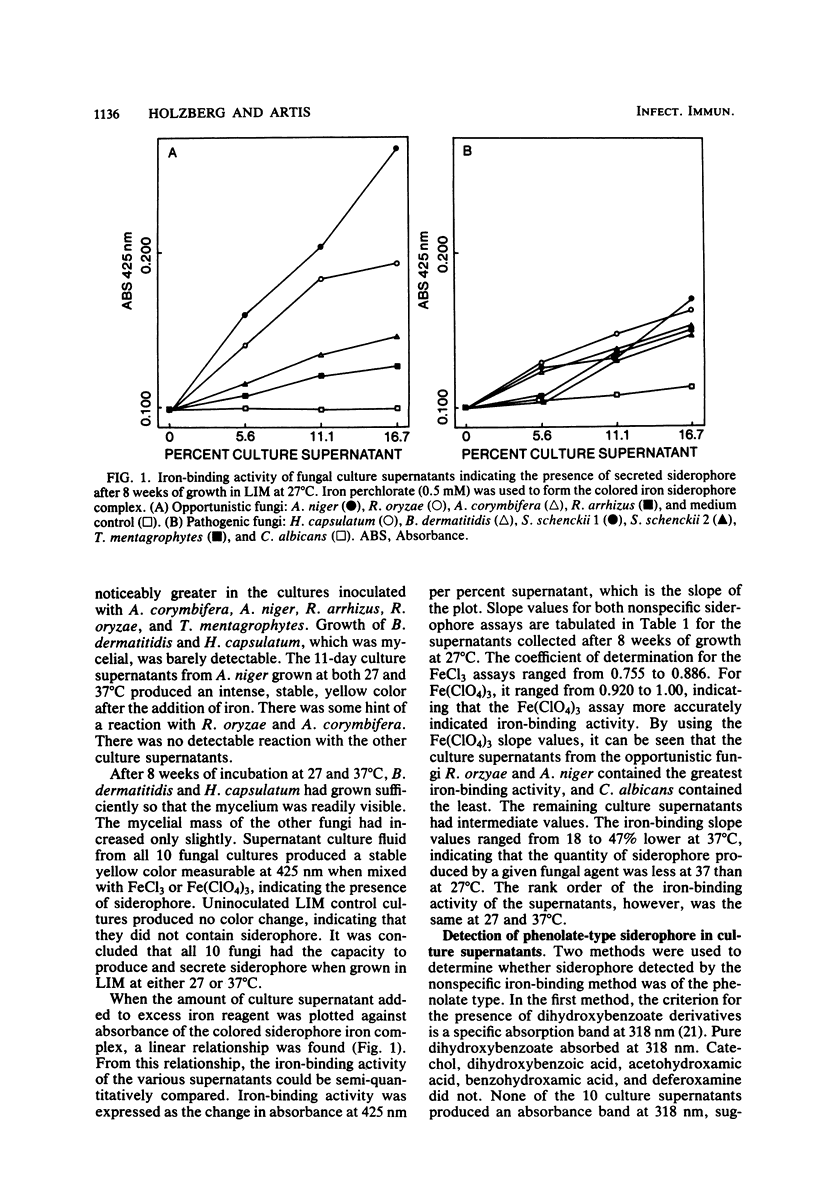
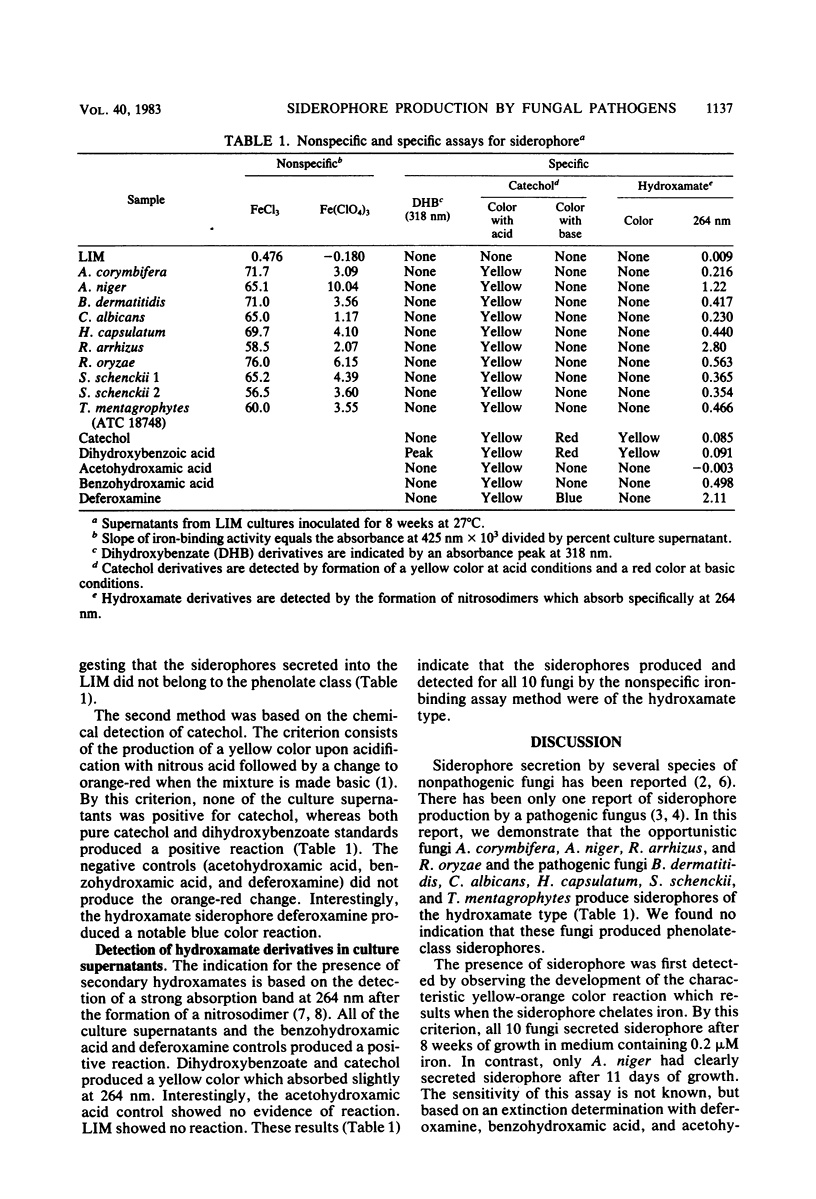
Abstract
It has been suggested that siderophores may function as virulence factors. There have been few studies on production of siderophores by opportunistic and pathogenic fungi. We examined siderophore production by Absidia corymbifera, Aspergillus niger, Rhizopus arrhizus, Rhizopus oryzae, Blastomyces dermatitidis, Histoplasma capsulatum, Sporothrix schenickii, Candida albicans, and Trichophyton mentagrophytes. Fungi were cultured at 37 and 27 degrees C in a chemically defined low-iron media (0.2 microM Fe). Culture supernatants were assayed for siderophores by two nonspecific methods [FeCl3 and Fe(ClO4)3] and three chemically specific assays (catechol, 2,3-dihydroxybenzoate, and hydroxamate). All fungi secreted siderophores. Only siderophores of the hydroxamate type were found. More siderophore was produced at 27 degrees C than at 37 degrees C. The present study adds eight fungi to the list of known siderophore producers and confirms siderophore production by H. capsulatum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt W. R. Identification of coprogen B and its breakdown products from Histoplasma capsulatum. Infect Immun. 1982 Mar;35(3):990–996. doi: 10.1128/iai.35.3.990-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt W. R., Underwood A. L., Appleton G. L. Hydroxamic acid from Histoplasma capsulatum that displays growth factor activity. Appl Environ Microbiol. 1981 Sep;42(3):560–563. doi: 10.1128/aem.42.3.560-563.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Granade T. C., Artis W. M. Antimycotic susceptibility testing of dermatophytes in microcultures with a standardized fragmented mycelial inoculum. Antimicrob Agents Chemother. 1980 Apr;17(4):725–729. doi: 10.1128/aac.17.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon A. H., Davis W. B., Arceneaux J. E., Byers B. R. Hydroxamate recognition during iron transport from hydroxamate-ion chelates. J Bacteriol. 1973 Sep;115(3):912–918. doi: 10.1128/jb.115.3.912-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. J., Warren R. A. Itoic acid synthesis in Bacillus subtilis. J Bacteriol. 1968 Feb;95(2):360–366. doi: 10.1128/jb.95.2.360-366.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATLEDGE C. RELATIONSHIP BETWEEN THE PRODUCTS OF AROMATIC BIOSYNTHESIS IN MYCOBACTERIUM SMEGMATIS AND AEROBACTER AEROGENES. Nature. 1964 Jul 25;203:428–429. doi: 10.1038/203428a0. [DOI] [PubMed] [Google Scholar]
- Reinhardt J. H., Allen A. M., Gunnison D., Akers W. A. Experimental human Trichophyton mentagrophytes infections. J Invest Dermatol. 1974 Nov;63(5):419–422. doi: 10.1111/1523-1747.ep12676579. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Roles of metallic ions in host-parasite interactions. Bacteriol Rev. 1966 Mar;30(1):136–151. doi: 10.1128/br.30.1.136-151.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Cox G. B., Gibson F. 2,3-Dihydroxybenzoate as a bacterial growth factor and its route of biosynthesis. Biochim Biophys Acta. 1967 Jul 25;141(2):319–331. doi: 10.1016/0304-4165(67)90106-7. [DOI] [PubMed] [Google Scholar]



