Abstract
Data on the bioavailability and toxicity of carbon nanotubes (CNTs) in the environment, and, in particular, on their interactions with vascular plants, are limited. We investigated the effects of industrial-grade multiwalled CNTs (75 wt% CNTs) and their impurities on alfalfa and wheat. Phytotoxicity assays were performed during both seed germination and seedling growth. The germinations of both species were tolerant of up to 2560 mg l−1 CNTs, and root elongation was enhanced in alfalfa and wheat seedlings exposed to CNTs. Remarkably, catalyst impurities also enhanced root elongation in alfalfa seedlings as well as wheat germination. Thus the impurities, not solely the CNTs, impacted the plants. CNT internalization by plants was investigated using electron microscopy and two-dimensional Raman mapping. The latter showed that CNTs were adsorbed onto the root surfaces of alfalfa and wheat without significant uptake or translocation. Electron microscopy investigations of internalization were inconclusive owing to poor contrast, so Fe3O4-functionalized CNTs were prepared and studied using energy-filter mapping of Fe3O4. CNTs bearing Fe3O4 nanoparticles were detected in the epidermis of one wheat root tip only, suggesting that internalization was possible but unusual. Thus, alfalfa and wheat tolerated high concentrations of industrial-grade multiwalled CNTs, which adsorbed onto their roots but were rarely taken up.
Keywords: plants, carbon nanotubes, nanotube uptake, nanotube phytotoxicity, Raman mapping, electron microscopy
1. Introduction
The extraordinary properties of carbon nanotubes (CNTs) [1] have inspired their use in biosensors [2], drug delivery vehicles and tumour imaging [3,4], electrically conductive polymers [5] and concrete reinforcement [6].
Despite the promise of CNTs, neither their interactions with biological systems nor their environmental fate are well understood. Their fibrous structure raises concerns that they might have effects similar to those of asbestos [7,8]. Observations of the interactions of CNTs with animals, made both in vitro and in vivo, are sometimes contradictory [1,3,7]. Similarly, studies assessing CNT toxicity and bioavailability in plants have reported positive [9,10], negative [9,11] and even neutral [12–14] effects on germination and growth. Besides the differences inherent among model species, variability exists between individual batches of CNTs, even those fabricated under identical conditions [15,16], and within batches, where CNT dimensions and degrees of surface functionalization vary [17]. Moreover, impurities derived from the catalyst used to produce the CNTs cause variability, even in purified batches, because they can be ‘protected’ from the CNT-purification processes by encapsulation within the tubes themselves [18], then mobilized and made bioavailable by preparation protocols [15], making it harder to attribute phytotoxicity solely to the CNTs. Hence, a comprehensive understanding of plant−CNT interactions requires both internalization and phytotoxicity assays.
Here, the phytotoxicity of up to 2560 mg l−1 of industrial-grade multiwalled CNTs (75 wt% CNTs), and of their catalytic impurities (25 wt% Fe/Al2O3), was investigated in two crop species, alfalfa and wheat. CNTs are expensive, and higher-purity materials cost more; hence, industrial-grade CNTs are especially desirable. This could increase the likelihood that metallic impurities associated with CNTs are released into the environment. Although studies have assessed the bioavailability and toxicity of metal-containing CNT impurities in mammalian cells [16,19], this, to our knowledge, is the first study to assess the effects of these impurities in whole organisms. The catalyst used in this study contained α-Al2O3 particles (125–150 µm), which are expected to be slightly soluble in the aqueous agar matrix, and Fe, which may be present as Fe0 nanoparticles, nano-Fe2O3 or -Fe3O4, or dissolved Fe2+ or Fe3+ ions.
Promising biotechnological applications for CNTs make their uptake by plants desirable. However, the small diameter and carbonaceous composition of CNTs hinder the study of CNT–plant interactions at the cellular level. New techniques, including fluorescence microscopy and vibrational spectroscopy, have been explored for the detection and tracking of CNTs in mammals [20,21] and plants [10,11].
To determine whether the plants internalized multiwalled CNTs, we used two techniques: Raman mapping was used to analyse cryogenized root cross-sections of plants exposed to 2560 mg l−1 industrial-grade multiwalled CNTs, and energy-filtered transmission electron microscopy (TEM) was used to detect industrial-grade multiwalled CNTs functionalized with Fe3O4 nanoparticles (Fe3O4–CNTs) in plant tissues.
2. Material and methods
2.1. Carbon nanotube synthesis, purification and characterization
CNTs were synthesized by the catalytic deposition of ethylene (4.5 SLPM) over Fe-impregnated non-porous alumina (Fe/Al2O3, 5 wt% Fe) at 650°C in a fluidized bed for 1 h [22]. Prior to synthesis, the catalyst was reduced with a mixture of N2 and H2 (H2 : N2 = 1 : 3, total flow = 4.5 SLPM) at 700°C for 1 h. As-synthesized CNTs were purified by microwave-assisted digestion (CEM MDS-81 oven). Typically, 1 g of as-synthesized material was placed in a Teflon vessel along with H2SO4 (40 ml, 5 M), and heated at 90 per cent power for 45 min. The CNT/acid mixture was vacuum filtered through an ethanol-wetted, 0.45 µm hydrophobic poly(tetrafluoroethylene) membrane and repeatedly washed with deionized (DI) water until the filtrate had neutral pH. This rendered industrial-grade CNTs (75 wt% CNT, 25 wt% catalytic impurities), which are labelled ‘purified CNTs’ here to distinguish them from as-synthesized CNTs.
To facilitate the detection and imaging of CNTs in plant tissues during in vivo uptake studies, purified CNTs were functionalized with Fe3O4 nanoparticles. Purified CNTs were mixed with poly(ethylene glycol) 200 (PEG, Merck Schuchardt OHG, mCNT : mPEG = 1 : 100) and enough iron salt (FeCl2•4H2O) to obtain a final Fe loading of 20 wt% on the CNTs. This formed a paste to which a NaOH–PEG mixture (mCNT : mNaOH = 1 : 1) was slowly added. The mixture was heated at 200°C in a conventional oven. After 14 h, excess Fe3O4 and PEG were eliminated by centrifuging, vacuum filtering and washing the Fe3O4–CNTs with DI water until a colourless supernatant was obtained and the filtrate had neutral pH.
CNTs were analysed by thermogravimetric analysis (TGA, TA Instruments SDT Q500, heating rate 10°C min−1 to 1000°C in air : nitrogen (3 : 2), total flow of 100 ml min−1), TEM (Philips CM12 TEM operated at 120 kV), Raman spectroscopy (Renishaw inVia Raman using an argon laser emitting at 514.5 nm, 10 accumulations, 20 s exposure time and 1% laser power), Fourier-transform infrared spectroscopy (FTIR; Bruker IFS66V Spectrometer; CNT-coated KBr crystal; resolution = 4 cm−1, 128 scans) and N2 adsorption/desorption isotherms measured at −196°C (Quantachrome Autosorb-1). The concentration of Fe in the CNTs was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES, Varian Vista AX). Residues from TGA samples were digested in aqua regia (HNO3 : HCl = 1 : 3) at 120°C for 2 h to extract the Fe. Quantitative Fe analysis was performed at 259.94 nm and compared with a calibration curve (correlation coefficient >0.9999) prepared using external standards (CHOICE Analytical).
2.2. Test plants
Alfalfa (Medicago sativa) and wheat (Triticum aestivum) were selected because they are model plant systems [23,24]. Wheat is also recommended by the guidelines for chemical testing proposed by the US Environmental Protection Agency [25] and the Organisation for Economic Co-operation and Development [26]. Alfalfa seeds were purchased from Greenpatch (Taree, Australia). Wheat seeds were donated by Professor Peter J. Sharp, Faculty of Agriculture, Food and Natural Resources, University of Sydney. To prevent fungal contamination, seeds were soaked in 1 vol% H2O2 for 15 min and washed five times with DI water immediately before use. Glassware was washed with acid prior to use. Single-use plastic containers were used in the phytotoxicity assays.
2.3. Phytotoxicity assays
2.3.1. Exposure media
Purified CNTs were sonicated in autoclaved DI water to form a stock suspension. Plant agar (Sigma-Aldrich) was warmed slightly to facilitate homogeneous mixing, then added to an appropriate quantity of the stock CNT suspension (final agar concentration 0.8% w/v). Scanning electron microscopy was used to verify the dispersion of the CNTs in agar (see electronic supplementary material, figure S1). Following preliminary assays, CNT–agar mixtures were prepared at nominal concentrations of 40−2560 mg l−1 CNTs.
The effects of catalyst impurities on plants were also tested; the catalyst was prepared as described above with mFe : mAl2O3 ∼ 1 : 4, the ratio found in the purified CNTs (vide supra). The purified CNTs contained 25 wt% catalyst, thus dispersions of catalytic impurity samples in agar were prepared at nominal concentrations of 10−640 mg l−1. For control experiments, agar was mixed with sterile DI water in the same ratio used for test samples.
2.3.2. Germination assay
Petri dishes (90 × 16 mm) were filled with 30 ml of test agar on which 16 (alfalfa) or 12 (wheat) seeds were placed, spaced approximately 15 mm apart. Plates were distributed randomly in a growth chamber (Contherm Scientific Ltd) at controlled temperature (12 h/12 h, 25°C/18°C) in the dark. The assay was terminated after 4 days, when 65 per cent of the control seeds had germinated and developed roots at least 20 mm long [25]. The number of germinated seeds (primary root ≥5 mm long [25]), root elongation for each seed, and pH of the medium were recorded. The germination index was calculated according to [27]:
 |
Standard deviations were calculated by comparing each replicate to its local mean.
2.3.3. Seedling exposure
Seeds were germinated in DI water at room temperature in the dark for 2 days. Four (alfalfa) or three (wheat) 20-mm-long seedlings were placed in flasks containing 60 ml CNT-agar. Flasks were distributed randomly in the growth chamber (12 h light/12 h dark, 25°C/18°C). Roots and shoots were measured after 6 days. Additionally, for alfalfa, a dicotyledon, the elongations of both cotyledons were measured; for wheat, a monocotyledon, leaf blade and coleoptile lengths were recorded. pH was noted.
2.4. Carbon nanotube uptake: microscopy
Two-day-old seedlings were transplanted into 600 ml beakers containing 200 ml of agar-solidified nutrient medium: 1-strength Hoagland medium (Hoagland's Basal No 2, Sigma-Aldrich) was adjusted to pH 5.8 with NaOH, mixed with plant agar and autoclaved for 15 min at 120°C. Warm agar was mixed with an aqueous suspension of purified CNTs or Fe3O4–CNTs to obtain a final concentration of 2560 mg l−1 CNTs. Control plants were grown in agar-solidified nutrient medium. Beakers were covered and incubated for 7 days in 12 h light/12 h dark at 25°C/18°C.
For microscopy analysis, four pieces were cut from the 5 mm region from the root tip and from either side of the first protruding lateral root. These were vacuum fixed in 6.25 vol% glutaraldehyde and 4 vol% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 30 min and then at ambient pressure overnight (4°C). Sections were washed with 0.1 M phosphate buffer and stained with 1% w/v aqueous OsO4 for 2 h, then with 1% w/v aqueous U(OAc)2 for 1 h, and finally dehydrated in an ethanol series (30, 50, 70, 90, 99, 100 vol%). Samples were infiltrated and embedded in Spurr's resin and oven-cured at 60°C for 24 h. Sections were cut using an ultramicrotome (Ultracut E1, Leica). For light microscopy (Nikon Eclipse E800), samples were cut into 0.1–0.5 µm sections and stained with 0.5% w/v toluidine blue in H2O. To search for CNTs in roots, 70 nm sections were cut with a diamond knife (Diamtome), transferred to copper grids and post-stained with 2% w/v aqueous U(OAc)2 and Reynolds' lead citrate [28] for 10 min each. Images were acquired using a JEOL 1400 TEM operated at 120 kV. For samples exposed to Fe3O4–CNTs, energy-filter maps for Fe were collected using a Philips CM120 Biofilter TEM at 120 kV and equipped with a Gatan Imaging Filter camera. Fe maps were acquired at the plasmon range of the electron energy loss spectra, in which Fe plasmon scattering is identified as 54 eV. A series of images was taken at the pre-edge of Fe (40 and 48 eV) and post-edge of Fe (64 eV), using a slit width of 8 eV, to calculate and remove background interference from Fe signals.
2.5. Carbon nanotube uptake: Raman spectroscopy
Plants were grown in the presence of purified multiwalled CNTs (2560 mg l−1) in agar (CNT–agar, prepared as described above) or in aqueous suspensions (CNT-water). For the latter, seedlings approximately 10 mm long were first grown in agar-solidified nutrient media without CNTs. After 7 days, the roots were submerged in a freshly prepared aqueous suspension of multiwalled CNTs (2560 mg l−1 CNT) for another 7 days. Controls were performed in DI water.
For the analysis of whole roots, plants were thoroughly rinsed and oven-dried at 50°C for 2 days. Roots were focused at 50× magnification and standard point spectra were collected as described above. Spectra recorded at three different points were averaged. For cross-section analyses, roots were harvested and thoroughly rinsed before being embedded and cryosectioned by the Histopathology Laboratory (University of Sydney). Root segments were fixed in cryostat specimen matrix (Tissue-Tek OCT Compound) by immersion in N2(l). Blocks were sectioned at −14°C in a Cryotome E Cryostat (Thermo Scientific). Sections of 7 µm were mounted onto glass slides and allowed to air-dry. Raman mapping mode was used to collect spectra of the cryosections over a spectral range of 1940–720 cm−1 (centre 1350 cm−1), using 1 accumulation and a 5 s exposure time. For data collection, the 50× microscope objective was used with a step size of 1.2 µm and X binning number of 1. Collected spectra were corrected and analysed using WiRE v. 3 software (Renishaw). Cosmic rays were removed using the neighbour cosmic ray removal method and false-colour maps were generated from the data using the signal-to-baseline method.
2.6. Statistical analysis
Mean and standard deviations were calculated from at least four replicate flasks/Petri dishes. Statistical significance between exposed plants and controls was determined using Student's t-test for non-equal variances or ANOVA, depending on the homogeneity of the variances, with statistical significance at p < 0.05.
3. Results and discusion
3.1. Carbon nanotube characterization
Thorough characterization of nanomaterials used in bio-assays is essential because their phytotoxicity and ability to penetrate tissues depends strongly on their physical and chemical properties [15,29,30], and the complex matrix formed by CNTs and their impurities necessitates a multitechnique approach. Table 1 summarizes the characterization of our multiwalled CNTs. Full characterization details, including thermogravimetric analysis, Raman and FTIR spectra, N2 adsorption–desorption isotherms, pore size distributions and elemental analysis of the impurities, are provided and discussed in the electronic supplementary material).
Table 1.
Summary of characterization data for as-synthesized, purified, and Fe3O4-functionalized carbon nanotubes.
| multiwalled carbon nanotubes |
|||||
|---|---|---|---|---|---|
| as-synthesized | purified | Fe3O4-functionalized | |||
| thermogravimetric analysis | puritya (wt%) | 25.4 | 75.2 | 67.2 | |
| max. oxidation Tb (°C) | 584.2 | 596.0 | 478.5 | ||
| FWHHc,d (°C) | 66.4 | 54.4 | 50.8 | ||
| inductively coupled plasma-atomic emission spectroscopy | catalyst impuritiese (wt%) | Fe | 1.4 ± 0.2 | 5.3 ± 0.3 | — |
| Al2O3 | 74.0 ± 1.0 | 19.7 ± 0.8 | — | ||
| transmission electron microscopy | external diameter (nm) | 12.1 ± 3.3 | 12.8 ± 3.8 | 10.9 ± 1.9 | |
| inner diameter (nm) | 5.8 ± 1.9 | 5.5 ± 1.7 | 5.4 ± 1.4 | ||
| adsorption/desorption isotherms | SSAf (m2 g−1) | 101.8 | 191.9 | 116.1 | |
| PSDg maximum (nm) | 3.12 | 2.78 | 2.46 | ||
| Raman spectroscopy | D band | position (cm−1) | 1349 | 1349 | 1350 |
| FWHHc (cm−1) | 48.4 | 55.0 | 5.2 | ||
| G band | position (cm−1) | 1585 | 1585 | 1585 | |
| FWHHc (cm−1) | 64.8 | 67.5 | 67.3 | ||
| D* band | position (cm−1) | 2694 | 2701 | 2701 | |
| FWHHc (cm−1) | 86.4 | 100.3 | 83.7 | ||
| ID/IGh | 1.2 | 1.2 | 1.2 | ||
aDetermined from the residual weight after combustion at 10°C min−1 to 1000°C.
bTemperature at which the rate of oxidation was greatest, as determined from the derivative weight loss profile.
cFWHH, Full-width at half height.
dFWHH of the largest oxidation peak in the derivative weight loss profile.
eResidues of the thermogravimetric analysis were digested in aqua regia for analysis.
fSSA, specific surface area, calculated using the Brunauer–Emmett–Teller model over P/P0 = 0.05–0.25.
gMaximum of the pore-size distribution calculated from the adsorption branch using the Barret–Joyner–Halenda method.
hRatio of the intensities of D band and G band. For details, see electronic supplementary material.
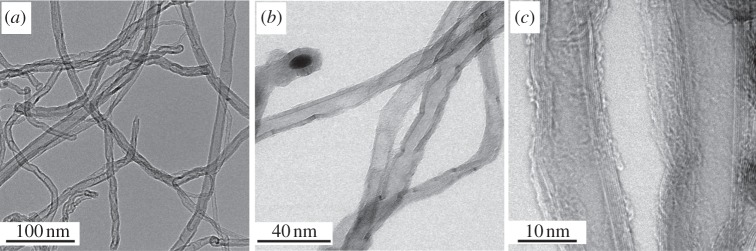
As-synthesized CNTs were purified by microwave digestion, rendering industrial-grade multiwalled CNTs that were 75 wt% pure (‘purified CNTs’). Neither structural damage nor loss of crystallinity were detected after purification. The similar thermogravimetric and derivative weight loss profiles (see electronic supplementary material, figure S2) measured for the as-synthesized and purified CNTs indicated that purification did not change the material structure. Identical Raman ID/IG ratios were observed for the two samples (ID/IG = 1.2, see electronic supplementary material, figure S5), further evincing that the quality of the CNTs did not change significantly upon acid purification. High-resolution TEM images showed multiwalled CNTs with well-defined walls (figure 1) and an outer diameter of 13 ± 4 nm. Some catalyst (Fe/Al2O3) particles were observed encapsulated in the purified CNTs, protected from acid purification. ICP-AES showed that the impurities (25 wt%) consisted of 5.3 wt% Fe and 19.7 wt% Al2O3 (see electronic supplementary material, figure S3). The FTIR spectra of the as-synthesized and purified CNT samples exhibited a ν(C=C) at approximately 1560 cm−1, confirming the graphitic structure of the CNT walls (see electronic supplementary material, figure S6). Additional functional groups were also detected on the CNT surfaces, and are discussed in detail in the electronic supplementary material. Briefly, these included alkyl groups (νas(C–H) = 2952 and 2922 cm−1; νs(C–H) ≈2870 cm−1 and 2850 cm−1) that characterize CNT defects [31–33], as well as ester groups (ν(C=O) = 1832 and 1730 cm−1) that indicated some surface oxygenation [33], which renders the CNTs somewhat hydrophilic.
Figure 1.
Transmission electron microscopy (TEM) images of acid-purified multiwalled carbon nanotubes (purified CNTs). (a,b) Multiwalled CNTs with few visible defects and containing a small number of encapsulated catalyst particles. (c) High-resolution TEM of the multiwalled CNTs.
3.2. Phytotoxicity assays
A seed's selectively permeable coat protects it from environmental hazards; however, once this is ruptured, the radicle (embryonic root) is exposed to the environment. Consequently, CNT phytotoxicity was assessed during both germination and seedling growth. As the CNTs contained 25 wt% catalytic impurities (see electronic supplementary material), control experiments containing only these impurities were also performed, at one-quarter of the nominal CNT concentration. This criterion was based on the extreme scenario in which all the catalytic impurities—free in the sample, contained between CNT aggregates and encapsulated in the CNTs (figure 1)—were bioavailable.
3.2.1. Germination
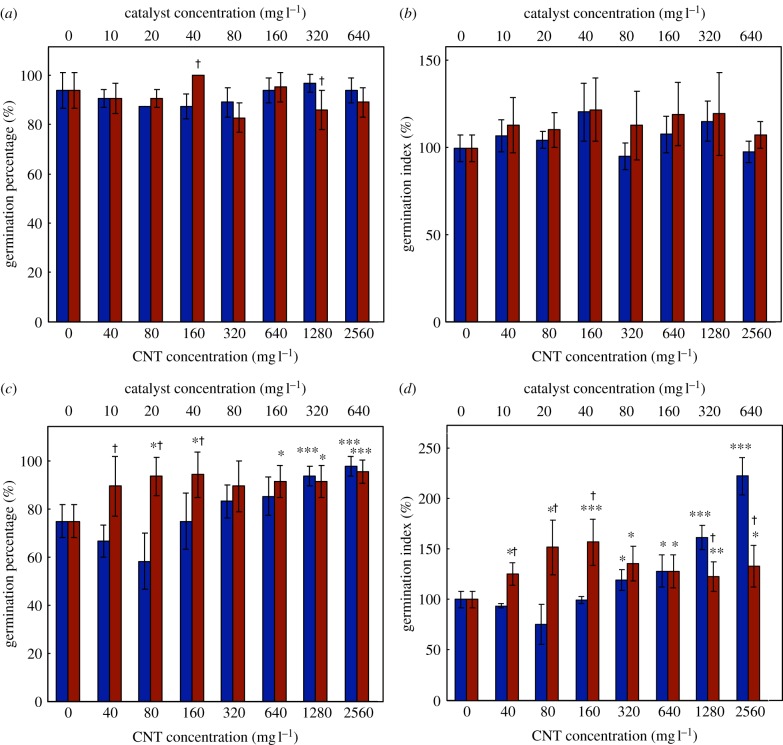
CNTs were not toxic to alfalfa or wheat germination under the conditions examined; both species tolerated them in high concentrations. Neither the germination percentage nor the germination index (GI) of alfalfa (figure 2a,b) was significantly affected by the CNT or catalyst treatments, compared with the controls. This complements reports stating that 2000 mg l−1 CNT did not affect the germination of lettuce, corn, cucumber, rape, radish or ryegrass [12], and that mung bean and Indian mustard seeds germinated normally in 40 mg l−1 CNT [14]. Conversely, 400 mg l−1 of a CNT−organic matter mixture reduced biomass and delayed flowering in rice seeds [11].
Figure 2.
Germination of alfalfa (a,b) and wheat (c,d) seeds incubated in a carbon nanotube–agar matrix (filled blue bar) and in the relevant catalytic impurities (filled red bar) (Fe and Al2O3). Seeds exhibiting a 5 mm radicle were recorded as germinated and the germination percentage was calculated from 16 (alfalfa) or 12 (wheat) seeds. The germination index (GI, see equation 2.1) combines information about seed germination and elongation. Abscissae represent nominal concentrations (*0.05 > p ≥ 0.01; **0.01 > p ≥ 0.005; ***p < 0.005; †statistically significant difference between CNT and catalyst treatments). (Online version in colour.)
For wheat, the germination percentage and GI (figure 2c,d) increased significantly in the presence of CNTs. At 2560 mg l−1 CNT, the germination percentage was higher than the controls (98 versus 75%), and the GI reached twice that of the controls. Enhanced germination has been observed at lower CNT exposures; tomato seed germination and seedling development improved dramatically upon exposure to 40 mg l−1 CNT [10]. No pH changes were observed during our assays (pH∼7), so changes in acidity were not responsible for the observed effects. Notably, the catalyst impurities alone strongly improved wheat germination percentage (figure 2c), exceeding the corresponding CNT effect in low-concentration treatments (40–160 mg l−1). This suggests that the enhanced germination percentage of wheat seeds in the presence of CNTs may be due to the catalyst residues rather than due to the CNTs themselves, and that the catalyst residues in a CNT sample are only partially bioavailable. Alternatively, the CNTs themselves may negatively impact germination percentage, and thus partially counteract the catalyst effect. The GI, on the other hand, was impacted more strongly by CNT concentration than the corresponding catalyst concentration at the highest concentrations. Thus, CNTs influenced root elongation in wheat; this was confirmed during seedling-growth experiments (vide infra).
Interestingly, wheat germination was more sensitive to CNTs than alfalfa germination was. As the larger wheat seed has a lower surface-to-volume ratio, the opposite result might have been expected. Indeed, Ma et al. [34] found that smaller seeds, e.g. lettuce, were more sensitive to nanoparticles than larger-seeded species such as wheat. In the present study, seed type may be the difference. Alfalfa produces hard seeds, with water-impermeable seed coats [35], so water uptake occurs primarily by the micropyle pore [36]; whereas white-grained wheat produces soft seeds. Thus, it is possible that CNTs penetrated the seed coat of wheat during water uptake and became bioavailable, but could not penetrate the seed coat of alfalfa; this would imply that the micropyle played a minor role in CNT bioavailability during alfalfa germination. This is particularly consistent with the observation that the catalyst impurities, which may have been partially solubilized, accounted for most of the increase in germination percentage in wheat.
3.2.2. Seedling elongation
Alfalfa and wheat seedlings grown in CNTs or catalyst impurities in agar appeared normal. No discolouration or swelling was detected, nor were symptoms of Fe excess (brown spotting, shoot bronzing, biomass reduction [37]). Above-ground organs (shoot, cotyledons, leaves or coleoptile) developed normally (see electronic supplementary material, figures S7–S11). Thus, in both cases, only root elongation was significantly enhanced compared with the controls (figure 3). The greatest effect was seen in roots of seedlings grown in 1280 mg l−1 CNTs, which were 70 per cent longer for alfalfa and 30 per cent longer for wheat, compared with the controls. At the highest CNT concentration, the roots were shorter than in the other CNT treatments, though still longer than the control plants. Similarly, both wheat and mung bean roots were more sensitive than their shoots to Cu nanoparticles [38]. CNTs have enhanced root elongation in cucumber and onion seedlings, but negatively impacted tomato, lettuce, carrot and cabbage [9]. However, the root growth enhancements in cucumber and onion were smaller than those we observed for alfalfa. A recent study on the genetic responses of tomato to CNTs suggested that they sensed CNTs as stress factors, and thus altered their gene expression and activated their growth [39]. This may explain the CNT-mediated growth enhancement we observed in alfalfa and wheat, although further research is needed. Unaltered pH values measured throughout the experiment (pH∼7) rule out acidity effects. Notably, in this and earlier [9,11,12] studies, the effects of CNTs on a given plant were independent of its genetic family (monocotyledon or dicotyledon).
Figure 3.
Root elongation of (a) alfalfa and (b) wheat seedlings. Twenty millimetre long seedlings were transplanted to the CNT−agar or catalyst−agar matrices and grown for 6 days. Abscissae represent nominal concentrations. (*0.05 > p ≥ 0.01; **0.01 > p ≥ 0.005; ***p < 0.005; †statistically significant difference between CNT and catalyst treatments; filled blue bar, carbon nanotubes; filled red bar, catalyst impurities). (Online version in colour.)
Catalyst impurities (Fe and Al2O3) also impacted seedling root elongation in both plants. Though alfalfa germination was unaffected (figure 2), the roots of alfalfa seedlings grown in the presence of the catalytic impurities showed enhanced growth compared with the controls (figure 3). Generally, the effects of catalytic impurities on alfalfa root growth were smaller or not significantly different from the effects of CNTs. Only at the highest catalyst concentration did the positive effect of catalytic impurities significantly exceed that of the CNTs. Thus, the impacts of the CNTs on alfalfa roots may be partially, but not entirely, attributed to their catalytic impurities.
On the other hand, despite the catalytic impurities having strongly impacted wheat germination (figure 2, vide supra), the catalyst impurities alone had no significant impact on the roots of wheat plants (figure 3b). Therefore, the mechanism by which catalytic impurities impact the growth of alfalfa seedlings did not operate in wheat at the concentrations studied (≤640 mg l−1).
The Fe and Al2O3 impurities were tested together, and either (or both) could have impacted germination and growth. Neither nano-Fe0 nor nano-Fe3O4 is toxic to pumpkin [40,41], but nano-Fe inhibits germination and growth in flax, ryegrass and barley [42], and nano-Fe3O4 inhibits root growth in Arabidopsis thaliana [43]. Both nano-Fe [40,44,45] and nano-Fe3O4 [41] have been taken up by exposed plants, so both can be bioavailable. Dissolved Al species can cause oxidative stress, interfere with the uptake and metabolism of essential elements, and cause morphological alterations in higher organisms [46–48]. In the present system, growth was actually enhanced in some cases, possibly because the impurities could have supplied the plant with Fe, a micronutrient, or have induced a stress response in the plants [39]. Potential impacts of the Al2O3 support may have been prevented by the poor solubility of Al species at the neutral pH of the growth medium; hydrated Al species are more soluble under acidic conditions.
3.3. Carbon nanotube internalization
Elemental analysis cannot detect CNTs in cells because of their carbonaceous composition. Low contrast in the electron microscope and the similarity of CNTs to cellular structures can result in their misidentification. Advances have been made using fluorescence microscopy in animal tissues [49,50], but the autofluorescence of vascular plant tissues generally blocks the visualization of CNTs. Only recently has vibrational spectroscopy been used to detect CNTs in plants. The Raman G peak was used to detect CNTs on the surface of longitudinal cuts of tomato seeds [10] and in tomato fruits of plants grown in CNT-rich media [39]. Lin et al. [11] confirmed by Fourier-transformed Raman spectroscopy that aggregates detected in rice were fullerene C70. However, the CNT Raman signal in rice plants exposed to multiwalled CNTs was too weak to demonstrate uptake [11]. To investigate CNT adsorption and internalization in alfalfa and wheat, we used Raman spectroscopy and electron microscopy.
CNT internalization by wheat and alfalfa was studied for plants grown in CNT–water or CNT–agar. Agar proved to be an adequate matrix for the preparation of CNT-enriched media, allowing the CNTs to be homogeneously distributed, and avoiding precipitation and aggregation. Thus agar ensures CNT bioavailability, and its viscous nature imitates soil-like environments better than water can, making it a valuable model for natural environments. CNT–water, on the other hand, offers less diffusion resistance, thus increasing the opportunities for CNTs to interact with the plants.
3.3.1. Raman spectroscopy
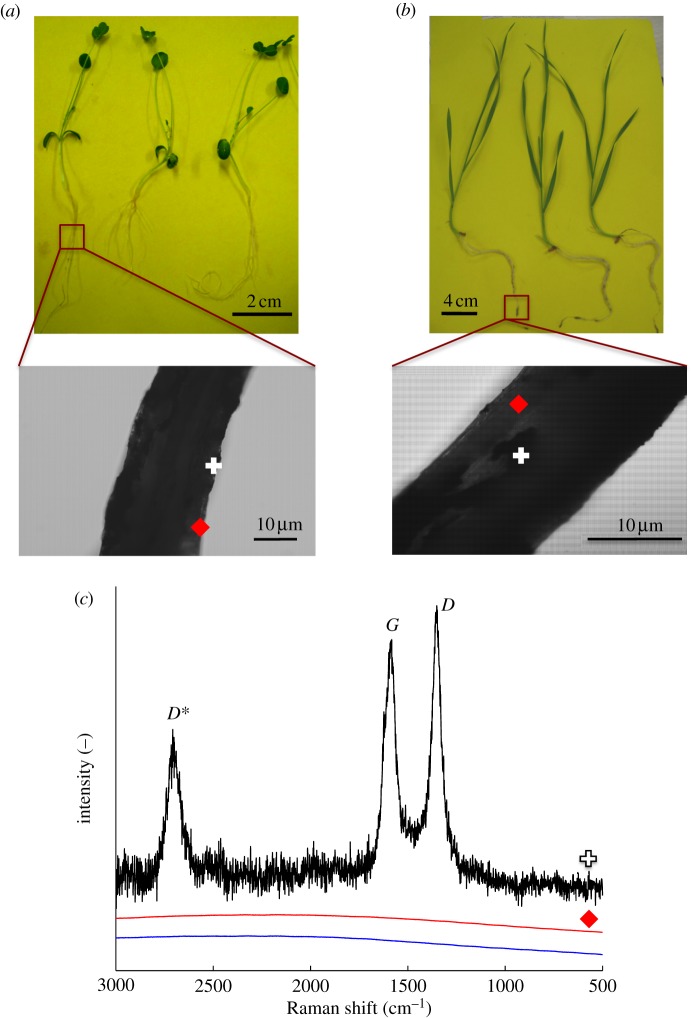
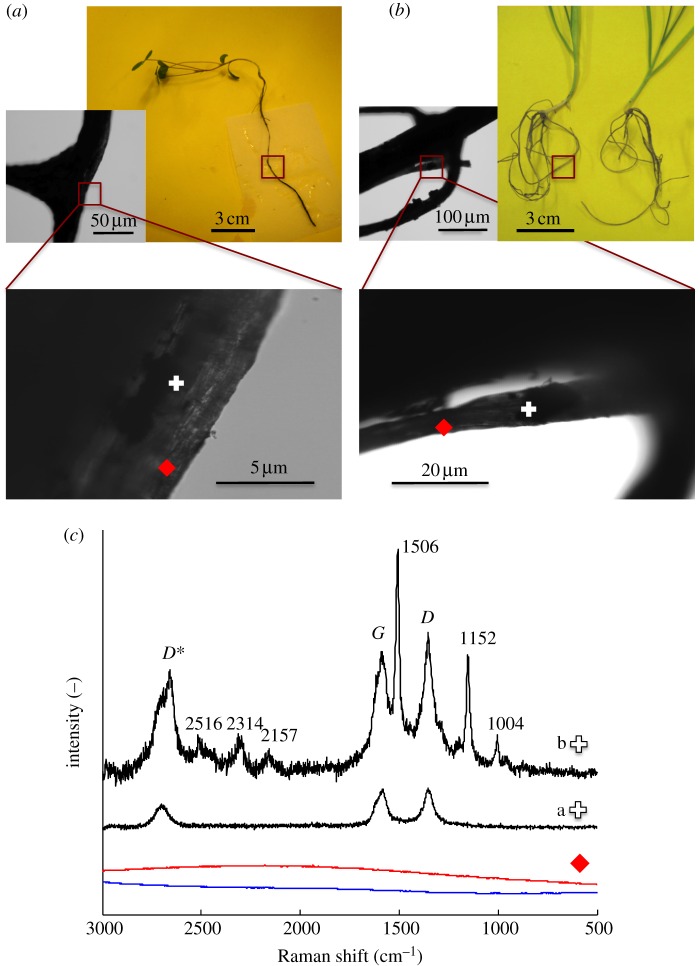
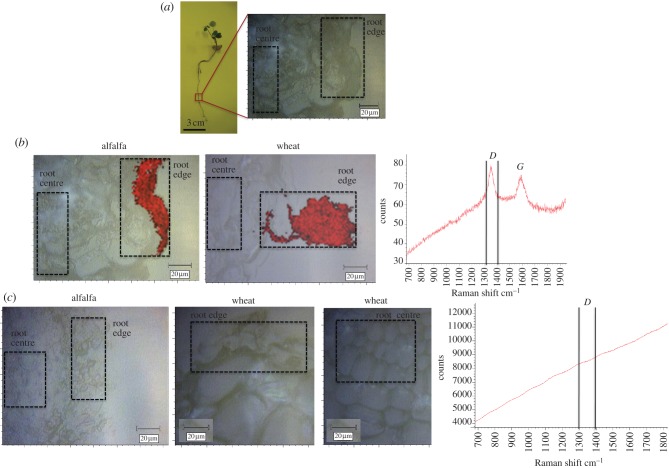
Raman spectroscopic signatures unique to CNTs (D, G and D* bands) were used to investigate the presence of CNTs in the plants. First, we attempted to trace purified CNTs in whole roots. Black deposits were present on the roots of plants grown in CNT–agar and –water, even after thorough washing, suggesting strong adsorption. Thus, both clear and dark spots on the roots were examined. No CNTs were detected at the clear spots of plants grown in CNT–agar (figure 4) or CNT-water (figure 5). The Raman spectroscopic signatures of CNTs were indeed found at the dark spots, confirming that the adsorbed material was CNTs (figures 4 and 5). However, additional Raman peaks were also present at the dark spots on the roots of plants grown in CNT–water (figure 5). These included signals for νas(NCS) at 2157 cm−1 and νs(NCS) at 1152 and 1004 cm−1, indicating the presence of isothiocyanate groups [51], which occur in the essential oils of plants such as alfalfa [52], Indian mustard [53] and rape [54]. Other signals, such as those at 2516, ≈2314 and 1506 cm−1, may have corresponded to these same compounds, or indicated functional groups on the CNT walls (i.e. defects). Thus, root exudates may have contributed to the bonding of CNTs to the roots, resulting in the irreversible adsorption of CNTs. The interaction of CNTs with cell wall biomolecules has been proposed as a mechanism by which CNTs induce hypersensitive response in plant cells [55]. The roots studied here appeared healthy by light microscopy (see electronic supplementary material, figure S12); however, reactive oxygen species were not assayed.
Figure 4.
Raman spectra of whole roots grown in agar containing multiwalled carbon nanotubes (CNTs). (a) Alfalfa; (b) wheat and (c) Raman spectra collected from alfalfa roots. In the ‘dark spot’ spectrum (plus symbol) the detection of D, G, and D* peaks indicate the presence of CNTs, consistent with the light microscopy images of the root surfaces. The absence of spectral features in the ‘clear spot’ spectrum (red diamond) indicates the absence of CNTs. The control spectrum (solid blue line) was collected from plants grown in agar without CNTs. Similar spectra were collected for wheat and alfalfa roots. (Online version in colour.)
Figure 5.
Raman spectra of whole roots grown in aqueous suspensions of multiwalled carbon nanotubes (CNTs). (a) Alfalfa; (b) wheat and (c) Raman spectra collected from alfalfa roots. In the ‘dark spot’ spectrum (plus symbol) the detection of D, G and D* peaks indicate the presence of CNTs, as shown in light microscopy images of the roots' surface. Additional peaks were detected in areas where CNTs were adsorbed to the roots' surface, though not in consistent amounts (compare, for example, spectra a and b, recorded from the same root). In the ‘clear spot’ spectrum (red diamond) the absence of spectral features indicates absence of CNTs. Control spectrum (solid blue line) was collected from plants grown without CNT. Similar spectra were collected for wheat and alfalfa roots. (Online version in colour.)
CNTs have been detected on the root surfaces of rice [11], wheat [13] and cucumber [9]; however, none of these internalized CNTs. Here, Raman spectroscopy identified the black aggregates on the root surfaces of alfalfa and wheat as CNTs (figures 4 and 5), but spectra of areas without visible aggregates were featureless. Therefore, CNTs were either adsorbed but not internalized, or were internalized but had Raman intensities insufficient for detection. To search for internalized CNTs, we pursued a Raman mapping technique that enabled the investigation of the planar inner section of the root tissue. Raman mapping has been used to trace CNTs in polymer−CNT composites [56–58], cancer cells [59] and mice [20,21], and to map CNT−quantum dot composites in tomato plants [60]. Here, root cross-sections were surveyed for the characteristic CNT bands, and false-colour maps were generated by calculating the signal-to-baseline ratio for the D band (1350 cm−1, figure 6). CNTs were found on the root surfaces of both alfalfa and wheat (figure 6b, area shown in red) grown in CNT-water. CNTs were associated with the root epidermis of alfalfa, but otherwise absent in the root tissue (figure 6b, ‘root centre’, colourless). In wheat, the CNTs covered a root hair and were seen on the root epidermis, but, similarly to alfalfa, were not detected in the survey of internal tissues (figure 6b). Thus compounds released by the plant, which probably contributed to CNT adsorption (vide supra), may also have prevented CNT penetration. Plants grown in CNT-agar showed no traces of CNTs in alfalfa or wheat roots (figure 6c). As CNTs were detected on the roots of whole-plant samples, embedding and cryosectioning may have caused material loss from CNT–agar-grown plants, where root exudates were not available to strengthen CNT adsorption. Overall, however, Raman mapping provided no evidence of CNT internalization in plants grown in CNT–agar or –water. Alimohammadi et al. [60] found CNT−quantum dots in the leaves of exposed tomato plants; the difference could have resulted from the nanomaterials and species studied, or from variations in the experimental conditions.
Figure 6.
Raman mapping of root cross-sections of plants grown in the presence of multiwalled carbon nanotubes (CNTs). (a) Root segments were fixed in cryostat specimen matrix by immersion in N2(l) and cryosectioned for analysis by Raman mapping. The edge and centre of the root cross-sections (as highlighted) were surveyed for the presence of the D band (1350 cm−1). (b) Plants grown in an aqueous suspension of multiwalled CNTs; red coloration indicates the presence of the D band, as shown in the Raman spectra. (c) Plants grown in agar doped with multiwalled CNTs; the lack of red coloration indicates the absence of multiwalled CNTs. (Online version in colour.)
3.3.2. Microscopy
A thorough search for purified CNTs within root cells was performed using electron microscopy; however, a lack of contrast and the small diameter of the CNTs precluded conclusive results. We therefore grew the plants in agar enriched with multiwalled CNTs functionalized with Fe3O4 nanoparticles. Though they could potentially alter the properties, bioavailability and plant interactions of the CNTs, the nanoparticles provided the opportunity to search for CNTs in plant tissues using Fe energy-filter mapping.1 TEM characterization of the Fe3O4-CNTs confirmed that they bore Fe3O4 nanoparticles, which were homogeneous in size and highly dispersed on the external nanotube surface (figure 7). The Fe3O4-CNTs had the same degree of wall graphitization (ID/IG = 1.2) and similar external diameter to purified CNTs (table 1), so nanoparticle addition had little or no effect on the physical properties of the CNTs (full characterization details for the Fe3O4-CNTs, including thermogravimetric analysis, Raman and FTIR spectra, N2 adsorption–desorption isotherms and pore size distributions, are provided in the electronic supplementary material).
Figure 7.
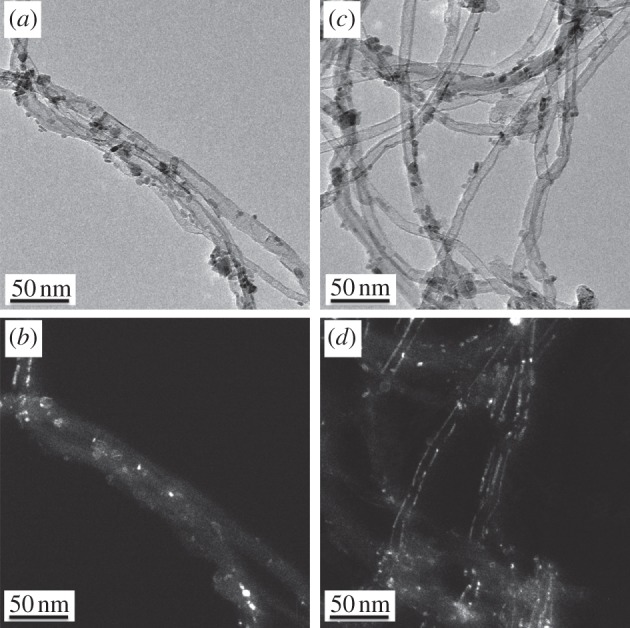
(a,c) Transmission electron microscopy (TEM) images of Fe3O4-functionalized multiwalled carbon nanotubes (CNTs). Fe3O4 nanoparticles were selectively deposited on the CNT walls. (b,d) Corresponding dark-field images highlight the crystallinity of the CNT walls and the attached Fe3O4 nanoparticles.
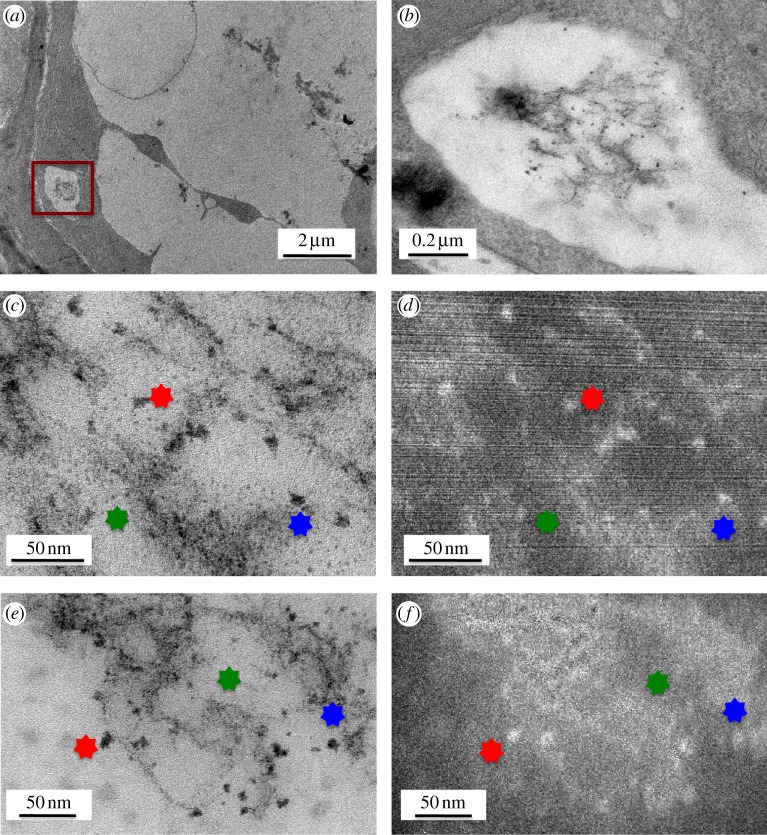
Consistent with the results of the Raman mapping studies, exhaustive exploration of the root tips and points of emergence of secondary roots of alfalfa plants exposed to Fe3O4-CNTs revealed no evidence of their internalization. In one exposed wheat root, however, TEM and high-resolution TEM images (figure 8) revealed tube-like features whose Fe energy-filter maps (figures 8d,f) highlighted white spheroids that suggested the presence of Fe. The Fe3O4-CNTs were observed in an epidermal cell of the root tip, and appeared to lie within a vacuole (figure 8).
Figure 8.
Transmission electron microscopy (TEM) images of wheat grown for 7 days in agar enriched with 2560 mg l−1 Fe3O4-functionalized carbon nanotubes. (a) Epidermal cell from the root tip of wheat. (b) Higher magnification image of the area highlighted in (a). (c,e) High-resolution TEM images of the area of interest and their corresponding Fe energy-filter maps for Fe (d,f, respectively). Owing to slight shifting during map acquisition, stars are used to indicate the same points in the images. (Online version in colour.)
Two-photon excitation microscopy has shown CNTs piercing, but not completely penetrating, the cell walls of wheat roots [13]. Aside from that result, microscopic techniques have provided limited evidence for the internalization of CNTs by live plants, largely because of the difficulties in imaging CNTs in cells. Here, attaching Fe3O4 nanoparticles to CNTs allowed them to be traced, and thus provided evidence that Fe3O4-CNTs crossed the cell wall and plasma membrane of one wheat cell. This result is preliminary and the penetration mechanism requires further investigation; however, the pores in wheat cell walls are larger (d ≤ 20 nm [61]) than the CNTs used in this study (d ∼ 12 nm; table 1), and may have provided a penetration pathway for appropriately oriented CNTs. That a wheat cell, but not an alfalfa cell, took up Fe3O4-CNTs may also reflect their different root types. Within 7 days, wheat develops several long, thin, seminal branched roots; whereas alfalfa develops one main root with a few short secondary roots. Thus, the wheat root system has larger surface area in contact with the test suspension [38]. Severe tissue damage, such as that seen in nanoparticle-exposed ryegrass [62], could also provide a penetration pathway; however our light-microscope images of alfalfa and wheat roots did not reveal extensive damage (see electronic supplementary material, figure S7). Minor, unobserved lesions cannot be excluded though, especially given the very low incidence of penetration.
4. Conclusions
Despite their promise, the environmental fate of CNTs, and their interactions with biological systems, have received limited study. We found that two crop species, alfalfa and wheat, were not damaged by multiwalled CNTs or their catalytic impurities (Fe species and Al2O3); rather, the latter enhanced their growth. Wheat germination was also promoted. More importantly, these plants tolerated high concentrations of industrial-grade CNTs, and even exhibited enhanced root development in their presence. In alfalfa, but not wheat, this could be partially attributed to the catalyst impurities.
No multiwalled-CNT uptake was observed in alfalfa roots. In wheat, Fe3O4-functionalized CNTs were detected in an epidermal root cell. Raman mapping showed that CNTs adsorbed onto alfalfa and wheat root surfaces without altering plant development or root-tissue morphology. The presence of functional groups such as carboxylic acids and isothiocyanates at the sites of CNT adsorption suggests that the CNTs were chemically bound to the plant cell walls.
Acknowledgements
P.M. thanks the Chilean Government Bicentennial Scholarship, Program for Australia and New Zealand, for financial support. The authors gratefully acknowledge L. Carter, T. Savage, V. Lo and J. Liu for assistance with vibrational spectroscopy, inductively coupled plasma spectroscopy, electron microscopy and CNT characterization, respectively, and A. I. Minett for helpful discussions.
Footnotes
The Fe atoms initially present in the CNTs, i.e. those derived from the residual catalyst, could not be imaged by energy-filter mapping because they were primarily contained within CNTs, and thus were not accessible to the electron beam.
References
- 1.Smart S. K., Cassady A. I., Lu G. Q., Martin D. J. 2006. The biocompatibility of carbon nanotubes. Carbon 44, 1034–1047 10.1016/j.carbon.2005.10.011 (doi:10.1016/j.carbon.2005.10.011) [DOI] [Google Scholar]
- 2.Mauter M. S., Elimelech M. 2008. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 42, 5843–5859 10.1021/es8006904 (doi:10.1021/es8006904) [DOI] [PubMed] [Google Scholar]
- 3.Ke P. C., Qiao R. 2007. Carbon nanomaterials in biological systems. J. Phys.: Condens. Matter 19, 373 101–373 125 10.1088/0953-8984/19/37/373101 (doi:10.1088/0953-8984/19/37/373101) [DOI] [Google Scholar]
- 4.Zavaleta C., de la Zerda A., Liu Z., Keren S., Cheng Z., Schipper M., Chen X., Dai H., Gambhir S. S. 2008. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Lett. 8, 2800–2805 10.1021/nl801362a (doi:10.1021/nl801362a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunlan J. C., Mehrabi A. R., Bannon M. V., Bahr J. L. 2004. Water-based single-walled-nanotube-filled polymer composite with an exceptionally low percolation threshold. Adv. Mater. 16, 150–153 10.1002/adma.200305409 (doi:10.1002/adma.200305409) [DOI] [Google Scholar]
- 6.Makar J. M., Chan G. W. 2009. Growth of cement hydration products on single-walled carbon nanotubes. J. Am. Ceram. Soc. 92, 1303–1310 10.1111/j.1551-2916.2009.03055.x (doi:10.1111/j.1551-2916.2009.03055.x) [DOI] [Google Scholar]
- 7.Donaldson K., Aitken R., Tran L., Stone V., Duffin R., Forrest G., Alexander A. 2006. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 92, 5–22 10.1093/toxsci/kfj130 (doi:10.1093/toxsci/kfj130) [DOI] [PubMed] [Google Scholar]
- 8.Klaine S. J., Alvarez P. J. J., Batley G. E., Fernandes T. F., Handy R. D., Lyon D. Y., Mahendra S., McLaughlin M. J., Lead J. R. 2008. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 27, 1825–1851 10.1897/08-090.1 (doi:10.1897/08-090.1) [DOI] [PubMed] [Google Scholar]
- 9.Cañas J. E., Long M., Nations S., Vadan R., Dai L., Luo M., Ambikapathi R., Lee E. H., Olszyk D. 2008. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 27, 1922–1931 10.1897/08-117.1 (doi:10.1897/08-117.1) [DOI] [PubMed] [Google Scholar]
- 10.Khodakovskaya M., Dervishi E., Mahmood M., Xu Y., Li Z., Watanabe F., Biris A. S. 2009. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3, 3221–3227 10.1021/nn900887m (doi:10.1021/nn900887m) [DOI] [PubMed] [Google Scholar]
- 11.Lin S., Reppert J., Hu Q., Hudson J. S., Reid M. L., Ratnikova T. A., Rao A. M., Luo H., Ke P. C. 2009. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5, 1128–1132 10.1002/smll.200801556 (doi:10.1002/smll.200801556) [DOI] [PubMed] [Google Scholar]
- 12.Lin D., Xing B. 2007. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 150, 243–250 10.1016/j.envpol.2007.01.016 (doi:10.1016/j.envpol.2007.01.016) [DOI] [PubMed] [Google Scholar]
- 13.Wild E., Jones K. C. 2009. Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ. Sci. Technol. 43, 5290–5294 10.1021/es900065h (doi:10.1021/es900065h) [DOI] [PubMed] [Google Scholar]
- 14.Ghodake G., Seo Y. D., Park D., Lee D. S. 2010. Phytotoxicity of carbon nanotubes assessed by Brassica juncea and Phaseolus mungo. J. Nanoelectron. Optoelectron. 5, 157–160 10.1166/jno.2010.1084 (doi:10.1166/jno.2010.1084) [DOI] [Google Scholar]
- 15.Guo L., Morris D. G., Liu X., Vaslet C., Hurt R. H., Kane A. B. 2007. Iron bioavailability and redox activity in diverse carbon nanotube samples. Chem. Mater. 19, 3472–3478 10.1021/cm062691p (doi:10.1021/cm062691p) [DOI] [Google Scholar]
- 16.Liu X., Gurel V., Morris D., Murray D. W., Zhitkovich A., Kane A. B., Hurt R. H. 2007. Bioavailability of nickel in single-wall carbon nanotubes. Adv. Mater. 19, 2790–2796 10.1002/adma.200602696 (doi:10.1002/adma.200602696) [DOI] [Google Scholar]
- 17.Hirsch A. 2002. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 41, 1853–1859 (doi:10.1002/1521-3773(20020603)41:11<1853::AID-ANIE1853>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 18.Ge C., et al. 2008. Quantitative analysis of metal impurities in carbon nanotubes: efficacy of different pretreatment protocols for ICPMS spectroscopy. Anal. Chem. 80, 9426–9434 10.1021/ac801469b (doi:10.1021/ac801469b) [DOI] [PubMed] [Google Scholar]
- 19.Liu S., Wei L., Hao L., Fang N., Chang M. W., Xu R., Yang Y., Chen Y. 2009. Sharper and faster ‘nano darts’ kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 3, 3891–3902 10.1021/nn901252r (doi:10.1021/nn901252r) [DOI] [PubMed] [Google Scholar]
- 20.Liu Z., Davis C., Cai W., He L., Chen X., Dai H. 2008. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl Acad. Sci. USA 105, 1410–1415 10.1073/pnas.0707654105 (doI:10.1073/pnas.0707654105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schipper M. L., Nakayama-Ratchford N., Davis C. R., Kam N. W. S., Chu P., Liu Z., Sun X., Dai H., Gambhir S. S. 2008. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotech. 3, 216–221 (doi:http://www.nature.com/nnano/journal/v3/n4/suppinfo/nnano.2008.68_S1.html.) [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Dunens O. M., Mackenzie K. J., See C. H., Harris A. T. 2008. Postsynthesis microwave treatment to give high-purity multiwalled carbon nanotubes. A.I.Ch.E. J. 54, 3303–3307 10.1002/aic.11641 (doi:10.1002/aic.11641) [DOI] [Google Scholar]
- 23.Bali R., Siegele R., Harris A. T. 2010. Biogenic separation, accumulation and cellular distribution of Cu, Co, and Ni in Medicago sativa under idealized conditions. Sep. Sci. Technol. 45, 1395–1401 10.1080/01496391003681014 (doi:10.1080/01496391003681014) [DOI] [Google Scholar]
- 24.López-Moreno M. L., de la Rosa G., Hernández-Viezcas J. A., Peralta-Videa J. R., Gardea-Torresdey J. L. 2010. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 58, 3689–3693 10.1021/jf904472e (doi:10.1021/jf904472e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Environmental Protection Agency 1996. Ecological effects test guidelines: Seed germination/root elongation toxicity test. Washington, DC: Prevention, Pesticides and Toxic Substances (7101) [Google Scholar]
- 26.OECD Guideline for testing of chemicals 2003. Terrestrial plant test: 208: Seedling emergence and seedling growth test. Paris: Organisation for Economic Co-operation and Development [Google Scholar]
- 27.Barrena R., Casals E., Colón J., Font X., Sánchez A., Puntes V. 2009. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75, 850–857 10.1016/j.chemosphere.2009.01.078 (doi:10.1016/j.chemosphere.2009.01.078) [DOI] [PubMed] [Google Scholar]
- 28.Reynolds E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 10.1083/jcb.17.1.208 (doi:10.1083/jcb.17.1.208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers K. W., Brown S. C., Krishna V. B., Wasdo S. C., Moudgil B. M., Roberts S. M. 2006. Research strategies for safety evaluation of nanomaterials. VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol. Sci. 90, 296–303 10.1093/toxsci/kfj099 (doi:10.1093/toxsci/kfj099) [DOI] [PubMed] [Google Scholar]
- 30.Santos A. R., Miguel A. S., Tomaz L., Malhó R., Maycock C., Vaz Patto M. C., Fevereiro P., Oliva A. 2010. The impact of CdSe/ZnS quantum dots in cells of Medicago sativa in suspension culture. J. Nanobiotechnol. 8, 24. 10.1186/1477-3155-8-24 (doi:10.1186/1477-3155-8-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C., Liang B., Ogino A., Wang X., Nagatsu M. 2009. Oxygen functionalization of multiwall carbon nanotubes by microwave-excited surface-wave plasma treatment. J. Phys. Chem. C 113, 7659–7665 10.1021/jp9012015 (doi:10.1021/jp9012015) [DOI] [Google Scholar]
- 32.Scheibe B., Borowiak-Palen E., Kalenczuk R. J. 2010. Oxidation and reduction of multiwalled carbon nanotubes—preparation and characterization. Mater. Charact. 61, 185–191 10.1016/j.matchar.2009.11.008 (doi:10.1016/j.matchar.2009.11.008) [DOI] [Google Scholar]
- 33.Zhao C., Ji L., Liu H., Hu G., Zhang S., Yang M., Yang Z. 2004. Functionalized carbon nanotubes containing isocyanate groups. J. Solid State Chem. 177, 4394–4398 10.1016/j.jssc.2004.09.036 (doi:10.1016/j.jssc.2004.09.036) [DOI] [Google Scholar]
- 34.Ma Y., Kuang L., He X., Bai W., Ding Y., Zhang Z., Zhao Y., Chai Z. 2010. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78, 273–279 10.1016/j.chemosphere.2009.10.050 (doi:10.1016/j.chemosphere.2009.10.050) [DOI] [PubMed] [Google Scholar]
- 35.Davies P. A. 1928. The effect of high pressure on the percentages of soft and hard seeds of Medicago sativa and Melilotus alba. Am. J. Bot. 15, 433–436 10.2307/2435804 (doi:10.2307/2435804) [DOI] [Google Scholar]
- 36.Deshpande S. S., Cheryan M. 1986. Microstructure and water uptake of Phaseolus and winged beans. J. Food Sci. 51, 1218–1223 10.1111/j.1365-2621.1986.tb13089.x (doi:10.1111/j.1365-2621.1986.tb13089.x) [DOI] [Google Scholar]
- 37.Nenova V. 2009. Growth and photosynthesis of pea plants under different iron supply. Acta Physiol. Plant. 31, 385–391 10.1007/s11738-008-0247-2 (doi:10.1007/s11738-008-0247-2) [DOI] [Google Scholar]
- 38.Lee W.-M., An Y.-J., Yoon H., Kweon H.-S. 2008. Toxicity and bioavailability of copper nanoparticles to the terrestial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 27, 1915–1921 10.1897/07-481.1 (doi:10.1897/07-481.1) [DOI] [PubMed] [Google Scholar]
- 39.Khodakovskaya M. V., de Silva K., Nedosekin D. A., Dervishi E., Biris A. S., Shashkov E. V., Galanzha E. I., Zharov V. P. 2011. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl Acad. Sci. USA 108, 1028–1033 10.1073/pnas.1008856108 (doi:10.1073/pnas.1008856108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Melendi P., et al. 2008. Nanoparticles as smart treatment-delivery systems in plants: assessment of different techniques of microscopy for their visualization in plant tissues. Ann. Bot. 101, 187–195 10.1093/aob/mcm283 (doi:10.1093/aob/mcm283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H., Han J., Xiao J. Q., Jin Y. 2008. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 10, 713–717 10.1039/b805998e (doi:10.1039/b805998e) [DOI] [PubMed] [Google Scholar]
- 42.El-Temsah Y. S., Joner E. J. 2010. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 27, 42–49 10.1002/tox.20610 (doi:10.1002/tox.20610) [DOI] [PubMed] [Google Scholar]
- 43.Lee C. W., Mahendra S., Zodrow K., Li D., Tsai Y.-C., Braam J., Alvarez P. J. J. 2010. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 29, 669–675 10.1002/etc.58 (doi:10.1002/etc.58) [DOI] [PubMed] [Google Scholar]
- 44.Cifuentes Z., Custardoy L., de la Fuente J., Marquina C., Ibarra M. R., Rubiales D., Pérez-de-Luque A. 2010. Absorption and translocation to the aerial part of magnetic carbon-coated nanoparticles through the root of different crop plants. J. Nanobiotechnol. 8, 26. 10.1186/1477-3155-8-26 (doi:10.1186/1477-3155-8-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corredor E., et al. 2009. Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol. 9, 45. 10.1186/1471-2229-9-45 (doi:10.1186/1471-2229-9-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rengel Z. 2004. Aluminium cycling in the soil–plant–animal–human continuum. BioMetals 17, 669–689 10.1007/s10534-004-1201-4 (doi:10.1007/s10534-004-1201-4) [DOI] [PubMed] [Google Scholar]
- 47.Roy A., Sharma A., Talukder G. 1988. Some aspects of aluminum toxicity in plants. Bot. Rev. 54, 145–178 10.1007/bf02858527 (doi:10.1007/bf02858527) [DOI] [Google Scholar]
- 48.Ma G., Rengasamy P., Rathjen A. J. 2003. Phytotoxicity of aluminium to wheat plants in high-pH solutions. Aust. J. Exp. Agr. 43, 497–501 10.1071/EA01153 (doi:10.1071/EA01153) [DOI] [Google Scholar]
- 49.Porter A. E., Gass M., Muller K., Skepper J. N., Midgley P. A., Welland M. 2007. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotech. 2, 713–717 10.1038/nnano.2007.347 (doi:10.1038/nnano.2007.347) [DOI] [PubMed] [Google Scholar]
- 50.Welsher K., Liu Z., Sherlock S. P., Robinson J. T., Chen Z., Daranciang D., Dai H. 2009. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotech. 4, 773–780 10.1038/nnano.2009.294 (doi:10.1038/nnano.2009.294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Socrates G. 2001. Infrared and Raman characteristic group frequencies: tables and charts, 3rd edn. New York, NY: Wiley [Google Scholar]
- 52.Park C. M., Taormina P. J., Beuchat L. R. 2000. Efficacy of allyl isothiocyanate in killing enterohemorrhagic Escherichia coli O157 : H7 on alfalfa seeds. Int. J. Food Microbiol. 56, 13–20 10.1016/S0168-1605(99)00202-0 (doi:10.1016/S0168-1605(99)00202-0) [DOI] [PubMed] [Google Scholar]
- 53.Morra M. J., Kirkegaard J. A. 2002. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 34, 1683–1690 10.1016/S0038-0717(02)00153-0 (doi:10.1016/S0038-0717(02)00153-0) [DOI] [Google Scholar]
- 54.Choesin D. N., Boerner R. E. J. 1991. Allyl isothiocyanate release and the allelopathic potential of Brassica napus (Brassicaceae). Am. J. Bot. 78, 1083–1090 10.2307/2444897 (doi:10.2307/2444897) [DOI] [Google Scholar]
- 55.Lin C., Fugetsu B., Su Y., Watari F. 2009. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 170, 578–583 10.1016/j.jhazmat.2009.05.025 (doi:10.1016/j.jhazmat.2009.05.025) [DOI] [PubMed] [Google Scholar]
- 56.Mayo J. D., Behal S., Adronov A. 2009. Phase separation of polymer-functionalized SWNTs within a PMMA/polystyrene blend. J. Polym. Sci., Part A: Polym. Chem. 47, 450–458 10.1002/pola.23161 (doi:10.1002/pola.23161) [DOI] [Google Scholar]
- 57.Radhakrishnan V. K., Davis E. W., Davis V. A. 2010. Influence of initial mixing methods on melt-extruded single-walled carbon nanotube–polypropylene nanocomposites. Polym. Eng. Sci. 50, 1831–1842 10.1002/pen.21696 (doi:10.1002/pen.21696) [DOI] [Google Scholar]
- 58.Zhou W., Pan K., Zhang L., Tian C., Fu H. 2009. Solar-induced self-assembly of TiO2-β-cyclodextrin-MWCNT composite wires. PCCP 11, 1713–1718 10.1039/b814529f (doi:10.1039/b814529f) [DOI] [PubMed] [Google Scholar]
- 59.Lamprecht C., Gierlinger N., Heister E., Unterauer B., Plochberger B., Brameshuber M., Hinterdorfer P., Hild S., Ebner A. 2012. Mapping the intracellular distribution of carbon nanotubes after targeted delivery to carcinoma cells using confocal Raman imaging as a label-free technique. J. Phys.: Condens. Matter 24, 164 206–164 209 10.1088/0953-8984/24/16/164206 (doi:10.1088/0953-8984/24/16/164206) [DOI] [PubMed] [Google Scholar]
- 60.Alimohammadi M., Xu Y., Wang D., Biris A. S., Khodakovskaya M. V. 2011. Physiological responses induced in tomato plants by a two-component nanostructural system composed of carbon nanotubes conjugated with quantum dots and its in vivo multimodal detection. Nanotechnology 22, 295101–295108 10.1088/0957-4484/22/29/295101 (doi:10.1088/0957-4484/22/29/295101) [DOI] [PubMed] [Google Scholar]
- 61.Chesson A., Gardner P. T., Wood T. J. 1997. Cell wall porosity and available surface area of wheat straw and wheat grain fractions. J. Sci. Food Agric. 75, 289–295 (doi:10.1002/(SICI)1097-0010(199711)75:3<289::AID-JSFA879>3.0.CO;2-R) [DOI] [Google Scholar]
- 62.Lin D., Xing B. 2008. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 42, 5580–5585 10.1021/es800422x (doi:10.1021/es800422x) [DOI] [PubMed] [Google Scholar]