Abstract
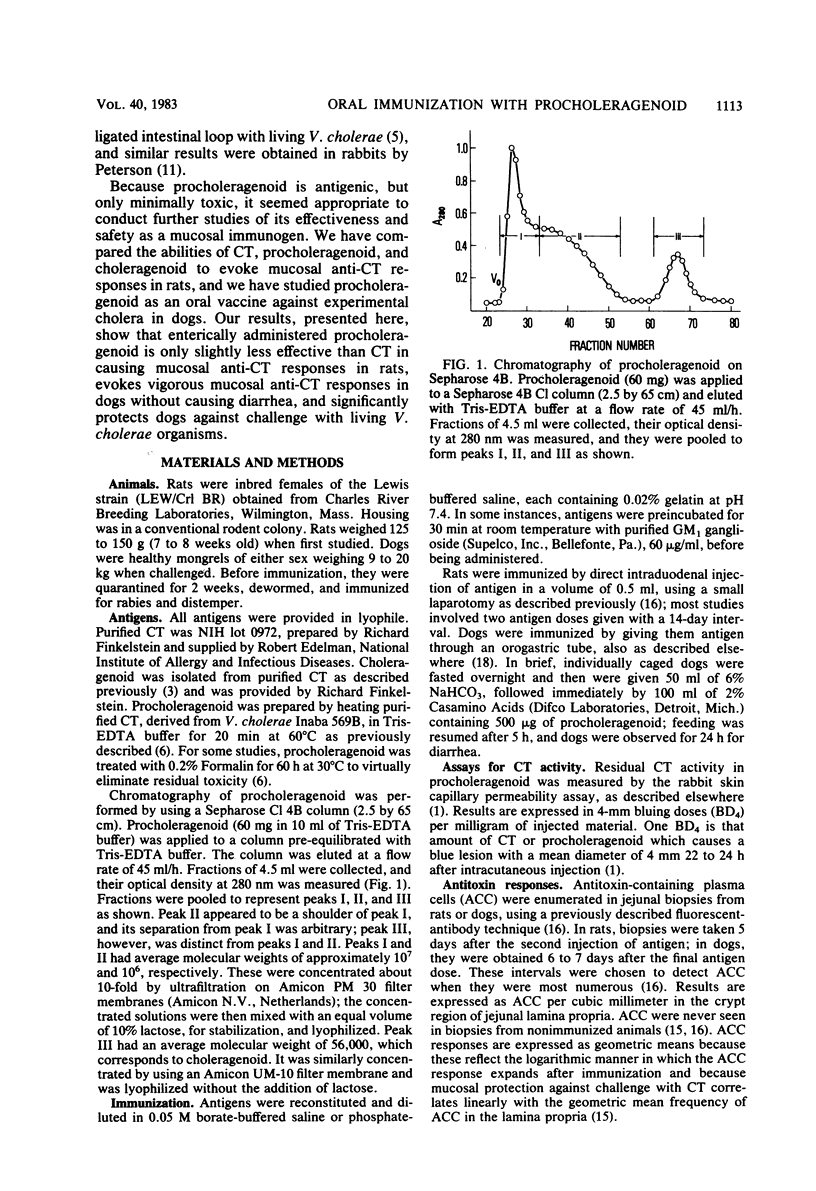
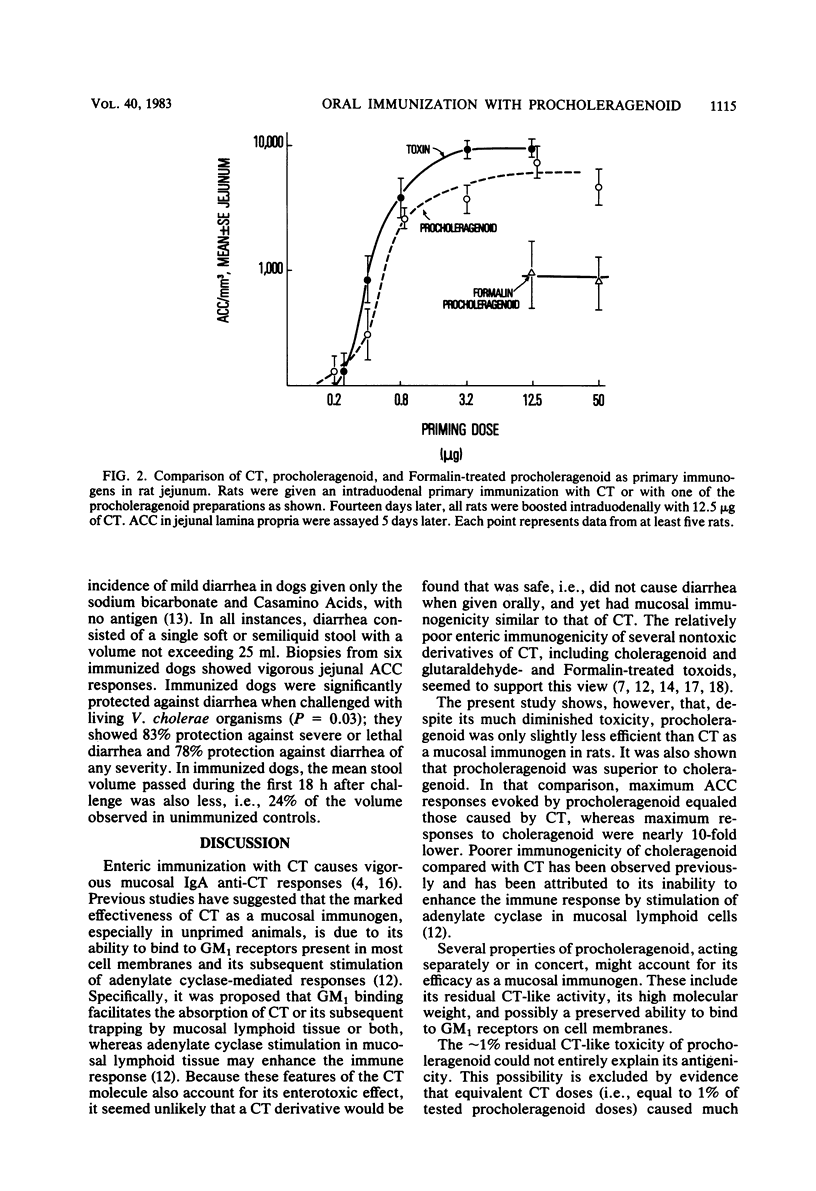
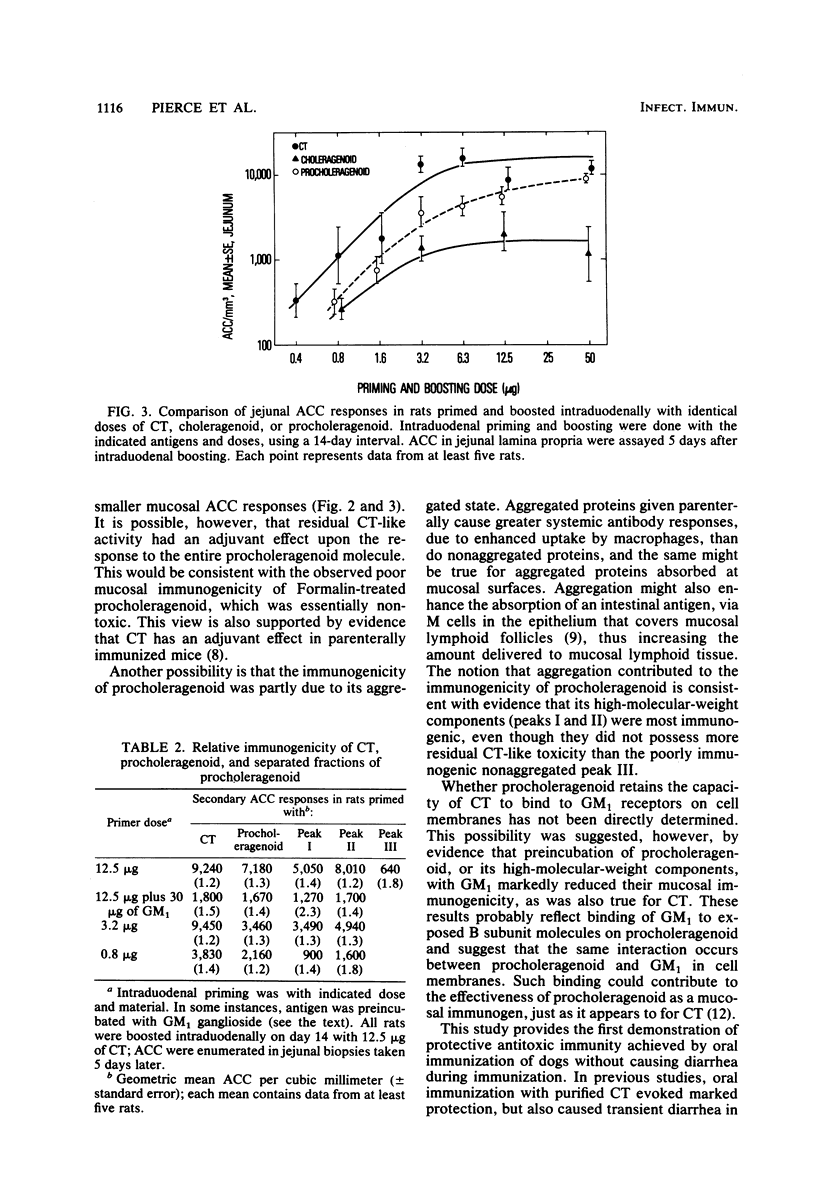
The immunogenicity and safety of procholeragenoid, a minimally toxic, heat-induced aggregate of cholera toxin (CT), were studied in enterically immunized rats and dogs. Although 99% less toxic than CT, procholeragenoid was only slightly less efficient in causing jejunal anti-CT responses in rats; in contrast, choleragenoid, the nontoxic B subunit pentamer of CT, was much less effective. The immunogenicity of procholeragenoid was due almost entirely to its large-molecular-weight components (MW = 10(6) to 10(7)) and was markedly reduced by preincubation with GM1 ganglioside or treatment with Formalin to eliminate residual toxicity. These findings suggest that molecular aggregation, binding to GM1 receptors on cell membranes, and stimulation of cellular adenylate cyclase each contributed to the effectiveness of procholeragenoid as a mucosal immunogen. In dogs, oral immunization with five 500-micrograms doses of procholeragenoid evoked vigorous anti-CT responses in jejunal mucosa without causing significant diarrhea. When subsequently challenged with virulent Vibrio cholerae, immunized dogs showed 83% protection against the development of severe or lethal diarrhea compared with non-immunized controls. These results confirm a protective role for mucosal antitoxin in experimental cholera and show that procholeragenoid is both safe and effective as an oral immunogen. Procholeragenoid, combined with other antigens of V. cholerae, may constitute a simple, safe, and effective oral vaccine for cholera.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E., Varallyay S., Inderbitzin T. M. Preparation of a purified antigenic cholera toxoid. Infect Immun. 1976 Jun;13(6):1692–1698. doi: 10.1128/iai.13.6.1692-1698.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Peterson J. W. Protection against experimental cholera by oral or parenteral immunization. Infect Immun. 1979 Nov;26(2):594–598. doi: 10.1128/iai.26.2.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Synergistic protection against experimental cholera by immunization with cholera toxoid and vaccine. Infect Immun. 1979 Nov;26(2):528–533. doi: 10.1128/iai.26.2.528-533.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Engel P. F. Antitoxic immunity to cholera in dogs immunized orally with cholera toxin. Infect Immun. 1980 Feb;27(2):632–637. doi: 10.1128/iai.27.2.632-637.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr Oral immunization of dogs with purified cholera toxin, crude cholera toxin, or B subunit: evidence for synergistic protection by antitoxic and antibacterial mechanisms. Infect Immun. 1982 Aug;37(2):687–694. doi: 10.1128/iai.37.2.687-694.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sircar B. K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978 Jul;21(1):185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Bonde G. Development of a vaccine against experimental cholera and Escherichia coli diarrheal disease. Infect Immun. 1981 May;32(2):534–541. doi: 10.1128/iai.32.2.534-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. I. Development of the model. J Infect Dis. 1969 Feb;119(2):138–149. doi: 10.1093/infdis/119.2.138. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



