Abstract
Background:
The metabolic syndrome (MES) is associated with a high risk of diabetes and cardiovascular disease. The aim of the present study was to determine the prevalence of the metabolic syndrome as well as cut-off points for waist circumference (WC) for diagnosis of MES in Zahedan, southeast Iran.
Methods:
Totally, 1802 people (735 men and 1067 women) with metabolic syndrome were surveyed according to National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and the International Diabetes Federation (IDF) criteria as well as obtained WC cut-off points for IDF criteria.
Results:
The prevalence of metabolic syndrome was higher in women than in men. In both sexes the prevalence increased with age. The prevalence of metabolic syndrome among 1802 individuals aged ≥19 years according to NCEP ATP III, IDF and IDF -AHA/NHLBI were 21.0% (15.4% in male, 24.9% female), 24.8 (20.0% in male, 28.1% in female) and 23.3% (19.7% in male, 25.8% in female), respectively. Low HDL-C (60.6%) and high WC (43.3%) were the most common components of the metabolic syndrome, followed by high triglycerides (32%), elevated glucose (17.1%) and high blood pressure (13%).
Conclusion:
Our data shows a high prevalence of MES in Zahedan, Southeast Iran, therefore, future health prevention strategies are required for the prevention of MES.
Keywords: Metabolic syndrome, Waist circumference, Epidemiology, Iran
Introduction
The term metabolic syndrome (MES), also known as the insulin resistance syndrome or syndrome X refers to a group of correlated disorders that include insulin resistance, glucose intolerance, obesity, dyslipidemia, and hypertension (1). It is well known that in the general population, metabolic syndrome (MES) is associated with increased cardiovascular morbidity (2), mortality (3) and high incidence of type 2 diabetes mellitus (4). Cardiovascular diseases (CVD) are one of the major causes of mortality in Iran (5). Although there are varying definitions for the MES by several health organizations, the basic components remain constant and include abdominal obesity, hypertension (HTN), atherogenic dyslipidemia, and glucose intolerance or diabetes. Metabolic syndrome by the NCEP definition is associated with a 1.7-fold increase in CVD risk (6).
Although there are some reports regarding the prevalence of MES in Iran (7–10), but the precise prevalence of it is unknown and may vary from area to area. The aim of the present study was to determine the prevalence of the metabolic syndrome in Zahedan, southeast Iran.
Materials and Methods
This population based descriptive cross-sectional study was performed on 1802 individuals (735 male, 1067 female) from September 2008 to March 2009 in Zahedan, southeast, Iran. The sample size calculation was done based on the assumption that the prevalence of MES to be approximately 33% according to the finding of Zabetian et al. (7). Samples were selected by random cluster sampling from 20 regions of Zahedan. The study was approved by the local Ethics Committee of Zahedan University of Medical Sciences and written informed consent was obtained from all subjects.
Face-to-face interviews using structured questionnaires the following were collected: demographic data (date of birth, contact information, medical history, current medications, family history, length of residence in the Zahedan); height (by a stadiometer using a centimeter scale), weight (by a clinical scale); waist circumference (by a tape measure just upper the superior iliac crest with the subject standing, at the end of normal expiration); blood pressure (by a mercury sphygmomanometer with the subject sitting); and 5 ml of venous blood drawn after 8–12 h fasting for laboratory tests.
The blood samples were collected in tubes and sera were obtained. Samples were stored at −80 ºC until further biochemical analysis. Biochemical analysis (fasting blood glucose, triglyceride, HDL-cholesterol) was assayed using a commercially available kit.
The prevalence of metabolic syndrome among 1802 individuals was determined according to NCEP ATP III (11), IDF (12) and IDF-AHA/NHLBI (13) criteria as shown in Table 1.
Table 1:
Criteria for diagnosis of the metabolic syndrome
| Criterion | ATP III panel * | IDF panel† | IDF -AHA/NHLBI* |
|---|---|---|---|
| Waist (cm) | |||
| Male | ≥102 | ≥94 | ≥94 |
| Female | ≥88 | ≥80 | ≥80 |
| HDL (mg/dL) | |||
| Male | <40 | <40 | <40 |
| Female | <50 | <50 | <50 |
| Triglyceride (mg/dL) | ≥150 | ≥150 | ≥150 |
| Fasting Glucose (mg/dL) | ≥100 | ≥100 | ≥100 |
| Blood pressure (mmHg) | ≥130/85 | ≥130/85 | ≥130/85 |
Three of five required.
Central adiposity required; two of subsequent four required.
Furthermore, we calculated the cut-off values for WC in men and women in our population for determination the prevalence of MES.
Statistical analysis was performed using SPSS version 17. Results are expressed as mean ±S.D. for quantitative variables and as frequency (%) for otherwise. A P value <0.05 was considered statistically significant.
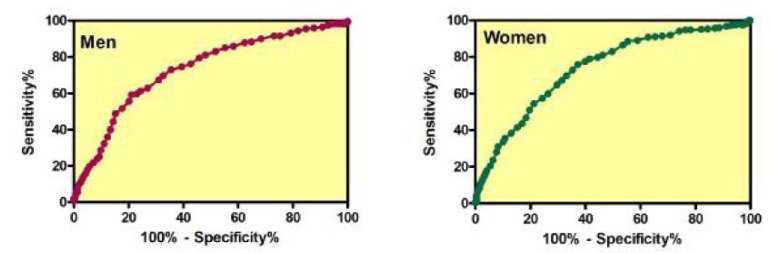
Receiver operating characteristic (ROC) curves were used to determine the cut-off values for WC (Fig. 1). The optimum cut-off value was determined by selecting the point that provided the greatest sum of sensitivity and specificity.
Fig. 1:
Receiver operator characteristic (ROC) curves analysis for determination of waist circumference (WC) for predicting the presence of at least two risk factors for metabolic syndrome in men and women. The area under the ROC curve for men and women was 0.73 and 0.74, respectively
Results
Clinical characteristics of our population individuals (n=1802; men=735, women=1067) is shown in Table 2. There was a significant differences regarding blood pressure (systolic and diastolic), weight, height, BMI, waist circumference, triglyceride, and HDL-C among men and women (P<0.05), while there was not significant differences regarding fasting blood glucose.
Table 2:
Clinical characteristic of the 1802 subjects
| Total (n=1802) | Mena (n=735) | Womenb (n=1067) | P value a versus b | |
|---|---|---|---|---|
| Age (yr) | 35.85±13.81 | 37.46±14.89 | 34.91±13.17 | <0.0001 |
| Systolic BP (mmHg) | 116.22 ±16.53 | 119.92±15.70 | 113.63±16.61 | <0.0001 |
| Diastolic BP (mmHg) | 74.02±11.95 | 76.43±11.47 | 72.35±11.99 | <0.0001 |
| Weight (kg) | 64.64±14.06 | 68.66±13.85 | 61.88±13.54 | <0.0001 |
| Height (cm) | 162.43±9.92 | 170.42±8.00 | 156.92±6.92 | <0.0001 |
| BMI (kg/m2) | 24.52±5.02 | 23.64±4.48 | 25.13±5.28 | <0.0001 |
| Waist (cm) | 86.45±14.63 | 87.91±14.19 | 85.44±14.84 | <0.0001 |
| Triglyceride (mg/dL) | 139.81±110.41 | 158.24±132.19 | 127.10±90.36 | <0.0001 |
| HDL-C (mg/dL) | 45.03±8.48 | 43.65±7.81 | 45.98±8.78 | <0.0001 |
| FBG (mg/dL) | 90.90±29.99 | 92.35±30.78 | 89.90±29.41 | 0.103 |
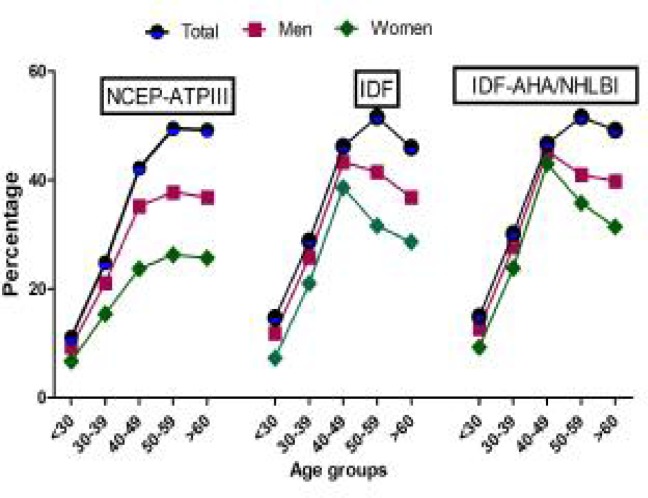
The prevalence of MES according to NCEP ATP III, IDF and IDF-AHA/NHLBI criteria is shown in Table 3. In our population the prevalence of MES was 21.0%, 24.8% and 23.3% based on NCEP ATP III, IDF and IDF-AHA/NHLBI criteria, respectively. In addition the prevalence of MES was significantly higher in women (NCEP ATP III=24.9%; IDF=28.1%, IDF-AHA/NHLBI=25.8) than men (NCEP ATP III=15.4%; IDF=20.0%; IDF-AHA/NHLBI=19.7). Age related increase in the prevalence of metabolic syndrome was observed in both of the genders (Fig. 2).
Table 3:
Prevalence of metabolic syndrome according to NCEP-ATPIII, IDF and IDF-AHA/NHLBI criteria
| Criteria | Total | Men | Women |
|---|---|---|---|
| NCEP ATP III (%) | 21.0 | 15.4 | 24.9 |
| IDF (%) | 24.8 | 20.0 | 28.1 |
| IDF-AHA/NHLBI (%) | 26.4 | 22.9 | 28.8 |
Fig. 2:
Prevalence of metabolic syndrome according to age and gender based on NCEP-ATPIII, IDF criteria and IDF-AHA/NHLBI
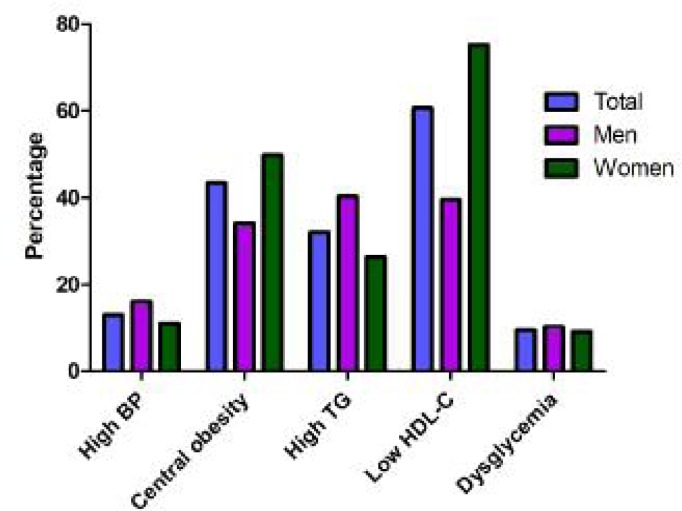
Percentage of the individual component of metabolic syndrome in our population was shown in Fig. 3. In our population, 13% had hypertension, 43.3% had central obesity, 32.0% had high triglyceride, 60.6% had low HDL-C and 17.1% had hyperglycemia.
Fig. 3:
Percentage of the individual components of metabolic syndrome in subjects
In women, abnormal BP, central obesity, high triglyceride, low HDL-C and hyperglycemia occurred in 11%, 49.8%, 26.4%, 75.2% and 16.4%, respectively. In men, abnormal BP, central obesity, high triglyceride, low HDL-C and hyperglycemia occurred in 16.1%, 34.0%, 40.3%, 39.5 % and 18.2%, respectively.
Overall, low serums HDL-C and central obesity were the most common risk factors, whereas hyperglycemia was the least common.
In addition we found that in our population, 16.2% of subjects had no risk factors, 35.3% of subjects had one, 23.5% had two, and 17.3% had three, 6.2% had four and 1.4% had five risk factors.
We found that the optimal cut-off point of WC for the prediction of at least two components of MES as defined by IDF was 93.5 cm for men (sensitivity=59.27%, specificity=78.85%) and 85.5 cm for women (sensitivity=75.79%, specificity=62.78%).
Discussion
The present study showed that the prevalence of MES was 21.0%, 24.8% and 23.3% based on NCEP-ATPIII, IDF and IDF-AHA/NHLBI, respectively.
Metabolic syndrome is defined as a cluster of metabolic risk factors including central obesity, glucose intolerance, dyslipidemia, and elevated blood pressure. The most widely accepted criteria are those proposed earlier (11). The IDF criteria for MES was based on the same parameters used in the NCEP criteria, the former identified central obesity as an essential component for MES (12).
There are some reports regarding the prevalence of MES in different parts of Iran. The prevalence of the metabolic syndrome was 6.5% among 15 to 17 year old adolescent girls in Mashhad, Iran (14), 50.8% in Iranian women aged ≥65 years who participated in the Tehran Lipid and Glucose Study (8), 34.7% based on the ATP III criteria (15), and 33.2% by the ATPIII criteria in men and women aged ≥20 years participated in the cross-sectional phase of the Tehran Lipid and Glucose Study (7). In addition, the prevalence of MES was 10.1% in a population-based cross-sectional study of 3036 Iranian adolescents (1413 boys and 1623 girls) 10 to 19 years (16), 39.9% (ATP III; male: 29.1% and female: 50.4%), 40.5% (IDF; male: 26% and female: 54.5%) in Greater Khorasan Province of Iran (17), 31.1% in adults population of Tehran (18), 27% in Tehran where WC cut-off point was 91.5 cm in men and 85.5 cm in women (10). Cut-off point of WC for predicting at least two other components of the metabolic syndrome as defined by the IDF was 89 cm for men and 91 cm for women in Iran (15). In the present study we found WC cut-off points of 93.5 cm for male and 85.5 cm for female which is different with former study (15). The prevalence of MES in the Irish population according to the ATPIII and IDF criteria was 13.2% and 21.4%, respectively (19), in urban Pakistan according to the IDF definition and modified ATP III criteria was 34.8% and 49%, respectively (20), among adult Qatari population was 26.5% and 33.7% (21) and 15–30% among Korean adults (22). The prevalence of metabolic syndrome is low in rural Japan as 4.6% in males and 4.2% in females (23). The investigators concluded that the lower prevalence of MES may be due to the consumption of traditional Japanese food.
One reason of such differences among various studies may be due to criteria used to define MES (e.g. ATP III vs. IDF, Waist to hip ratio vs. abdominal obesity) but most probably might be due to heterogeneity of population.
Prevalence of MES based on IDF criteria was higher than NCEP ATPIII criteria because of high dependence of diagnosis of MES to abdominal obesity in IDF criteria. The higher rate of MES in women may be largely due to the lower physical activity which may also explain the higher prevalence of MES in aged individuals.
In conclusion, the findings of our population-based study showing a high prevalence of the metabolic syndrome in Southeast of Iran which has implications for diabetes and cardiovascular prevention programs.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This study was supported by a research grant from Zahedan University of Medical Sciences. The authors would like to thank all subjects who willingly participated in the study. The authors declare that there is no conflict of interests.
References
- 1.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 2.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–90. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Ruotsalainen S, Moilanen L, Lepisto P, Laakso M, Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28:857–64. doi: 10.1093/eurheartj/ehl524. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 5.Sarraf-Zadegan N, Boshtam M, Malekafzali H, Bashardoost N, Sayed-Tabatabaei FA, Rafiei M, Khalili A, Mostafavi S, Khami M, Hassanvand R. Secular trends in cardiovascular mortality in Iran, with special reference to Isfahan. Acta Cardiol. 1999;54:327–33. [PubMed] [Google Scholar]
- 6.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 7.Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. 2007;77:251–7. doi: 10.1016/j.diabres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hadaegh F, Zabetian A, Tohidi M, Ghasemi A, Sheikholeslami F, Azizi F. Prevalence of metabolic syndrome by the Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions and their association with coronary heart disease in an elderly Iranian population. Ann Acad Med Singapore. 2009;38:142–9. [PubMed] [Google Scholar]
- 9.Hadaegh F, Esmaillzadeh A, Azizi F. Metabolic risks in individuals with normal body mass index and normal waist circumference. Eur J Cardiovasc Prev Rehabil. 2007;14:200–7. doi: 10.1097/01.hjr.0000230096.73579.64. [DOI] [PubMed] [Google Scholar]
- 10.Esteghamati A, Ashraf H, Rashidi A, Meysamie A. Waist circumference cut-off points for the diagnosis of metabolic syndrome in Iranian adults. Diabetes Res Clin Pract. 2008;82:104–7. doi: 10.1016/j.diabres.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.International Diabetes Federation The IDF consensus worldwide definition of the metabolic syndrome [article online] www.idf.org.
- 13.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and international association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 14.Mirhosseini NZ, Yusoff NA, Shahar S, Parizadeh SM, Mobarhen MG, Shakery MT. Prevalence of the metabolic syndrome and its influencing factors among adolescent girls in Mashhad, Iran. Asia Pac J Clin Nutr. 2009;18:131–6. [PubMed] [Google Scholar]
- 15.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32:1092–7. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esmaillzadeh A, Mirmiran P, Azadbakht L, Etemadi A, Azizi F. High prevalence of the metabolic syndrome in Iranian adolescents. Obesity (Silver Spring) 2006;14:377–82. doi: 10.1038/oby.2006.50. [DOI] [PubMed] [Google Scholar]
- 17.Nezhad MA, Ghayour-Mobarhan M, Parizadeh SM, Safarian M, Esmaeili H, Khodaei GH, Kazemi-Bajestani SM, Hosseini SJ, Jooya M, Ferns GA. Metabolic syndrome: its prevalence and relationship to socioeconomic parameters in an Iranian population. Nutr Metab Cardiovasc Dis. 2008;18:e11–2. doi: 10.1016/j.numecd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Azizi F, Salehi P, Etemadi A, Zahedi-Asl S. Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract. 2003;61:29–37. doi: 10.1016/s0168-8227(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 19.Waterhouse DF, McLaughlin AM, Sheehan F, O’Shea D. An examination of the prevalence of IDF- and ATPIII-defined metabolic syndrome in an Irish screening population. Ir J Med Sci. 2009;178:161–66. doi: 10.1007/s11845-008-0269-1. [DOI] [PubMed] [Google Scholar]
- 20.Hydrie MZ, Shera AS, Fawwad A, Basit A, Hussain A. Prevalence of metabolic syndrome in urban Pakistan (Karachi): comparison of newly proposed International Diabetes Federation and modified Adult Treatment Panel III criteria. Metab Syndr Relat Disord. 2009;7:119–24. doi: 10.1089/met.2008.0055. [DOI] [PubMed] [Google Scholar]
- 21.Bener A, Zirie M, Musallam M, Khader YS, Al-Hamaq AO. Prevalence of metabolic syndrome according to Adult Treatment Panel III and International Diabetes Federation criteria: a population-based study. Metab Syndr Relat Disord. 2009;7:221–9. doi: 10.1089/met.2008.0077. [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Park CY, Oh SW, Yoo HJ. Prevalence of obesity and metabolic syndrome in Korean adults. Obes Rev. 2008;9:104–7. doi: 10.1111/j.1467-789X.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto A, Nishimura R, Suzuki N, Matsudaira T, Taki K, Tsujino D, Miyashita Y, Ebara F, Ishikawa S, Tajima N. Low prevalence of metabolic syndrome and its components in rural Japan. Tohoku J Exp Med. 2008;216:69–75. doi: 10.1620/tjem.216.69. [DOI] [PubMed] [Google Scholar]