30.Guyer M. S., Reed R. R., Steitz J. A., Low K. B. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb. Symp. Quant. Biol. 45, 135–140 10.1101/SQB.1981.045.01.022 (doi:10.1101/SQB.1981.045.01.022) [DOI] [PubMed] [Google Scholar]
- 31.Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.008 10.1038/msb4100050 (doi:10.1038/msb4100050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J. 2001. Molecular cloning: a laboratory manual, 3rd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- 33.Long S., Reed J. W., Himawan J., Walker G. C. 1988. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J. Bacteriol. 170, 4239–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J. H. (ed.) 1972. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 35.Biggs C. A., Lant P. A. 2000. Activated sludge flocculation: on-line determination of floc size and the effect of shear. Water Res. 34, 2542–2550 10.1016/S0043-1354(99)00431-5 (doi:10.1016/S0043-1354(99)00431-5) [DOI] [Google Scholar]
- 36.Rasband W. 2008. ImageJ. See http://rsb.info.nih.gov/ij/. [Google Scholar]
- 37.Cheng H. P., Walker G. C. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180, 5183–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loewus F. A. 1952. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 24, 219 10.1021/ac60061a050 (doi:10.1021/ac60061a050) [DOI] [Google Scholar]
- 39.Tuinier R., de Kruif C. G. 1999. Phase separation, creaming, and network formation of oil-in-water emulsions induced by an exocellular polysaccharide. J. Colloid Interface Sci. 218, 201–210 10.1006/jcis.1999.6327 (doi:10.1006/jcis.1999.6327) [DOI] [PubMed] [Google Scholar]
- 40.Cheng H. P., Walker G. C. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS–ChvI two-component regulatory system. J. Bacteriol. 180, 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao S.-Y., Luo L., Har K. J., Becker A., Rüberg S., Yu G.-Q., Zhu J.-B., Cheng H.-P. 2004. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J. Bacteriol. 186, 6042–6049 10.1128/JB.186.18.6042-6049.2004 (doi:10.1128/JB.186.18.6042-6049.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller P., Keller M., Weng W. M., Quandt J., Arnold W., Pühler A. 1993. Genetic analysis of the Rhizobium meliloti exoYFQ operon: ExoY is homologous to sugar transferases and ExoQ represents a transmembrane protein. Mol. Plant Microbe Interact. 6, 55–65 [DOI] [PubMed] [Google Scholar]
- 43.York G. M., Walker G. C. 1998. The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J. Bacteriol. 180, 4184–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadasivan L., Neyra C. A. 1985. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J. Bacteriol. 163, 716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poon W. C. K. 2002. The physics of a model colloid polymer mixture. J. Phys. Condens. Matter 14, R859–R880 10.1088/0953-8984/14/33/201 (doi:10.1088/0953-8984/14/33/201) [DOI] [Google Scholar]
- 46.Rinaudi L. V., González J. E. 2009. The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J. Bacteriol. 191, 7216–7224 10.1128/JB.01063-09 (doi:10.1128/JB.01063-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres P. S., et al. 2007. Controlled synthesis of the DSF cell–cell signal is required for biofilm formation and virulence in Xanthomonas campestris. Environ. Microbiol. 9, 2101–2109 10.1111/j.1462-2920.2007.01332.x (doi:10.1111/j.1462-2920.2007.01332.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vroege G. J., Lekkerkerker H. N. W. 1993. Theory of the isotropic–nematic–nematic phase separation for a solution of bidisperse rodlike particles. J. Phys. Chem. 97, 3601–3605 10.1021/j100116a026 (doi:10.1021/j100116a026) [DOI] [Google Scholar]
- 49.Schwarz-Linek J., Dorken G., Winkler A., Wilson L. G., Pham N. T., French C. E., Schilling T., Poon W. C. K. 2010. Polymer-induced phase separation in suspensions of bacteria. Europhys. Lett. 89, 68003 10.1209/0295-5075/89/68003 (doi:10.1209/0295-5075/89/68003) [DOI] [Google Scholar]
- 50.Schwarz-Linek J., Winkler A., Wilson L. G., Pham N. T., Schilling T., Poon W. C. K. 2010. Polymer-induced phase separation in Escherichia coli suspensions. Soft Matter 6, 4540–4549 10.1039/C0SM00214C (doi:10.1039/C0SM00214C) [DOI] [Google Scholar]
- 51.Marenduzzo D., Finan K., Cook P. R. 2006. The depletion attraction: an underappreciated force driving cellular organization. J. Cell Biol. 175, 681–686 10.1083/jcb.200609066 (doi:10.1083/jcb.200609066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim E.-H., Chow W. S., Horton P., Anderson J. M. 2005. Entropy-assisted stacking of thylakoid membranes. Biochim. Biophys. Acta 1708, 187–195 10.1016/j.bbabio.2005.03.011 (doi:10.1016/j.bbabio.2005.03.011) [DOI] [PubMed] [Google Scholar]
- 53.Brader J. M., Dijkstra M., Evans R. 2001. Inhomogeneous model colloid–polymer mixtures: adsorption at a hard wall. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 63, 041405 10.1103/PhysRevE.63.041405 (doi:10.1103/PhysRevE.63.041405) [DOI] [PubMed] [Google Scholar]
- 54.Dinsmore A. D., Warren P. B., Poon W. C. K., Yodh A. G. 1997. Fluid–solid transitions on walls in binary hard-sphere mixtures. Europhys. Lett. (EPL) 40, 337–342 10.1209/epl/i1997-00468-4 (doi:10.1209/epl/i1997-00468-4) [DOI] [Google Scholar]
- 55.Caetano-Anollés G., Wall L. G., De Micheli A. T., Macchi E. M., Bauer W. D., Favelukes G. 1988. Role of motility and chemotaxis in efficiency of nodulation by Rhizobium meliloti. Plant Physiol. 86, 1228–1235 10.1104/pp.86.4.1228 (doi:10.1104/pp.86.4.1228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pesavento C., Becker G., Sommerfeldt N., Possling A., Tschowri N., Mehlis A., Hengge R. 2008. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev. 22, 2434–2446 10.1101/gad.475808 (doi:10.1101/gad.475808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz-Linek J., Valeriani C., Cacciuto A., Cates M. E., Marenduzzo D., Morozov A. N., Poon W. C. K. 2012. Phase separation and rotor self-assembly in active particle suspensions. Proc. Natl Acad. Sci. USA 109, 4052–4057 10.1073/pnas.1116334109 (doi:10.1073/pnas.1116334109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg H. C. 1993. Random walks in biology. Princeton, NJ: Princeton University Press [Google Scholar]
- 59.Bailey J. E., Ollis D. F. 1986. Biochemical engineering fundamentals. New York, NY: McGraw-Hill [Google Scholar]