Abstract
The cell migration plays a crucial role in a variety of physiological and pathological processes and can be regulated by the cell–substrate interactions. We found previously that the poly(sodium 4-styrenesulphonate) (PSS)/poly(diallyldimethylammonium) chloride (PDADMAC) multilayers post-treated in 1–5 M NaCl solutions result in continuous changes of their physico-chemical properties such as thickness, chemical composition, surface charge, swelling ratio and wettability. In this study, the responses of human smooth muscle cells (SMCs) on these salt-treated multilayers, particularly the governing factors of cellular migration that offer principles for designing therapeutics and implants, were disclosed. The cell migration rate was slowest on the 3 M NaCl-treated multilayers, which was comparable with that on tissue culture plates, but it was highest on 5 M NaCl-treated multilayers. To elucidate the intrinsic mechanisms, cell adhesion, proliferation, adhesion and related gene expressions were further investigated. The SMCs preferred to attach, spread and proliferate on the PSS-dominated surfaces with well-organized focal adhesion and actin fibres, especially on the 3 M NaCl-treated multilayers, while were kept round and showed low viability on the PDADMAC-dominated surfaces. The relative mRNA expression levels of adhesion-related genes such as fibronectin, laminin and focal adhesion kinase, and migration-related genes such as myosin IIA and Cdc42 were compared to explain the different cellular behaviours. These results reveal that the surface chemistry and the swelling of the salt-treated multilayers govern the cell migration behaviours.
Keywords: cell migration, polyelectrolyte multilayers, post treatment, smooth muscle cells, swelling
1. Introduction
One of the major challenges in regenerative medicine and tissue engineering is to optimize the material–cell interactions, and upon successful optimization, cell adhesion, proliferation, migration and differentiation can be modulated in a controlled manner. Cell adhesion, proliferation and differentiation behaviours on substrates are influenced by surface physico-chemical properties such as topography or roughness, hydrophobicity and hydrophilicity, charge, chemical groups, types of ligands and stiffness [1–6]. However, cell migration behaviours on different materials, which are essential for understanding the tissue regeneration process and for developing regenerative materials, are less understood. Previous studies show that cell migration is mediated through interactions with the surface-bound extracellular matrix proteins, growth factors and small ligands, such as collagen [7], fibronectin [8], laminin [9], VEGF [10], bFGF [11] and RGD [12,13]. Besides, cell migration also can be dominated by the chemical and physical properties of the substrates. For example, Webb et al. found that the migration rate of MC3T3-E1 osteoblasts is significantly slower on the thiol surface compared with the oxidized thiol, quaternary amine and methyl surfaces [14]. Pelham & Wang [6] and Lo et al. [15] showed that cell motility can be governed purely by the substrate rigidity. In general, cells on flexible substrates display a larger migration rate and can move from the soft region towards the rigid side of the substrate. More recently, we found that the cell mobility rate is increased initially along with the increase in poly(ethylene glycol) (PEG) amount on the surface, and reaches maximum values at a moderate grafting mass between 300 and 500 ng cm−2 [16].
Among the various surface engineering methods, the layer-by-layer assembly, refined by Decher in the 1990s [17], can be diversely used to tailor substrate properties and is particularly suitable to address material–cell interactions [18,19]. For example, different deposition parameters of the polyelectrolyte multilayers (PEMs), such as the type of polyelectrolytes [20,21], temperature [22], pH [23] and salt concentrations [24,25], have great impacts on hydration, swelling and mobility of the polymer chains. Consequently, the chemical composition, stability, surface functional groups, thickness, stiffness, hydration degree and surface roughness are varied, which in turn show different performance in terms of cell adhesion, proliferation and differentiation [23,26–28]. So far, both cell attractive and cell resistive PEMs have been made by using different building blocks [29–31]. Moreover, Picart et al. [32,33] found that the cell adhesion, proliferation and differentiation also strongly depend on the mechanical properties of PEMs. Those multilayers containing natural polysaccharides such as poly(l-lysine)/hyaluronan are usually highly hydrated, and thereby can resist cell adhesion. By contrast, the cells can adhere onto the cross-linked PEMs as a result of stiffness enhancement.
Multilayers composed of strong polyelectrolytes of poly(sodium 4-styrenesulphonate) (PSS)/poly(diallyldimethylammonium) chloride (PDADMAC) have been employed either alone or by integration of other components so as to modify the surface of the biomaterials. For example, gelatin was coated on the PSS/PDADMAC multilayers-modified nanofibres to improve L929 mouse fibroblast adhesion [34]. The PSS/PDADMAC multilayers also are used to regulate primary hepatocytes, which prefer to attach and spread on PSS but not on the PDADMAC surface, in a protein-free medium [2,35]. Mendelsohn et al. [26] compared the PSS/PDADMAC multilayers constructed with and without extraneous salt, and found that the PEMs assembled with salt are more cytophobic owing to a much less dense ionic cross-linking character. Recently, we found that the physico-chemical properties of the PSS/PDADMAC multilayers could be effectively modulated by post-treatment with different concentrations of NaCl solutions [36]. For the multilayers-1 M and multilayers-2 M (the multilayers post-treated with 1 and 2 M NaCl solutions, respectively), the original structures and properties of the multilayers are mostly retained, with a PDADMAC-dominated and positively charged surface and larger swelling ratios in water. The hardly swollen multilayers-3 M, by contrast, have a PSS-dominated and negatively charged surface as a result of a larger loss of PDADMAC. For the multilayers-4 and 5 M, PSS becomes abundant on the surface and the swelling ratios are quite high, especially for the multilayers-5 M, which shows the highest hydration extent in water. These salt-treated multilayers with different physico-chemical properties (chemical composition, surface charge and hydration degree) are expected to show different performances in regulating cell migration as will be shown in this pioneering study, obtaining a comprehensive view of the parameters governing cell migration.
The human vascular smooth muscle cells (SMCs) are essential for blood vessels, especially during vessel remodelling in physiological conditions such as pregnancy and exercise, or after vascular injury [37]. In some vascular surgeries, SMC migration may induce thrombosis and intimal hyperplasia, especially revascularization for small diameter blood vessel tissue engineering [38]. Hence, regulation of SMC motility on biomaterials is critical to the performance of blood-contacting implants and vascular tissue engineering scaffolds. For example, SMCs display a distinct morphology in softer scaffolds with more fibre looping, resulting in a stronger cell migration ability and enhanced healing effect in a rat abdominal wall replacement model [39].
In this work, the physico-chemical properties of the salt-treated multilayers in phosphate-buffered saline (PBS) and cell-culture medium will be studied, in which the cells are cultured and thereby these properties are intrinsically tied with the cell responses. The migration behaviours of SMCs are then monitored in situ on these salt-treated multilayers. Cell adhesion, organization of focal adhesions and cytoskeleton, as well as adhesion and migration-related gene expression are characterized to reveal the mediating mechanism. This study will clarify cellular migration behaviours on substrates with different physico-chemical properties, which is of paramount importance to elucidate the factors governing cell migration and in turn to apply the principles to the development of novel biomaterials.
2. Experimental section
2.1. Materials
Polyethyleneimine (PEI, Mw = 25 kDa), PDADMAC (Mw = 200–350 kDa), PSS (Mw = 70 kDa), fluorescein diacetate (FDA) and propidum iodide (PI) were obtained from Sigma-Aldrich. The water used in this work was purified by a Milli-Q water system (Millipore, USA). All the polyelectrolytes were prepared to a final concentration of 1 mg l−1 aqueous solutions. PEI was dissolved in water, and PSS and PDADMAC were supplemented with 1 M NaCl. Quartz, glass and silicon wafers were cleaned in piranha solution (7 : 3 v/v% H2SO4/H2O2). After rinsed with water, they were dried under a smooth stream of N2.
2.2. Multilayer assembly
To ensure the successful adsorption, a precursory layer of PEI was deposited on the silicon wafers. PSS and PDADMAC were then alternately assembled by auto-dipping at 20°C. Between alternate exposures to the two kinds of polymer solutions for 20 min, there were three rinses with 0.1 M NaCl solutions for 3 min. In the last step, the films were immersed in triple-distilled water for at least 5 min to eliminate the adsorbed salt. A total of seven bilayers were assembled and the multilayers are expressed as (PSS/PDADMAC)7.
2.3. Post-treatment of the multilayers in NaCl solutions
The (PSS/PDADMAC)7 multilayers were incubated in 1–5 M NaCl solutions at room temperature for 2 h and were then rinsed with water and dried under a smooth stream of N2, respectively. Because the PEMs were assembled in the 1 M NaCl solution, the physico-chemical properties of the pristine PEMs are identical to the multilayers-1 M [36,40].
2.4. Spectroscopic ellipsometry
The thickness of the multilayers was determined in air and liquid (water or PBS) from a spectroscopic ellipsometer (model M2000D, J. A. Woollam Inc., Lincoln, NE, USA) at an incident angle of 75° within a wavelength range of 300–1700 nm and 300–1100 nm, respectively. The thickness was calculated from the ellipsometric parameters, Δ and ψ, using a Cauchy model. The swelling ratio was defined as the ratio of multilayer thicknesses in wet and dry states, respectively.
2.5. Water contact angle measurement
The surface static contact angles of the multilayers were measured directly by dropping 1 μl of ultrapure water using a Krüss DSA 100 system at ambient temperature.
2.6. Surface charge properties
The surface zeta potentials of the multilayers were measured in 10 mM NaCl, using Delsa Nano Series zeta potential/submicron particle size analysers (Beckman Coulter, USA), via electrophoresis technology.
2.7. Cell culture
The human vascular SMCs were obtained from the Cell Bank of Typical Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cells were maintained in a regular growth medium consisting of high-glucose DMEM (Gibco, USA) supplemented with 10 per cent foetal bovine serum (FBS, Sijiqin Inc., Hangzhou, China), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin and cultured at 37°C in a 5 per cent CO2 humidified environment.
2.8. Cell migration
Before cell culture, the salt-treated multilayers were sterilized under UV light for 1 h. The SMCs were then plated on the salt-etched multilayers at a low cell density (5 × 103 cells cm−2) in order to minimize the influence of cell–cell interactions. Approximately 12 h after the cell plating in 10 per cent FBS DMEM, the cell migration was in situ recorded using a time-lapse phase-contrast microscope (IX81, Olympus) equipped with an incubation chamber (37°C and 5% CO2 humidified atmosphere).
The SMC trajectories were reconstructed from the centre positions of individual cells over the whole observation time. The cell migration distance (S) and displacement (D) was calculated by Imagepro Plus software according to the following equations at 1 h time intervals over the observation time of 24 h (t). Here, the SMC trajectories and migration rate (ν = S/t) are calculated only from viable cells, because the dead cells are unable to spread and migrate. Hence, the fraction of migrated cells is equal to the ratio of live cells (for detail, see §2.9 cell adhesion).
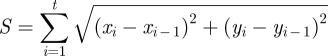 |
and
where xi (or yi) and xi − 1 (or y i − 1 ) represent x-axis (or y-axis) coordinates of cell centre positions at the i and i−1 time, respectively. x0 and y0 values are set as 0. At least 15 cells were calculated for each sample.
2.9. Cell adhesion
The cells were seeded on the multilayer surfaces at a density of 2.5 × 104 cm−2 with or without 10 per cent FBS. Four hours later, the slides were rinsed with PBS. After the cells were stained by a mixture of 5 μg ml−1 FDA (for living cells) and 20 μg ml−1 PI (for dead cells) for 20 min, they were gently rinsed with PBS for two times and subjected to livability analysis under a fluorescence microscope (PBS was streamed down along the wall of the well to avoid washing away the dead cells). The ratio of live cells is defined as the (live cell number/total cell number) × 100%, which also can be regarded as the fraction of migrated cells. For the cell adhesion study, the cells cultured for 4 h in 10 per cent FBS growth medium were stained by FDA and counted under the fluorescence microscope. A total of 40 observations from 10 positions on each of four samples were adopted to calculate the ratio of live cells on each type of multilayers.
2.10. Cell proliferation
Cell proliferation was characterized by cell number, MTT (3-(4,5-dimethyl) thiazol-2-yl-2,5-dimethyl tetrazolium bromide) and cell cycle assays. Briefly, the cells were plated at a density of 2.5 × 104 cm−2. Four hours, 24 h and 48 h later, the cell number was determined by stochastic count after FDA staining. A total of 40 observations from 10 positions on each of four samples were adopted to calculate the cell number. For the MTT assay, after the cells were cultured on the multilayers at determined time intervals, 50 µl MTT (5 mg ml−1) was added to each well. Four hours later, the dark blue formazan crystals generated by the mitochondrial dehydrogenase in the cells were dissolved by dimethyl sulphoxide (DMSO), and the absorbance at 570 nm was measured by a microplate reader (Model 550, Bio Rad). The results were averaged from four parallel samples. For quantification of the cell cycle, after culture on the multilayers for 24 h, the cells were carefully rinsed twice by PBS, and then trypsinized and fixed overnight in 75 per cent ethanol at −20°C. After the treatments with a mixture of RNase, PI and Trition X-100 for 30 min, the samples were analysed by flow cytometry (FACS Calibur, Becton Dickinson BD) with excitation at 488 nm and a 560 nm band pass filter for red fluorescence of PI. A total of 1 × 104 cells were analysed for each sample, and the results were averaged from four parallel samples.
2.11. Organization of focal adhesions and cytoskeleton
Fluorescent staining of actin, vinculin and cell nuclei was carried out to study the cell morphology, spreading area and focal adhesion. Briefly, after culture in the medium containing 10 per cent FBS for 4 or 24 h, the SMCs were carefully washed with PBS and then fixed for 30 min with 4 per cent paraformaldehyde at 37°C. The cells were further treated for 10 min with 0.5% (v/v) Triton X-100/PBS at 4°C so as to increase the permeability of the cell membrane. After rinsing with PBS for three times, they were incubated in 1 per cent bovine serum albumin (BSA)/PBS for 30 min and then in a mouse monoclonal antibody against human vinculin (Sigma) for 1 h. After washing twice in 1 per cent BSA/PBS, they were further stained with FITC-labelled goat anti-mouse IgG (Beyotime, China), rhodamine phalloidin (Invitrogen) and DAPI (Sigma) for 1 h, followed by three washes in PBS. A total of 60 cells on each type of multilayers were observed under a confocal laser scanning microscope (CLSM, LSM 510, Carl Zeiss). The images were analysed with LSM Image Browser software to determine the cell spreading extent and average cell area.
2.12. Real-time RT-PCR analysis
Real-time quantitative reverse transcription polymerase chain reaction (real-time RT-PCR) analysis was conducted to examine the expression profiles of adhesion and migration-specific genes for fibronectin, laminin, focal adhesion kinase (FAK), myosin IIA and Cdc42 in the SMCs. Briefly, after the cells were cultured on the multilayers for 24 h, total RNA was extracted using Trizol reagent (Invitrogen, USA), according to the manufacturer's instructions and quantified by using a biophotometer (Eppendorf, Germany). In each sample, 2 mg RNA was used for reverse transcription under standard conditions using M-MLV Reverse Transcriptase cDNA synthesis kit (Promega, USA). The resulting cDNA was used as a template in subsequent PCR amplifications. The primer sequences used in this study are listed in table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous reference housekeeping gene. The real-time PCR reactions were carried out with the SYBR Premix Ex-Taq Kit (Takara, Japan) and iQ qPCR system (Bio Rad, USA). The relative gene expression values were calculated with the comparative DDCT (threshold cycle) method, and normalized to the housekeeping gene.
Table 1.
Primer sequences and PCR product lengths for fibronectin, laminin, FAK, myosin IIA, Cdc42 and GAPDH.
| gene | primer sequence | length |
|---|---|---|
| fibronectin | 5′ CCTGGCACTTCTGGTCAGCAAC 3′ | 133 bp |
| 5′ CCTACATTCGGCGGGTATGGTC 3′ | ||
| laminin | 5′ CACCTATGTGCGTCTCAAGTTCC 3′ | 170 bp |
| 5′ GCTGCTCGTCCCCTCCTGT 3′ | ||
| FAK | 5′ CTCCTGGTGCAATGGAGCGAGTAT 3′ | 183 bp |
| 5′ GCAGGTGACTGAGGCGGAATC 3′ | ||
| myosin IIA | 5′ CGAAGAGGTAGATGGCAAAGCGGAT 3′ | 105 bp |
| 5′ GGGAGGCTGTGGTGTCTGTCT 3′ | ||
| Cdc42 | 5′ GCTGGGACTACAGGTCATCATCAGAT 3′ | 106 bp |
| 5′ CAACAGCACCATCGCCCACAACA 3′ | ||
| GAPDH | 5′ CTGCTCCTCCTGTTCGACAGT 3′ | 100 bp |
| 5′ CCGTTGACTCCGACCTTCAC 3′ |
2.13. Statistical analysis
The data are expressed as mean ± standard deviation (s.d.). The statistical significance between groups was determined by one-way analysis of variance (ANOVA) in the Origin software. The Tukey means comparison method was performed, and the statistical significance was set as p < 0.05.
3. Results
3.1. Physico-chemical properties of salt-treated multilayers
The salt post-treatment is a simple, quick, nontoxic and versatile way to modulate the structures and properties of PSS/PDADMAC multilayers. The physico-chemical properties of the multilayers treated with NaCl solutions of different concentrations are summarized in table 2. The molar ratios of PSS/PDADMAC multilayers were enhanced along with the increase in salt concentrations. Meanwhile, the surface chemistry was changed from positive PDADMAC domination below 2 M NaCl to negative PSS domination above 3 M NaCl. The zeta potentials of the multilayers treated with 1, 2, 3, 4 and 5 M salt solutions were +40, +25, −47, −39 and −29 mV, respectively [36]. The absolute values of the zeta potentials were obviously decreased after the multilayers were incubated in a serum-containing medium owing to the adsorption of serum proteins. However, no charge reversal was found, and the values kept around +15–20 mV for the multilayers-1 and 2 M, and -15 mV for the multilayers-3, 4 and 5 M, respectively. The water contact angles were initially decreased along with the increase of salt concentrations until 3 M NaCl (32°), and then increased again (52° for the multilayers-5 M). The overall roughness (RMS) of the multilayers in PBS was varied within a small range, i.e. 11.0 ± 1.7, 4.1 ± 1.3, 3.6 ± 2.2, 6.4 ± 0.1 and 4.6 ± 0.4 nm for the multilayers-1, 2, 3, 4 and 5 M, respectively [36]. The dry thicknesses of the multilayers treated with 1, 2, 3, 4 and 5 M NaCl solutions measured by ellipsometry were 182, 172, 142, 89 and 30 nm, respectively. The thickness in air was confirmed further by atomic force microscopy, verifying the accuracy of the ellipsometry data [41]. Therefore, significant mass loss occurred along with the increase in NaCl concentrations. After the multilayers were immersed in DMEM/10 per cent FBS medium for 2 h, their dry thicknesses increased to 218, 197, 158, 104 and 59 nm, respectively, confirming the serum protein absorption on the multilayers. The larger amount of protein adsorption on the multilayers-1 M might be attributed to the fact that the proteins could penetrate into the bulk of the positively charged and highly swollen PEMs [42]. After immersion in cell culture medium for 2 d, the thicknesses of the multilayers were not reduced, suggesting that the multilayers were stable in vitro and suitable for cell culture. After salt treatment, the swelling ratios of the multilayers in both water and PBS declined along with the increase of salt concentration until 3 M, and then increased significantly at higher concentrations of salt solutions (table 2). The swelling ratio in water also fits well with the results obtained by QCM measurement in a previous report [36]. This trend was unchanged after the multilayers were immersed in the DMEM/10 per cent FBS medium, but the absolute values of the swelling ratios were reduced slightly because some of the water in the multilayers was replaced after protein adsorption [43].
Table 2.
The physico-chemical properties of (PSS/PDADMAC)7 multilayers after being treated by 1, 2, 3, 4 and 5 M NaCl, respectively.
| samplesa | 1 M | 2 M | 3 M | 4 M | 5 M |
|---|---|---|---|---|---|
| surface-dominated polyelectrolytes | PDADMAC | PDADMAC | PSS | PSS | PSS |
| zeta potentials (mV) | +41 ± 10 | +26 ± 8 | −47 ± 9 | −38 ± 9 | −29 ± 3 |
| zeta potentials in DMEM/10% FBS (mV) | +21 ± 5 | +11 ± 5 | −15 ± 5 | −13 ± 4 | −16 ± 4 |
| water contact angle (°) | 64 ± 6 | 52 ± 8 | 32 ± 7 | 47 ± 10 | 52 ± 8 |
| roughness in PBS (nm) | 11.0 ± 1.7 | 4.1 ± 1.3 | 3.6 ± 2.2 | 6.4 ± 0.1 | 4.6 ± 0.4 |
| dry thickness (nm) | 182 ± 2 | 172 ± 1 | 142 ± 1 | 89 ± 2 | 30 ± 5 |
| thickness in PBS (nm) | 428 ± 21 | 255 ± 3 | 185 ± 1 | 188 ± 14 | 154 ± 74 |
| swelling ratio in PBS | 2.3 ± 0.1 | 1.5 ± 0 | 1.3 ± 0 | 2.1 ± 0.2 | 5.1 ± 0.2 |
| swelling ratio in water | 5.4 ± 1.4 | 3.4 ± 1.4 | 1.3 ± 0 | 4.3 ± 1.4 | 7.7 ± 1.3 |
| dry thickness after immersion in DMEM/10% FBS (nm) | 218 ± 4 | 197 ± 14 | 158 ± 9 | 104 ± 9 | 59 ± 6 |
| swelling ratio in DMEM/10% FBS | 2.0 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.4 | 4.6 ± 0.4 |
| dry thickness after incubation in DMEM/ 10% FBS for 2 days (nm) | 210 ± 14 | 192 ± 12 | 161 ± 11 | 118 ± 8 | 68 ± 10 |
aThe data of surface-dominated polyelectrolytes, zeta potentials, roughness in PBS, dry thicknesses and swelling ratios in water were cited from Han et al. [36]. To test the physico-chemical properties of the multilayers in cell culture environment, the salt-treated multilayers were immersed into DMEM/10% FBS for 4 h at 37°C, if not otherwise stated.
3.2. Cell migration
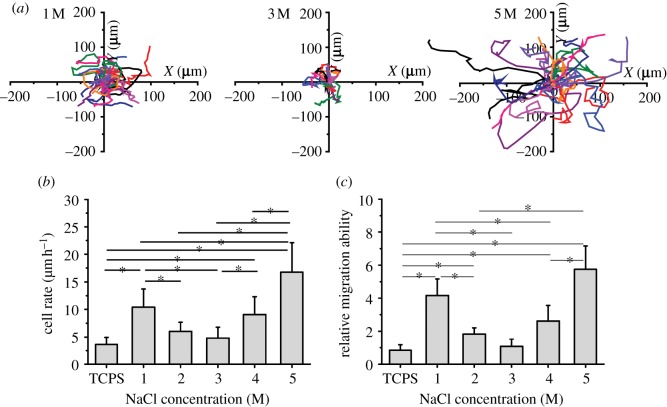
Cell migration is a dynamic process of cell adhesion and detachment on a surface. A time-lapse phase-contrast microscope was used to record the in situ cell migration trajectories during 24 h culture (figure 1a), according to which the cell migration rate was calculated (figure 1b). The cell movement on the multilayers was random, which was confirmed by the almost 0 net cell migration velocities in x- and y-directions (see the electronic supplementary material, figure S1). Because the dead cells do not contribute to the migration, they are not considered in the migration study. Namely, the fraction of migrated cells is equal to the live-cell ratio shown in figure 2a. After 24 h, almost all the cells on the multilayers-1, 2, 4 and 5 M migrated a longer distance (S) than their characteristic length (L; for detail see the electronic supplementary material, figure S2), namely the fraction of S > L was 100 per cent. This fraction dropped to 28 per cent and 40 per cent on TCPS and the multilayers-3 M, respectively. Moreover, the fractions of cells whose migration displacement (D) was larger than their characteristic length (D > L) were 60, 64, 0, 80 and 85 per cent on the multilayers-1, 2, 3, 4 and 5 M, respectively. The fraction on TCPS was 32 per cent. The migration rate (figure 1b) was slowest on the multilayers-3 M (4.8 ± 2 µm h−1), which was closest to that on the TCPS (3.6 ± 1.3 µm h–1) and the multilayers-2 M (6.0 ± 1.7 µm h−1) (p > 0.05). Cell migration rate was significantly improved to approximately 9–10 µm h−1 on the multilayers-1 M and the multilayers-4 M (p < 0.05), and reached the highest value of 16.8 ± 5.3 µm h−1 on the multilayers-5 M (p < 0.05).
Figure 1.
Cell migration on the salt-treated (PSS/PDADMAC)7 multilayers. (a) Random cell migration trajectories on the multilayers post-treated with 1, 3 and 5 M NaCl solutions, respectively. (b) Cell migration rates averaged from greater than or equal to 15 cells on TCPS and the salt-treated multilayers. (c) The relative migration ability of cells on TCPS and the salt-treated multilayers. The relative migration ability is defined as the ratio of cell migration distance to its characteristic length. Asterisks indicate significant difference, which is determined by one-way analysis of variance (ANOVA) in the Origin software. The statistical significance of asterisks is set as p < 0.05 and the Tukey means comparison method is performed.
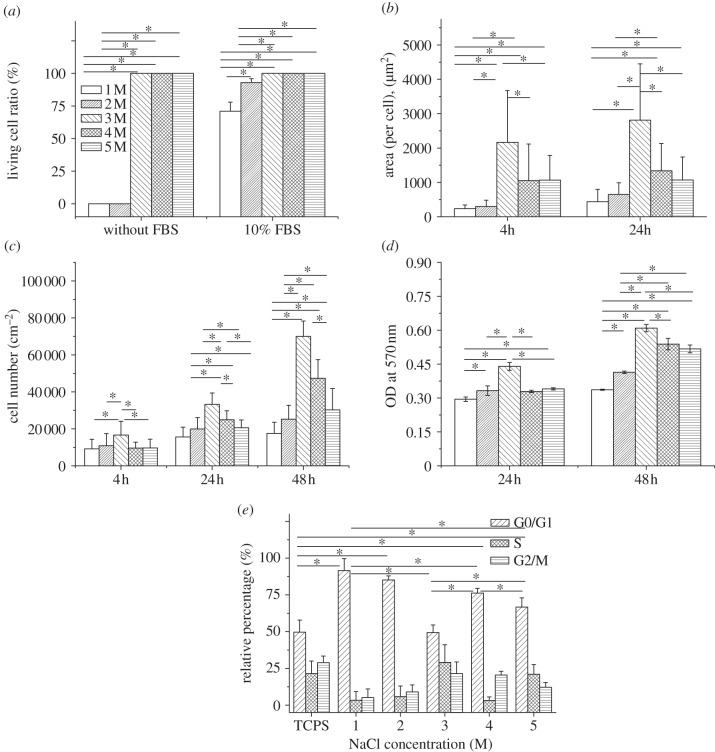
Figure 2.
SMCs adhesion and proliferation on the multilayers post-treated with different NaCl solutions. (a) The ratio of living SMCs cultured on the multilayers for 4 h with and without FBS, respectively. The live and dead cells were distinguished by FDA and PI staining. (b) Cell spreading area, (c) cell number and (d) OD value of the SMCs on the multilayers after being cultured in DMEM/10 per cent FBS for different periods of time. (e) Cell cycle analysis of G0/G1, S and G2/M phases of SMCs incubated for 24 h on TCPS and the multilayers post-treated with 1, 2, 3, 4 and 5 M salt solutions, respectively. Asterisks indicate significant difference, which is determined by one-way analysis of variance (ANOVA) in the Origin software. The statistical significance of asterisks is set as p < 0.05 and the Tukey means comparison method is performed.
In addition, the relative cell migration ability (S/L) was introduced because the cells had a different characteristic length on the various salt-treated multilayers (see the electronic supplementary material, figure S2). As shown in figure 1c, in general, the relative cell migration ability had a similar alteration trend to that of the cell migration rate (figure 1b), except that the range of variation was enlarged. After 24 h migration, the cells on the multilayers-3 M moved a similar distance to their characteristic length (see the electronic supplementary material, figure S2, approx. 100 µm), which is similar to those on the TCPS and the multilayers-2 M (p > 0.05). By contrast, the relative cell migration ability was significantly enhanced on the multilayers-4 M (p < 0.05), and further enhanced to a greater extent on the multilayers-1 M and multilayers-5 M (p < 0.05) with a ratio of 4.2 ± 1.0 and 5.7 ± 1.4 (see the electronic supplementary material, figure S2, approx. 50 µm and approx. 75 µm), respectively. All the results reveal that the cell mobility on the salt-treated multilayers is reduced initially until 3 M, and then increased again. The lowest cell mobility is found on the multilayers-3 M, which is similar to that on the TCPS.
3.3. Cell adhesion
Cell adhesion has a significant influence on cell migration, because the SMCs must attach to the substrate and then form protrusion to migrate. In order to explore the mechanism of cell migration, the adhesion of SMCs on the multilayers was quantitatively examined in the presence and absence of FBS [44]. In the absence of serum, two sharp contrast results were obtained (figure 2a): all the cells were dead on the PDADMAC-dominated surfaces (the multilayers-1, 2 M), but were alive on the PSS-dominated surfaces (the multilayers-3, 4, 5 M). In the presence of 10 per cent FBS, the live cell ratios on the multilayers-1 and 2 M were improved to 71 ± 7% and 93 ± 3%, respectively, while kept unchanged (100%) on the PSS-dominated surfaces (p < 0.05) (figure 2a). In all other experiments, the results were obtained in the presence of 10 per cent FBS to ensure adequate cell viability.
Cell spreading was further observed and quantified by cell adhesion area after cell seeding for 4 h (see the electronic supplementary material, figure S3 and figure 2b). The SMCs kept round on all the PDADMAC-dominated surfaces, with very small spreading areas, i.e. 235 ± 106, and 301 ± 181 µm2 per cell on the multilayers-1 and 2 M, respectively. By contrast, the cell spreading area on the multilayers-3 M (2168 ± 1508 µm2) was largest, and then decreased to 1053 ± 964 µm2 and 1067 ± 722 µm2 (p < 0.05) on the multilayers-4 and 5 M, respectively. After the culture time was extended to 24 h, the cell spreading area on all the multilayers was improved to some extent, but the relative values between each other kept unchanged (figure 2b).
3.4. Cell proliferation
The cell proliferation was quantified by cell number (figure 2c), MTT assay (figure 2d) and cell cycle analysis (figure 2e). During the first 4 h, the cell numbers on all the multilayers were very close to each other, except a significant higher number on the multilayers-3 M (p < 0.05) (figure 2c). After 24 and 48 h, the cell numbers were increased on all the sample surfaces, but were more significant on the multilayers treated with 3, 4 and 5 M NaCl solutions (p < 0.05). Again, the multilayers-3 M had a significantly larger number of cells regardless of the culture time (p < 0.05). The cell viability showed a similar pattern as that of the cell number, i.e. highest on the multilayers-3 M at both 24 and 48 h (p < 0.05). However, compared with its doubled cell number, the viability (figure 2d) was increased only about 25 per cent. This inconsistency is likely caused by the larger number of cells, the so-called inhibitory effect by cell confluence [45].
The cell cycle is a series of events that can induce cell division and duplication, comprising interphase (gap1, G1), synthesis (S), gap2 (G2) and mitosis (M) phases [46]. Generally, three stages are discerned: G0/G1, S and G2/M. Only one set (2n for SMCs) and duplicate set (4n) of DNAs are present during the G0/G1 and G2/M phases, respectively, whereas an intermediate amount (2n–4n) can be found during S phase [47]. The cells cultured on the TCPS for 24 h displayed a large portion of S and G2/M phases (figure 2e), suggesting active DNA synthesis and cell duplication. On all the sample surfaces, only the cells cultured on the multilayers-3 M exhibited a very similar distribution of cell phases as those on the TCPS (p > 0.05), demonstrating its good cell compatibility. By contrast, the cells cultured on the multilayers-1 and 2 M were mostly trapped in the G0/G1 phase, although their S and G2/M phases were increased slightly at a higher NaCl concentration. When the cells were cultured on the multilayers-4 and 5 M, their G0/G1 phase were further decreased but still significantly higher than those of cells cultured on the TCPS and the multilayers-3 M (p < 0.05).
3.5. Organization of focal adhesions and cytoskeleton
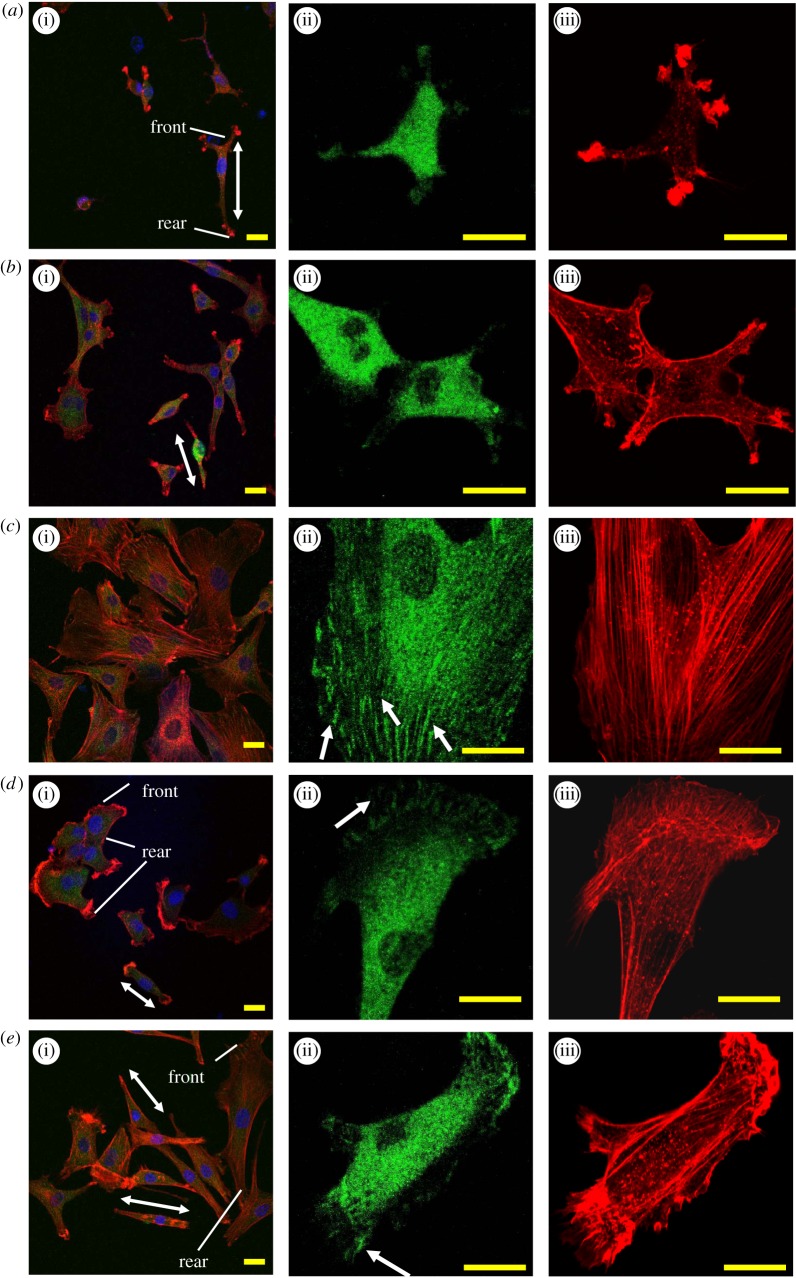
The physical links between the outside and inside of a cell are mainly tuned by integrins [48]. The focal adhesions contain a high concentration of activated and engaged integrins. Basically, the cell migration rate is inversely correlated with the cell focal adhesions, which are tuned by cytoskeleton reorganization and intracellular signalling, and are commonly required for the other cellular events such as cell adhesion, spreading, survival, proliferation and differentiation [49,50]. The focal adhesions after cell migration for 12 h (or seeding for 24 h) were investigated by staining vinculin (associated with focal adhesions) and F-actin (the major membrane-cytoskeletal protein in focal adhesions; figure 3). On the multilayers-3 M, some large vinculin clusters (figure 3c(ii), arrow indicated) were clearly visible, indicating that many focal adhesion plaques in the cells are assembled and then matured to be more stable structures, which can also be observed for the well spreading cells cultured on TCPS or glass [51]. The actin filaments of cells on the multilayers-3 M were clustered and distributed throughout the entire area of the cell, displaying actin stress fibres of high tension (figure 3c(iii)). Some focal adhesion plaques could also be observed on the multilayers-4 and 5 M (figure 3d(ii),e(ii), arrow indicated), but most of them preferentially localized to the leading edge. Moreover, actin stress fibres as those on the multilayers-3 M could not be observed (figure 3d(iii),e(iii)), but the formation of actin branches could be observed at the cell leading edge, which can drive the formation of a broad lamellipodia and protrusion in the direction of migration. In contrast, neither distinct focal adhesion plaques nor stressed actin fibres were observed on the multilayers-1 and 2 M (figure 3a,b). However, actin polymerization was formed to produce some cell protrusions, especially on the multilayers-1 M.
Figure 3.
Morphology and focal contact formation of the SMCs cultured on the salt-treated multilayers for 24 h. Representative CLSM images of SMCs on the multilayers treated with (a) 1, (b) 2, (c) 3, (d) 4 and (e) 5 M NaCl solutions, respectively. (a(i)–e(i)) Merge fluorescence images of vinculin (green), actin (red) and nucleus (blue). (a(ii)–e(ii)) and (a(iii)–e(iii)) vinculin and actin in a single cell, respectively. Arrows in (c(ii)), (d(ii)) and (e(ii)) indicate areas of large focal adhesion plaques. Two-head arrows indicate the polarity of the SMCs, and the texts illustrate the cell front (cell leading edge) and rear.
3.6. Gene expression
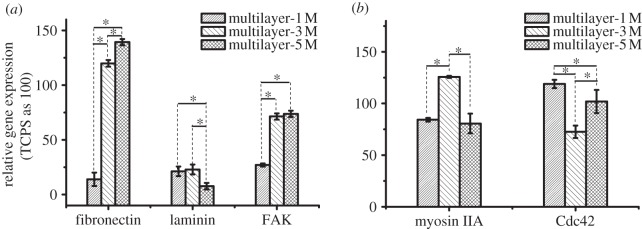
The cellular response is mediated by the gene expression. Fibronectin and laminin are two major extracellular matrix glycoproteins governing cell adhesion, spreading and migration, and FAK is an intracellular kinase localizing to focal adhesions and regulating both cellular adhesion and antiapoptotic survival signalling [52,53]. Figure 4a shows that the relative mRNA levels of fibronectin, laminin and FAK for the cells cultured on the multilayers-1 M were markedly decreased to 14 per cent, 21 per cent and 27 per cent of those on TCPS (p < 0.05), respectively. The mRNA levels of laminin and FAK were obviously decreased for the cells cultured on the multilayers-3 and 5 M, but the mRNA level of fibronectin was enhanced compared with that on TCPS.
Figure 4.
Gene expression analysis of SMCs cultured on TCPS and the salt-treated multilayers for 24 h. (a) Cell adhesion-related genes of fibronectin, laminin and FAK, and (b) migration-related genes of myosin IIA and Cdc42 detected by real-time quantitative RT-PCR. The data were normalized to those control cells cultured on TCPS. Asterisks indicate significant difference at p < 0.05.
Myosins comprise a family of ATP-dependent motor proteins and are best known for their roles in muscle contraction and involvement in a wide range of other eukaryotic motility processes. Myosin II is the sub-family and responsible for producing tension between the front and the rear of a cell from the interaction with actin filaments [54,55]. Cdc42 is active toward the leading edge of migrating cells and regulates cell polarity [56]. Both inhibition and global activation of Cdc42 can disrupt the directionality of migration [57]. Figure 4b shows that the mRNA levels of myosin IIA, an important protein in myosin II family, was significantly enhanced for the cells cultured on the multilayers-3 M but significantly weakened for the cells cultured on the multilayers-1 and 5 M (p < 0.05). The mRNA level of Cdc42 was obviously decreased for the cells cultured on the multilayers-3 M but slightly increased for the cells cultured on the multilayers-1 and 5 M (p < 0.05).
4. Discussion
The earlier-mentioned results demonstrate a V-shape pattern of SMC migration rate or relative migration ability and other related cellular events as a function of salt concentration during multilayer treatment. The SMCs migrate slowest on the multilayers-3 M, which are closest to those on TCPS, with the best spreading morphology and abundant focal adhesion plaques. By contrast, the cells migrate fastest on the multilayers-5 M. Migration can be modulated by many factors, including the existence of soluble and immobilized chemical cues and substrate properties [7,15,58]. Taking all the results into consideration, the multilayer structures and properties such as surface chemistry, zeta potential, hydrophilicity and swelling may have significant influences on the different cell performance. Here the surface morphology shall not be taken into consideration because of its smaller variation (smaller than 10 nm) and the less relevant fact of monotonous decrease of surface roughness. Also, Lampin et al. reported that the roughness has less influence on cell migration [59].
Usually, cell motility is directly influenced by the formation and presence of focal adhesions. In order to migrate, a cell is required to form new adhesion points on its front (cell leading edge), and simultaneously to release the old ones in its rear [54]. These new adhesion points driven by actin polymerization are regarded as protrusions, which can extend and become lamellipodia or filopodia, and then be stabilized through focal adhesions [60]. Hence, for the cells cultured on the multilayers-4 and 5 M, the actin fibres were polymerized at the cell leading edge, accompanying the formation of focal adhesion plaques (shown by large vinculin clusters). These results reveal that the lamellipodia are formed and the focal adhesion plaques serve as traction sites for migration, both of which improve the cell migration rate (figure 3d,e). By contrast, for the cells cultured on the multilayers-3 M, many larger focal adhesion plaques were observed inside the cells (figure 3c(ii), arrow indicated), and the actin stress fibres crossed through the whole cell. The focal adhesions assembled significantly in most area of the cells can cause tight adhesion, disrupt release of the rear, and thereby inhibit cell motility and result in the slowest migration rate [50]. This result is consistent with the fact that the over-expression of vinculin reduces cell motility, whereas generally the cells expressing lower vinculin migrate much faster [61]. However, for the cells on the multilayers-1 and 2 M, the cell protrusions driven by actin polymerization and low expression of vinculin may promote cell movement to some extent (figure 3a,b), but their weak focal adhesions impair cell motility and cause a slower migration rate than that on the multilayers-5 M. Therefore, cell motility can be largely improved at moderate focal adhesion.
However, why are the focal adhesions significantly different among the cells cultured on the salt-treated multilayers? The cell adhesion results reveal that all the SMCs cultured on the PSS-dominated surfaces have better performance than those on the PDADMAC-dominated surfaces (figure 2), confirming that the surface chemistry takes a major role in cell response such as live-cell ratio, spreading area, cell adhesion number and proliferation. In the serum-free culture medium, all the cells were dead on the PDADMAC-dominated surfaces (figure 2a; multilayers-1 and 2 M). It is known that the unbound segments of positive PDADMAC may extrude into the medium, and severely disturb the structures and functions of cell membranes as a result of combination with the negative membrane proteins and phospholipids, leading to severe cytotoxicity [62,63]. In the culture medium containing serum proteins, some of the unassociated segments of PDADMAC might be blocked by the adsorbed proteins, leading to the reduction of cytotoxicity. Nevertheless, the positively charged surface after protein adsorption still causes cell death to some extent (table 2) and the most severe down regulation of FAK (figure 4a). This result is also in good agreement with that of the cell cycle analysis (figure 2e), because the cells cultured on the PDADMAC-dominated multilayers are mostly trapped in the G0/G1 phase. Therefore, the cells that spread poorly on these surfaces produce a low level of genes for FAK and fibronectin (figure 4a), leading to the lower expression of focal adhesion-related proteins, i.e. vinculin (figure 3a,b). By contrast, the negatively charged surfaces dominated by PSS can interact with the cells and proteins in a more friendly way, and better maintain the natural conformation of the serum proteins. Thus, the cells produce a higher level of genes for fibronectin and FAK (figure 4a), and the focal adhesion-related proteins (figure 3c,d).
The surface chemistry has substantial influences on cell behaviours. Many surfaces with specific chemical compositions have been prepared and used to elucidate their influences on cell adhesion and migration. The extracellular matrix (ECM) proteins, peptides and cell growth factors such as collagen [7], fibronectin [8], laminin [9], VEGF [10], bFGF [11] and RGD [12,13] can bind to the receptors on the cell surface, initiate intracellular signalling pathways and finally regulate cell functions. Therefore, they are widely used to enhance the cell adhesion and other functions. Other synthetic surfaces with different functional groups can also regulate cell behaviours. For example, human fibroblasts adhere stronger on carboxyl and amine than methyl, polyethylene glycol (PEG) and hydroxyl-terminated surfaces [64]. The osteoblasts migrate slower on thiol surfaces compared with oxidized thiol, quaternary amine and methyl surfaces [14]. In this study, we found that surfaces with positive charge, which induce cell apoptosis, can reduce cell mobility. Here, apparently the sulphonate group plays a major role in good cell adhesion and spreading.
With the same surface chemistry, the SMCs still show the best focal adhesion and spreading on the multilayers-3 M than on the other two PSS-dominated surfaces (multilayers-4 and 5 M). Other factors must come into play. Previous works demonstrate that surface wettability has a significant impact on cell behaviour, as evidenced by the fact that the cells usually grow well on a substrate with a contact angle of 50–70° [65]. In this study, the water contact angle of the multilayers-5 M is near 50°, but the cell growth is not as good as that on the multilayers-3 M (contact angle of 32°). Therefore, the wettability of the multilayers is not a major governing factor in our system. Besides, the thickness and stiffness/swelling may have significant influences on the cell behaviours. A stiffer underlying film has a smaller critical mechanosensing thickness for cells [66]. For example, when the PSS/PDADMAC multilayers are assembled in water with a low hydrated structure, the critical mechanosensing thickness for cells is less than 80 nm [67]. However, this critical thickness is enlarged to 1–2 μm or more on the soft gels [68]. In other words, the compositions of the multilayers play an important role too. Taking into the fact that cell adhesion on the thinner multilayers-5 M was poorer than that on the thicker multilayers-3 M, one can conclude that the swelling ratio or stiffness of the salt-treated multilayers plays a stronger role although the effect of thickness on cell migration cannot be absolutely excluded. In principle, the anchorage-dependent cells such as SMCs must adhere onto a substrate with enough strength before any other cellular events. On very soft surfaces such as the highly hydrated and swollen one, the substrate cannot provide strong enough adhesion force for the cells to anchor and spread. By contrast, a rigid substrate can provide enough adhesion force [6,69]. As shown in table 2, the multilayers-3 M displays the smallest swelling ratio, implying that it has the strongest stiffness in the culture medium. Therefore, the smallest swelling behaviour of the multilayers-3 M should play a major role in the best cell adhesion and proliferation, and the most obvious focal adhesion plaques in the cells. A previous study found that on the polyacrylamide hydrogel with a mechanical gradient and different concentrations of collagen (type I), the chemical signals have a more dominant role in regulating fibroblasts movement [67]. The present results demonstrate, however, that both the surface chemistry and swelling property are the governing factors for cell migration.
The cycle of cell migration can be divided into four phases: polarization, protrusion, traction and disassembly. When the cells extend their protrusions, cell polarity must take place, implying a difference in the front and rear of the cell [50,54]. Hence, besides focal adhesion, the expression of related genes on polarization and protrusion was analysed. Cdc42 can promote actin polymerization and then regulate cell polarity. As a master regulator of cell polarity, Cdc42 in cells cultured on the multilayers-1 and 5 M is upregulated (figure 4b), inducing cell polarity and formation of protrusions in the cell front (figure 3a,e, texts and two heads arrows). Hence, more obvious lamellipodia on the multilayers-1 and 5 M can be observed, which should be responsible for the faster cell migration. Comparatively, the expression level of Cdc42 in the cells cultured on the multilayers-3 M is reduced, implying the weak polarization and restriction on formation of protrusions in the cell front. This is consistent with the immunostaining results (figure 3c(i)). Compared with those normally polarized cells with ruffled and fan-shaped protrusions at the leading edge and a traction point at the rear, the front and rear in these low polarized cells are not easily distinguished, which can explain the slower mobility on the multilayers-3 M. The cells on the multilayers-3 M display upregulated myosin IIA level, which is consistent with their highly spreading morphology because myosin II can promote the generation of tension between the front and back of a cell [54,55].
5. Conclusion
The study of cell migration behaviours on biomaterials surface is of paramount importance because it can reveal many physiological and pathological events and eventually guide the design of biomaterials with better performance in tissue regeneration. In this work, the physico-chemical properties of the PSS/PDADMAC multilayers were modulated by post-treatment with 1–5 M NaCl solutions. These resulting thin films were stable in the cell culture medium. We found that both the surface chemistry and swelling property, but not the surface roughness and surface wettability, play primary roles in cell migration.
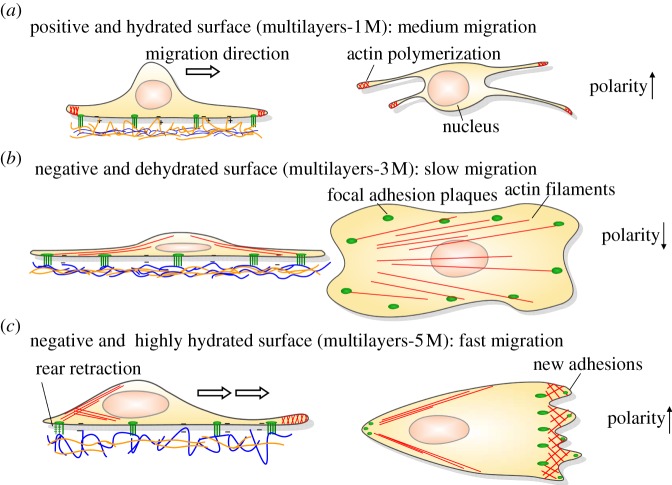
On the positively charged surface (figure 5a, the multilayers-1 and 2 M), the SMCs have lower viability and smaller spreading extent owing to the surface cytotoxicity generated by the interactions of positively charged polymer chains and negatively charged cell membrane. Hence, the expression level of vinculin, FAK and fibronectin is reduced. Actin polymerization occurs in the front of lamellipodia, and thereby the cell polarity takes place and Cdc42 is upregulated. As a result of the weaker adhesion and enhanced polarity, SMCs have a moderate migration rate. The cells prefer to spread and proliferate on the PSS-dominated surfaces that are negatively charged (multilayers-3, 4 and 5 M), showing higher expression of fibronectin and FAK. In particular, the dehydrated multilayers-3 M can provide a strong adhesion force for the cells to anchor, so that the cell actin filaments distribute throughout the cells with well-organized focal adhesion plaques, resulting in stronger cell adhesion (figure 5b). The downregulation of Cdc42 causes lower cell polarity. Hence, the cells display the slowest cell mobility, which is comparable with that on TCPS control. The same negatively charged but highly hydrated surface such as the multilayers-5 M, however, can effectively promote cell mobility (figure 5c). Actin fibres are formed at the cell leading edge, yielding broad lamellipodia and protrusions in the direction of migration. Meanwhile, most of the focal adhesion plaques localize on the leading edge, which is consistent with the upregulation of Cdc42, leading to a stronger forward force. Therefore, the cells migrate fastest on this surface.
Figure 5.
Scheme of the SMCs migration behaviours on the multilayers with different surface charge and swelling properties. (a) The SMCs have a moderate migration rate on the positively charged and hydrated surface (the multilayers-1 M). (b) The SMCs migrate slowest on the negatively charged and dehydrated surface (the multilayers-3 M). (c) The SMCs migrate fastest on the negatively charged and highly hydrated surface (the multilayers-5 M).
These basic principles may provide a profound understanding of cell–materials interactions and new guidelines for the design of advanced biomaterials in regenerative medicine. For example, in some vascular surgeries, the excessive migration of SMCs results in restenosis and intimal hyperplasia, especially for small diameter blood vessel tissue engineering. In this situation, dehydrated and negatively charged surfaces such as the multilayers-3 M could be used to reduce the cell migration rate. In other cases, stronger SMC migration ability on biomaterials can enhance tissue regeneration such as in an abdominal wall replacement model. In this regard, highly swelling and negatively charged surfaces such as the multilayers-5 M could be an optimal choice.
Acknowledgements
This study was financially supported by the Natural Science Foundation of China (20934003), and the National Basic Research Program of China (2011CB606203).
References
- 1.Sagvolden G., Giaever I., Pettersen E. O., Feder J. 1999. Cell adhesion force microscopy. Proc. Natl Acad. Sci. USA 96, 471–476 10.1073/pnas.96.2.471 (doi:10.1073/pnas.96.2.471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidambi S., Lee I., Chan C. 2004. Controlling primary hepatocyte adhesion and spreading on protein-free polyelectrolyte multilayer films. J. Am. Chem. Soc. 126, 16286–16287 10.1021/ja046188u (doi:10.1021/ja046188u) [DOI] [PubMed] [Google Scholar]
- 3.Kidambi S., Udpa N., Schroeder S. A., Findlan R., Lee I., Chan C. 2007. Cell adhesion on polyelectrolyte multilayer coated polydimethylsiloxane surfaces with varying topographies. Tissue Eng. 13, 2105–2117 10.1089/ten.2006.0151 (doi:10.1089/ten.2006.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrotra S., Lynam D., Maloney R., Pawelec K. M., Tuszynski M. H., Lee I., Chan C., Sakamoto J. 2010. Time controlled protein release from layer-by-layer assembled multilayer functionalized agarose hydrogels. Adv. Funct. Mater. 20, 247–258 10.1002/adfm.200901172 (doi:10.1002/adfm.200901172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler A., Bacakova L., Newman C., Hategan A., Griffin M., Discher D. 2004. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 86, 617–628 10.1016/S0006-3495(04)74140-5 (doi:10.1016/S0006-3495(04)74140-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelham R. J., Wang Y. L. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA 94, 13 661–13 665 10.1073/pnas.94.25.13661 (doi:10.1073/pnas.94.25.13661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raines E. W. 2000. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 81, 173–182 10.1046/j.1365-2613.2000.00155.x (doi:10.1046/j.1365-2613.2000.00155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith J. T., Tomfohr J. K., Wells M. C., Beebe T. P., Kepler T. B., Reichert W. M. 2004. Measurement of cell migration on surface-bound fibronectin gradients. Langmuir 20, 8279–8286 10.1021/la0489763 (doi:10.1021/la0489763) [DOI] [PubMed] [Google Scholar]
- 9.Dertinger S. K. W., Jiang X. Y., Li Z. Y., Murthy V. N., Whitesides G. M. 2002. Gradients of substrate-bound laminin orient axonal specification of neurons. Proc. Natl Acad. Sci. USA 99, 12 542–12 547 10.1073/pnas.192457199 (doi:10.1073/pnas.192457199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L. Y., Ratner B. D., Sage E. H., Jiang S. Y. 2007. Endothelial cell migration on surface-density gradients of fibronectin, VEGF, or both proteins. Langmuir 23, 11 168–11 173 10.1021/la701435x (doi:10.1021/la701435x) [DOI] [PubMed] [Google Scholar]
- 11.DeLong S. A., Moon J. J., West J. L. 2005. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 26, 3227–3234 10.1016/j.biomaterials.2004.09.021 (doi:10.1016/j.biomaterials.2004.09.021) [DOI] [PubMed] [Google Scholar]
- 12.DeLong S. A., Gobin A. S., West J. L. 2005. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J. Control Release 109, 139–148 10.1016/j.jconrel.2005.09.020 (doi:10.1016/j.jconrel.2005.09.020) [DOI] [PubMed] [Google Scholar]
- 13.Guarnieri D., De Capua A., Ventre M., Borzacchiello A., Pedone C., Marasco D., Ruvo M., Netti P. A. 2010. Covalent immobilized RGD gradient on PEG hydrogel scaffold influences cell migration parameters. Acta Biomater. 6, 2532–2539 10.1016/j.actbio.2009.12.050 (doi:10.1016/j.actbio.2009.12.050) [DOI] [PubMed] [Google Scholar]
- 14.Webb K., Hlady V., Tresco P. A. 2000. Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces. J. Biomed. Mater. Res. 49, 362–368 (doi:10.1002/(SICI)1097-4636(20000305)49:3<362::AID-JBM9>3.0.CO;2-S) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo C. M., Wang H. B., Dembo M., Wang Y. L. 2000. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 10.1016/S0006-3495(00)76279-5 (doi:10.1016/S0006-3495(00)76279-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Mao Z., Gao C. 2011. Controlling the migration behaviors of vascular smooth muscle cells by methoxy poly (ethylene glycol) brushes of different molecular weight and density. Biomaterials 33, 810–820 [DOI] [PubMed] [Google Scholar]
- 17.Decher G. 1997. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277, 1232–1237 10.1126/science.277.5330.1232 (doi:10.1126/science.277.5330.1232) [DOI] [Google Scholar]
- 18.Richert L., Lavalle P., Vautier D., Senger B., Stoltz J. F., Schaaf P., Voegel J. C., Picart C. 2002. Cell interactions with polyelectrolyte multilayer films. Biomacromolecules 3, 1170–1178 10.1021/bm0255490 (doi:10.1021/bm0255490) [DOI] [PubMed] [Google Scholar]
- 19.Tang Z. Y., Wang Y., Podsiadlo P., Kotov N. A. 2006. Biomedical applications of layer-by-layer assembly: from biomimetics to tissue engineering. Adv. Mater. 18, 3203–3224 10.1002/adma.200600113 (doi:10.1002/adma.200600113) [DOI] [Google Scholar]
- 20.Elbert D. L., Herbert C. B., Hubbell J. A. 1999. Thin polymer layers formed by polyelectrolyte multilayer techniques on biological surfaces. Langmuir 15, 5355–5362 10.1021/la9815749 (doi:10.1021/la9815749) [DOI] [Google Scholar]
- 21.Hubsch E., Ball V., Senger B., Decher G., Voegel J. C., Schaaf P. 2004. Controlling the growth regime of polyelectrolyte multilayer films: changing from exponential to linear growth by adjusting the composition of polyelectrolyte mixtures. Langmuir 20, 1980–1985 10.1021/la0361870 (doi:10.1021/la0361870) [DOI] [Google Scholar]
- 22.Tan H. L., McMurdo M. J., Pan G. Q., Van Patten P. G. 2003. Temperature dependence of polyelectrolyte multilayer assembly. Langmuir 19, 9311–9314 10.1021/la035094f (doi:10.1021/la035094f) [DOI] [Google Scholar]
- 23.Thompson M. T., Berg M. C., Tobias I. S., Rubner M. F., Van Vliet K. J. 2005. Tuning compliance of nanoscale polyelectrolyte multilayers to modulate cell adhesion. Biomaterials 26, 6836–6845 10.1016/j.biomaterials.2005.05.003 (doi:10.1016/j.biomaterials.2005.05.003) [DOI] [PubMed] [Google Scholar]
- 24.Dubas S. T., Schlenoff J. B. 2001. Swelling and smoothing of polyelectrolyte multilayers by salt. Langmuir 17, 7725–7727 10.1021/la0112099 (doi:10.1021/la0112099) [DOI] [Google Scholar]
- 25.Guzman E., Ritacco H., Rubio J. E. F., Rubio R. G., Ortega F. 2009. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyl-dimethylammonium chloride) and poly(4-styrene sulfonate of sodium). Soft Matter 5, 2130–2142 10.1039/b901193e (doi:10.1039/b901193e) [DOI] [Google Scholar]
- 26.Mendelsohn J. D., Yang S. Y., Hiller J., Hochbaum A. I., Rubner M. F. 2003. Rational design of cytophilic and cytophobic polyelectrolyte multilayer thin films. Biomacromolecules 4, 96–106 10.1021/bm0256101 (doi:10.1021/bm0256101) [DOI] [PubMed] [Google Scholar]
- 27.Hillberg A. L., Holmes C. A., Tabrizian M. 2009. Effect of genipin cross-linking on the cellular adhesion properties of layer-by-layer assembled polyelectrolyte films. Biomaterials 30, 4463–4470 10.1016/j.biomaterials.2009.05.026 (doi:10.1016/j.biomaterials.2009.05.026) [DOI] [PubMed] [Google Scholar]
- 28.Hu Y., Cai K., Luo Z., Jandt K. D. 2010. Layer-by-layer assembly of β-stradiol loaded mesoporous silica nanoparticles on titanium substrates and its implication for bone homeostasis. Adv. Mater. 22, 4146–4150 10.1002/adma.201000854 (doi:10.1002/adma.201000854) [DOI] [PubMed] [Google Scholar]
- 29.Swiston A. J., Cheng C., Um S. H., Irvine D. J., Cohen R. E., Rubner M. F. 2008. Surface functionalization of living cells with multilayer patches. Nano Lett. 8, 4446–4453 10.1021/nl802404h (doi:10.1021/nl802404h) [DOI] [PubMed] [Google Scholar]
- 30.Hu Y., Cai K., Luo Z., Zhang R., Yang L., Deng L., Jandt K. D. 2009. Surface mediated in situ differentiation of mesenchymal stem cells on gene-functionalized titanium films fabricated by layer-by-layer technique. Biomaterials 30, 3626–3635 10.1016/j.biomaterials.2009.03.037 (doi:10.1016/j.biomaterials.2009.03.037) [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y. B., Gao C. Y., He T., Liu X. Y., Shen J. C. 2003. Layer-by-layer assembly to modify poly(l-lactic acid) surface toward improving its cytocompatibility to human endothelial cells. Biomacromolecules 4, 446–452 10.1021/bm025723k (doi:10.1021/bm025723k) [DOI] [PubMed] [Google Scholar]
- 32.Richert L., Boulmedais F., Lavalle P., Mutterer J., Ferreux E., Decher G., Schaaf P., Voegel J. C., Picart C. 2004. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules 5, 284–294 10.1021/bm0342281 (doi:10.1021/bm0342281) [DOI] [PubMed] [Google Scholar]
- 33.Schneider A., et al. 2006. Polyelectrolyte multilayers with a tunable Young's modulus: influence of film stiffness on cell adhesion. Langmuir 22, 1193–1200 10.1021/la0521802 (doi:10.1021/la0521802) [DOI] [PubMed] [Google Scholar]
- 34.Dubas S. T., Kittitheeranun P., Rangkupan R., Sanchavanakit N., Potiyaraj P. 2009. Coating of polyelectrolyte multilayer thin films on nanofibrous scaffolds to improve cell adhesion. J. Appl. Polym. Sci. 114, 1574–1579 10.1002/app.30690 (doi:10.1002/app.30690) [DOI] [Google Scholar]
- 35.Kidambi S., Sheng L. F., Yarmush M. L., Toner M., Lee I., Chan C. 2007. Patterned co-culture of primary hepatocytes and fibroblasts using polyelectrolyte multilayer templates. Macromol. Biosci. 7, 344–353 10.1002/mabi.200600205 (doi:10.1002/mabi.200600205) [DOI] [PubMed] [Google Scholar]
- 36.Han L., Mao Z., Wuliyasu H., Wu J., Gong X., Yang Y., Gao C. 2012. Modulating the structure and properties of poly (sodium 4-styrenesulfonate)/poly (diallyldimethylammonium chloride) multilayers by concentrated salt solutions. Langmuir 28, 193–199 10.1021/la2040533 (doi:10.1021/la2040533) [DOI] [PubMed] [Google Scholar]
- 37.Owens G. K., Kumar M. S., Wamhoff B. R. 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 10.1152/physrev.00041.2003 (doi:10.1152/physrev.00041.2003) [DOI] [PubMed] [Google Scholar]
- 38.Hong Y., Ye S. H., Nieponice A., Soletti L., Vorp D. A., Wagner W. R. 2009. A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and phospholipid polymer blend. Biomaterials 30, 2457–2467 10.1016/j.biomaterials.2009.01.013 (doi:10.1016/j.biomaterials.2009.01.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashizume R., Fujimoto K. L., Hong Y., Amoroso N. J., Tobita K., Miki T., Keller B. B., Sacks M. S., Wagner W. R. 2010. Morphological and mechanical characteristics of the reconstructed rat abdominal wall following use of a wet electrospun biodegradable polyurethane elastomer scaffold. Biomaterials 31, 3253–3265 10.1016/j.biomaterials.2010.01.051 (doi:10.1016/j.biomaterials.2010.01.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han L. L., Zhou J., Gong X., Gao C. Y. 2009. Solvent-assisted polymer micro-molding. Chinese. Sci. Bull. 54, 2193–2204 10.1007/s11434-009-0212-5 (doi:10.1007/s11434-009-0212-5) [DOI] [Google Scholar]
- 41.Han L. L., Zhou J., Gong X., Yang J., Gao C. Y. 2010. Force-free patterning of polyelectrolyte multilayers under solvent assistance. Macromol. Mater. Eng. 295, 716–725 10.1002/mame.201000008 (doi:10.1002/mame.201000008) [DOI] [Google Scholar]
- 42.Salloum D. S., Schlenoff J. B. 2004. Protein adsorption modalities on polyelectrolyte multilayers. Biomacromolecules 5, 1089–1096 10.1021/bm034522t (doi:10.1021/bm034522t) [DOI] [PubMed] [Google Scholar]
- 43.Tristan F., Palestino G., Menchaca J. L., Perez E., Atmani H., Cuisinier F., Ladam G. 2009. Tunable protein-resistance of polycation-terminated polyelectrolyte multilayers. Biomacromolecules 10, 2275–2283 10.1021/bm900453s (doi:10.1021/bm900453s) [DOI] [PubMed] [Google Scholar]
- 44.Allen L. T., et al. 2006. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 27, 3096–3108 10.1016/j.biomaterials.2006.01.019 (doi:10.1016/j.biomaterials.2006.01.019) [DOI] [PubMed] [Google Scholar]
- 45.Muller G., Behrens J., Nussbaumer U., Bohlen P., Birchmeier W. 1987. Inhibitory-action of transforming growth-factor-beta, on endothelial-cells. Proc. Natl Acad. Sci. USA 84, 5600–5604 10.1073/pnas.84.16.5600 (doi:10.1073/pnas.84.16.5600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Kooten T. G., Whitesides J. F., von Recum A. F. 1998. Influence of silicone (PDMS) surface texture on human skin fibroblast proliferation as determined by cell cycle analysis. J. Biomed. Mater. Res. 43, 1–14 (doi:10.1002/(SICI)1097-4636(199821)43:1<1::AID-JBM1>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- 47.Mao J. S., Cui Y. L., Wang X. H., Sun Y., Yin Y. J., Zhao H. M., De Yao K. 2004. A preliminary study on chitosan and gelatin polyelectrolyte complex cytocompatibility by cell cycle and apoptosis analysis. Biomaterials 25, 3973–3981 10.1016/j.biomaterials.2003.10.080 (doi:10.1016/j.biomaterials.2003.10.080) [DOI] [PubMed] [Google Scholar]
- 48.Stevens M. M., George J. H. 2005. Exploring and engineering the cell surface interface. Science 310, 1135–1138 10.1126/science.1106587 (doi:10.1126/science.1106587) [DOI] [PubMed] [Google Scholar]
- 49.Lee J., Chu B. H., Chen K. H., Ren F., Lele T. P. 2009. Randomly oriented, upright SiO2 coated nanorods for reduced adhesion of mammalian cells. Biomaterials 30, 4488–4493 10.1016/j.biomaterials.2009.05.028 (doi:10.1016/j.biomaterials.2009.05.028) [DOI] [PubMed] [Google Scholar]
- 50.Wong J. Y., Velasco A., Rajagopalan P., Pham Q. 2003. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir 19, 1908–1913 10.1021/la026403p (doi:10.1021/la026403p) [DOI] [Google Scholar]
- 51.Lou P. J., Chiu M. Y., Chou C. C., Liao B. W., Young T. H. 2010. The effect of poly (ethylene-co-vinyl alcohol) on senescence-associated alterations of human dermal fibroblasts. Biomaterials 31, 1568–1577 10.1016/j.biomaterials.2009.11.048 (doi:10.1016/j.biomaterials.2009.11.048) [DOI] [PubMed] [Google Scholar]
- 52.Colognato H., Yurchenco P. D. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213–234 (doi:10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R) [DOI] [PubMed] [Google Scholar]
- 53.Pankov R., Yamada K. M. 2002. Fibronectin at a glance. J. Cell Sci. 115, 3861–3863 10.1242/jcs.00059 (doi:10.1242/jcs.00059) [DOI] [PubMed] [Google Scholar]
- 54.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. 2003. Cell migration: integrating signals from front to back. Science 302, 1704–1709 10.1126/science.1092053 (doi:10.1126/science.1092053) [DOI] [PubMed] [Google Scholar]
- 55.Fournier M. F., Sauser R., Ambrosi D., Meister J. J., Verkhovsky A. B. 2010. Force transmission in migrating cells. J. Cell Biol. 188, 287–297 10.1083/jcb.200906139 (doi:10.1083/jcb.200906139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh R. E., Kurokawa K., Ohba Y., Yoshizaki H., Mochizuki N., Matsuda M. 2002. Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell Biol. 22, 6582–6591 10.1128/MCB.22.18.6582-6591.2002 (doi:10.1128/MCB.22.18.6582-6591.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Etienne-Manneville S., Hall A. 2002. Rho GTPases in cell biology. Nature 420, 629–635 10.1038/nature01148 (doi:10.1038/nature01148) [DOI] [PubMed] [Google Scholar]
- 58.Carter S. B. 1967. Haptotaxis and mechanism of cell motility. Nature 213, 256. 10.1038/213256a0 (doi:10.1038/213256a0) [DOI] [PubMed] [Google Scholar]
- 59.Lampin M., WarocquierClerout R., Legris C., Degrange M., SigotLuizard M. F. 1997. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 36, 99–108 (doi:10.1002/(SICI)1097-4636(199707)36:1<99::AID-JBM12>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 60.Lauffenburger D. A., Horwitz A. F. 1996. Cell migration: a physically integrated molecular process. Cell 84, 359–369 10.1016/S0092-8674(00)81280-5 (doi:10.1016/S0092-8674(00)81280-5) [DOI] [PubMed] [Google Scholar]
- 61.Fernandez J. L. R., Geiger B., Salomon D., Benzeev A. 1992. Overexpression of vinculin suppresses cell motility in BALB/c 3T3 cells. Cell Motil. Cytoskel. 22, 127–134 10.1002/cm.970220206 (doi:10.1002/cm.970220206) [DOI] [PubMed] [Google Scholar]
- 62.Fischer D., Li Y. X., Ahlemeyer B., Krieglstein J., Kissel T. 2003. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24, 1121–1131 10.1016/S0142-9612(02)00445-3 (doi:10.1016/S0142-9612(02)00445-3) [DOI] [PubMed] [Google Scholar]
- 63.Murata H., Koepsel R. R., Matyjaszewski K., Russell A. J. 2007. Permanent, non-leaching antibacterial surfaces. 2: how high density cationic surfaces kill bacterial cells. Biomaterials 28, 4870–4879 10.1016/j.biomaterials.2007.06.012 (doi:10.1016/j.biomaterials.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 64.Faucheux N., Schweiss R., Lutzow K., Werner C., Groth T. 2004. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 25, 2721–2730 10.1016/j.biomaterials.2003.09.069 (doi:10.1016/j.biomaterials.2003.09.069) [DOI] [PubMed] [Google Scholar]
- 65.Brocchini S., James K., Tangpasuthadol V., Kohn J. 1997. A combinatorial approach for polymer design. J. Am. Chem. Soc. 119, 4553–4554 10.1021/ja970389z (doi:10.1021/ja970389z) [DOI] [Google Scholar]
- 66.Maloney J. M., Walton E. B., Bruce C. M., Van Vliet K. J. 2008. Influence of finite thickness and stiffness on cellular adhesion-induced deformation of compliant substrata. Phys. Rev. E 78, 041923. 10.1103/PhysRevE.78.041923 (doi:10.1103/PhysRevE.78.041923) [DOI] [PubMed] [Google Scholar]
- 67.Hale N. A., Yang Y., Rajagopalan P. 2010. Cell migration at the interface of a dual chemical–mechanical gradient. Acs Appl. Mater. Interfaces 2, 2317–2324 10.1021/am100346k (doi:10.1021/am100346k) [DOI] [PubMed] [Google Scholar]
- 68.Sen S., Engler A. J., Discher D. E. 2009. Matrix strains induced by cells: computing how far cells can feel. Cell. Mol. Bioeng. 2, 39–48 10.1007/s12195-009-0052-z (doi:10.1007/s12195-009-0052-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Discher D. E., Janmey P., Wang Y. L. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 10.1126/science.1116995 (doi:10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]