Abstract
Background:
The aim of this study was to detect the prognostic significance of survivin level and the expression of total p53 in acute lymphoblastic leukemia (ALL) and its correlation to patients' outcome.
Methods:
Sixty two children newly diagnosed with acute lymphoblastic leukemia were treated with chemotherapy and followed up for 2 years or until death. Twenty apparently healthy volunteers with matched age and sex were taken as control. Survivin protein was measured by quantitative sandwich enzyme immunoassay and total human p53 was measured by Flow cytometry in peripheral blood at diagnosis and at complete remission.
Results:
A highly significant elevation (P<0.0001) was found in survivin protein and total p53 levels in acute lymphoblastic leukemia children patients at diagnosis compared to controls. At complete remission a significant decrease of the two indices were found in ALL patients compared to those at diagnosis (P<0.0001). Survivin protein and total p53 was significantly higher in non-survived compared to survived group (P<0.0001 & P=0.016, respectively). A positive correlation was found between survivin level and total human p53 level in children with ALL (r=0.501 & P<0.0001).
Cconclusion:
survivin protein is related to anti-apoptotic proteins and its high expression lead to unsuccessful treatment of ALL. Survivin and TP53 are new prognostic tools in ALL, independent of age and sex.
Keywords: Survivin, p53, Acute lymphoblastic leukemia, Gene expression, ELISA, Flow cytometry
Introduction
Acute lymphoblastic leukemia (ALL) is characterized by the clonal proliferation and accumulation of malignant blast cells in the bone marrow and peripheral blood (1). It is the most common malignancy diagnosed in children, representing nearly one third of all pediatric cancers. The annual incidence of acute lymphoblastic leukemia is approximately 9–10 cases per 100,000 populations in childhood. The peak incidence occurs in children aged 2–5 years (2).
Apoptosis is an active biological mechanism leading to programmed cell death. During the last decade, complex networks of pro- and anti-apoptotic proteins that strictly govern the regulation of apoptosis pathways have been identified (3, 4). Apoptosis is known to play an important role in the cellular response to genotoxic stress. Therefore, loss of apoptotic response in tumor cells is thought to be one of the mechanisms involved in malignant progression and resistance to chemotherapy (5).
P53 is a tumor suppressor protein that in humans is encoded by the TP53 gene located on the short arm of chromosome 17 (6). P53 is a transcription factor that plays a key role in both cell cycle arrest and apoptosis. While p53 levels are kept low in unstressed cells, they rapidly increase in response to stressors, such as DNA damage. P53 will then become activated through posttranslational modifications and tetramerization following genotoxic or cytotoxic stress (7, 8). P53 has many anticancer mechanisms, and plays a role in apoptosis, genetic stability, and inhibition of angiogenesis. In its anti-cancer role, p53 works through several mechanisms. It can activate DNA repair proteins when DNA has sustained damage, induce growth arrest by holding the cell cycle at the G1/S regulation point on DNA damage recognition and can initiate apoptosis if DNA damage proves to be irreparable (9).
Inhibitor of apoptosis proteins (IAP) is an important gene family responsible for apoptosis regulation. Consistent with their ability to block the common pathway of apoptosis, IAPs suppress multiple cell death stimuli initiated via the extrinsic, i.e. death receptor, or intrinsic, i.e. mitochondrial, apoptotic pathways (10). IAP has been defined eight different inhibitor of apoptosis family of proteins members so far: NAIP, XIAP, c-IAP1, c-IAP2, Ts-XIAP, ML-IAP, Apollon and Survivin. There are common molecular structures of these members.
Survivin gene is localized at chromosome 17 and has 4 exons and 3 introns. Survivin is an onco-fetal protein; it is expressed in embryonic life and in various malignant tumors (11).
The chemotherapeutic drug doxorubicin, a DNA-damaging agent, activates a p53-survivin signaling pathway inducing cell cycle arrest and apoptosis in childhood acute lymphoblastic leukemia (ALL) (12). Tumor suppressor p53 regulates the expression of the inhibitor of apoptosis protein survivin, which may play a role in pathological process of cancer (13).
The aim of this study was to investigate the protein level of survivin and the expression of total p53 and the prognostic role of these proteins in children patients with ALL.
Materials and Methods
Sixty two children with newly diagnosed ALL were enrolled in this study. Patients, newly diagnosed in 2009–2010, were selected from Children Hospital Mansoura University. They were 37 (59.7%) males and 25 (40.3%) females with age range from 2 to 18 years. A written consent was taken from parents of children.
All patients received chemotherapy according to Linker-Protocol:
-Induction (Daunorubicin 50 mg/m2 IV days 1–3; Vincristine 1.4 mg/m2 IV days 1, 8, 15, and 22; L-asparaginase 6000 IU/m2 IM days 17–28; Prednisone 60 mg/m2 divided into 3 doses PO days 1–14).
-Consolidation cycle 1, 3, 5, 7 (Daunorubicin 50 mg/m2 IV days 1 and 2; Vincristine 1.4 mg/m2 IV days 1 and 8; Prednisone 60 mg/m2 divided into 3 doses PO days 1–14; L-asparaginase 12000 IU/m2 IM days 2, 4, 7, 9, 11, and 14).
-Consolidation cycle 2, 4, 6, 8 (Teniposide 165 mg/m2 IV days 1, 4, 8, and 11; Cytarabine 300 mg/m2 IV days 1, 4, 8, and 11).
-Consolidation cycle 9 (Methotrexate 690 mg/m2 IV over 42 hours; Leucovorin 15 mg/m2 every 6 hours for 12 doses beginning at 42 hours from starting).
All patients were regularly followed-up with intervals of a few months in an outpatient clinic. Patients were observed over 2 years or until death. Twenty three of them relapsed shortly (3–5 months after complete remission) and died within the follow up time. Twenty apparently healthy volunteers with matched age ranged from 3–16 year and matched sex (12 males and 8 females) were taken as control. Other factors that may interfere with the results were excluded including drugs or inflammatory disorders. Three ml of peripheral venous blood samples were taken, in sterile test tubes with heparin, from healthy subjects and ALL patients before start of therapy and after achievement of complete remission.
Survivin protein assessment was done using the Quantikine Human Survivin immunoassay sandwich-type solid phase ELISA (R&D Systems, Minneapolis, United States of America). A monoclonal antibody specific for Survivin has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any Survivin present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for Survivin is added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Survivin bound in the initial step. The color development is stopped and the intensity of the color is measured. The minimum detectable dose (MDD) of survivin is 4.44 pg/mL.
Flow cytometric analysis of p53 proteins was performed on the mononuclear cells after Ficoll sedimentation. Immuno-staining was carried out using the mouse monoclonal antibody Allophycocyanine-conjugated mouse anti-human p53 against p53 protein (R&D Systems “UK & Europe”). A FACSCalibur (BECTON DICKINSON) flow cytometer was used for analysis and the data were collected in the list mode. P53 labeling, measured in the fluorescence detector (FL) forward scatter (FSC) and side scatter (SSC) were collected using linear scales. The fluorescence signals were collected using logarithmic scales. Data acquisition and analysis by Cell Quest program (the magnitude of the signal was measured by using cell TM DNA experiment document user's guide ‘02-61539-00’) were performed on 104 viable cells. Expression was evaluated as cell percent (The number of stained cells minus the number of cells stained by irrelevant negative control).
Data were statistically analyzed using SPSS program, standard version 10. Quantitative data were presented as mean ± standard deviation, Student’s t-test and ANOVA were used to compare between means. Correlation between variables was done using Pearson’s correlation study. P ≤ 0.05 was considered to be statistically significant.
Results
Table 1 shows a significant elevation (P<0.001) in both survivin level and p53% in total ALL patients at diagnosis compared to control group.
Table 1:
Survivin levels and p53 % in ALL patients and control group
| Groups | Survivin (pg/ml) | p53% |
|---|---|---|
| Control (n=20) | 28.3 ± 9.65 | 4.79 ± 2.08 |
| Patients at Diagnosis (n=62) | 143.27 ± 56.15 | 12.41 ± 7.11 |
| ALL (L1) at diagnosis (n=19) | 86.05 ± 34.87 | 11.47 ± 3.57 |
| ALL (L2) at diagnosis (n=28) | 147.64 ± 34.24 | 11.61 ± 5.7 |
| ALL (L3) at diagnosis (n=15) | 207.60 ± 81.61 | 15.09 ± 11.46 |
| Survived Patients at diagnosis (n=39) | 118.38 ± 46.13 | 10.75 ± 4.64 |
| Patients at remission (n=39) | 35.79 ± 12.09 | 3.62 ± 1.39 |
| Non survived Patients at diagnosis (n=23) | 185.47 ± 45.90 | 15.22 ±9.48 |
A significant decrease of the two indices (P<0.001) is observed in patients at remission compared to survived patients at diagnosis. The comparison between survived and non-survived patients at diagnosis resulted in high significant elevation in survivin level (P<0.001) and p53% (P=0.016) in non-survived compared to survived patients.
Regarding ALL subtypes, there are a highly significant elevation in survivin levels in L3 in comparison with L2 and with L1 (P<0.001 for each), in contrast there are no significant differences in TP53 levels concerning the different sub-types of ALL (P>0.05 for each).
On the other hand, there are no correlation between age and either survivin and p53 levels in patients at diagnosis. Also the comparison between male and female patients regarding survivin and p53 levels shows no significant difference.
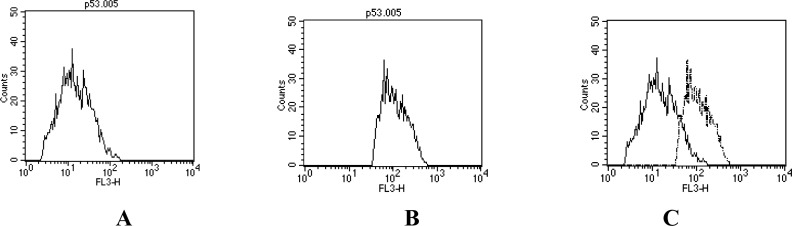
Figure 1 shows a positive correlation between p53 expression and survivin level in ALL children at diagnosis (r= 0.501 & P<0.001). The Flow cytometry data analysis of p53 expression in acute leukemia peripheral blood was shown in Fig. 2.
Fig. 1:
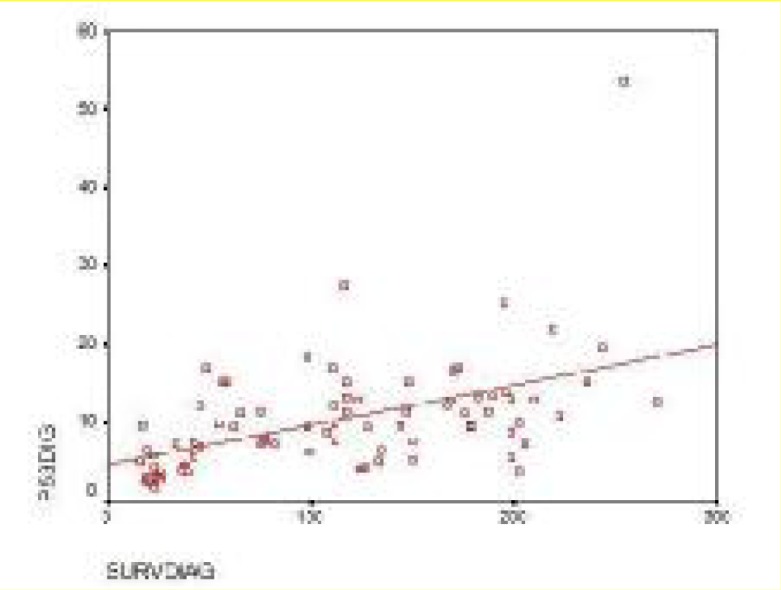
Correlation between Survivin level and P53 % of ALL children at diagnosis
Fig. 2:
Flow cytometry data analysis of P53 expression in acute leukemia peripheral blood A: Negative control sample, B: Sample labeled with anti-human P53 APC C: Overlapping of A and B
Discussion
Numerous immunophenotypic features have been examined for their potential prognostic significance in predicting treatment outcome in leukaemias. These include immunophenotypic subgroups of acute lymphoblastic leukaemia (ALL), expression of individual surface antigens or combined immunophenotypic features, and more recently, molecules mediating the multidrug resistance phenotype or being involved in the regulation of drug-induced apoptosis (14).
Survivin is the smallest member of the inhibitor of apoptosis family of proteins (IAPs), implicated in the preservation of cell viability, directly inhibiting caspase-3 and -7 activity and regulating the cell cycle in G2/M phase (15). Survivin is rarely expressed in terminally differentiated adult tissues but they appear to be highly upregulated in most cancers (16). Particulary in lung cancer, expression levels of survivin appear to relate with disease prognosis (17, 18). There has been considerable interest in survivin from various viewpoints of biomedical research. The complexity of subcellular localization, the evidence for a dual role in both apoptosis and cell division, and the far-reaching consequences of overexpression in cancer point to a critical role of survivin at the interface between cell proliferation and apoptosis, dramatically exploited in cancer (19).
Our study shows a highly significant elevation of protein survivin level in children with ALL compared to control group and this elevation decreased at remission and approach the control level. Survivin expressions have been studied in some solid tumors (gastric, colorectal, pancreatic, hepatocellular cancers and sarcomas) and found to be related with higher proliferative markers and lower apoptotic index. In most of these studies survivin has been found to be a bad prognostic indicator (20, 21). Survivin expression has been studied also in hematologic neoplasias (22–24). In a study covering 222 cases with non-Hodgkin’s lymphoma (NHL), Survivin expression has been found in 60% of the cases and 5 year overall survival has been found as 40% vs 54% (P = 0.02) in patients with survivin expression than in those without expression (25). In a recent study Oto et al., 2007 (26) show that survivin was expressed in 83.8 % of 74 patients with acute leukemia and conclude that its expression is a bad prognostic indicator and survivin negativity shows good clinical outcome in acute leukemia. With these studies, it has been shown that survivin expression is frequent in malignant tumors and is a new target for therapy.
As regards p53, the present study revealed that there was a highly significant increase in the expression levels of TP53 in children with ALL at diagnosis compared to control group. At remission its level returns to control range. Zolota et al., 2007 (27) revealed that p53 protein expression can be detected in 81% of AML samples and the results reported by Kurotaki et al., 2000 (28) suggest that the suppression of apoptosis associated with enhanced p53 accumulation increases the probability of developing leukemia in myelodysplastic syndrome. This is concordant with a study including 43 bone marrow samples of children with leukemia, p53 protein was expressed in 12 of them and p53 positive cells in a majority of children with unfavorable prognostic features suggests that dysfunction of the p53-dependent cell growth control have a role in the development of high risk leukemias (29).
P53 is an extremely unstable protein due to its degradation by the proteasome after binding to its major negative regulator protein, murine double minute 2 (MDM2) which acts as ubiquitin ligase that is induced by p53 in a feedback loop (30). However, in cells exposed to genotoxic stress, p53 protein conformation changes, escapes from MDM2 interaction, becomes accumulated and turn it into an active transcription factor (31, 32). Previous studies have demonstrated a correlation between the p53 protein expression detected by immunohistochemical methods and mutation of the gene (33, 34). Some changes found in malignant breast tumors, such as the presence of mutated p53 protein, both nuclear and cytoplasmic staining for p53 protein was detected, and the percentage of positive malignant tumors was 34% (35). These results are in agreement with the results of other investigators that showed positive immunohistochemical (IHC) reaction for p53 protein in the range of 22 – 45% (36, 37). P53 was heterogeneously expressed and phosphorylated in AML patient samples and could accumulate following DNA damage (38). It is the most frequently inactivated protein in human cancer, and more than 50% of all solid tumors carry a mutation in the TP53 gene (39).
As regards, the comparison between survived and non-survived ALL children patients at diagnosis revealed a highly significant increase in survivin level and p53 expression in non-survived compared to survived patients.
Regarding p53, our result indicated that the overexpression of this gene is correlated to poor prognosis and associated with unfavorable outcome in pediatric ALL patients. In a study, ALL patients with p53 mutations had a 3.8-fold increase in risk of death than those patients without p53 mutations (40). These findings suggest that p53 mutation is associated with poor clinical outcome that is characterized by (a) a shortened duration of survival after first relapse; (b) a reduced response to reinduction therapy; (c) a shortened duration of first remission; and, hence, (d) an overall decreased duration of survival and increased risk of death. Since the inactivation of p53 in cancer has been associated with poor survival, refractory disease, and chemoresistance (41), p53 gene therapy have been designed to restore p53 function (42). Survivin is one of the anti-apoptotic molecules, poor indicator of overall survival in acute leukemias (26). Nevertheless, survivin is highly expressed in dividing cells and cancer-derived cell lines and has become a valid target for anti-cancer drugs, including those that use antisense approaches (43).Targeted deletion of the murine survivin gene revealed a critical role for this protein in the cell cycle through regulation of the spindle formation during mitosis (44). Survivin is strongly up-regulated in angiogenically-stimulated endothelium in vitro and in vivo. Increased surviving expression protected endothelial cells from apoptosis during the proliferative and remodeling phases of angiogenesis (45). Evasion of apoptosis and the ability to proliferate uncontrollably are two of the molecular traits found perhaps in all human cancers. Positioned at the interface between the regulation of apoptosis and the control of cell proliferation, survivin is described a molecule that is expressed in most human cancers encouraging new studies demonstrate that it may be possible to exploit the survivin pathway for cancer diagnosis and therapy (19).
A positive correlation between survivin level and TP53 expression was found in children ALL at diagnosis. This is in agreement with the result of Hui et al., (46), they stated that there was a positive correlation between survivin and p53 expression in HCC. Baytekin et al., (47) suggest that survivin expression may be positively regulated by mutant p53 in renal cell carcinoma (RCC), and this expression may have an impact on resistance to chemotherapy in RCC. This positive correlation indicates that cooperation between survivin and p53 may play a certain role in occurrence and /or development ALL. In addition, survivin was identified as one of the target genes suppressed by wild type p53. Irrespective of a direct or indirect mechanism for p53 suppression of survivin gene transcription, expression of survivin strongly counteracted p53-dependent apoptosis. Whereas loss of survivin caused by wild type p53 contributed, at least in part, to p53-dependent apoptosis (48).
In conclusion, survivin and TP53 expression have a synergetic bad prognostic indicator, independent of age and sex, in children with acute lymphoblastic leukemia. Survivin protein is related to anti-apoptotic proteins and its high expression lead to unsuccessful treatment of ALL.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
The authors declare that there is no conflict of interests.
References
- 1.Harrison CJ. Acute lymphoblastic leukaemia. Best Pract Res Cl Ha. 2001;14:593–607. doi: 10.1053/beha.2001.0156. [DOI] [PubMed] [Google Scholar]
- 2.Ribera JM, Oriol A. Acute lymphoblastic leukemia in adolescents and young adults. Hematol Oncol Clin North Am. 2009;23(5):1033–42. doi: 10.1016/j.hoc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 4.Nachmias B, Ashhab Y, Bucholtz V, Drize O, Kadouri L, Lotem M, et al. Caspase-mediated cleavage converts livin from an antiapoptotic to a pro-apoptotic factor: implications for drug-resistant melanoma. Cancer Res. 2003;63:6340–9. [PubMed] [Google Scholar]
- 5.Arrends MJ, Willie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–4. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- 6.Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, et al. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991;252(5013):1708–11. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- 7.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 8.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Biotechnology Information The p53 tumor suppressor protein. Genes and Disease. United States National Institutes of Health Retrieved -05-28 2008 [Google Scholar]
- 10.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 11.Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med. 2001;7:542–7. doi: 10.1016/s1471-4914(01)02243-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Gu L, Li F, Zhu Y, Woods WG, Findley HW. DNA damage induces a novel p53-survivin signaling pathway regulating cell cycle and apoptosis in acute lymphoblastic leukemia cells. J Pharmacol Exp Ther. 2002;303(1):124–31. doi: 10.1124/jpet.102.037192. [DOI] [PubMed] [Google Scholar]
- 13.Zhu N, Gu L, Findley HW, Chen C, Dong JT, Yang L, et al. KLF5 Interacts with p53 in regulating survivin expression in acute lymphoblastic leukemia. J Biol Chem. 2006;281(21):14711–8. doi: 10.1074/jbc.M513810200. [DOI] [PubMed] [Google Scholar]
- 14.Schabath R, Ratei R, Ludwig W. The prognostic significance of antigen expression in leukaemia. Best Pract Res Cl Ha. 2003;16(4):613–28. doi: 10.1016/s1521-6926(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 15.Goyal L. Cell death inhibition: keeping caspases in check. Cell. 2001;104:805–8. doi: 10.1016/s0092-8674(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 16.Duffy MJ, O’Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett. 2007;249:49–60. doi: 10.1016/j.canlet.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Wang L, Jiang GN, He WX, Ding JA. The role of survivin on overall survival of non-small cell lung cancer, a meta-analysis of published literatures. Lung Cancer. 2008;61:91–6. doi: 10.1016/j.lungcan.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Karanikas V, Tsohas S, Boukas K, Kerenidi T, Nakou M, Dahabreh J, et al. Co-expression patterns of tumor-associated antigen genes by non-small cell lung carcinomas: implications for immunotherapy. Cancer Biol Ther. 2008;7:345–52. doi: 10.4161/cbt.7.3.5424. [DOI] [PubMed] [Google Scholar]
- 19.Altieri DC. Survivin in apoptosis control and cell cycle regulation in cancer. Prog Cell Cycle Res. 2003;5:447–52. [PubMed] [Google Scholar]
- 20.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 21.Tracey L, Pérez-Rosado A, Artiga MJ, et al. Expression of the NF KB targets BCL2 and BIRC5/survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206:123–34. doi: 10.1002/path.1768. [DOI] [PubMed] [Google Scholar]
- 22.Ansell SM, Arendt BK, Grote DM, Jelinek DF, Novak AJ, Wellik LE, et al. Inhibition of survivin expression suppresses the growth of aggressive non-Hodgkin's lymphoma. Leukemia. 2004;18:616–23. doi: 10.1038/sj.leu.2403281. [DOI] [PubMed] [Google Scholar]
- 23.Carter BZ, Mak DH, Schober WD, et al. Regulation of survivin expression through Bcr-Abl/MAPK cascade: targeting survivin overcomes imatinib resistance and increases imatinib sensitivity in imatinib-responsive CML cells. Blood. 2006;107:1555–63. doi: 10.1182/blood-2004-12-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlette EJ, Medeiros LJ, Goy A, Lai R, Rassidakis GZ. Survivin expression predicts poorer prognosis in anaplastic large-cell lymphoma. J Clin Oncol. 2004;22:1682–8. doi: 10.1200/JCO.2004.10.172. [DOI] [PubMed] [Google Scholar]
- 25.Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, et al. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921–5. [PubMed] [Google Scholar]
- 26.Oto OA, Paydas S, Tanriverdi K, Seydaoglu G, Yavuz S, Disel U. Survivin and EPR-1 expression in acute leukemias: prognostic significance and review of the literature. Leuk Res. 2007;31(11):1495–501. doi: 10.1016/j.leukres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Zolota V, Siriniana C, Melachrinoua M, Symeonidisb A, Bonikosa D. Expression of the regulatory cell cycle proteins p21, p27, p14, p16, p53, mdm2, and cyclin E in bone marrow biopsies with acute myeloid leukemia. Correlation with patients’ survival. Pathol Res Pract. 2007;203:199–207. doi: 10.1016/j.prp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Kurotaki H, Tsushima Y, Nagai K, Yagihashi S. Apoptosis, bcl-2 expression and p53 accumulation in myelodysplastic syndrome, myelodysplastic-syndrome-derived acute myelogenous leukemia and de novo acute myelogenous leukemia. Acta Haematol. 2000;102:115–23. doi: 10.1159/000040984. [DOI] [PubMed] [Google Scholar]
- 29.Gustafsson B, Christenson B, Hjalmar V, Winiarski J. Cellular expression of MDM2 and p53 in childhood leukemias with poor prognosis. Med Pediatr Oncol. 2000;34(2):117–24. doi: 10.1002/(sici)1096-911x(200002)34:2<117::aid-mpo9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promote the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 31.Collot-Teixeira S, Bass J, Denis F, Ranger-Rogez S. Human tumor suppressor p53 and DNA viruses. Rev Med Virol. 2004;14:301–19. doi: 10.1002/rmv.431. [DOI] [PubMed] [Google Scholar]
- 32.Abdelmoula-Souissi S, Rekik L, Gargouri A, Mokdad-Gargouri R. High-level expression of human tumour suppressor P53 in the methylotrophic yeast: Pichia pastoris. Protein Expres Purif. 2007;54:283–8. doi: 10.1016/j.pep.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Maestro R, Dolcetti R, Gasparotto D, et al. High frequency of P53 gene alteration associated with protein expression in human squamous cell carcinoma of the larynx. Oncogene. 1992;7(6):1159–66. [PubMed] [Google Scholar]
- 34.Somers KD, Merrick A, Lopez ME, et al. Frequent P53 mutations in head and neck cancer. Cancer Res. 1992;52(21):5997–6000. [PubMed] [Google Scholar]
- 35.Sirotkovic-Skerlev M, Krizanac S, Kapitanovic S, Husnjak K, Unusic J, Pavelic K. Expression of c-myc, erbB-2, p53 and nm23-H1 gene product in benign and malignant breast lesions: Coexpression and correlation with clinicopathologic parameters. Exp Mol Pathol. 2005;79:42–50. doi: 10.1016/j.yexmp.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Cattoretti G, Rilke F, Andreola S, D’Amato L, Delia D. P53 expression in breast cancer. Int J Cancer. 1988;41:178–83. doi: 10.1002/ijc.2910410204. [DOI] [PubMed] [Google Scholar]
- 37.Davidoff AM, Humphrey PA, Iglehart JD, Marks JR. Genetic basis for p53 overexpression in human breast cancer. Proc Natl Acad Sci USA. 1991;88:5006–10. doi: 10.1073/pnas.88.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irish J, Anensen N, Hovland R, Skavland J, Børresen-Dale A, Bruserud O, et al. Flt3 Y591 duplication and Bcl-2 overexpression are detected in acute myeloid leukemia cells with high levels of phosphorylated wild-type p53. Blood. 2007;109(6):2589–96. doi: 10.1182/blood-2006-02-004234. [DOI] [PubMed] [Google Scholar]
- 39.Hollstein M, Sidransky D, Vogelstein B, Harris CC. P53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 40.Diccianni M, Yu J, Hsiao M, Mukherjee S, Shao L, Yu A. Clinical significance of P53 mutations in relapsed T-cell acute lymphoblastic leukemia. Blood. 1994;84(9):3105–12. [PubMed] [Google Scholar]
- 41.Bykov VJ, Wiman KG. Novel cancer therapy by reactivation of the P53 apoptosis pathway. Ann Med. 2003;35:458–65. doi: 10.1080/07853890310017152. [DOI] [PubMed] [Google Scholar]
- 42.Roth JA, Grammer SF. Gene replacement therapy for non-small cell lung cancer: a review. Hematol Oncol Clin North Am. 2004;18:215–29. doi: 10.1016/s0889-8588(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 43.Wright CW, Duckett CS. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J Clin Invest. 2005;115:2673–8. doi: 10.1172/JCI26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh WK, et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199:399–410. doi: 10.1084/jem.20032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275(13):9102–5. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 46.Hui W, Zan Y, Wang X, Kang H, Guan H, Ma X. Expression of Survivin, p53 and its relationship with apoptosis, proliferation in hepatocellular carcinoma(HCC) Journal of Nanjing Medical University. 2008;22(4):255–9. [Google Scholar]
- 47.Baytekin F, Tuna B, Mungan U, Aslan G, Yorukoglu K. Significance of P-glycoprotein, P53, and survivin expression in renal cell carcinoma. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.09.001. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 48.Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21(17):2613–22. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]