Abstract
Severe heat-shock to bone cells caused during orthopaedic procedures can result in thermal damage, leading to cell death and initiating bone resorption. By contrast, mild heat-shock has been proposed to induce bone regeneration. In this study, bone cells are exposed to heat-shock for short durations occurring during surgical cutting. Cellular viability, necrosis and apoptosis are investigated immediately after heat-shock and following recovery of 12, 24 h and 4 days, in osteocyte-like MLO-Y4 and osteoblast-like MC3T3-E1 cells, using flow cytometry. The regeneration capacity of heat-shocked Balb/c mesenchymal stem cells (MSCs) and MC3T3-E1s has been investigated following 7 and 14 day's recovery, by quantifying proliferation, differentiation and mineralization. An immediate necrotic response to heat-shock was shown in cells exposed to elevated temperatures (45°C, 47°C and most severe at 60°C). A longer-term apoptotic response is induced in MLO-Y4s and, to a lesser extent, in MC3T3-E1s. Heat-shock-induced differentiation and mineralization by MSCs. These findings indicate that heat-shock is more likely to induce apoptosis in osteocytes than osteoblasts, which might reflect their role as sensors detecting and communicating damage within bone. Furthermore, it is shown for the first time that mild heat-shock (less than equal to 47°C) for durations occurring during surgical cutting can positively enhance osseointegration by osteoprogenitors.
Keywords: thermal necrosis, apoptosis, heat-shock, bone, surgical cutting, mineralization
1. Introduction
Surgical procedures, such as brain, orthopaedic joint replacement and spinal surgery, critically rely on specialized instrumentation to provide surgeons with access to bones and organs of the body. Such procedures require surgeons to cut through bone tissue to gain access to underlying organs and joints, while causing minimal damage [1]. During surgical cutting, bone and surrounding soft tissues are exposed to mechanical abrasion and elevated temperatures, which are generated by the cutting process. Elevated temperatures lead to thermal necrosis and apoptosis of bone cells, as well as damage to surrounding soft tissue, bone marrow and cells crucial for post-operative healing [2–6]. As a direct response to necrotic and apoptotic cellular death [2], thermally damaged bone tissue and cells are subsequently resorbed [5–8], and as such, temperature elevations are likely to affect patient outcome by delaying healing [3–5,7]. It has been shown that post-surgical healing of osteotomies in rabbit tibia is largely dependent on the duration of cutting, which is tightly linked with the degree of temperature elevation [8–10], and that in vivo remodelling of rabbit tibial bone exposed to 53°C within a thermal chamber for 1 min did not begin until three to five weeks after heat injury [4].
Elevated temperatures during orthopaedic surgery induce bone tissue damage, the extent of which is dependent on the temperature itself and the duration of exposure [3–7,11]. It is generally accepted that the threshold for the occurrence of morphological bone tissue damage is 47°C for 1 min. Longer durations and higher temperatures result in irreversible damage to bone, which is subsequently resorbed and replaced with connective tissue [5]. Depending on the extent of heat-shock, responses are triggered at the cellular level, leading to cell death by either necrosis or apoptosis. Cellular necrosis is the immediate response to severe injury occurring within minutes of cellular insult and is characterized by cell swelling and loss of membrane integrity, ultimately triggering an inflammatory response. Apoptosis is a highly regulated and coordinated process beginning hours after injury, involving the systemic disassembly of the structural and functional components of the cell, which are packaged into membrane-bound vesicles and phagocytosed, eliminating an associated inflammatory response [2,12–14]. It has been reported that rat calvarial osteoblasts exposed to severe heat-shock (48°C for 10 min) demonstrated irreversible responses of cell necrosis and apoptosis after 12 h recovery, whereas less severe insult (more than or equal to 45°C for 10 min) lead to transient reversible responses such as disruption of the actin cytoskeleton [2]. However, continuous surgical cutting of bone rarely lasts more than 1 min and the precise effect of thermal elevations for such short durations have not been investigated. Therefore, there is a distinct need for a profile of cellular viability, necrosis and apoptosis for time and temperature combinations that occur during surgical cutting procedures.
It has been reported that exposure of human MSCs to mild thermal elevations, 42.5°C for 1 h, enhanced the extent of osteoblastic differentiation by up to 42 per cent [15]. Furthermore, conditioned media from heat-treated (42°C for 1 h) human foetal osteoblasts promoted osteogenesis of MSCs and was accredited to secreted factors in the heat-treated media [16]. Such studies suggest that slight temperature elevation for long durations can actually induce a positive cellular response, enhancing tissue regeneration. Whether such responses occur during the shorter durations of temperature elevation experienced during surgical cutting is unknown.
The ideal outcome of surgical cutting is to remove bone tissue while minimizing cellular damage, but also to leave a cut surface that is favourable for cell attachment and new matrix deposition to facilitate effective healing post-operatively [1] or formation of a strong bond between the implant and surrounding bone (osseointegration). Unfavourable outcomes include connective tissue formation around the implant, which can affect the long-term anchorage of the implant by delaying formation of a mature bone interface [5,7,17,18]. To date, the optimal cutting criteria to minimize thermal necrosis and apoptosis and to synergistically enhance tissue regeneration by inducing mineralization are unknown.
The objectives of this study were to expose MSCs, osteoblasts and osteocytes to elevated temperatures for the durations that occur during orthopaedic cutting procedures in order to investigate: (i) the immediate and long-term effects of heat-shock on bone cell necrosis, apoptosis and viability; and (ii) the regeneration capability of osteoprogenitor cells following heat-shock.
2. Material and methods
2.1. Cell culture
Two bone cell-lines (osteocyte-like MLO-Y4 and osteoblast-like MC3T3-E1 cells) and Balb/c mouse MSCs were used in this study. Murine-derived MLO-Y4 cells (a gift from Prof. Lynda Bonewald, San Antonio) possess many similar characteristics with primary osteocytes such as low expression of alkaline phosphatase (ALP) and producing numerous dendritic processes [19]. MLO-Y4 cell cultures were maintained on collagen-coated flasks in α-modified minimal essential medium (α-MEM) supplemented with 2.5 per cent foetal bovine serum (FBS, HyClone Laboratories Inc.), 2.5 per cent iron-supplemented calf serum (FCS, HyClone Laboratories Inc.), 2 mM l-glutamine, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin. MC3T3-E1 osteoblast-like cells are a good model for osteoblast studies, as they express high amounts of ALP, produce minerals and have the ability to differentiate into osteocytes [20]. MC3T3-E1 cultures were maintained in α-MEM supplemented with 5 per cent FBS, 2 mM l-glutamine, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin.
Balb/c primary MSC cultures were obtained and characterized according to the protocols of Peister et al. and as previously described [21,22]. Briefly, femurs and tibiae were removed from 8–10-week old female and male mice, and cultured in RPMI-1640 medium supplemented with 9 per cent FBS, 9 per cent horse serum (HS), 100 U ml−1 penicillin, 100 g ml−1 streptomycin and 2 M l-glutamine. The ends of the bones were clipped off, and bones were centrifuged at 400g for 2 min. The cell pellets were plated onto a T175 flask for 24 h, following which the flask was washed with sterile phosphate-buffered solution (PBS). Media were replenished and cells were cultured until large colonies were observed (approx. 4 days), after which cells were replated and cultured for a further 10 days. Balb/c MSCs for experiments were maintained in Iscoves MEM supplemented with 10 per cent FBS, 10 per cent HS, 2 mM l-glutamine, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin until confluent. The osteogenic, chondrogenic and adipogenic potential of these MSCs was confirmed as previously described [21].
MC3T3-E1s and MSCs were maintained in either expansion or osteogenic culture conditions to investigate the regeneration capacity following heat-shock. The expansion culture media were supplemented with 5 mM β-glycerophosphate, 50 μg ml−1 ascorbic acid and 10 nM dexamethasone to imitate osteogenic culture conditions.
All cells were maintained in 75 cm2 tissue culture flasks at 37°C in a 5 per cent CO2-humidified atmosphere. Once confluent, cells were detached using trypsin–EDTA, seeded at a density of 104 cells cm−2 and pre-incubated for 24 h prior to heat exposure experiments. All chemicals and reagents were purchased from Sigma-Aldrich, unless otherwise stated.
2.2. Heat exposure experiments
Balb/c MSC, MLO-Y4 and MC3T3-E1 cells were exposed to preheated media at either 37°C (control), 45°C, 47°C or 60°C, and maintained on a hot plate at these temperatures for 30 s or 1 min. On the basis of in vivo studies, 60°C was chosen to be an extreme case where widespread cellular death with limited recovery was expected to occur, whereas 47°C was taken as the threshold for cellular damage initiation [4,6]. The temperature accuracy of the hot plate is ±0.3°C, and the uniformity of temperature distribution on the surface of the plate is ±0.2°C. A thermocouple was inserted into an unused well to monitor the temperature of the preheated media, and this temperature measurement was accurate to ±1°C for the duration of exposure. After heat exposure, cells were returned to normal culture conditions in a CO2 incubator at 37°C and cultured for varying recovery periods of 0, 12, 24 h, 4, 7 and 14 days.
2.3. Fluorescent microscopy
After each recovery period, Balb/c MSC, MLO-Y4 and MC3T3-E1 cells exposed to heat-shock were fixed in 4 per cent paraformaldehyde for 15 min and permeabilized in 0.1 per cent Triton X-100 for 5 min. The actin cytoskeleton was stained by incubating the cell in PBS containing 1 per cent fluorescein isothiocyanate (FITC)-conjugated phalloidin for 45 min at room temperature. The coverslips were mounted with Vectashield mounting media with 4,6-diamidino-2-phenylindole (DAPI) nuclear counter stain. Fluorescence was visualized with an Olympus BX51 upright fluorescent microscope at 20× magnification.
2.4. Flow cytometry
Analysis of apoptosis and necrosis in cells immediately after exposure and following recovery periods of 12, 24 h and 4 days was performed by staining with propidium iodide (PI) and Annexin V-FITC (ImmunoTools GmbH, Germany) using a flow cytometer (BD FACS CANTO). PI stains cells that have lost membrane integrity (a feature of necrosis), whereas annexin V-FITC stains phosphatidylserine (PS) (a phospholipid that translocates from inside to outside the lipid membrane during apoptosis). Live cells are double negative for annexin V-FITC and PI, apoptotic cells are annexin V-FITC positive and PI negative, and advanced apoptotic and necrotic cells are positive for both annexin V-FITC and PI.
Floating cells were collected, and adherent cells were detached by incubation with trypsin-EDTA for 2 min. Cells were washed and resuspended in 500 μl of annexin V buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCL, 1 mM MgCl2, 1.8 mM CaCl2) [23]. Annexin V-FITC (7 μg ml−1) was added, and samples were incubated for 15 min on ice and in the dark. Subsequently, 4 μl of 20 μg ml−1 PI was added to each sample, vortexed briefly. Stained cells were subjected to flow cytometric analysis. Annexin V staining was detected in the FL1 channel, and PI was detected in the FL2 channel. A minimum of 10 000 events were acquired, and cell debris was excluded from analysis by appropriate forward and right angle scatter threshold settings.
2.5. Determination of alkaline phosphatase activity
ALP expression indicates differentiation along the osteoblastic lineage and is an early marker of mineralization. ALP content was quantified using a colorimetric assay that uses p-nitrophenyl phosphate (p-NPP) as a phosphate substrate. To quantify intracellular ALP, cell lysate was prepared using three cycles of freeze–thaw in deionized distilled water. Extracellular ALP secretion was analysed from samples of spent media collected from cells immediately after heat treatment and following recovery periods of 7 and 14 days, and stored at −80°C until use. A 40 μl sample of cell lysate or spent media was added to a 96-well plate, and the volume was brought up to 80 μl with deionized water (ddH2O), to which 50 μl of 5 mM p-NPP solution (Sigmafast p-NPP) was added. After incubation at room temperature for 1 h, absorbance was measured at 405 nm using an atomic absorbance spectrophotometer (Wallac Victor, PerkinElmer Life Science). Specific ALP activity was quantified against a standard curve of 0–80 nM p-NPP, and expressed as U ml−1.
2.6. Quantification of mineralization
After recovery periods of 7 and 14 days, MC3T3-E1 and Balb/c cells were washed gently with PBS and fixed with 4 per cent paraformaldehyde for 15 min. The mineralized matrix was quantified by alizarin red S staining methods [15,24]. Alizarin red S (1 mole) binds to two moles of calcium, forming an alizarin red S–calcium complex. Briefly, samples were stained with 40 mM alizarin red S (pH 4.2) for 15 min at room temperature on an orbital rotator. Samples were rinsed gently three times with distilled water to remove any unbound solution and then quantified by incubation with preheated 10% (wt/vol) cethylpyridium chloride for 20 min on an orbital rotator (Gyro-rocker SSL3, Stuart Scientific). Absorbance was measured at 562 nm using an atomic absorbance spectrophotometer (Wallac Victor). Using a standard curve, the absorption reading was converted to calcium ion concentration, and results were normalized to cell number to estimate calcium concentration per cell.
2.7. DNA content
DNA content was determined following recovery periods of 7 and 14 days, using a Hoechst DNA assay [25]. Briefly, cells were subjected to cell lysis by three cycles of freeze–thawing in molecular grade water. The Hoechst 33258 (Sigma) working dye solution was prepared by adding 20 ml of Hoechst buffer (100 mM Tris, 2 M NaCl and 10 mM EDTA) to 30 ml of dH2O and 5 µl of 1 mg ml−1 Hoechst dye, and the solution was protected from light. One hundred and forty microlitres of the cell lysate was mixed with 60 µl of Hoechst working solution and incubated in the dark for 10 min. The fluorescence was measured at an emission of 460 nm and an excitation of 365 nm using an atomic absorbance spectrophotometer (Wallac Victor). DNA content was determined by using a known amount of purified calf thymus DNA as the standard.
2.8. Statistics
Experiments were conducted in triplicate and repeated, giving n = 6 per group. Data are expressed as a mean ± s.d. Statistical differences between groups were determined using an ANOVA-crossed factor model, defined using the general linear model ANOVA function. Tukey's test method for comparison between treatments at each time point was used to determine statistical significance defined as p ≤ 0.05 (Minitab v. 16).
3. Results
3.1. Effects of heat-shock on cellular morphology
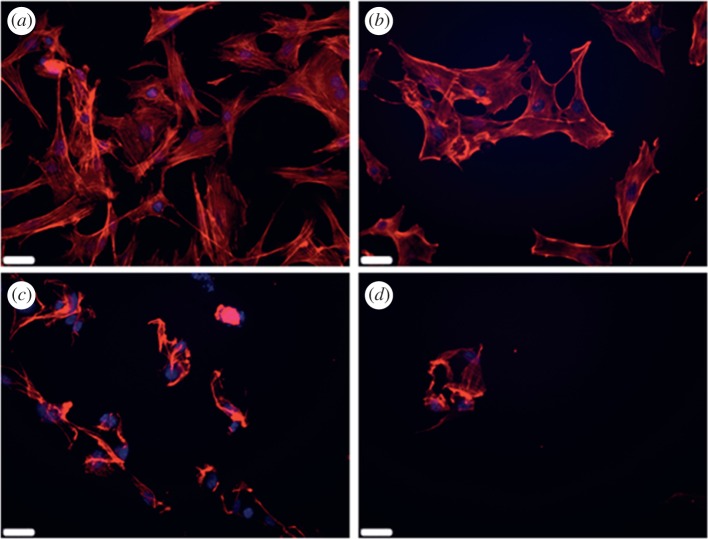
The effects of heat-shock on cell morphology and structure after recovery of 24 h can be seen clearly in figure 1. MC3T3-E1s cultured at 37°C (control) show a confluent cell layer with a spread morphology, characteristic of healthy cells. Cells exposed to 45°C for 1 min have a slightly reduced cell number, but the cells present have a spread morphology. Cells exposed to 47°C for 1 min show a reduced cell population and areas of highly concentrated staining in the cytoskeleton, which suggests blebbing of the membrane with some visible detaching and rounded cell bodies, characteristic of necrosis and apoptosis. A similar response is seen in MC3T3-E1s exposed to 60°C, having a largely depleted cell number compared with the control.
Figure 1.
Actin filament (phalloidin) and nucleus stained (DAPI) MC3T3-E1s following 24 h recovery after heat-treatment of (a) 37°C, (b) 45°C, (c) 47°C and (d) 60°C heat-shock for 1 min. Scale bars: (a–d) 32 µm. (Online version in colour.)
3.2. Effects of heat-shock on MC3T3-E1 cellular viability, necrosis and apoptosis
3.2.1. Cell viability
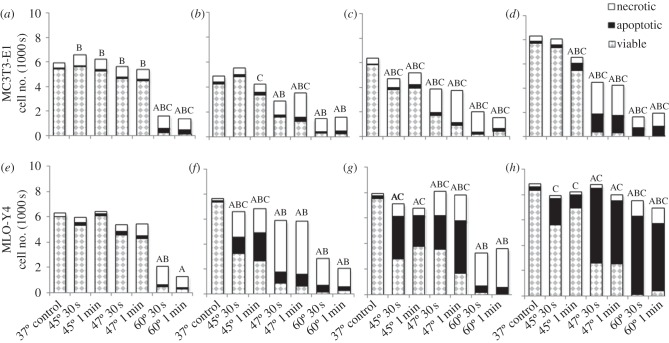
MC3T3-E1s exposed to heat-shock show a degree of responses varying with temperature and duration of exposure. The number of viable MC3T3-E1s is significantly decreased immediately after cells are exposed to 60°C for 30 s and 1 min compared with the control (figure 2a). After 12 h, viability is significantly reduced after exposure to 47°C and 60°C for 30 s and 1 min compared with the control (figure 2b). After 24 h, a significant decrease in viable MC3T3-E1s is observed after exposure to all elevated temperatures and durations compared with control (37°C; figure 2c). At 4 days, MC3T3-E1 viability is significantly decreased following exposure to heat-shock more than equal to 45°C for 1 min (figure 2d).
Figure 2.
Effects of 45°C, 47°C, 60°C heat-shock for 30 s and 1 min with 37°C as control, on viability of (a–d) MC3T3-E1 and (e–h) MLO-Y4 cells with less than 1, 12, 24 h and 4 days recovery. Statistical significance between groups exposed to heat-shock compared with the control—‘A’ indicating statistical significance in number of viable cells, ‘B’ indicating statistical significance in necrotic cells and ‘C’ indicating statistical significance in apoptotic cells (p ≤ 0.05).
3.2.2. Cell necrosis
A significant initial necrotic response is observed after exposure of cells to all elevated temperatures for all durations, compared with the control (figure 2a). After 12 h, a significantly increased necrotic MC3T3-E1 population is observed after exposure to 47°C and 60°C for 30 s and 1 min compared with the control (figure 2b). Following 24 h, a significantly increased necrotic MC3T3-E1 population is observed in cells exposed to heat-shock at all elevated temperatures and durations (figure 2c). At 4 days, MC3T3-E1s exposed to more than equal to 45°C for 1 min exhibit a significantly increased necrotic cell population (figure 2d).
3.2.3. Cell apoptosis
An immediate (less than 1 h) significant increase in the number of apoptotic MC3T3-E1s is seen after exposure to 60°C for 30 s and 1 min compared with the control (figure 2a). After 12 h recovery, a significant increase in the number of apoptotic MC3T3-E1s was observed only in cells exposed to 45°C and 47°C for 1 min compared with the control (figure 2b). Following 24 h recovery, a significantly increased apoptotic cell population is observed in all MC3T3-E1s exposed to all elevated temperatures and durations compared with control (37°C; p ≤ 0.0166; figure 2c). After 4 days, the apoptotic cell population is significantly increased in MC3T3-E1s exposed to heat-shock more than equal to 45°C for 1 min compared with control (37°C; p = 0.0001; figure 2d).
3.3. Effects of heat-shock on MLO-Y4 cellular viability, necrosis and apoptosis
3.3.1. Cell viability
An immediate significant decrease in the number of viable MLO-Y4s is seen after exposure to 60°C for 30 s and 1 min compared with the control (figure 2e). After 12 and 24 h recovery, a significant reduction in the number of viable MLO-Y4s is seen after exposure to all elevated temperatures and durations compared with control (37°C; p ≤ 0.0001; figure 2f,g). Following a recovery period of 4 days, a significant reduction in viability is seen in cells exposed to 47°C and 60°C for 30 s and 1 min compared with the control (figure 2h).
3.3.2. Cell necrosis
An initial necrotic response is observed in MLO-Y4s exposed to 60°C for 30 s p = 0.0041, less than 1 h after exposure (figure 2e). After 12 h recovery, a significant increase in necrotic MLO-Y4s is seen after exposure to all elevated temperatures and durations compared with the control (37°C; p ≤ 0.0003; figure 2f). Following 24 h recovery, a significant increase in the necrotic response is seen in cells exposed to 47°C and 60°C for 30 s and 1 min compared with the control (figure 2g). After 4 days recovery, MLO-Y4 cell necrosis is significantly increased after exposure to severe heat-shock of 60°C for 30 s and 1 min compared with the control (figure 2h).
3.3.3. Cell apoptosis
A minimal initial apoptotic response is observed in MLO-Y4s less than 1 h after exposure. No significant difference in cell apoptosis was observed in any group exposed to elevated temperature immediately after exposure compared with control (figure 2e). After 12 h recovery, a significant increase in apoptotic MLO-Y4 cells is seen after exposure to 45°C for 30 s and 1 min compared with the control (figure 2f). After 24 h, the apoptotic response is more severe with a significant increase in apoptosis for cells exposed to 45°C and 47°C for 30 s and 1 min compared with the control, p = 0.0001 (figure 2g). After 4 days recovery, a significantly higher apoptotic cell population is observed in all MLO-Y4s exposed to all elevated temperatures and durations compared with control, p ≤ 0.03 (figure 2h).
3.4. Effects of heat-shock on osteogenic regeneration of bone cells
Differentiation and mineralization capacity of Balb/c MSCs and MC3T3-E1s was investigated by analysing intracellular and extracellular ALP expression, and alizarin red quantification of cells cultured in osteogenic or expansion culture conditions.
3.4.1. Alkaline phosphatase activity
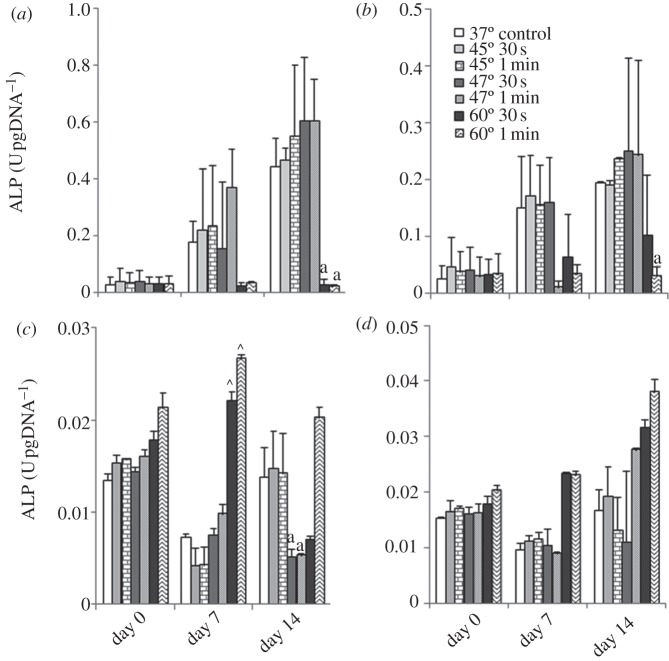
Intracellular ALP expression by Balb/c MSCs shows a similar trend for all treatment groups: the cells cultured in osteogenic conditions have consistently higher ALP expression than cells cultured in expansion media. After 14 days recovery, intracellular ALP expression was significantly reduced in Balb/c MSCs cultured under osteogenic conditions after exposure to 60°C for 30 s and 1 min compared with control (figure 3a), but also for Balb/c MSCs cultured in expansion media after exposure to 60°C for 1 min (figure 3b).
Figure 3.
Effects of 45°C, 47°C and 60°C heat-shock for 30 s and 1 min with 37°C as control, on intracellular alkaline phosphatase production of (a,b) Balb/c MSCs and (c,d) MC3T3-E1s cultured in (a,c) osteogenic and (b,d) expansion media with 0, 7 and 14 days recovery at 37°C. Statistical difference is indicated between groups exposed to heat-shock compared with the control as follows: arrowheads denote at day 7, and ‘a’ denotes at day 14 (p ≤ 0.05).
When cultured in osteogenic conditions and following 7 days recovery, intracellular ALP was significantly increased in cells exposed to 60°C for 30 s and 1 min compared with the control. After 14 days, there is a statistic difference in intracellular ALP expression in MC3T3-E1 cells cultured in osteogenic conditions and expose to 47°C for 30 s and 1 min (figure 3c). When cultured in expansion media, no significant difference is seen between any of the elevated temperature groups at any time point (figure 3d).
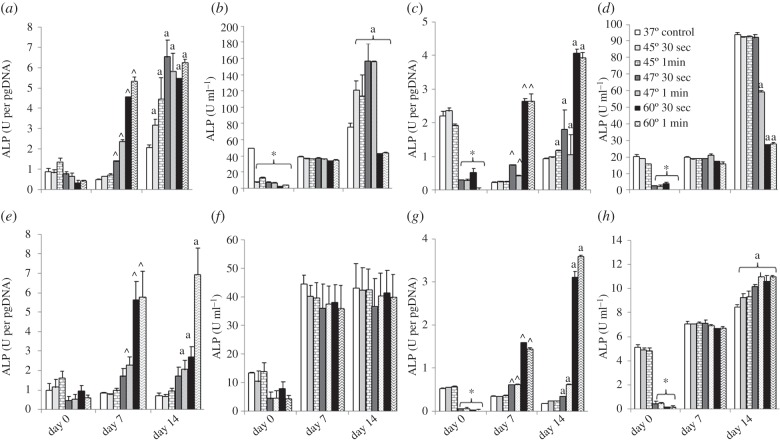
Extracellular ALP expression by Balb/c MSCs show a similar trend for all treatment groups, cells cultured in osteogenic conditions being consistently higher than cells cultured in expansion media. Following 7 days recovery, ALP expression was significantly increased in cells exposed to 47°C and 60°C for 30 s and 1 min compared with the control. After 14 days, ALP per cell is significantly higher in all cells exposed to elevated temperatures compared with the control, p ≤ 0.0005.
Focusing on the total ALP expression of the cell population, an immediate significant reduction of ALP ml−1 is observed in all groups exposed to heat-shock and cultured under osteogenic conditions (p = 0.0001; figure 4b). By 14 days, ALP ml−1 is significantly increased in cells exposed to 45°C and 47°C for 30 s and 1 min, and significantly decreased in cells exposed to 60°C for 30 s and 1 min compared with control, p = 0.0001.
Figure 4.
Effects of 45°C, 47°C and 60°C heat-shock for 30 s and 1 min with 37°C as control, on extracellular alkaline phosphatase expression of Balb/c MSCs (a–d) and MC3T3-E1s (e–h) cultured in osteogenic (a,b,e,f) and expansion media (c,d,g,h). Normalized to DNA content (a,c,e,g) and alkaline phosphatase content per millilitre (b,d,f,h) with 0, 7 and 14 days recovery at 37°C. Statistical significance between groups exposed to heat-shock compared with the control: asterisks denote at day 0; arrowheads denote at day 7 and ‘a’ denotes at day 14 (p ≤ 0.05).
Extracellular ALP expression of Balb/c MSCs cultured in expansion media show a similar trend to cells cultured in osteogenic conditions. An immediate decrease in ALP expression per cell is seen in cells exposed to 47°C and 60°C for 30 s and 1 min, p ≤ 0.0001 compared with the control (figure 4c). After 7 days recovery, a significant increase in ALP expression per cell is seen in cells exposed to 47°C and 60°C for 30 s and 1 min, p ≤ 0.0162 compared with the control. At 14 days, a significant increase in ALP expression per cell is observed in all cells exposed to elevated temperatures more than equal to 45°C for 1 min, p ≤ 0.0006 compared with the control.
An immediate decrease in total ALP expression is seen in cells exposed to 47°C and 60°C for 30 s and 1 min, p ≤ 0.0001 compared with the control, (figure 4d). Total ALP expression of the cell population after 14 days recovery is significantly reduced in cells exposed to temperatures more than equal to 47°C for 1 min compared with control, p = 0.0001.
Following 7 and 14 days recovery, extracellular ALP expression of MC3T3-E1s exposed to 47°C for 1 min and 60°C for 30 s and 1 min and cultured in osteogenic conditions was statistically increased compared with the control, p ≤ 0.0234 (figure 4e). No significant difference is seen in total ALP expression per millilitre is observed for any treatment group at any time point (figure 4f).
When cultured in expansion media, extracellular ALP expression of MC3T3-E1s was immediately significantly reduced after cells were exposed to 47°C and 60°C for 30 s and 1 min, p ≤ 0.0001 (figure 4g). Similar to cells cultured in osteogenic conditions, after 7 and 14 days recovery, a significant increase in ALP secretion per cell is seen after exposure to 47°C and 60°C for 30 s and 1 min, p ≤ 0.0001.
An initial significant decrease in overall ALP secretion per millilitre is observed in cells exposed to 47°C and 60°C for 30 s and 1 min (figure 4h). After 14 days recovery, ALP ml−1 is significantly increased in MC3T3-E1s exposed to all treatment groups compared with the control, p ≤ 0.0002.
3.4.2. Calcium deposition
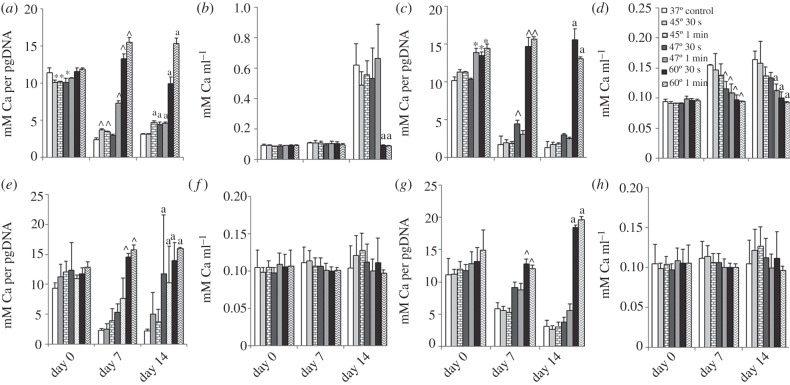
Under osteogenic conditions, calcium deposition per picogram DNA of Balb/c MSCs is significantly lower immediately after exposure to 45°C for 30 s and 1 min and 47°C for 30 s (p ≤ 0.0002; figure 5a). At 7 days, calcium deposition is significantly increased in cells exposed to 45°C for 30 s and 1 min, 47°C for 1 min and 60°C for 30 s and 1 min compared with the control. Similarly, at 14 days, calcium deposition is significantly increased in cells exposed to 45°C for 1 min, 47°C and 60°C for 30 s and 1 min compared with the control.
Figure 5.
Effects of 45°C, 47°C and 60°C heat-shock for 30 s and 1 min with 37°C as control, on calcium production (alizarin red) of (a–d) Balb/c MSCs and (e–h) MC3T3-E1s cultured in (a,b,e,f) osteogenic and (c,d,g,h) expansion media. Normalized to DNA content (a,c,e,g) and calcium content per millilitre (b,d,f,h) with 0, 7 and 14 days recovery at 37°C. Statistical significance between groups exposed to heat-shock compared with the control: asterisks denote at day 0; arrowheads denote at day 7 and ‘a’ denotes at day 14 (p ≤ 0.05).
There is no significant difference in the total calcium deposition per millilitre immediately after heat-shock or following 7 days recovery; however, at 14 days, calcium production is significantly decreased in cells exposed to 60°C for 30 s and 1 min compared with the control.
Calcium deposition of Balb/c MSCs cultured in expansion media shows a similar trend to the cells cultured in osteogenic media when normalized to pg DNA (figure 5c). An immediate increase in calcium deposition is seen in cells exposed to more than equal to 47°C for 1 min. After 7 days, a significant increase in calcium deposition is seen in MSCs exposed to 47°C for 30 s and 60°C for 30 s and 1 min, p = 0.0001. At 14 days, a significant increase is observed in cells exposed to 60°C for 30 s and 1 min, p = 0.0001.
The total calcium deposition per millilitre is significantly decreased in cells exposed to 47°C and 60°C for 30 s and 1 min, compared with the control after 7 days, p ≤ 0.01. Similarly, at 14 days, there is a significant decrease in calcium deposition per millilitre in cells exposed to 47°C for 1 min, 60°C for 30 s and 1 min compared with the control.
Under osteogenic conditions, calcium deposition per pg DNA of MC3T3-E1 cells exposed to 60°C for 30 s and 1 min is significantly higher than control, following 7 days recovery (figure 5e). After 14 days, calcium production per cell is significantly increased in cells exposed to 47°C and 60°C for 30 s and 1 min compared with the control. There is no significant difference in the overall calcium deposition per millilitre at any time point (figure 5f).
When cultured in expansion media, a similar trend is seen as osteogenic conditions (figure 5g). Following 7 and 14 days recovery, calcium deposition per picogram DNA is significantly higher in cells exposed to 60°C for 30 s and 1 min compared with control, p = 0.0001. There is no significant difference in the overall calcium deposition per millilitre at any time point.
4. Discussion
This study provides a novel insight into the immediate and long-term cellular responses to elevated temperatures experienced in bone tissue during surgical cutting. The effects of heat-shock on bone cell viability and regeneration capacity are shown to be dependent on the degree of temperature elevation, the duration of exposure and the phenotype of the cell. The biological response of bone cells to elevated temperatures is characterized by an immediate necrotic response followed by later-stage apoptosis. Furthermore, is it shown that these biological responses are minimized if heat-shock is maintained below a critical temperature of less than equal to 47°C for 1 min. This study reports that osteoblast-like cells are more susceptible to heat-induced cellular death than osteocyte-like cells. When heat-shock is minimized to 45°C for 1 min for MLO-Y4 cells, complete recovery (as indicated by percentage necrosis, viability and population size) occurs after 4 days, whereas MC3T3-E1 cells can withstand the same temperature only for 30 s. Most interestingly, it is shown that after 14 days recovery an increase in intracellular ALP production, indicating osteogenic differentiation, occurs in MSCs exposed to mild heat-shock (less than or equal to 47°C for 1 min) as well as increased ALP secretion and calcium deposition of MSCs and MC3T3-E1 cells after exposure to heat-shock.
In vivo studies have identified 47°C as the threshold temperature at which morphological bone tissue damage occurs [5]. ALP denaturation has been reported to occur at 56°C [26] and when bone is heated to 60°C, obvious bone tissue necrosis occurs without signs of vascular recovery over a follow-up period of 100 days [4,5]. Irreparable tissue damage was reported for temperatures greater than or equal to 70°C for 1 min [27]. The threshold for bone tissue injury has been defined as 47°C for 1 min, based on histological observations of cortical necrosis in rabbit bone [5]. These studies suggest that in vivo exposure of bone tissue to cutting temperatures of 47°C for 1 min resulted in bone resorption after 30 days, and proposed that this resorption would have a negative effect on the long-term anchorage of orthopaedic implants [4,5]. The findings of the current study suggest that minimizing temperature elevation to less than 47°C reduces the number of necrotic and apoptotic cells. It is interesting to speculate on the differences in timing of initiation of apoptosis and necrosis observed in our studies. Apoptosis is a complex tightly regulated sequence, whereby the cell systematically degrades its functional and structural constituents [2]. The time period of initiation of apoptotic cell death to final-stage fragmentation can be several hours, depending on the severity of the damage, cell type and apoptotic pathway [12], and is accredited to the time involved in pro-apoptotic protein synthesis. However, an immediate form of preprogrammed apoptosis can also be triggered in less than 30 min, whereby all the necessary cellular components are constitutively synthesized and only need to be activated [28]. The authors propose that temperature elevations induced in this study are activating both immediate apoptosis (more than 1 h) and later-stage apoptosis pathways (less than 4 h).
The findings of the current study also suggest that minimizing temperature elevation to less than 47°C enhances tissue regeneration by inducing mineralization of MC3T3-E1 cells as well as differentiation and mineralization of MSCs. This observed regenerative response is in agreement with previous studies that showed that exposure of osteoprogenitor cells to mild heat-shock (39–42.5°C)-induced differentiation along the osteoblastic line and enhanced mineralized nodule formation [15,24]. In these studies, however, the duration of exposure to elevated temperatures is much longer than what is likely to occur during surgical cutting procedures. The results of the current study propose that there is a synergistic effect of minimizing heat-shock to less than 47°C for 1 min, which minimizes cell mortality and also induces mineralized matrix production. These findings provide a profile of cellular reactions that may inform the design of future surgical instrumentation, whereby post-operative healing can be optimized to reduce patient recovery time and the need for revision operations.
Interestingly, an additional response of cells exposed to severe heat-shock (60°C for 30 s and 1 min) is found, whereby ALP production and calcium deposition is largely increased (figure 5). These findings might be accredited to hypermineralization of the cells. Hypermineralization of osteocyte lacunae (micropetrosis) is a phenomenon described by Frost in 1960, and was proposed to occur during or after osteocyte death [29–31]. A recent study reported that osteocyte lacunae in aged bone showed an increase in hypermineralized calcium phosphate occlusions as well as a decrease in osteocyte lacunar density, compared with younger bone [32]. These studies suggest a strong link between bone cell death and mineralization, which agrees with our results.
The use of the cell-lines MLO-Y4 and MC3T3-E1 is a possible limitation to this study. However, both cell-lines have been shown to be appropriate representatives of primary osteocytes and osteoblasts, and are widely used to study bone cell biology in vitro [19,20,33,34]. A further limitation is that cells were cultured in monolayer on tissue culture plastic, which means that they are organized two-dimensionally, whereas in vivo osteocytes are embedded within a three-dimensional collagen and mineral matrix. Depending on the location of the bone cells within the bone tissue, the health and condition of the bone, the mineralized matrix may alter the direct exposure of cells to temperature. Therefore, while temperatures experienced by bone tissue have been shown to reach 60°C [35], it is not yet known whether such temperatures are indeed experienced by the cells embedded within the mineralized bone matrix. Nonetheless, these studies provide a direct insight into the effects of elevated temperature over short durations on cell viability and tissue regeneration. Future studies are required to fully characterize the temperatures experienced by bone cells in vivo.
Temperature elevations during surgical cutting are difficult to avoid, and are dependent on a number of factors, including force applied, speed of cut, health of bone, geometry of blade, depth of cut and irrigation technique [3,7]. Previous studies have identified bone tissue damage at 47°C and have taken this temperature to be the threshold for thermal necrosis [5]. Our results suggest that this threshold is slightly lower than previously identified (45°C), but although a certain amount of cellular damage is indeed initiated when cells are exposed to temperatures above the control of 37°C, this is minimal (figure 2). Therefore, while temperature elevations above 37°C are inevitable, our study shows that as long as temperatures are maintained less than equal to 45°C, there is a synergistic effect of minimizing necrosis and apoptosis, but also enhanced tissue regeneration that may in fact accelerate the healing process. Cooling of the surgical tool and surrounding bone by means of irrigation can limit temperature elevations to less than 10°C during surgical procedures [36], and maintain temperatures below the values associated with bone necrosis [7]. Therefore, based on our results, cooling may have a positive effect on tissue regeneration by minimizing temperature elevations to below 47°C. However, irrigation by manual methods in clinical situations leads to inconsistent cooling with often only the superficial bone experiencing the cooling effect [37], and as such, all cells may not be protected from harmful temperature elevations.
It is intriguing to speculate on the mechanisms by which heat-shock induces necrosis and apoptosis in bone cells. It has been previously shown that an upregulation of the heat-shock protein HSP70 occurs in osteoblasts heat-treated to 45°C and 48°C for 10 min [2]. HSP70 is directly involved in the apoptotic process by having a protective role in stress responses [38]. The protective mechanism of HSP70 has been shown to have the ability to repair cellular damage and prevent apoptosis in rat calvarial osteoblasts treated to 45°C but not in cells treated to 48°C [2]. The findings of this study provide a novel insight into the precise effects of thermal injury occurring during surgical cutting procedures, whereby bone cells appear to be much more sensitive than previously reported [2] with significant changes in cell viability and regeneration occur after very short durations to temperature elevations (30 s and 1 min). Previous understanding of cellular responses to thermal insult has been obtained from longer duration experiments (10 min) [2], which are not representative of continuous surgical cutting of bone tissue. Additionally, this study examines the longer-term outcome following short periods of thermal injury and observe a distinct apoptotic response in MLO-Y4 cells that initiates 24 h after cells were exposed to 45°C and 47°C. A continued apoptotic response is observed after 4 days recovery, which could be accredited to the systematic degradation of the apoptotic cell and the formation of smaller membrane-bound vesicles (apoptotic bodies). These apoptotic bodies are rapidly removed in vivo by phagocytic cells, and therefore the inflammatory response is minimized [13].
Interestingly, MLO-Y4s were found to be more resilient to heat-shock than MC3T3-E1s. Osteocytes are terminally differentiated osteoblasts that are embedded within a mineralized bone matrix. Owing to their position within the bone and their complex communication network, it has been widely accepted that osteocytes are sensory cells that can control and regulate mineralization in response to mechanical stimuli or tissue damage [33,39,40]. A recent study examined whether cells, having undergone mechanical damage in the form of cell process rupture, emitted biochemical signals to neighbouring cells to initiate a response to damage [41]. It was demonstrated that when MLO-Y4 cell networks were subjected to mechanical damage (160–800 μm planar, crack-like defects), altered production of cytokines known to regulate bone remodelling (receptor activator of NFκB ligand, osteoprotegerin) occurred depending on the extent of damage and recovery time following application of injury [41]. This study proposed that in spite of an injured cell's impaired viability, microcracks were detected by surrounding non-injured cells, which can signal to initiate the repair response in neighbouring cells [41]. The findings of this study suggest that cellular damage induced due to temperature elevations might stimulate bone remodelling in a similar manner. Furthermore, these results report for the first time that MLO-Y4s are more robust than MC3T3-E1s under heat-shock. It is proposed that this difference reflects the role of osteocytes as sensory cells embedded within bone tissue, detecting damage and stimulating a response in neighbouring cells.
In conclusion, the results of this study indicate that an immediate necrotic response to heat-shock occurs in bone cells exposed to elevated temperatures and show, for the first time, that if the level of heat-shock is above a critical level, then an additional longer-term apoptotic response is observed up to 4 days recovery. When heat-shock is minimized to 45°C for 1 min for MLO-Y4 cells and 45°C for 30 s for MC3T3-E1 cells, populations showed recovery, with viability and population size comparable to control by 4 days. At higher temperatures (60°C), an immediate depletion in cell population occurs without recovery up to 14 days. Interestingly, osteocyte-like cells are reported to be more resilient to heat treatment than osteoblasts, which may reflect their role as sensors embedded within bone tissue. Furthermore, heat-shock was found to induce differentiation and mineralization by osteoprogenitor cells. There is a distinct need for a thorough biological understanding to allow continual innovation of surgical instrumentation to enhance post-surgical patient outcome. The results of this study indicate that mild heat-shock might positively affect healing by inducing osseointegration of orthopaedic implants and thereby may improve patient outcome.
Acknowledgements
The MLO-Y4 cell-line was received as a gift from Professor Lynda Bonewald (School of Dentistry, University of Missouri, Kansas City, MO, USA). The authors acknowledge funding from the National University of Ireland Galway Fellowship Scheme, the National University of Ireland Travelling Scholarships in Engineering, the European Research Council (ERC) (under grant no. 258992; BONEMECHBIO). We also acknowledge Alessandro Natoni and the NCBES flow Cytometry Facility and Georgina Shaw for the Balb/c MSCs.
Study design and planning: E.D., M.H., C.C., D.T. and LMcN. Data collection and analysis: E.D., M.H., L.McN. Manuscript preparation and editing: E.D., M.H., C.C., D.T. and L.McN. D. Tallon and C. Casey are employees of Stryker Corporation.
References
- 1.Leucht P., Lam K., Kim J.-B., Mackanos M. A., Simanovskii D. M., Longaker M. T., Contag C. H., Schwettman H. A., Helms J. A. 2007. Accelerated bone repair after plasma laser corticotomies. Ann. Surg. 246, 140–150 10.1097/01.sla.0000258559.07435.b3 (doi:10.1097/01.sla.0000258559.07435.b3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S., Chien S., Brånemark P.-I. 1999. Heat shock-induced necrosis and apoptosis in osteoblasts. J. Orthop. Res. 17, 891–899 10.1002/jor.1100170614 (doi:10.1002/jor.1100170614) [DOI] [PubMed] [Google Scholar]
- 3.Lundskog J. 1972. Heat and bone tissue. An experimental investigation of the thermal properties of bone and threshold levels for thermal injury. Scand. J. Plastic Reconstr. Surg. 9, 1–80 [PubMed] [Google Scholar]
- 4.Eriksson A., Albrektsson T., Grane B., McQueen D. 1982. Thermal injury to bone: a vital-microscopic description of heat effects. Int. J. Oral Surg. 11, 115–121 10.1016/S0300-9785(82)80020-3 (doi:10.1016/S0300-9785(82)80020-3) [DOI] [PubMed] [Google Scholar]
- 5.Eriksson A. R., Albrektsson T. 1983. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J. Prosthet. Dent. 50, 101–106 10.1016/0022-3913(83)90174-9 (doi:10.1016/0022-3913(83)90174-9) [DOI] [PubMed] [Google Scholar]
- 6.Eriksson A. R., Albrektsson T., Magnusson B. 1984. Assessment of bone viability after heat trauma: a histological, histochemical and vital microscopic study in the rabbit. Scand. J. Plastic Reconstr. Surg. Hand Surg. 18, 261–268 10.3109/02844318409052849 (doi:10.3109/02844318409052849) [DOI] [PubMed] [Google Scholar]
- 7.Toksvig-Larsen S., Ryd L. 1989. Temperature elevation during knee arthroplasty. Acta Orthop. 60, 439–442 10.3109/17453678909149314 (doi:10.3109/17453678909149314) [DOI] [PubMed] [Google Scholar]
- 8.Albrektsson T. 1980. The healing of autologous bone grafts after varying degrees of surgical trauma. A microscopic and histochemical study in the rabbit. J. Bone Joint Surg. Br. 62, 403–410 [DOI] [PubMed] [Google Scholar]
- 9.Albrektsson T., Brånemark P.-I., Hansson H.-A., Lindström J. 1981. Osseointegrated titanium implants: requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 52, 155–170 10.3109/17453678108991776 (doi:10.3109/17453678108991776) [DOI] [PubMed] [Google Scholar]
- 10.Albrektsson T., Linder L. 1981. Intravital, long-term follow-up of autologous experimental bone grafts. Arch. Orthop. Trauma Surg. 98, 189–193 10.1007/BF00632976 (doi:10.1007/BF00632976) [DOI] [PubMed] [Google Scholar]
- 11.Abouzgia M. 1997. Temperature rise during drilling through bone. Int. J. Oral Maxillofac. Implants 12, 342–352 [PubMed] [Google Scholar]
- 12.Ziegler U. 2004. Morphological features of cell death. Physiology 19, 124–128 10.1152/nips.01519.2004 (doi:10.1152/nips.01519.2004) [DOI] [PubMed] [Google Scholar]
- 13.Noble B. S. 2003. Bone microdamage and cell apoptosis. Eur. Cells Mater. 6, 46–56 [DOI] [PubMed] [Google Scholar]
- 14.Noble B. S., Reeve J. 2000. Osteocyte function, osteocyte death and bone fracture resistance. Mol. Cell. Endocrinol. 159, 7–13 10.1016/S0303-7207(99)00174-4 (doi:10.1016/S0303-7207(99)00174-4) [DOI] [PubMed] [Google Scholar]
- 15.NØRgaard R., Kassem M., Rattan S. I. S. 2006. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann. NY Acad. Sci. 1067, 443–447 10.1196/annals.1354.063 (doi:10.1196/annals.1354.063) [DOI] [PubMed] [Google Scholar]
- 16.Ye C. P., Heng B. C., Liu H., Toh W. S., Cao T. 2007. Culture media conditioned by heat-shocked osteoblasts enhances the osteogenesis of bone marrow-derived mesenchymal stromal cells. Cell Biochem. Funct. 25, 267–276 10.1002/cbf.1330 (doi:10.1002/cbf.1330) [DOI] [PubMed] [Google Scholar]
- 17.Augustin G., Davila S., Mihoci K., Udiljak T., Vedrina D., Antabak A. 2008. Thermal osteonecrosis and bone drilling parameters revisited. Arch. Orthop. Trauma Surg. 128, 71–77 10.1007/s00402-007-0427-3 (doi:10.1007/s00402-007-0427-3) [DOI] [PubMed] [Google Scholar]
- 18.Larsson S., Stadelmann V. A., Arnoldi J., Behrens M., Hess B., Procter P., Murphy M., Pioletti D. P. 2012. Injectable calcium phosphate cement for augmentation around cancellous bone screws. In vivo biomechanical studies. J. Biomech. 45, 1156–1160 10.1007/s00402-007-0427-3 (doi:10.1007/s00402-007-0427-3) [DOI] [PubMed] [Google Scholar]
- 19.Kato Y., Windle J. J., Koop B. A., Mundy G. R., Bonewald L. F. 1997. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 12, 2014–2023 10.1359/jbmr.1997.12.12.2014 (doi:10.1359/jbmr.1997.12.12.2014) [DOI] [PubMed] [Google Scholar]
- 20.Sudo H., Kodama H.-A., Amagai Y., Yamamoto S., Kasai S. 1983. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 96, 191–198 10.1083/jcb.96.1.191 (doi:10.1083/jcb.96.1.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birmingham E., Niebur G. L., McHugh P. E., Barry F. P., McNamara L. M. 2012. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater. 23, 13–27 [DOI] [PubMed] [Google Scholar]
- 22.Peister A., Mellad J. A., Larson B. L., Hall B. M., Gibson L. F., Prockop D. J. 2004. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103, 1662–1668 10.1182/blood-2003-09-3070 (doi:10.1182/blood-2003-09-3070) [DOI] [PubMed] [Google Scholar]
- 23.Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545–1556 10.1084/jem.182.5.1545 (doi:10.1084/jem.182.5.1545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shui C., Scutt A. 2001. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J. Bone Miner. Res. 16, 731–741 10.1359/jbmr.2001.16.4.731 (doi:10.1359/jbmr.2001.16.4.731) [DOI] [PubMed] [Google Scholar]
- 25.Singh D. N., Kumar V. 2010. A proliferation of myoblast skeletal cells on three-dimensional supermacroporous cryogels. Int. J. Biol. Sci. 6, 371–381 10.7150/ijbs.6.371 (doi:10.7150/ijbs.6.371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews L. S., Hirsch C. 1972. Temperatures measured in human cortical bone when drilling. J. Bone Joint Surg. Am. 54, 297–308 [PubMed] [Google Scholar]
- 27.Berman A. T., Spence J. R., Yanicko D. R., Sih G. C., Zimmerman M. R. 1984. Thermally induced bone necrosis in rabbits: relation to implant failure in humans. Clin. Orthop. Relat. Res. 186, 284–292 [PubMed] [Google Scholar]
- 28.Godar D. E. 1999. Light and death: photons and apoptosis. J. Invest. Dermatol. 4, 17–23 10.1038/sj.jidsp.5640175 (doi:10.1038/sj.jidsp.5640175) [DOI] [PubMed] [Google Scholar]
- 29.Frost H. M. 1960. Micropetrosis. J. Bone Joint Surg. Am. 42, 144–150 [PubMed] [Google Scholar]
- 30.Krane S. M. 2001. Death and taxes: glucocorticoids and bone cell apoptosis. BoneKEy Osteovision, March 22 10.1138/2001019 (doi:10.1138/2001019) [DOI] [Google Scholar]
- 31.Carpentier V. T., Wong J., Yeap Y., Gan C., Sutton-Smith P., Badiei A., Fazzalari N. L., Kuliwab J. S. 2012. Increased proportion of hypermineralized osteocyte lacunae in osteoporotic and osteoarthritic human trabecular bone: implications for bone remodeling. Bone 50, 688–694 10.1016/j.bone.2011.11.021 (doi:10.1016/j.bone.2011.11.021) [DOI] [PubMed] [Google Scholar]
- 32.Busse B., Djonic D., Milovanovic P., Hahn M., Püschel K., Ritchie R. O., Djuric M., Amling M. 2010. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 9, 1065–1075 10.1111/j.1474-9726.2010.00633.x (doi:10.1111/j.1474-9726.2010.00633.x) [DOI] [PubMed] [Google Scholar]
- 33.Bonewald L. F. 2011. The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 10.1002/jbmr.320 (doi:10.1002/jbmr.320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quarles L. D., Yohay D. A., Lever L. W., Caton R., Wenstrup R. J. 1992. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J. Bone Miner. Res. 7, 683–692 10.1002/jbmr.5650070613 (doi:10.1002/jbmr.5650070613) [DOI] [PubMed] [Google Scholar]
- 35.Sugita N., Osa T., Mitsuishi M. 2009. Analysis and estimation of cutting-temperature distribution during end milling in relation to orthopedic surgery. Med. Eng. Phys. 31, 101–107 10.1016/j.medengphy.2008.05.001 (doi:10.1016/j.medengphy.2008.05.001) [DOI] [PubMed] [Google Scholar]
- 36.Krause W. R., Bradbury D. W., Kelly J. E., Lunceford E. M. 1982. Temperature elevations in orthopaedic cutting operations. J. Biomech. 15, 267–275 10.1016/0021-9290(82)90173-7 (doi:10.1016/0021-9290(82)90173-7) [DOI] [PubMed] [Google Scholar]
- 37.Karmani S. 2006. The thermal properties of bone and the effects of surgical intervention. Curr. Orthop. 20, 52–58 10.1016/j.cuor.2005.09.011 (doi:10.1016/j.cuor.2005.09.011) [DOI] [Google Scholar]
- 38.Sapozhnikov A. M., Ponomarev E. D., Tarasenko T. N., Telford W. G. 1999. Spontaneous apoptosis and expression of cell surface heat-shock proteins in cultured EL-4 lymphoma cells. Cell Prolif. 32, 363–378 10.1111/j.1365-2184.1999.tb01354.x (doi:10.1111/j.1365-2184.1999.tb01354.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. 2007. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metabol. 5, 464–475 10.1016/j.cmet.2007.05.001 (doi:10.1016/j.cmet.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 40.Hazenberg J., Freeley M., Foran E., Lee T., Taylor D. 2006. Microdamage: a cell transducing mechanism based on ruptured osteocyte processes. J. Biomech. 39, 2096–2103 10.1016/j.jbiomech.2005.06.006 (doi:10.1016/j.jbiomech.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 41.Mulcahy L. E., Taylor D., Lee T. C., Duffy G. P. 2011. RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone 48, 182–188 10.1016/j.bone.2010.09.014 (doi:10.1016/j.bone.2010.09.014) [DOI] [PubMed] [Google Scholar]