Abstract
Purpose.
Serratia marcescens is frequently isolated from lenses of patients with contact lens-associated corneal infiltrates. In the current study, we examined the role of toll-like receptors (TLRs) and interleukin-1 receptor type 1 (IL-1R1) in S. marcescens–induced corneal inflammation and infection.
Methods.
The central corneal epithelium of C57BL/6 and gene knockout mice was abraded, and 1 × 107 S. marcescens were added in the presence of a silicone hydrogel contact lens, and we examined corneal inflammation by confocal microscopy and neutrophil enumeration. Viable bacteria were quantified by colony-forming units (CFU).
Results.
S. marcescens induced neutrophil recruitment to the corneal stroma, and increased corneal thickness and haze in C57BL/6 mice. Conversely, CFU was significantly lower by 48 hours post infection. In contrast, MyD88−/−, IL-1R−/−, TLR4−/−, and TLR4/5−/− corneas infected with S. marcescens had significantly increased CFU, indicating impaired clearance. However, there was no significant difference in CFU among C57BL/6, TIRAP−/−, and TRIF−/− mice. Tobramycin-killed S. marcescens induced corneal inflammation in C57BL/6 mice, which was impaired significantly in MD-2−/− mice and in C57BL/6 mice pretreated topically with the MD-2 antagonist eritoran tetrasodium.
Conclusions.
S. marcescens induces corneal inflammation by activation of TLR4/MD-2/MyD88 and the IL-1R1/MyD88 pathways, which are potential therapeutic targets for inhibition of S. marcescens-induced corneal inflammation.
S. marcescens clinical isolates activate TLR4 and TLR5 in the cornea, resulting in CXC chemokine production and neutrophil recruitment, and limiting bacterial growth. We also demonstrate that blocking TLR activation is a potential therapeutic target for S. marcescens-induced corneal inflammation.
Introduction
Corneal inflammation is an important adverse event in extended wear silicone hydrogel contact lens users. Clinical manifestations include contact lens acute red eye (CLARE), infiltrative keratitis (IK), and contact lens peripheral ulcers (CLPU) where patients may experience acute pain, redness, and photophobia.1 With over 34 million contact lens wearers in the United States and 140 million worldwide, even a relatively small percentage of contact lens wearers with these clinical manifestations translates to a large total number of affected individuals.1 In contrast to corneal infection, bacteria are not cultured from the corneal surface, but patients with CLARE, CLPU, or IK are likely to have bacteria on the lens, which in the static contact lens wearing environment exposes the corneal surface to bacteria and bacterial products.
Although the initial stimulus for development of sterile infiltrates is not known, it seems reasonable to propose that activation of resident cells in the cornea by these bacterial products can initiate an inflammatory response by activating pathogen recognition molecules on corneal epithelial cells or resident macrophages and dendritic cells. We and others have used murine models to demonstrate that toll-like receptors (TLRs) are present at the corneal surface, and that exposure to killed bacteria or bacterial products stimulates production of pro-inflammatory and chemotactic cytokines that mediate recruitment of neutrophils to the corneal stroma.2–6 Although not shown directly in human disease, at least two sets of studies are consistent with this hypothesis: firstly, TLRs are expressed on human corneal epithelial cells and their activation induces production of neutrophil chemokines,7–11 and secondly, corneal biopsies from patients with CLPU reveal the presence of neutrophils.12
Gram-negative and Gram-positive bacteria are recovered from contact lenses of patients with corneal infiltrates, including Pseudomonas, Staphylococcus, and Serratia species.13–16 S. marcescens is a Gram-negative bacteria found commonly in food, water, and soil. However, it also is opportunistic, and can cause nosocomial infections. S. marcescens also can form biofilm by regulating fimbriae expression, which is more resistant to antibiotics and disinfectants.15,17
In the current study, we show that S. marcescens clinical isolates activate TLR4 and TLR5 in the cornea, induce CXC chemokine production and recruit neutrophils to the corneal stroma, killing the bacteria, and causing elevated stromal thickness and haze. However, under conditions where the TLR/interleukin-1 receptor type 1 (IL-1R1) signaling pathway is impaired, S. marcescens can replicate in the cornea and cause severe keratitis.
Materials and Methods
S. marcescens Strains and Growth Conditions
S. marcescens clinical isolates 056, 094, 087, 080, and 156 were obtained from patients with contact lens-associated corneal infiltrates at the University Hospitals Case Medical Center Eye Institute. Bacteria were grown in 20 mL of brain heart infusion broth (BHI; Difco, Detroit, MI) at 37°C for 18 hours, subcultured 1:100 in fresh BHI, and grown to an optical density (OD) of 0.17 at 650 nm to obtain a log-phase culture (approximately 108 colony-forming units [CFU]/mL). Bacteria were washed three times with PBS (Life Technologies, Grand Island, NY) and centrifuged at 5000 revolutions per minute (rpm) for 5 minutes, and then resuspended in PBS to achieve the final inoculum. CFU were quantified by plating track dilutions onto BHI agar.
Source of Mice
C57BL/6 and IL-1R1−/− mice (6 to 8 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). TLR4−/−, MyD88−/−, and TRIF−/− mice were obtained with permission from S. Akira (Research Institute for Microbial Diseases, Osaka University, Japan), TIRAP−/− mice were obtained from Ruslan Medzhitov, and TLR5−/− mice were obtained from Richard Flavell, both from the Howard Hughes Medical Institute, Yale University, New Haven, Connecticut. MD-2−/− mice were obtained from K. Miyaki, University of Tokyo, and TLR4/5−/− mice were generated at the CWRU animal facility. All of these gene knockout mouse strains are on a C57BL/6 background. Animals were housed under specific pathogen-free conditions in microisolator cages, and maintained according to institutional guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
S. marcescens–Induced Keratitis
Mice were anesthetized by intraperitoneal injection of 0.4 mL 2,2,2,-tribromoethanol (1.2%), and the central corneal epithelium was abraded with three parallel contiguous scratches 1 mm in length using a 26-gauge needle as shown previously.2,3 A 2 μL bacterial suspension containing the appropriate inoculum was applied to the scarified cornea.2 A sterile trephine (Miltex, Tuttlingen, Germany) was used to generate a 2 mm diameter punch of a silicone hydrogel contact lens (lotrafilcon A; CIBA Vision, Duluth, GA), which was placed over the central abraded cornea to maintain contact between the bacteria and the ocular surface as described previously.7 The lenses remained in place for 2 hours until the animals recovered from anesthesia. For trauma controls, corneas were abraded and given topical sterile PBS. Infection of C57BL/6 corneas with 1 × 106, 1 × 107, or 1 × 108 live bacteria showed a dose response for stromal thickness, stromal haze and CFU (see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/11/7382/suppl/DC1). We used the intermediate 1 × 107 bacterial inoculum in all studies.
In Vivo Confocal Microscopy
Analysis of cellular infiltration was accomplished using the in vivo ConfoScan3 microscope system (Nidek Technologies, Fremont, CA), as described previously.2,7 Briefly, mice were anesthetized and immobilized on a secure platform. A 40× objective was placed on the corneal surface, using transparent gel (Genteel; Novartis Ophthalmic, Duluth, GA) as a medium between the corneal surface and the objective, and use of the Navis software (Lucent Technologies, Murry Hill, NJ) allowed us to capture images with numerical values for light intensity every 0.5 μm, and store them as a stack for analysis of corneal thickness and haze. Stromal thickness was defined as the area between the basal epithelium and corneal endothelium, and stromal haze was calculated from the light intensity of each image of the corneal stroma. To obtain these values, a series of intensity values for each image of the corneal stroma was saved to a computer spreadsheet (Excel; Microsoft, Redmond, WA) and exported into Prism (GraphPad Software, San Diego, CA) to generate a curve using the “curve and regression” function. The total area under the curve, representing total thickness and light intensity, was calculated and used as a measure of the stromal haze.
Quantification of Viable Bacteria in the Cornea
Whole eyes were homogenized under sterile conditions using the Mixer Mill MM300 (Retsch, Inc., Newtown, PA) at 33 Hz for 4 minutes. A 20 μL aliquot of the eye homogenate was serially diluted 1:10 in sterile PBS. Serial 10-fold dilutions of the samples were plated onto BHI agar (Difco), and the plates were incubated overnight at 37°C. The number of viable bacteria in an individual eye was determined by counting individual colonies on plates that contained the various dilutions.
Histologic Examination of Corneas
Mice were sacrificed at 24 and 48 hours post treatment. The eyes were enucleated immediately, fixed in 10% neutral buffered formalin for 24 hours, and then stored in 70% ethanol until sectioning. Sections 5 μm thick were cut and stained with Gill's hematoxylin for 3 to 5 minutes following deparaffinization, and then dipped into acetic acid and placed in Shandon bluing solution (ThermoShandon, Waltham, MA) and counterstained with Eosin Y solution. The slides then were dehydrated and mounted under Permount medium.
Quantification of Neutrophils in the Cornea by Immunohistochemistry
Eyes were enucleated and fixed in formaldehyde, and embedded in paraffin. The 5 μm sections then were washed with PBS, and incubated 2 hours with antineutrophil antibody NIMP-R14 hybridoma supernatant in 1% fetal calf serum (FCS)/PBS. Sections then were incubated 45 minutes with FITC conjugated rabbit anti-rat antibody (Vector Laboratories, Burlingame, CA), and the total number of neutrophils per 5 μm section was enumerated by direct counting of fluorescence-labeled neutrophils as described previously.7
Bone Marrow Macrophages
Total bone marrow cells were derived as described previously.18 Mice were euthanized, and the femur and tibia were removed and centrifuged to extract the marrow. The pellet was washed in PBS and resuspended in 1 mL of sterile RBC lysis buffer for 5 minutes. Bone marrow cells then were washed twice with Dulbecco's modified Eagle's medium (DMEM), and incubated in DMEM containing 10% fetal bovine serum (FBS) and 30% L929 conditioned medium for 10 days to allow differentiation into macrophages.18 The cells were plated at 1 × 105/100 μL per well, and stimulated with either LPS (100 ng/mL), Pam3Cys (100 ng/mL), or Serratia (056 strain) killed in 0.6% tobramycin solution.19 Macrophages were incubated 6 hours, and supernatants were collected for cytokine analysis as described in our previous studies.18
Cytokine Analysis
To measure cytokine production in infected corneas, individual corneas were dissected and homogenized in 0.2 mL PBS. A 25 μL aliquot was assayed by ELISA according to the manufacturer's directions (R&D Systems, Minneapolis, MN). Cytokine levels in the supernatants of stimulated bone marrow–derived macrophages also were measured by ELISA.
Statistics
Statistical significance was calculated by either Student's t-test or one-way ANOVA with Tukey post-analysis using Prism (GraphPad Software). A value of < 0.05 between groups was considered significant.
Results
S. marcescens Activation of Bone Marrow Derived Macrophages
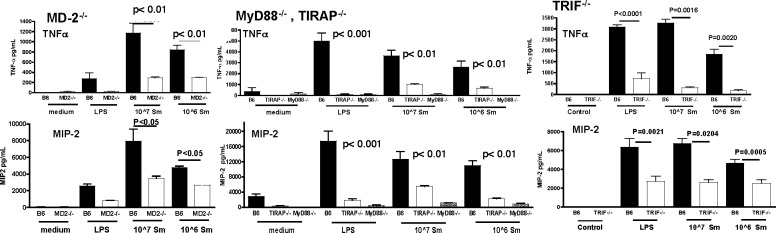
Our previous studies showed P. aeruginosa activation of TLR4/MD-2 LPS receptor signals through the TIRAP/MyD88 and the TRAM/TRIF-dependent pathways.20 To determine if S. marcescens also activates both pathways, macrophages were derived from bone marrow cells isolated from C57BL/6 and gene knockout mice, and incubated with tobramycin-killed S. marcescens. After 6 hours, TNF-α and CXCL2/MIP-2 were quantified by ELISA.
As shown in Figure 1 (left panels), compared to unstimulated macrophages, LPS and killed S. marcescens induced TNF-α and CXCL2/MIP-2 production by C57BL/6 macrophages. However, S. marcescens incubation with MD-2−/− macrophages resulted in significantly lower production of both cytokines, indicating that S. marcescens activates this receptor. Figure 1 (center and right panels) also demonstrates that TIRAP−/− and TRIF−/− macrophages produced significantly less TNF-α and CXCL2/MIP-2 compared to C57BL/6 macrophages, whereas cytokine production was completely ablated in MyD88−/− macrophages. As an additional control, cytokine production induced by the TLR2/1 ligand Pam3Cys was impaired in MyD88−/− macrophages, but not in MD-2−/−, TIRAP−/−, or TRIF−/− macrophages compared to C57BL/6 macrophages (data not shown).
Figure 1. .
MD-2 and adaptor molecule use in macrophages stimulated with killed S. marcescens. 1 × 105 bone marrow macrophages from C57BL/6, MD-2−/−, MyD88−/−, TIRAP−/−, or TRIF−/− mice were incubated with LPS, or with 1 × 107 or 1 × 106 tobramycin-killed S. marcescens clinical isolate (056). After 6 hours, supernatants were removed and cytokine production was measured by ELISA. Data are mean ± SD and represent two repeat experiments.
Together, these findings indicate that S. marcescens induces TLR4/MD-2 activation by signaling through both the MyD88 and TRIF-dependent pathways.
MyD88 Regulates Corneal Inflammation and Infection Caused by S. marcescens
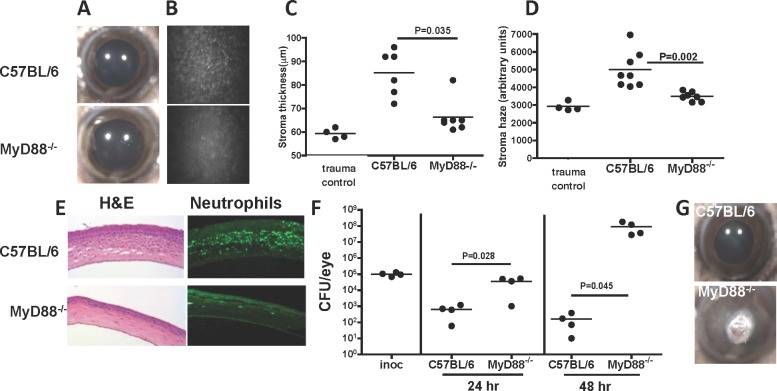
To determine the role of MyD88 in S. marcescens–induced corneal inflammation and infection, C57BL/6 mice were abraded by three parallel scratches, and 1 × 107 live S. marcescens clinical isolate (056) in 2 μL PBS were added topically to the cornea. A 2 mm diameter punch from a silicone hydrogel contact lens was then placed on the corneal surface for 2 hours while the mice remained anesthetized, and corneas were examined after 24 hours by in vivo confocal microscopy (Confoscan; Nidek Technologies). The number of surviving bacteria was determined by CFU.
As shown in the representative images in Figure 2A, there was no apparent corneal opacification in either C57BL/6 or MyD88−/− mice when examined by light microscopy 24 hours after infection. However, when examined by in vivo confocal microscopy, there was a pronounced cellular infiltrate in the central corneal stroma of C57BL/6, but not MyD88−/− mice (Fig. 2B). Quantification of these images throughout the cornea showed increased corneal thickness and haze in C57BL/6 corneas infected with S. marcescens compared to naïve (untreated) controls (Figs. 2C, 2D). However, these values were significantly lower in infected MyD88−/− compared with C57BL/6 corneas, and were similar to naïve and trauma controls. We also tested five S. marcescens clinical isolates and found no differences in corneal thickness or haze (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/11/7382/suppl/DC1). Further, stromal thickness and haze values following corneal abrasion and addition of sterile PBS (trauma control) were not significantly different from naïve mice (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/11/7382/suppl/DC1).
Figure 2. .
The role of MyD88 in S. marcescens–induced corneal infection. Corneas of C57BL/6 and MyD88−/− mice were abraded, and 1 × 107 S. marcescens clinical isolate (056) in 2 μL PBS were added topically. A 2 mm diameter punch from a silicone hydrogel contact lens then was placed on the corneal surface for 2 hours. Corneal images were taken by light microscopy, and corneal thickness and haze (B–E) were examined using the ConfoScan3 microscope system. (A) Representative corneas 24 hours after infection (original magnification is ×20). (B) Representative Confoscan images of the central corneal stroma (original magnification is ×200). (C, D) Corneal thickness and haze measured by Confoscan. Trauma controls were abraded and exposed to sterile PBS. (E) Representative 5 μm corneal sections stained with hematoxylin and eosin or immunostained with the anti-mouse neutrophil Ab NIMP R14. (F) CFU at 24 and 48 hours post infection. Inoc: CFU after 2 hours on the ocular surface, representing the inoculum). (G) Representative C57BL/6 and MyD88−/− corneas 48 hours post infection. Data points represent individual corneas; the experiment was repeated twice with similar results.
As neutrophils are the predominant infiltrating cell type in corneal infiltrates and in LPS-induced corneal inflammation,2,7 we next quantified the number of neutrophils in the corneal stroma after infection with S. marcescens. Corneal sections were immunostained with NIMPR-14, which reacts with murine neutrophils, and the number of positive cells per section was counted by fluorescence microscopy. Figure 2E shows histology and NIMPR14-stained corneal sections from C57BL/6 and MyD88−/− corneas 24 hours after infection. There were significantly less infiltrating cells overall, and fewer NIMPR14+ neutrophils in MyD88−/− compared to C57BL/6 corneas (Fig. 2E). Together these findings demonstrated that MyD88 is essential for corneal inflammation caused by S. marcescens.
As corneas were infected with live bacteria, we also assessed the role of MyD88 on bacterial growth and survival. Figure 2F shows CFU after S. marcescens infection. In C57BL/6 corneas, bacterial numbers decreased compared to the starting inoculum (after the 2 hours exposure while the lens is on the ocular surface) until they were no bacteria detected (after 72 hours, data not shown). In marked contrast, CFU recovered from MyD88−/− mice were significantly higher than C57BL/6 corneas at each time point, and at 48 hours were elevated above day 1 MyD88−/− corneas, indicating that instead of being killed, S. marcescens is replicating in the corneas of these mice. As a consequence of bacterial replication associated with impaired neutrophil infiltration, MyD88−/− corneas at 48 hours had more opacification than C57BL/6 mice (Fig. 2G), resulting in corneal perforation (not shown).
We concluded from these studies that MyD88 regulates neutrophil recruitment to the cornea and bacterial killing, and that in the absence of MyD88, S. marcescens is able to replicate in the cornea and can cause corneal opacification.
IL-1R1 Regulates S. marcescens Corneal Infection
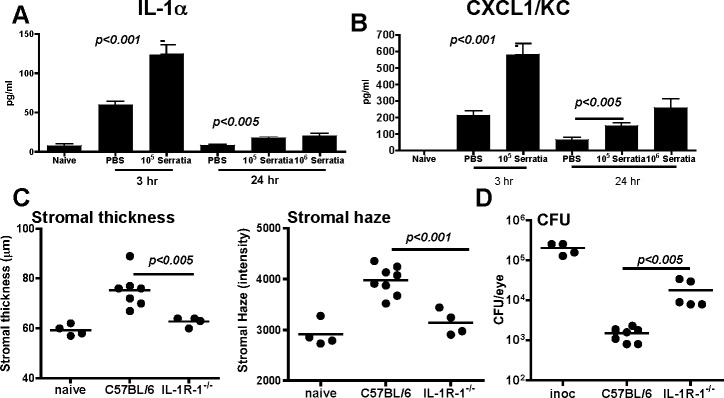
MyD88-dependent signaling is initiated by IL-1R1 in addition to TLRs,21 and in P. aeruginosa corneal infection, IL-1α is produced in the cornea.20 To determine if IL-1α and the neutrophil chemokine CXCL1/KC are produced after S. marcescens infection, C57BL/6 mice were euthanized 3 hours after infection, corneas were dissected and homogenized, and cytokine production was measured by ELISA.
Figures 3A and 3B show that after 3 hours, IL- 1α and CXCL1 were elevated in response to trauma/PBS compared to naïve corneas. However, both cytokines were elevated significantly after infection with S. marcescens, and were reduced 24 hours after infection. To examine the role of IL-1R1 in S. marcescens–induced corneal inflammation, corneas of C57BL/6 and IL-1R1−/− mice were abraded and infected with S. marcescens as before, and after 24 hours, corneal inflammation and bacterial survival were assessed. As shown in Figure 3C, stromal haze and thickness values were significantly lower in IL-1R1−/− compared to C57BL/6 corneas. Conversely, CFU were significantly elevated in IL-1R1−/− corneas (Fig. 3D). These findings are similar to MyD88−/− mice, thereby indicating that the IL-1R1/MyD88 pathway has an important role in neutrophil recruitment, corneal inflammation, and consequently bacterial growth and survival.
Figure 3. .
The role of IL-1R1 in S. marcescens–induced corneal inflammation and infection. Corneas of C57BL/6 and IL-1R1−/− mice were abraded and infected with S. marcescens as before. IL-1α and CXCL1/KC production was assessed after 3 and 24 hours (A, B). Data are mean ± SD of three replicate values. Stromal thickness, haze, and bacterial survival (CFU) were examined at 24 hours post infection (C, D). Data points represent individual corneas, and all data are representative of three repeat experiments.
TLR4−/− and TLR4/5−/− Mice Have an Impaired Ability to Regulate S. marcescens Growth in Infected Corneas
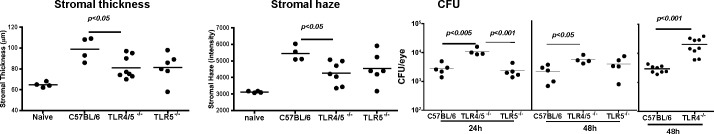
As S. marcescens are flagellate Gram-negative bacteria, we next examined the role of TLR4 and TLR5 in corneal inflammation and survival of S. marcescens. Corneas of C57BL/6, TLR4−/−, TLR5−/−, and TLR4/5−/− mice were abraded, and were infected with S. marcescens as before. Corneal thickness and haze were measured 24 hours post infection, and CFU were assessed after 24 and 48 hours.
Figure 4 shows significantly lower corneal thickness and haze values in TLR4/5−/− mice compared to C57BL/6 mice, whereas TLR5−/− values were not significantly different from C57BL/6 mice. Conversely, CFU at 24 hours was significantly higher in TLR4/5−/− mice compared to C57BL/6 and TLR5−/− mice, and between C57BL/6 and TLR4/5−/− mice after 48 hours. CFU were significantly higher in TLR4−/− than in C57BL/6 mice. Together, these findings are consistent with an essential role for TLR4 rather than TLR5 in S. marcescens corneal infection and inflammation.
Figure 4. .
The role of TLR4 and TLR5 in S. marcescens–induced corneal inflammation and infection. Corneas of C57BL/6, TLR4−/−, TLR5−/−, and TLR4/5−/− mice were abraded and infected with live S. marcescens as before. After 24 and 48 hours, stromal thickness and haze, and bacterial survival (CFU) were assessed. Data points represent individual corneas. This experiment was repeated twice with similar results.
Adaptor Molecules TRIF and TIRAP Have a Redundant Role in S. marcescens Corneal Infection
In addition to MyD88, the TIR-containing adaptor molecules Mal/TIRAP and TRIF are important in directing the host response in TLR4/MD-2–dependent cell signaling.21 TIRAP is required for recruitment of MyD88 to the cytoplasmic tail of TLR4, and TRIF signaling is independent of MyD88. Further, we showed previously that MyD88 and TRIF pathways have a role in P. aeruginosa keratitis.20
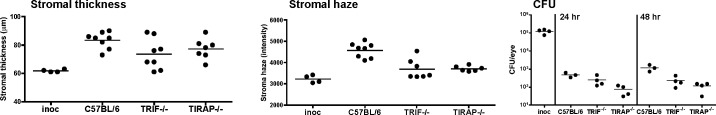
To determine if TRIF and TIRAP also regulate S. marcescens–induced corneal inflammation, C57BL/6, TIRAP−/−, and TRIF−/− corneas were abraded and infected with S. marcescens as before. Stromal thickness and haze were quantified after 24 hours, and CFU were examined after 24 and 48 hours. Figure 5 shows that there were no significant differences in stromal thickness or haze among infected C57BL/6, TIRAP−/−, and TRIF−/− corneas. Further, in contrast to MyD88−/− IL-1R1−/−, and TLR4/5−/− mice, CFU was not significantly elevated in TIRAP−/− or TRIF−/− corneas, indicating that although the TLR4 pathway is important, TRIF and TIRAP have redundant roles in S. marcescens keratitis.
Figure 5. .
The role of TRIF and TIRAP in S. marcescens–induced corneal inflammation and infection. C57BL/6, TIRAP−/−, and TRIF−/− corneas were abraded and infected with S. marcescens as before. Stromal thickness and haze values are shown for 24 hours post infection. CFU was quantified 24 and 48 hours post infection. Data points represent individual corneas, and are representative of two repeat experiments. There were no significant differences among the groups.
S. marcescens–Induced Corneal Inflammation Is Dependent on MD-2
As TLR4 has a role in S. marcescens infection, and MD-2 antagonism inhibits corneal inflammation induced by antibiotic killed P. aeruginosa,7 we examined the role of MD-2 and the effect of MD-2 antagonism in S. marcescens–induced corneal inflammation. S. marcescens were killed by tobramycin, and either used to stimulate corneal inflammation in C57BL/6 and MD-2−/− mice, or C57BL/6 corneas were treated with the MD-2 antagonist eritoran tetrasodium before adding killed S. marcescens.
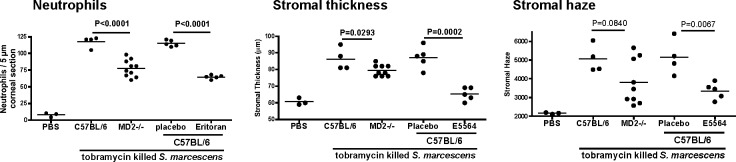
As shown in Figure 6, neutrophil recruitment, and stromal thickness and haze were significantly lower in MD-2−/− mice, and in eritoran tetrasodium–treated C57BL/6 corneas. These findings indicated that targeting MD-2 is a critical mediator of S. marcescens–induced inflammation.
Figure 6. .
The role of MD-2 in S. marcescens–induced corneal inflammation. (A) Corneas of C57BL/6 and MD-2−/− mice were abraded and 1 × 107 tobramycin-killed S. marcescens were placed on the corneal surface as before. (B) Corneas of C57BL/6 mice were abraded and 10 μL eritoran tetrasodium (E5564) or vehicle was added for 1 hour prior with a contact lens before stimulation with killed bacteria. After 24 hours, stromal thickness and haze were measured as before, and the number of neutrophils per corneal section was determined after immunostaining with NIMPR14. Data points represent individual corneas, and the experiment was repeated twice with similar results.
Discussion
S. marcescens corneal infection of immunocompetent mice was found to be self-limiting and associated with relatively mild, transient inflammation. This is consistent with the symptoms of contact lens–associated corneal infiltrates where these organisms have been isolated.13–16 Although S. marcescens produces several cytotoxins, including the zinc metalloproteinase serralysin that can induce an inflammatory response,22,23 this apparently is not sufficient to protect the bacteria in the presence of a robust host response. This is in marked contrast to P. aeruginosa, which causes severe corneal opacification and ulceration due to rapid, uncontrolled growth of the bacteria.24 The P. aeruginosa type III secretion system, in which exotoxins are injected into host cells and either kills the cells or facilitates bacterial replication.25 We, and others showed that the type III secretion system, including ExoS, ExoT, and ExoU, contributes to survival of bacteria in the cornea and severity of disease by inducing apoptosis in neutrophils.26–29 Further, P. aeruginosa mutants that do not express type III secretion are cleared rapidly in immunocompetent mice, but not in MyD88−/−, TLR4−/−, or TLR4/5−/− mice.26
In our study, we found that S. marcescens induces production of CXCL1 and IL-1α in the cornea within 3 hours of infection, which is similar to P. aeruginosa.7,20 As CXCL1 has neutrophil chemotactic activity, and IL-1α can activate vascular endothelial cells, neutrophils are recruited rapidly to the corneal stroma following infection. S. marcescens is killed rapidly in the cornea following neutrophil infiltration, which is what we observed with P. aeruginosa Type III secretion mutants. In both cases, fewer neutrophils are required to kill the bacteria, and there is less corneal opacification. This scenario is consistent with the less severe disease caused by S. marcescens (corneal infiltrates) compared to P. aeruginosa (severe microbial keratitis and corneal ulceration). Hence, the presumed sterile corneal infiltrative responses seen with extended wear contact lenses in which S. marcescens is isolated is likely due to either bacteria that penetrate the corneal stroma and are killed, or to exposure to products of dead bacteria, such as LPS and flagellin.
In contrast to immune competent individuals, S. marcescens causes nosocomial infections in immune compromised individuals and in neonates.30,31 Therefore, we examined the role of TLR and IL-1R signaling in regulating S. marcescens corneal infection. We reasoned that S. marcescens signaling would be similar to that induced by P. aeruginosa in which LPS activates TLR4 on macrophages through TIRAP/MyD88 and TRIF, and flagellin activates TLR5 through MyD88, leading to NFκB translocation to the nucleus, and expression of neutrophil chemotactic cytokines, and IL-1α and IL-1β.20 These pro-inflammatory cytokines then mediate further cell activation through IL-1R1 and MyD88.
Using isolated macrophages from gene knockout mice, we found that S. marcescens activates TLR4/MD-2 through the TIRAP/MyD88 and TRIF pathways. In vivo data support these observations, as mice deficient in MyD88, IL-1R1, or TLR4 showed impaired neutrophil recruitment and were less able to regulate S. marcescens corneal infection. As MyD88 is the common adaptor molecule for TLR4, TLR4/5, and IL-1R1 signaling,28 infection of these gene knockout mice resulted in uncontrolled bacterial growth and rapid corneal perforation, whereas other gene knockout mice showed less susceptibility. The role of multiple receptors and signaling pathways to a single organism most likely explains the intermediate phenotypes of infected TLR4, TLR4/5, and IL-1R1 mice, and the absence of observable phenotypes in mice deficient in TLR5, TIRAP, or TRIF. Although yet to be determined, the nonredundant role of TLR4 in the presence of flagellin and TLR5 likely is due to the highly sensitive response of the TLR4/MD-2 complex, which can detect picomolar levels of bacterial endotoxin.26 Taken together, these data supported the concept that S. marcescens–induced corneal inflammation is similar to that of P. aeruginosa. Therefore, a similar approach could be taken to limit the inflammatory response associated with S. marcescens–induced corneal infiltrates.
Although our studies on P. aeruginosa showed an essential role for macrophages in early cytokine production,20 we also reported that corneal epithelial cells express TLR4 constitutively, but that MD-2 mRNA, cell surface expression LPS responsiveness are dependent on IFN-γ/STAT1 signaling.32 As corneal epithelial cells express TLR5, MyD88, and TRIF,11,18 it is likely that these cells also contribute to the corneal inflammation caused by S. marcescens LPS and flagellin.
MD-2 expression on corneal epithelial cells and resident macrophages is an attractive target to inhibit corneal inflammation. To this end, we showed that corneal inflammation induced by LPS or tobramycin-killed P. aeruginosa is significantly reduced following topical application of a specific MD-2 competitive inhibitor eritoran tetrasodium.7 Our finding that this MD-2 inhibitor also blocked inflammation induced by killed S. marcescens indicated that the approach of blocking TLR activation together with topical antibiotics may have broad application for treatment or prevention of corneal infiltrates associated with contact lens wear.
Supplementary Material
Acknowledgments
Scott Howell and Catherine Doller in the Visual Sciences Core Facility assisted with this study.
Footnotes
Supported by NIH Grants RO1EY14362 (EP) and P30EY11373 (EP), by The Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation, and by an Alcon Research Institute award (EP).
These authors contributed equally to this work presented here and therefore should be regarded as equivalent authors.
Disclosure: R. Zhou, None; R. Zhang, None; Y. Sun, None; S. Platt, None; L. Szczotka-Flynn, None; E. Pearlman, None
References
- 1.Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84:257–272 [DOI] [PubMed] [Google Scholar]
- 2.Johnson AC, Heinzel FP, Diaconu E, et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595 [DOI] [PubMed] [Google Scholar]
- 3.Khatri S, Lass JH, Heinzel FP, et al. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest Ophthalmol Vis Sci. 2002;43:2278–2284 [PubMed] [Google Scholar]
- 4.Schultz CL, Buret AG, Olson ME, Ceri H, Read RR, Morck DW. Lipopolysaccharide entry in the damaged cornea and specific uptake by polymorphonuclear neutrophils. Infect Immun. 2000;68:1731–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz CL, Morck DW, McKay SG, Olson ME, Buret A. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp Eye Res. 1997;64:3–9 [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564). Invest Ophthalmol Vis Sci. 2009;50:1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hozono Y, Ueta M, Hamuro J, et al. Human corneal epithelial cells respond to ocular-pathogenic, but not to nonpathogenic-flagellin. Biochem Biophys Res Commun. 2006;347:238–247 [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254 [DOI] [PubMed] [Google Scholar]
- 12.Holden BA, Reddy MK, Sankaridurg PR, et al. Contact lens-induced peripheral ulcers with extended wear of disposable hydrogel lenses: histopathologic observations on the nature and type of corneal infiltrate. Cornea. 1999;18:538–543 [PubMed] [Google Scholar]
- 13.Szczotka-Flynn L, Debanne SM, Cheruvu VK, et al. Predictive factors for corneal infiltrates with continuous wear of silicone hydrogel contact lenses. Arch Ophthalmol. 2007;125:488–492 [DOI] [PubMed] [Google Scholar]
- 14.Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007;84:247–256 [DOI] [PubMed] [Google Scholar]
- 15.Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009;28:918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens. 2010;36:116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanks RM, Stella NA, Kalivoda EJ, et al. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol. 2007;189:7262–7272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–3996 [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83 [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Karmakar M, Roy S, et al. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384 [DOI] [PubMed] [Google Scholar]
- 22.Kwak J, Lee K, Shin DH, et al. Biochemical and genetic characterization of arazyme, an extracellular metalloprotease produced from Serratia proteamaculans HY-3. J Microbiol Biotechnol. 2007;17:761–768 [PubMed] [Google Scholar]
- 23.Kida Y, Inoue H, Shimizu T, Kuwano K. Serratia marcescens serralysin induces inflammatory responses through protease-activated receptor 2. Infect Immun. 2007;75:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa). Chem Immunol Allergy. 2007;92:185–194 [DOI] [PubMed] [Google Scholar]
- 25.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E. ExoS and ExoT ADP ribosyltransferase activities mediate Pseudomonas aeruginosa keratitis by promoting neutrophil apoptosis and bacterial survival. J Immunol. 2012;188:1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44:3892–3898 [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, Evans DJ, Fleiszig SM. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci. 2003;44:5220–5227 [DOI] [PubMed] [Google Scholar]
- 29.Tam C, Lewis SE, Li WY, Lee E, Evans DJ, Fleiszig SM. Mutation of the phospholipase catalytic domain of the Pseudomonas aeruginosa cytotoxin ExoU abolishes colonization promoting activity and reduces corneal disease severity. Exp Eye Res. 2007;85:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arslan U, Erayman I, Kirdar S, et al. Serratia marcescens sepsis outbreak in a neonatal intensive care unit. Pediatr Int. 2010;52:208–212 [DOI] [PubMed] [Google Scholar]
- 31.Buffet-Bataillon S, Rabier V, Betremieux P, et al. Outbreak of Serratia marcescens in a neonatal intensive care unit: contaminated unmedicated liquid soap and risk factors. J Hosp Infect. 2009;72:17–22 [DOI] [PubMed] [Google Scholar]
- 32.Roy S, Sun Y, Pearlman E. Interferon-gamma-induced MD-2 protein expression and lipopolysaccharide (LPS) responsiveness in corneal epithelial cells is mediated by Janus tyrosine kinase-2 activation and direct binding of STAT1 protein to the MD-2 promoter. J Biol Chem. 2011;286:23753–23762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.