Abstract
The nutrients are able to interact with molecular mechanisms and modulate the physiological functions in the body. The Nutritional Genomics focuses on the interaction between bioactive food components and the genome, which includes Nutrigenetics and Nutrigenomics. The influence of nutrients on f genes expression is called Nutrigenomics, while the heterogeneous response of gene variants to nutrients, dietary components and developing nutraceticals is called Nutrigenetics. Genetic variation is known to affect food tolerances among human subpopulations and may also influence dietary requirements and raising the possibility of individualizing nutritional intake for optimal health and disease prevention on the basis of an individual’s genome. Nutrigenomics provides a genetic understanding for how common dietary components affect the balance between health and disease by altering the expression and/or structure of an individual’s genetic makeup. Nutrigenetics describes that the genetic profile have impact on the response of body to bioactive food components by influencing their absorption, metabolism, and site of action.
In this way, considering different aspects of gene–nutrient interaction and designing appropriate diet for every specific genotype that optimize individual health, diagnosis and nutritional treatment of genome instability, we could prevent and control conversion of healthy phenotype to diseases.
Keywords: Nutritional genomics, Nutrigenomics, Nutrigenetics, Genetic variation
Introduction
With the completion of human genome sequencing and entering the-Omics area, the new term “Nutritional Genomics” tends to replace the former “nutrient-gene interactions” (1). It has been demonstrated that numerous genetic polymorphisms can influence protein structure function. The Nutritional genomic area includes two parts: first Nutrigenomics that is the study of interaction between dietary components and the genome, and the regulating changes in proteins and other metabolism; second Nutrigenetics that identify the response to dietary components with regard to genetic differences (2).
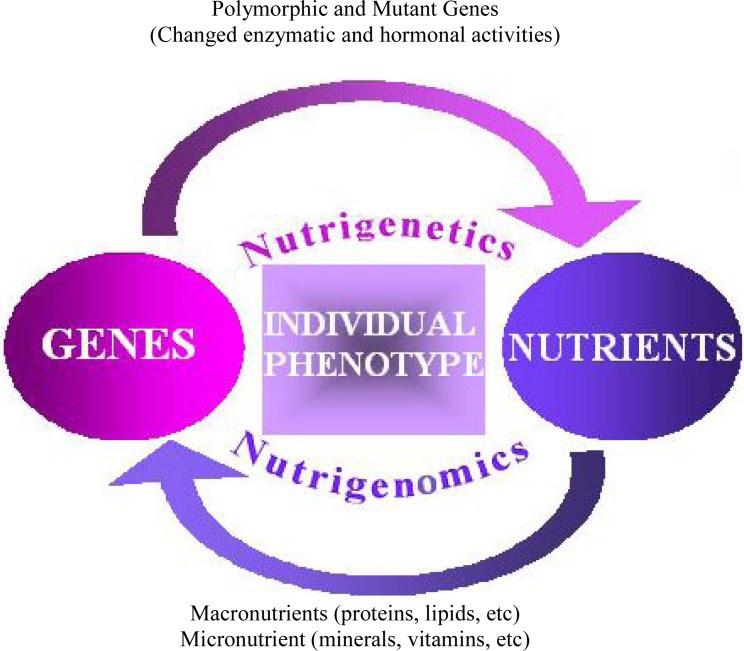
Nutrients are as environmental factors can interact with genetic material. It has been clearly demonstrated that DNA metabolism and repair depend on a wide range of dietary factors that act as cofactors or substrates in metabolic pathway, but much less is known about the impact of cofactors and/or micronutrients deficiency or excess on the fidelity of DNA replication and repair (3). Although the nutrients can influence the development of a particular phenotype, the response to a specific nutrient that determined by the individual genotype has also to be considered (Fig. 1).
Fig.1:
Nutrigenomics and Nutrigenetics are resulted from genes and nutrients interaction.
Genetic responses involve: effect on genome evolution, mutation, selection, programming, viability, gene expression, chromosome stability, signal transduction and metabolic pathways, protein synthesis and structure, epigenetic events, chronic diseases.
Nutritional responses involve: effect on nutrients absorption, nutrients utilization and requirement, food/nutrient tolerance, and food atopies
The central role of genetic code in determining genome stability and related health outcomes such as developmental defects, degenerative diseases, and cancer is well-established (4). The etiology of complex chronic diseases obviously relates to both environmental and genetic factors (5). Specifically, the “fetal basis of adult disease” or “early origins hypothesis” postulates that nutrition and other environmental factors during prenatal and early postnatal development influence gene expression and cellular plasticity, which can alter susceptibility to adult diseases (cardiovascular diseases, diabetes, obesity. etc) (6).
The concept of nutrients effects on DNA stability, repair and on the different gene expression processes, recently became more prominent in nutritional science (7). Numerous dietary components can alter genetic and epigenetic events and therefore influence health (8).
SNPs (single nucleotide polymorphisms) are the most common genetic variation, occur at about 500–2000 bp throughout the human genome, and normally found in at least 1% of the population (5). Many human studies have demonstrated the evidence for interaction between SNPs in various genes and the metabolic response to the diet. Moreover, SNPs analysis provides a potential molecular tool for investigating the role of nutrition in human health, diseases and identification of optimal diets (9)
Nutrients and genome interact at two levels: 1) Nutrients can induce or repress gene expression thereby altering individual phenotype. 2) Conversely, single nucleotide polymorphisms can alter the bioactivity of important metabolic pathways and mediators and influence the ability of nutrients to interact with them (Fig. 1).
Nutritional Genomics
The interaction between the nutrients and cellular/genetic processes is being referred to as “nutritional Genomics” (10). This term describes the interface of biochemistry genomics, human nutrition, understanding of reactions and interactions at the molecular genomic levels (11). The conceptual basis for this genomic research can be summarized with the following five principles: 1) Common dietary chemicals act on the human genome, either directly or indirectly, to alter gene expression and/or structure. 2) Under certain circumstances and in some individuals, diet can be a serious risk factor for a number of diseases. 3) Some diet-regulated genes (and their normal, common variants) are likely to play a role in the onset, incidence, progression, and/or severity of chronic diseases.
4) The degree to which diet influences the balance between healthy and disease states may depend on an individuals genetic background. 5) Dietary intervention based on knowledge of individual nutritional requirement, nutritional status, and genotype (i.e., “individualized nutrition”) can be used to prevent, relieve, or cure chronic disease.
Nutrigenetics
Nutrigenetics term was used first time by Dr R.O Brennan in 1975 in his book Nutrigenetics (12). Nutrigenetics points to understanding how the genetic background of an individual impact to the diet (13).
The study of gene-nutrient interaction is a developing area of science. This idea that adverse diet/genome interaction can cause disease is not new and the unsuitable diet for any individual genotype could be a risk factor for monogenetic and polygenetic disease (10, 14). Genetic polymorphisms can influence response to environmental elements, such as enzymatic activities changes that affect circulating concentrations and ultimately the effectiveness of chemicals and their metabolites (5). Furthermore, metabolic disorders are other examples of influence of the genetic variations to diet such as PKU, defects associated with long chain fatty acid oxidation, iron absorption (haemochromatosis), which can be reasonably well managed with dietary restrictions (15).
As mentioned earlier SNPs study can be categorized in the field of Nutrigenetics. Some specific examples of the association between SNPs and specific food components such as enzymes deficiency are reviewed in this article. For example, different mutations in galactose-1-phosphate uridyltransferase (GALT) gene (14–18), phenylalanine hydroxylase gene (19, 20), and Glucose-6-phosphate dehydrogenize (G6PD) gene (21–24) resulted in Galactosemia, Phenylketonuria (PKU), and Favism diseases, respectively. Other examples of enzymes polymorphisms include Lactase-phlorizin hydrolase gene (LPH) polymorphisms that show how SNPs alter gene expression. This polymorphism is in the upstream of the lactase-phlorizin hydrolase gene (LPH) associated with hypolactasia and changes tolerance to dietary lactose (milk sugar, LPH hydrolyzes lactose into glucose and galactose) and allows different expression of the LPH (25, 26).
Glutathione peroxide gene polymorphism is another example. The association between selenium supplementation and reduced incidence of liver, colon, prostate, and lung cancer in human has been shown. However, no individuals may respond equally. Glutathione peroxide is a selenium-dependent enzyme that acts as an antioxidant enzyme. Polymorphism at codon 198 of human glutathione peroxides results in a subsituation of proline to leucine amino acid, and has been associated with an increase risk of lung cancer. Investigators shown that persons with (Pro/Lue) genotype were at 80% greater risk for lung cancer and (Lue/Lue) genotypes were at 130% greater risk compared risk those with the (Pro/Pro) genotype. The leucine-coding allele was less responsive to increased activity because of selenium supplementation as compared with the prolin-containing allele (8).
Manganese super oxide dismutase (MnSOD) is a mitochondrial enzyme that plays a key role in detoxification of reactive oxygen species. A polymorphism valine to alanin subsituation in in this enzyme alters its transport into mitochondria, which has been associated with increased risk of breast cancer (8).
Methylen tetrahydrofulate redoctase (MTHFR) enzyme catalyzes the reaction that produces 5-methyl tetrahydrofolate. The one-carbon units are carried on N-5 or N10 of tetrahydrofolate. One-carbone metabolism is needed for the denovo synthesis of purine nucleotides and thymidilate and for the remethylation of homocyisteine to methionine. With methionine adenylation S-adenosylmethionine (SAM) is formed, which is a cofactor for numerous methylation reactions such as DNA methylation that affect gene regulation (27). For the MTHFR gene tow important SNPs has been well recognized: C677T (cytosine-to-thymidine subsituation resulting in the conversion of an alanine to valine) and A1298C (adenine-to-cytosine subsituation resulting in the conversion of an alanine to glotamic acid). The C677T polymorphism is the most common variant that occurs as homozygous T/T in 5–10% of the and as heterozygous C/T genotypes up to 40% general population (28). The presence of C677T or A1298C mutations is associated with reduction in MTHFR enzyme activity and impairs folate accumulation, which may cause increases homocysteine concentration in plasma, a risk factor for venous thromboembolic and ischemic arterial diseases (2).
Another polymorphism of MTHFR gene is Ala222Val that affects folate metabolism. It increases the conversion of dUMP to dTMP and leads to more folate-dependent thymidine biosynthesis and folate deficiency (27). This polymorphism is a risk factor for spontaneous abortions and decreased fetal viability, thus maternal folate supplementation can be useful for individuals with this polymorphism (29).
MTHFR is also involved in maintenance genomic CpG methylation patterns and prevention of DNA strand breaks, these mutations are associated with increased risk of neural tube defects and some types of cancer (27).
Changes in the concentration of folate (the MTHFR substrate) and riboflavin (the MTHFR cofactor) can modulate the activity of MTHFR gene (28). Generally, folic acid supplementation can help the negative health effect of these SNPs with decrease in plasma homocysteine levels (2, 27, 28).
Enzymes that utilize and metabolize vitamin B12 have been associated with NTDs, increased risk of Down syndrome and colon cancer. For example, a common polymorphism in the HFE gene (Cys282Tyr) is associated with iron storage disease hereditary haemochromatosis, leading to an iron accumulation in the liver, heart and endocrine glands. This protein is an important regulator of cellular iron homeostasis and has role in intestinal iron absorption by regulating the interaction of the transferrin receptor with transferrin (27).
Cytochrome P450s (CYPs) enzymes play a central role in the oxidative biotransformation of steroids, prostaglandins, nutrients, drugs, chemicals and carcinogens. Several dietary factors can alter the expression of CYP isoforms. CYP1A2 plays an essential role in the metabolism of wide range of drug and chemical substances. For example, CYP1A2 activates dietary carcinogens such as aromatic amines, but also detoxifies compounds such as caffeine. Low-activity CYP1A2 genotype with an increased risk of myocardial infarction suggests that this enzyme detoxify a substance, which may be an important risk factor in the population. Indeed, individuals with a low-activity CYP1A2 genotype are at a greater risk of coffee-associated heart disease. As caffeine is the main substance in coffee and is detoxified by CYP1A2, it may be an important risk factor for heart disease in certain population (5).
Glutathione S transferase (GST) enzyme is a superfamily of enzymes that play an important role in the detoxification of several dietary compounds. GSTM1, GSTT1 and GSTP1 are isoforms of this enzyme. The GSTM1 and GSTT1 null genotype have been associated with both an increased and a decreased risk of some types of cancers such as breast cancer (5, 30). Some components such as dietary isothiocyanates that are found in cruciferous vegetables are eliminated with GSTs enzymes. Indeed, protective effect of the GSTM1 null genotype on colon and lung cancer has been related to lower urinary excretion of glutathione-conjugated phytochemicals indicating they are not rapidly excreted. GSTT1 plays a similar role to GSTM1 in eliminating beneficial phytochemicals found in cruciferous vegetables. Moreover, in vegetables rich in phytochemicals such as isothiocyanates the expression of GSTs is increased conjugating them to more water-soluble forms that are easily excreted (5).
Endothelial nitric oxide synthase (eNOS) is synthesized from the amino acid L-argenine by NO synthase (NOS). The eNOS is expressed in the endothelium and produces NO that diffuses to vascular smooth muscle cell, where it increases the concentration of cGMP, leading to vascular relaxation. NO has central role in the pathogenesis of coronary spasm and atherogenesis. Several polymorphisms of eNOS may be associated with specific phenotype. For example, a Glu298Asp polymorphism in the eNOS gene has been associated with ischemic heart disease, myocardial infarction, and coronary spasm (30).
Genetic polymorphisms in catechol-O-methyltransferase, sulfotransferase, and UDP-glucuronosyltransferase result in differences in enzymatic activity. These enzymes metabolize some of dietary compounds. For example, green tea was associated with a lower risk of breast cancer only in women with the low-activity allele for catechol-O-methyltransferase. This enzyme catalyzes the methylation of catechins (a polyphenolic antioxidant plant secondary metabolite) in green tea making them more quickly eliminated (5).
Apolipoprotein E (ApoE) gene has three different alleles (ε2, ε3, ε4). Persons with ε4 variant respond to a high-fat diet negatively with an increased risk for coronary heart disease (CHD). In these individuals, low-fat diet should be useful (2). Moreover, there is an important relationship between allelic variants in the ApoA1/C3/ A4/A5 genes and the effect of dietary fats on lipoprotein metabolism and CVD (cardio vascular diseases) risk. Linkage disequilibrium within Apo A1/C3/A4/A5 cluster has been represented to affect plasma lipid concentration and CVD risk. Apolipoprptein A-1 is and is a key component of high-density lipoprotein particles (HDL). The locus of gene encoding APOA-1 is on chromosome 11q and highly polymorph and has a specific SNP in its promoter region (19, 20). An Adenin/Guanin subsituation in the promoter region (−75bp) of the ApoA1 gene is common in different populations. The presence of A allele (A/A and A/G) has been associated with incresed HDL-cholesterol. Moreover, mild increase in APOA-1 concentrations in subjects with the G/G genotype was observed (28, 30). APOA-5 gene is also an important regulator of triglyceride (TG)-rich lipoprotein (TRL) metabolism (30).
One of the Vitamin D receptor (VDR) polymorphism is Fok1. Individuals with F allele have three amino acids more than those without F allele in their VDR. The Ff or ff genotype is associated with 51% and 84% greater risk of colorectal cancer, respectively. Individuals that consumed low calcium and fat diet have more than double risk of colorectal cancer, specifically in persons with ff genotype rather than Ff genotype (8). VDR polymorphisms have been also associated with childhood and adult’s asthma (27).
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptor supper family that plays an essential role in fatty acid oxidation, glucose, and extracellular lipid metabolism. PPARs are the best-known fatty-acid-regulated nuclear receptors. One of the three members of the PPARs family regulates many genes involved in fatty acid metabolism. PPR-α (PPARA) plays a central role in lipid oxidation and inflammation, whereas PPAR-γ is involved in adipocytes differentiation, glucose and lipid storage, and inflammation. PPAR-δ (also known as PPAR-β), may has a crucial role in development, lipid metabolism, and inflammation. These receptors bind to fatty acid and regulate the expression of genes involved in fatty acid transport and metabolism. PPARs family also involve in activation of about 300 genes (31).
The PPAR-α gene has a polymorphism at codon 162 (Lue162Val) that has been associated with changes in total cholesterol, LDL-associated cholesterol, and Apo B concentrations. The less common V162 allele is associated with significantly higher serum concentration of total cholesterol, LDL cholesterol, Apo B, and Apo C-III than in carriers of L162 allele, especially in men (20). For individuals with the common L162 allele, increased intake of polyunsaturated fatty acids (PUFAs) had little effect on fasting triacylglycerol concentrations. In those with the less common V162 allele, however, fasting triacylglycerol concentrations fell abundantly with increasing PUFA intake (32).
Nutrigenomics
Nutrigenomics aims to identify the effects of several nutrients, including macronutrients and micronutrients on the genome (13) and explores the interaction between genes and nutrients or food bioactives and their effects on human health (33). The influence of nutrients on the transcription activity, gene expression, and heterogeneous response of gene variants is also referred to as “Nutrigenomics”.
Nutrigenomics also describes the use of functional genomic tools to study a biological system to understanding of how nutritional molecules affect metabolic pathways and homeostatic control. This branch of science will reveal the optimal diet form within a series of nutritional changes, whereas Nutrigenetics will yield critically important information that assist clinicians in identifying the optimal diet for a given individual, i.e. personalized nutrition (13). Transcriptomics, proteomics, and metabolomics are also technologies that apply in Nutrigenomics research (33).
According to numerous studies, nutrients can alter the expression of genes at the level of gene regulation, signal transduction, chromatin structure and protein function (33).
Epidemiological studies show association between food intake and the incidence and severity of chronic diseases (34, 35). A large number of nutrition related pathologies (obesity, metabolic syndromes, type 2 diabetes, CVD, and some types of cancers) are polygenic and multifactorial and their onset and progression are related to multiple genes and their variants as well as several environmental factors, especially the diet (30).
Dietary chemicals can affect gene expression directly or indirectly. At the cellular level nutrients may act as ligands for transcription factor receptors (36, 37) or be metabolized by primary or secondary metabolic pathways, thereby altering concentrations of substrates or intermediates, and finally positively or negatively affect signal pathways (38–40).
Transcription factors (TFs) are one of the key molecules through with nutrients can alter the gene expression. One of the most important groups of nutrient sensors is PPARs TFs with 48 members in the human genome. The majority of receptors in this superfamily bind nutrients, their metabolites, and influences expression of specific genes involved in numerous metabolic process in the liver, including fatty acid oxidation, ketogenesis, gluconeogenesis, amino acid metabolism, cellular proliferation, and acute-phase response (41). For example, the fatty acids palmitic (16:0), oleic (18:1n9), linoleic (18:2n6), and arachidonic acid (20:4n6) (42–45), and the eicosanoids, 15deoxy-δ12, 14prostaglandinJ2 and 8-(S) hydroxyeicosatraenoic acid, are ligands for PPAR-δ (46–48). These nuclear receptors act as sensors for fatty acids. Lipid sensors usually heterodimerize with retinoid receptor, whose ligand is derived from another dietary chemical, vitamin A, and hyperforin, bind directly to nuclear receptors and influence gene expression (Table 1). The liver X receptor-α (binding cholesterol metabolites), bind as a heteromers to specific nucleotide sequence (response elements) in the promoter regions of a large member of genes. During ligand binding, nuclear receptors undergo a conformational change that results in coordinated dissociation of corepressors and recruitment of coactivator proteins to prepare transcriptional activation (41). Thereby, a number of genes are induced such as those involved in fatty acid oxidation or fatty-acid storage, depending on the cellular metabolic state (31). In metabolically active organs, such as the liver, intestine, and adipose tissue, these TFs act as nutrient sensors by changing the level of DNA transcription of specific genes in response to nutrients changes (41).
Table 1:
Nuclear receptors and dietary ligands. Umbers in parentheses indicate percent activity after ligand binding relative to estradiol (36, 37, 50, 52, 53)
| Regulation | Receptor | Type | Endogenous ligand | Dietary ligand |
|---|---|---|---|---|
| Endocrine: hormonal lipids: feedback paradigm | Estrogen | ERα ERβ |
17β-Esteradiol (100) 17β-Esteradiol (100) Progestrone |
Genisteine (4) |
| Progestrone | Testoterone | Genisteine (87) | ||
| Androgen | 5α -dihydrotestosterone | Endogenous metabolism cholestrol precursor | ||
| Androgen | Aldosterone | |||
| Glucocorticoid | Cortisol | |||
| Mixed paradigm | Retinoic acid | RARα | All-trans retinoic acid | Vitamin A |
| RARβ | All-trans retinoic acid | Vitamin A | ||
| RARγ | All-trans retinoic acid | Vitamin A | ||
| Thyroid | TRα TRβ |
Iodine Iodine |
||
| Vitamin D | 1,25-dihydroxyvitamin D | Vitamin D/Sunshine | ||
| Ecdisone | Cholesterol derivatives | Cholesterol | ||
| Lipid sensors: dietary lipids: feed-forward paradigm | Retinoid X | Cis-9-retinoic acid | Docsahexaenoic acid | |
| PPAR | PPARα PPARβ PPARδ |
FA FA/eicosanoids ? |
Pristinic/phytanic Pristinic/phytanic |
|
| Pregnan X | Estrogen Progesterone Progesterone |
Hyperforin Coumesterol Genisteine |
||
| Liver X | Oxysterols | Cholesterol metabolites | ||
| Famosoid X | Bile acids |
Dietary chemicals indirectly regulate some of TFs. The sterol regulatory element binding proteins (SREBPs), for example, are activated by protease cleavage, an event regulated by low levels of oxy sterols and changes in insulin/ glucose and PUFAS (49). The carbohydrate-responsive element-binding protein (chREBP) is a large TF, activated in response to high glucose levels, and is regulated by reversible phosphorylation events (50). This DNA binding protein serves as an effector of lipogenic gene expression (51).
Moreover, dietary chemicals can directly affect signal transduction pathways. For example, green tea contains the polyphenol, 11-epigallocatechin-3-gallate (EGCG) that EGCG inhibits tyrosine phosphorylation of Her-2/neu receptor and epidermal growth factor receptor that reduces signaling via the phosphatidyl inositol 3-kinase (PI-3)-AKt kinase-NF-kB pathway. Activation of the NF-kB pathway is associated with some types of breast cancer (50, 52, 53).
PUFAs such as n-3 and n-6 are other micronutrients, which are also referred to as omega-3 and omega-6 fatty acids, may influence gene expression. Animal studies have demonstrated that PUFA intake can modulate the gene expression of several enzymes involved in lipid and carbohydrate metabolism. A significant interaction has also been shown for the PPARA Lue162Val polymorphism n-6 PUFA intake. Individuals with the less common V162 allele, increased n-6 PUFA intake is associated with a marked reduction in triacylglycerol concentration, whereas this association is not observed in L162 carriers. Conversely, in L162 and V162 carriers n-3 PUFA intakes results in triacylglycerol concentrations reduction (32).
Approximately 40 micronutrients are needed in the human diet. Suboptimal intakes of specific micronutrients have been associated with CVD (Vit B, E, and carotenoids), cancer (folate, carotenoids), neural tube defects (folate), and bone mass (Vit D) (54). B6, B12 and folate deficiencies, for example, are associated with increased serum homocysteine levels. Hyperhomocysteinemia is a risk factor and marker for coronary artery disease. Deficiency of Vit B12, folic acid, B6, niacin, C or E, iron or zinc appears to imitate radiation in damaging DNA by causing single and double-strand breaks, oxidative lesions, or both (55), (Table 2).
Table 2:
Examples of the role and effect of specific micronutrients deficiencies on genomic stability (56, 57)
| Micronutrients | Role in genomic stability | Consequence of deficiency |
|---|---|---|
| Vits C and E | Prevention of DNA and lipid oxidation. | Increased baseline level of DNA strand breaks, chromosome breaks, oxidative DNA lesions and lipid peroxide adducts on DNA. |
| Vit D | Antioxidant activity by increasing glutathione level in normalm cell, inducuction apoptosis in cancer cells. | |
| Folate and Vits B2, B6, B12 | Maintenance methylation of DNA, synthesis of dTMP from dUMP and efficient recycling of folate. | uracil misincorporation in DNA, increased chromosome breaks and DNA hypomethylation. |
| Niacin, Nicotinic acid | Required as substrate for poly (ADP- ribose) polymerase which is involved in cleavage and rejoining of DNA and telomere length Maintenance and DNA repair. | Increased level of unrepaired nicks in DNA, increased chromosome breaks and rearrangement, sensitivity of mutagens. |
| Zinc, Manganese and Selenium | Zn, required as a cofactor for Cu/Zn supperoxid dismutase, endonuclease IV, P53 function, DNA replication and Zinc finger proteins such as poly (ADP- ribose) polymerase. Mn, required as a component of mitochondrial Mn superoxide dismutase. Se, required as a component of peroxidases e.g. glutathione peroxidase. |
Increased DNA breaks and oxidation, elevated chromosomal damage rate. |
| Iron | Required as a component of ribonucleotide reductase and mitochondrial cytochromes. | Reduced DNA repair capacity, increased propensity for oxidative damage to mitochondrial DNA. |
| Magnesium, Calcium | Mg, required as a cofactor for a variety of DNA polymerases, in nucleotide excision Repaire, base excision repair and mismatch Repair, essencial for microtubule Polymerization and chromosome segregation. Ca, plays an important role in chromosome Segregation and is required for apoptosis. | Reduced fidelity of DNA replication, reduced DNA repaire capacity, chromosome segregation Errors,survival of genomically aberrant cells. |
Nutrient deficiencies are more important than radiation because of constancy of exposure to milieu promoting DNA damage (58–60). For example, folate deficiency breaks chromosomes due to substantial incorporation of uracil in human DNA (4 million uracil/cell) (61).
Amino acids can play the role of nutritional signals in the modulation of expression of particular genes. Studies have shown that cells can detect variants in amino acid levels and respond by mechanism as control of transcription, mRNA stabilization ,as well as by up or down regulation of translation initiation (62). For example, in human cells amino acid L-tryptophan in supraphysiologic concentrations is a powerful inducer of collagenase gene expression at a transcriptional level. The increase in collagenase mRNA levels was reversible, time and L-tryptophan dose-dependent (63).
Simple and complex carbohydrates have differential effects on blood glucose concentrations. Foods with a high glycemic index (GI) would increase insulin production and, decrease synthesis of insulin receptors. High glucose concentration also induces the transcription of several genes of the glycolytic and lipogenic pathways (64).
Therefore, dietary chemicals are regularly ingested and are involved indirectly and directly in regulation gene expression, it follows that a subset of genes regulated by diet must be involved in disease initiation, progression, and severity (65, 66).
Nutritional epigenetics
The term “epigenetics” is used to gene expression that occurs without changes in the DNA sequence. Epigenetic regulation plays an important role in development and is needed to gain stable expression or repression of genes in specific cell types or at defined developmental stages (67). Epigenetic changes may influence cell cycle control, DNA damage, apoptosis, invasion, imprinting, and aging (8).
Epigenetic events can be modified by bioactive food components (Table 3).
Table 3:
Some nonessential nutrients and bioactive food components that can alter genetic and epigenetic events (8)
| Nutrient group | Example |
|---|---|
| Phytochemicals | Isothiocyanates, allyl sulfur Carotenoids, flavonoids, indoles, |
| Zoochemicals | Conjugated linoleic acid, n-3 fatty acids |
| Fungochemicals | β-glucans, lentinan, schizophylan, and other compounds in mushrooms. |
| Bacteriochemicals | Equol, butyrate, and other compounds formed from gastrointestinal flora fermentation |
A majority of regulatory proteins including DNA methyltransferases, methyl-cytosine guanine dinucleotide binding proteins, histon-modifing enzymes, chromatin-remodeling factors, and their multimolecular complexes are involved in the overall epigenetic process (8). The best studied epigenetic modification is DNA methylation and in the mammals genome occurs at many of cytosine residues that are followed by guanine residue (CpG islands) and in most cases methylation in these regions induces gene repression. However, this phenomenon can lead to the expression of neighboring genes (67). Studies identify that DNA methylation is dependent on bioactive food components ranging from alcohol to zinc (8) (Table 4).
Table 4:
| Micronutrients | |
|---|---|
| Alcohol | Genistein |
| Arsenic | Methionine |
| Betaine | Nickel |
| Cadmium | Polyphenol |
| Choline | Selenium |
| Coumestrol | Vitamin A |
| Equol | Vitamin B6 |
| Fiber | VitaminB12 |
| Folate | Zinc |
Several dietary factors may influence the provision of methyl groups available for the formation of S-adenosylmethinine. Moreover, dietary factors may modulate the use of methyl group by processes including change in DNA methyltransferase activity. The methyl groups’ status depends on B vitamins as cofactors including folate, Vit B12, and Vit B6 (29). The folate-dependent biosynthesis of nucleotide precursors for DNA synthesis and of SAM for genome methylation is dependent on the availability of many vitamins including B12, B6, niacin, riboflavin and minerals (zinc, cobalt). Therefore, folate-mediated one-carbon metabolism mediates communication between the cellular nutrient environment and regulation of the genome. Impairments in one-carbon metabolism and the SAM cycle induced by nutritional deficiencies and/or SNPs in genes that encode folate-dependent enzymes, alter genome methylation patterns and gene expression levels. Disruptions in folate metabolism are common and increase risk cancers, cardiovascular disease, neurological disorders and developmental anomalies such as spina bifida, cleft palate, and spontaneous abortion. Therefore, folate supplementation can reduce the risk of these disorders developing (69).
DNA methylation patterns may effect on the response to bioactive food components and thereby account for differences in response in normal and neoplastic cells (70).
Genome health and disease prevention
It is clear that even the small damages in the genome can cause crucial effects in whole human life. DNA metabolism and repair is depending on a variety of dietary factors that act as cofactors or substrates. Nutritional requirements is important for the prevention of DNA oxidation (i.e. antioxidants such as carotenoids, Vit E and C), prevention of uracil incorporation into DNA (i.e. folate), maintenance methylation of CpG in DNA (methionine, cholin, folate and vitamin B12), as cofactors or as components of DNA repair enzymes (Zn, Mg), maintenance of telomere length (niacin, folate) (56, 69, 70).
Many chronic diseases are polygenic and result from interaction between genes and environmental factor. Dietary intervention based on nutritional requirement, nutritional status, and genotype (i.e., “individualized nutrition”), can be used to prevent, control or treatment of chronic disease such as cardiovascular diseases (CVD), metabolic syndromes, and cancer (41). These disorders are partly mediated by chronic exposure to certain food components. Fore example, the association between amount of calories (35), the levels and types of vitamins (71), fat (72), and carbohydrates with atherosclerosis, diabetes, obesity, cancer, hypertension, and other chronic diseases is demonstrated (73).
Genome damage and nutritional deficiency
As mentioned earlier, nutritional status influences genome stability and deficiency of certain micronutrients can result in critical damages in the genome. Studies have shown that at least nine micronutrients (Vit E, Ca, folate, retinol, nicotinic acid, β-caroten, riboflavin, pantothenic acid, and biotin) affect genome stability in human in vivo (4). Folate and vitamin B12 are need for DNA replication, repair and maintenance of DNA methylation patterns. Both in vivo and in vitro studies with human cells clearly show that folate and vitamin B12 deficiencies and elevated plasma homocysteine are associated with the expression of chromosomal fragile sites, chromosomal breaks, excessive uracile in DNA and DNA hypomethylation. Nicotinic acid (niacin) also plays a fundamental role in chromosome integrity and reduction of cancer risk (Table 2) (70).
Reactive oxygen species (ROS) such as highly reactive hydroxyl radical and superoxide radical contributes to DNA damage. Antioxidants (Vit C and E) and enzymes such as superoxid dismutase, catalase and glutathionperoxidase may control lipid and protein oxidation induced by ROS (3).
Since developmental, degenerative diseases and aging are partly caused by DNA damage, defining optimal requirements of key minerals and vitamins for preventing nuclear and mitochondrial DNA damage is important (70).
Infertility is another consequence of genome damage on human health. Genome damage results from specific micronutrient deficiencies may cause developmental defects in the fetus or increased risk of cancer in the child. For example, inadequate Vit C intake results in increased oxidation of sperm DNA; folate deficiency increases risk of NTDs and genome damage. Increased risk of childhood leukemia in children with mothers who did not intake enough folic acid supplementation during pregnancy has demonstrated. Additionally, zinc deficiency induces oxidative damage to DNA and impairs DNA repair, which has a teratogenic effects (70, 74).
Telomere and nutritional status
Telomeres are nucleoprotein structures that cap the ends of chromosomes, and maintain chromosome stability. Degeneration of telomeres leads to whole chromosomal instability, and chromosomal fusion and therefore gene amplification, an important risk factor for cancer (3). Folate and nicotinic acid deficiency increased oxidative stress and telomere dysfunction. Under folate deficiency uracil is incorporated into DNA instead of thymidine, leading to chromosome breakage. Similarly, oxidative stress causes telomere shortening. Folate and other methyl donors such as Vit B12, cholin, and methionin have an important role in maintenance methylation of cytosine. Defects in the DNA methylation can cause excessive telomere elongation and homologous recombination between telomeres and telomeres fusion. Hypomethylation or hypermethylation of the CpG islands is in the promoter of telomerase may cause excessive expression of telomerase or silence the gene respectively (3).
Telomere shortening has been observed in number of conditions including obesity, psychological stress, immune dysfunction, cancer, and CVD. In vitro studies antioxidant treatment has been found to prevent telomere damage (3).
Conclusions
Nutritional genomics elucidate the interaction among nutrients, metabolic intermediates, and the mammalian genome. The response to bioactive food components is dependent on genetic background (Nutrigenetics effects) that can influence absorption and metabolism targets or sites of action. Likewise, the response to food components depends on DNA methylation and other epigenetic events. The ability of bioactive food components to influence gene expression patterns (nutrigenomics effects) is also a factor in determining the overall response. Finally, bioactive food components may influence protein synthesis, degradation, and posttranslational modification. Understanding the interrelationships among human genetics diversity, genome function, and dietary components will enable precise manipulation of genome function and stability throughout the life cycle for optimal human health and disease prevention.
With greater insight in to the gene-nutrient interaction, alterations in diet and single nutrient interventions may help us to better protect against cancer, decrease the occurrence of cardiovascular and other chronic diseases, and perhaps increase human longevity.
Ethical Considerations
All ethical issues including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc have been completely observed by the author.
Acknowledgments
The authors are thankful to Prof. A. Jazayery, Dept. of Nutrition, school of Public Health and Dr. H. Sadighi, Genetic Clinic, for their critical comments and corrections. The authors declare that they have no conflicts of interest.
References
- 1.Shils ME, Shike M, Ross AC, Caballero B, J-Cousins R. Modern Nutrition in Health and Disease. 10th ed. lippincott Williams A wolters Kluwer Company; Philadelphia: 2006. chapter: 39. [Google Scholar]
- 2.Subbiah MT. Nutrigenetics and nutraceuticals: the next wave riding on personalized medicine. Transl Res. 2007;149(2):55–61. doi: 10.1016/j.trsl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Bull C, Fenech M. Genome health nutrigenomics and nutrigenetics: nutritional requirements for chromosomal stability and telomere maintenance at the individual level. Proc Nutr Soc. 2008;67(2):146–56. doi: 10.1017/S0029665108006988. [DOI] [PubMed] [Google Scholar]
- 4.Fenech M. Genome health nutrigenomics and nutrigenetics-diagnosis and nutritional treatment of genome damage on an individual basis. Food Chem Toxicol. 2008;46(4):1365–70. doi: 10.1016/j.fct.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 5.El-Sohemy A. Nutrigenetics. Forum Nutr. 2007;60:25–30. doi: 10.1159/000107064. [DOI] [PubMed] [Google Scholar]
- 6.Dolinoy DC, Jirtle RL. Environmental epigenetics in human health. Environ Mol Mutagen. 2008;49(1):4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- 7.Paoloni-Giacobino A, Grimble R, Pichard C. Genetics and nutrition. Clin Nutr. 2003;22(5):429–35. doi: 10.1016/s0261-5614(03)00064-5. [DOI] [PubMed] [Google Scholar]
- 8.Trujillo E, Davis C, Milner J. Nutrigenomics, Proteomics, Metabolomics, and the Practice of Dietetics. J Am Diet Assoc. 2006;106(3):403–13. doi: 10.1016/j.jada.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson LR. Nutrigenomics: integrating genomics approaches into nutrition research. Mol Diag Ther. 2006;10(2):101–8. doi: 10.1007/BF03256449. [DOI] [PubMed] [Google Scholar]
- 10.Kaptur J, Raymond LR. Nutritional genomics: the next frontier in the Post genomic era. Physiol Genomics. 2004;16:167–77. doi: 10.1152/physiolgenomics.00107.2003. [DOI] [PubMed] [Google Scholar]
- 11.Milner JA. Molecular Targets for Bioactive Food Components. J Nutr. 2004;134(9):2492S–2498S. doi: 10.1093/jn/134.9.2492S. [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Experi Biol Med. 2010;235:785–95. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- 13.David M. Mutch, Walter Wahli, Gary Williamson. Nutrigenomics and nutrigenetics: the emerging faces of Nutrition. FASEB J. 2005;19(12):1602–16. doi: 10.1096/fj.05-3911rev. [DOI] [PubMed] [Google Scholar]
- 14.Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased Km): relevance to genetic disease and polymorphisms. Am J clin Nutr. 2002;75(4):616–58. doi: 10.1093/ajcn/75.4.616. [DOI] [PubMed] [Google Scholar]
- 15.Palou A. From nutrigenomics to personalized nutrition. Genes Nutr. 2007;2(1):5–7. doi: 10.1007/s12263-007-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farhud DD, Shalileh M. (In press). Guidline of nutritional regimens for Galactosemia, Dept. of Experimental Education.
- 17.Krauss RM. Dietary and genetic effects on LDL heterogeneity. World Rev Nutr Diet. 2001;89:12–22. doi: 10.1159/000059791. [DOI] [PubMed] [Google Scholar]
- 18.Ko DH, Chang HE, Song SH, Park KU, Kim JQ, Kim MC, Song YH, Hong YH, Lee DH, Song J. Molecular and biochemical characterization of the GALT gene in Korean patients with galactose-1-phosphate uridyltransferase deficiency. Clin Chim Acta. 2010. (in press). [DOI] [PubMed]
- 19.Farhud DD, Shalileh M. Phenylketonuria and its dietary therapy in children. Iranian J Pediatric. 2008;18(suppl 1):88–98. [Google Scholar]
- 20.Brigeli S, Flohe R, Joost HG. Nurtigenomics. 2006. (Translated in press, by Farhud DD and Athari S).
- 21.Farhud DD, Sadighi H, Amirshahi P, Tavakkoli FSH. Serum level measurements of Gc, Cp, IgG, IgA and IgM in patients with Favism in Iran. Iranian J Public Health. 1993;22:1–4. [Google Scholar]
- 22.Farhud DD, Yazdanpanah L. G6PD deficiency. Iranian J Public Health. 2008;37(4):1–18. [Google Scholar]
- 23.Farhud DD, Amirshahi P. Ahaptoglobinemia in Favism patients from Iran. Acta Anthropogenet. 1985;9(4):206–13. [PubMed] [Google Scholar]
- 24.Hedayat SH, Farhud DD, Montazami K, Ghadiryan P. The pattern of bean consumption, laboratory findings in patients with Favism, G6PD deficiency, and a control group. J Trop Pediatr. 1981;27(2):110–13. doi: 10.1093/tropej/27.2.110. [DOI] [PubMed] [Google Scholar]
- 25.Lucock Mark. Molecular Nutrition and Genomics. 2007. (Translated in press, by Farhud DD and Zarif Yeganeh M).
- 26.Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30(2):233–7. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 27.Stove Patrick J. Influence of human genetic variation on nutritional requirements. Am J Clin Nutr. 2006;83(2):436S–42S. doi: 10.1093/ajcn/83.2.436S. [DOI] [PubMed] [Google Scholar]
- 28.Virgili F, Perozzi G. How Does Nutrigenomics Impact Human Health? IUBMB Life. 2008;60(5):341–44. doi: 10.1002/iub.85. [DOI] [PubMed] [Google Scholar]
- 29.Stover Patrick J. Nutritional genomics. Physiol Genomics. 2004;16(2):161–5. doi: 10.1152/physiolgenomics.00204.2003. 15. [DOI] [PubMed] [Google Scholar]
- 30.Iacoviello L, Santimone I, Latella MC, de Gaetano G, Donati MB. Nutrigenomics: a case for the common soil between cardiovascular disease and cancer. Genes Nutr. 2008;3(1):19–24. doi: 10.1007/s12263-008-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ordovas JM. Nutrigenetics, Plasma Lipids, and Cardiovascular Risk. J Am Diet Assoc. 2006;106(7):1074–81. doi: 10.1016/j.jada.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Ordovas JM. Genetic interactions with diet influence the risk of cardiovascular disease. Am J Clin Nutr. 2006;83(2):443S–446S. doi: 10.1093/ajcn/83.2.443S. [DOI] [PubMed] [Google Scholar]
- 33.El-Sohemy A. The Science of Nutrigenomics. Health Law Review. 2008;16(3):5–8. [Google Scholar]
- 34.Willett W. Isocaloric diets are of primary interest in experimental and epidemiological Studies. Int J EPidemiol. 2002;31(3):694–5. [PubMed] [Google Scholar]
- 35.Jenkins DJA, Kendall ccw, Ransom TPP. Dietary fiber, the evolution of the human diet and Coronary heart disease. Nutr Res. 1998;18:633–52. [Google Scholar]
- 36.Dauncey MJ, White P, Burton KA, Katsumata M. Nutrition hormone receptor- gene interactions: implications for development and disease. Proc Nutr Soc. 2001;60(1):63–72. doi: 10.1079/pns200071. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs MN, Lewis DF. Steroid hormone receptors and dietary ligands: a selected review. Proc Nutr Soc. 2002;61(1):105–22. doi: 10.1079/pns2001140. [DOI] [PubMed] [Google Scholar]
- 38.Clarke SD. Nutrient regulation of gene and protein expression. Curr opin Clin Nutr Metab care. 1999;2(4):287–9. doi: 10.1097/00075197-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Eastwood MA. A molecular biological basis for the nutritional and Pharmacological benefits of dietary plants. QJM. 2001;94(1):45–8. doi: 10.1093/qjmed/94.1.45. [DOI] [PubMed] [Google Scholar]
- 40.Lin SJ, Guarentel Nicotinamide adenine dinucleotid, a metabolic regulator of transcription, longevity and disease. Curr opin, cell Biol. 2003;15(2):241–6. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 41.Afman L, Müller M. Nutrigenomics: From Molecular Nutrition to Prevention of Disease. J Am Diet Assoc. 2006;106(4):569–76. doi: 10.1016/j.jada.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Auwerx J. Regulation of gene expression by fatty acids and fibric acid derivatives: an integrative role for Peroxisome Proliferator activated receptors. The Belgian Endocrine Society lecture. Horm Res. 1992;38(5–6):269–77. doi: 10.1159/000182557. [DOI] [PubMed] [Google Scholar]
- 43.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta- oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(5):879–87. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 44.Göttlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid -activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1992;89(10):4653–57. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferators and fatty acids. Mol Endocrinol. 1992;6(10):1634–41. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- 46.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14- Prostaglandin J2 is a Ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 47.Kliewer SA, lenhard Jm, willsan TM, Patel I, Morris DC, Lehmann JM. A Prostaglandin J2 metabolite binds Peroxisome Proliferator-activated receptor gamma and Promotes adipocyte differentiation. Cell. 1995;83(5):813–19. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 48.Kliewer SA, Xu HE, lambert MH, Willson TM. Peroxisome Proliferator-activated receptors: from genes to Physiology. Recent Prog Horm Res. 2001;56:239–63. doi: 10.1210/rp.56.1.239. [DOI] [PubMed] [Google Scholar]
- 49.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin - 3 -gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin cancer Res. 2001;7(12):4220–29. [PubMed] [Google Scholar]
- 50.Uyeda K, Yamashita H, Kawaguchi T. Carbohydrate responsive element-binding protein (chREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol. 2002;63(12):2075–80. doi: 10.1016/s0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 51.Uyeda1 Kosaku, Repa Joyce J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metabolism. 2006;4:107–10. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Edwards PA, Tabor D, Kast HR, Ven-kateswaran A. Regulation of gene expression by SREBP and SCAP. Biochim Biophys Acta. 2000;1529(1–3):103–13. doi: 10.1016/s1388-1981(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 53.Nobel S, Abrahmsen L, Oppermann U. Metabolic conversion as a Pre-receptor control Mechanism for lipophilic hormones. Eur J Biochem. 2001;268(15):4113–25. doi: 10.1046/j.1432-1327.2001.02359.x. [DOI] [PubMed] [Google Scholar]
- 54.Fairfield KM, Fletcher RH. Vitamins For chronic disease Prevention in adults: Scientific review. JAMA. 2002;287(23):3116–26. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]
- 55.Ames BN. DNA damage from micro-nutrient deficiencies is likely to be a major cause of cancer. Mutat Re. 2001;(1–2):475. 7–20. doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 56.Fenech M. Nutrition and genome health. Forum Nutr. 2007;60:49–65. doi: 10.1159/000107067. [DOI] [PubMed] [Google Scholar]
- 57.Pianetti S, Guo S, Kavanagh KT, So-nenshein GE. Green tea polyphenol epigallocatechin -3 gallate inhibits Her-2 /neu signaling, Proliferation, and transformed Phenotype of breast cancer cell. Cancer Res. 2002;62(3):652–55. [PubMed] [Google Scholar]
- 58.Ames BN, Gold LS. Paracelsus to parascience: the environmental cancer distraction. Mutat Res. 2000;447(1):3–13. doi: 10.1016/s0027-5107(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 59.Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer. 2002;2(9):694–704. doi: 10.1038/nrc886. [DOI] [PubMed] [Google Scholar]
- 60.Blount BC, mack MM, Wehr CM, Mac Gregor JT, Hiatt RA, Wang G, Wickrama-singhe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation in to human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acod Sci, USA. 1997;94(7):3290–95. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jefferson LS, Kimball SR. Amino acid regulation of gene expression. J Nutr. 2001;131(9 Suppl):2460S–6S. doi: 10.1093/jn/131.9.2460S. [DOI] [PubMed] [Google Scholar]
- 62.Varga J, Li L, Mauviel A, Jimenez SA. L-Tryptophan in supraphysiologic concentrations Stimulates collagenase gene expression in human skin fibroblasts. Lab Invest. 1994;70(2):183–91. [PubMed] [Google Scholar]
- 63.Augustin LS, Franceschi S, Jenkins DJ, Kendall CW, La Vecchia C. Glycemic index in choronic disease: a review. Eur J clin Nutr. 2002;56(11):1049–71. doi: 10.1038/sj.ejcn.1601454. [DOI] [PubMed] [Google Scholar]
- 64.Kaput J, Swartz D, paisley E, Mangian H, Daniel WL, Visek WJ. Diet- disease interactions at the molecular level: an experimental paradigm. J Nutr. 1994;124(8 Suppl):1296 S–305 S. doi: 10.1093/jn/124.suppl_8.1296S. [DOI] [PubMed] [Google Scholar]
- 65.Park EI, paisley EA, Mangian HJ, Swartz DA, Wu MX, O’Morchoe PJ, Behr SR, Visek WJ, Kaput J. Lipid level and type alter stearoyl coA desaturase mRNA abundance differently in mice with distinct susceptibilities todiet–influenced diseases. J Nutr. 1997;127(4):566–73. doi: 10.1093/jn/127.4.566. [DOI] [PubMed] [Google Scholar]
- 66.Ross SA. Nutritional genomic approaches to cancer prevention research. Experimental oncology. 2007;29(4):250–6. [PubMed] [Google Scholar]
- 67.Stover PJ, Caudill MA. Genetic and Epigenetic Contributions to Human Nutrition and Health: Managing Genome-Diet Interactions. 2008. [DOI] [PMC free article] [PubMed]
- 68.Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med. 2004;229:988–995. doi: 10.1177/153537020422901002. [DOI] [PubMed] [Google Scholar]
- 69.Fenech M. The Genome Health Clinic and Genome Health Nutrigenomics concepts: diagnosis and nutritional treatment of genome and epigenome damage on an individual basis. Mutagenesis. 2005;20(4):255–69. doi: 10.1093/mutage/gei040. [DOI] [PubMed] [Google Scholar]
- 70.Fairfield KM, Fletcher RH. Vitamins For chronic disease Prevention in adults: Scientific review. JAMA. 2002;287(23):3116–26. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]
- 71.Willet WC. Balancing life-style and genomics research for disease Prevention. Science. 2002;296(5568):695–8. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 72.Genkins DJ, Kendall cw, Augustin LS, et al. Glycemic index: overview of implication in health and Disease. Am J clin Nutr. 2002;76:266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 73.Kumar D. From evidence-based medicine to genomic medicine. Genomic Med. 2007;1(3–4):95–104. doi: 10.1007/s11568-007-9013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fenech M, Ferguson LR. Vitamins/minerals and genomic stability in humans. Mut Res. 2001;475:1–6. doi: 10.1016/s0027-5107(01)00069-0. [DOI] [PubMed] [Google Scholar]