Abstract
Background
Dermatophytes are the most common causative agents of superficial mycoses. Species identification of these fungi is important from therapeutic and epidemiological point of wive. Traditional approaches for identification of dermatophytes at the species level, relying on macroscopic and microscopic features of the colonies, usually are time-consuming and unreliable in many circumstances. Recently a broad varieties of rapid and accurate DNA-based techniques were successfuly utilized for species delineation of dermatophytes.
Methods:
The ITS1-5.8S-ITS2 region of rDNA from various reference strains of dermatophyte species were amplified using the universal fungal primers ITS1 and ITS4.The PCR products were digested by a single restriction enzyme, MvaI. The enzyme was evaluated in both in silico and practical PCR-RFLP assay to find the exact differentiating restriction profiles for each species. To validate the standardized PCR-RFLP system, all tested strains were subjected to sequencing and sequence analysis.
Results:
The obtained RFLP patterns were specific for many species including T. interdigitale, T. rubrum, T. violaceum, M. persicolor, M. audouinii, M. nanum (A. obtusum) and E. floccosum but were similar for some closely related species such as M. canis / M. ferrugineum. Sequencing of the ITS1-5.8S-ITS2 fragment from all type strains affirmed the RFLP findings.
Conclusion:
It was practically revealed that the ITS-PCR followed by MvaI-RFLP is a useful and reliable schema for identification and differentiation of several pathogenic species and can be used for rapid screening of even closely related species of dermatophytes in clinical and epidemiological settings.
Keywords: Dermatophytes, Identification, ITS, PCR-RFLP
Introduction
Dermatophytes are a group of specialized molds, affecting the superficial keratinized structures (skin, hair and nails) of human and animal hosts, producing dermatophytosis, commonly referred to as ‘ringworm’ or tinea. They are classified in three genera, Epidermophyton, Microsporum, and Trichophyton containing three ecological groups of anthropophilic, zoophilic and geophilic species (1, 2). Dermatophytes are the most common agents of cutaneous fungal infections worldwide(3, 4). Infections are contagious and represent a significant public health problem in many parts of the world. Dermatophytosis is not a reportable disease but is a matter of concern because of its contagiousness nature (5).
Correct identification of dermatophytes at the species level is useful for differentiating between dermatophytosis and dermatomycosis (6), to control of environmental and animal sources of infection and help for developing the preventive strategies (7). From clinical point of view, for definition of species and performance of an epidemiologic study it is important to have a reliable method for identification of dermatophytes (8). Species-level identification of these fungi classically relies on macro and micro morphological features of the colonies on general and specific culture media and on some biochemical and physiological complementary tests (1). However, in many circumstances phenotypic characteristics overlap between species, and many isolates have atypical nature in primary isolation thus attempt for final identification is time consuming and requires expertised personel on microscopical properties (2, 9–11). By development of PCR technology, a wide variety of molecular techniques such as RAPD-PCR, Nested-PCR, PCR-RFLP, PCR-EIA, Real-time PCR and microarray technology were employed as possible alternatives for routine identification of fungi including dermatophytes (2).
At the present study, the ITS1-5.8S-ITS2 fragment of ribosomal DNA gene (rDNA) in the dermatophyte species were used as a reliable marker for species identification. We retrieved the reliable sequences of internal transcribed spacers (ITS) regions from GenBank, then computationally (in-silico) and practically subjected them to a polymerase chain reaction-restriction enzyme (PCR-RE) assay for identifying nearly all pathogenic dermatophyte species. Additionally,we amplified and digested the DNA target in some reference dermatophyte strains to confirm the method. We prepared a relatively perfect restriction fragment lemgh polymorphism (RFLP) barcode by using only a single enzyme and believe that it could be useful for clinical and epidemiological aims.
Material and Methods
Virtual restriction enzyme digestion
The complete sequence of internal transcribed spacers 1 and 2 regions and the 5.8S ribosomal DNA subunit flanked these regions were retrieved fromNCBI (http://www.ncbi.nlm.nih.gov/nuccore). The sequences were edited by MEGA 4 software (MEGA version 4, Tamura, Dudley, Nei, and Kumar 2007), and sujected to popular sequence alignment tool of ClustalW. The arranged sequences were then exported to DNASIS software (Hitachi DNASIS® MAX v3.0) and subjected to digital digestion with 610 restriction enzymes included in the software. The enzymes with the best discriminatory power were selected and species-specific RFLP profiles were determined.
Reference strains
To assess the actual feasibility and applicability of the in silico PCR-RFLP findings, twenty five reference strains of different dermatophyte species were prepared from two reference collections: NBRC (NITE Biological Resource Center) and JCM (Japan Collection of Microorganisms) and used in the study. The strains were T. mentagrophytes (NBRC 5974, NBRC 5809, NBRC 5466), T. mentagrophytes var. interdigitale (NBRC 5812), Arthroderma. vanbreuseghemii (JCM 1891), A. benhamiae (JCM 1885), M. persicolor (NBRC 5975), T. tonsurans (NBRC 5928, NBRC 5945), T. equinum (NBRC 31610), T. rubrum (NBRC 5808, NBRC 5467), T. violaceum (NBRC 31064), E. floccosum (NBRC 9045), M. canis (NBRC 9182), M. ferrugineum (NBRC 6081, NBRC 5831), M. audouinii (NBRC 6074), A. obtusum (JCM 1907), A. uncinatum (NBRC 31978), T. schoenleinii (NBRC 8192, NBRC 8191), M. cookei (NBRC 7862), and M. gypseum (NBRC 8228, NBRC 5948). Strains inoculated into the plates containing Potato Dextrose Agar (PDA) medium (Difco, USA) and incubated at 28ºC for 2–3 weeks till the colonies came up.
DNA extraction
DNA was extracted from the strains using the method described by Makimura et al. (12). Briefly, amount (approximately 5 cubic millimeter) of a fresh colony was placed in lysis buffer (200 mM Tris-HCl, pH 7.5, 25 mM EDTA, 0.5% w/v SDS, 250 mM NaCl), and crushed with a conical grinder. Samples were incubated for 20 min at 100°C and mixed with 150 μl of 3.0 M sodium acetate, kept at −20°C for 10 min and centrifuged at 12,000 g for 10 min. The supernatants were extracted once with phenol chloroform iso-amyl alcohol (25:24:1), and subsequently extracted once again with chloroform. DNA was precipitated with an equal volume of iso-propanol, washed with 300 μl of 70% ethanol, dried and suspended in 50 μl of ultrapure water. The final solution was kept at −20 ºC until using as template for PCR.
Primers and PCR condition
The universal fungal primers, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (11) were used to amplify the entire ITS rDNA region in the standard strains. Amplification was carried out by a PCR mixture contained 2.5 μl of 10X reaction buffer, 200 μM of dNTPs mixture, 0.125 μl of Taq polymerase (5 U/μl), 30 pmol of each forward and reverse primers, 1 μl of DNA template solution and enough ultrapure water up to a final volume of 25 μl. Each reaction mixture was preheated to 94ºC for 6 minutes, then PCR performed by the following protocol: 35 cycles of 30 seconds at 94ºC, 30 seconds at 58ºC and 1 min at 72ºC; a final extension at 72ºC for 10 min and followed by cooling at 4ºC.
Restriction digestion of the PCR products
The amplified products were subjected to digestion with MvaI Fast digest (Fermentas Life Sciences, Lithuania) for 10 min at 37ºC. The reaction mixture contained 10 μl of PCR amplicons, 0.5 μl of the enzyme, 1.5 μl of 10X buffer and 3 μl of water to a final volume of 15 μl.
Detection of amplified products and restriction digestion
PCR amplicons were separated by running the 5 μl of products in a 1.5% (w/v) agarose gel incorporated with 2 μl ethidium bromide and electrophoresed in TBE (90mM Tris, 90 mM boric acid, 2 mM EDTA) at 100V for 60 min. A 10 μl aliquot of restriction digestion products were separated by running in a 2% agarose gel. A 100 base pair (bp) ladder was used as DNA molecular weight marker in each run. The gels were visualized using gel documentation system and recorded photographically, then were compared with the profiles obtained by in silico analysis.
Sequencing and multiple alignments
All reference strains which preliminarily identified by PCR-RFLP, were sequenced by both ITS1 and ITS4 primers using an automated DNA Sequencer (ABI PRISM™ ABI-3730 Genetic Analyzer, PE Applied Biosystem). For final identification, the obtained consences sequences were compared with the Dermatophytes ITS DNA barcode database (http://www.cbs.knaw.nl/dermatophytes/BioloMICSID.aspx). Alignment of the obtained edited forward and reverse sequences was conducted using BioEdit software: (http://www.mbio.ncsu.edu/bioedit/bioedit.html)
Results
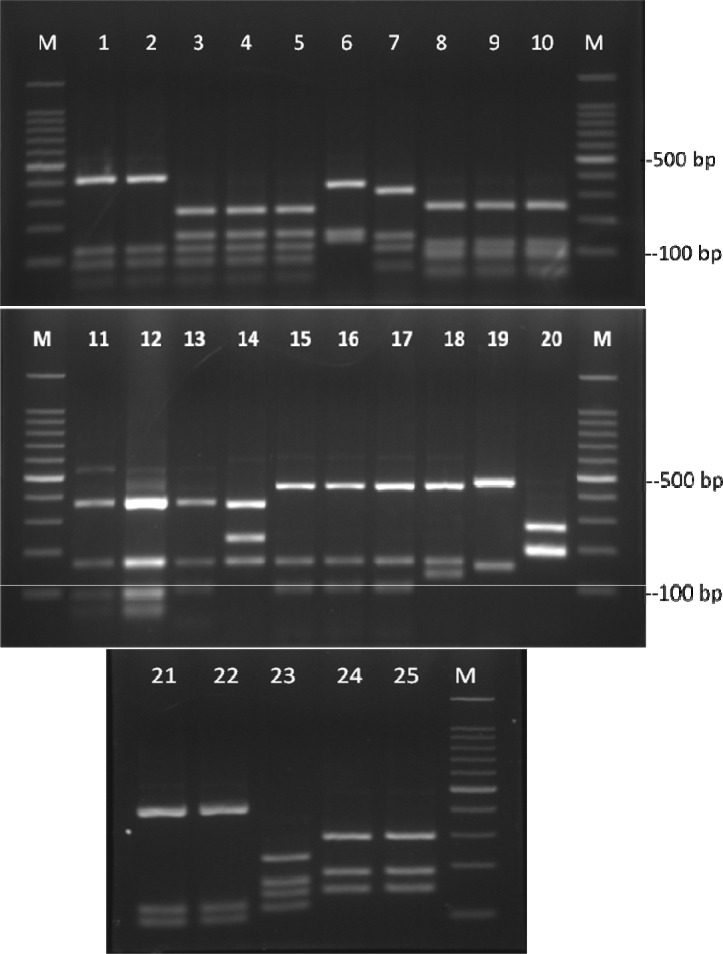
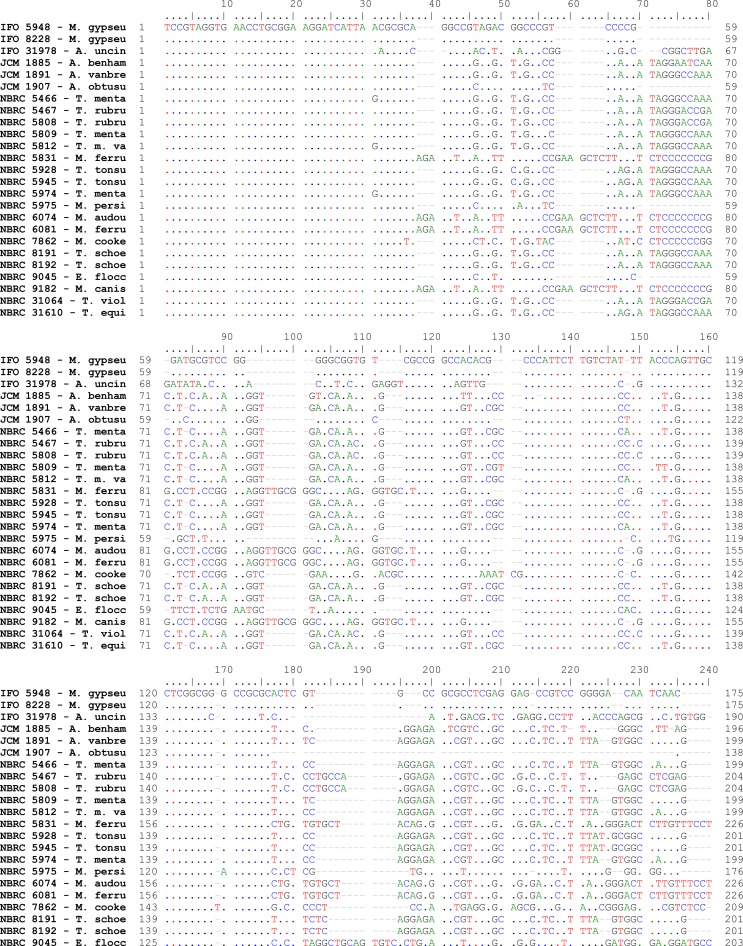
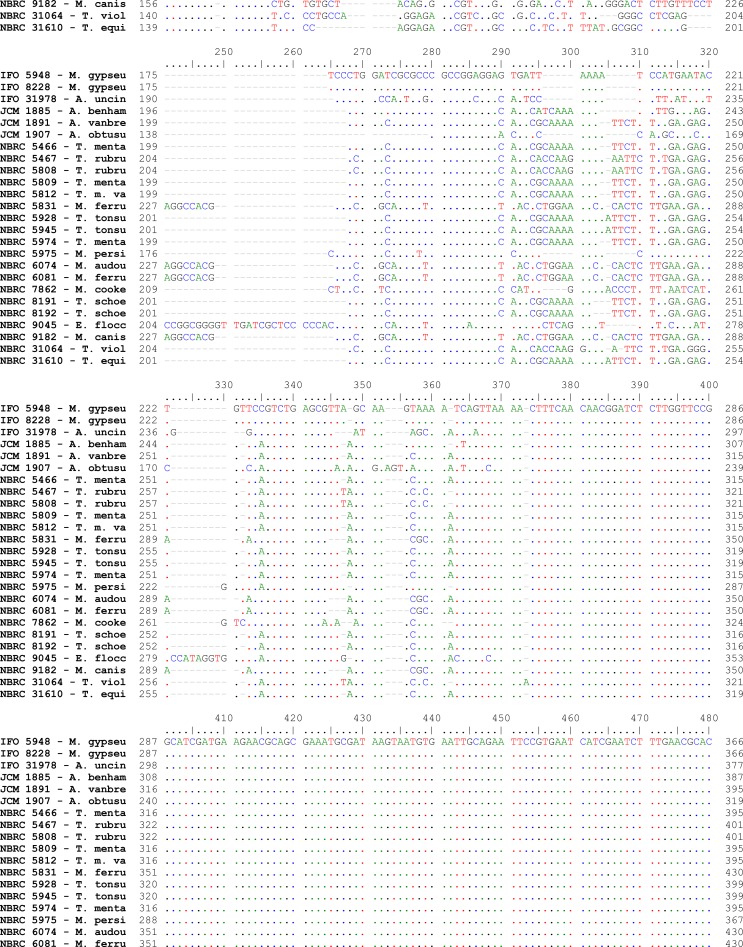
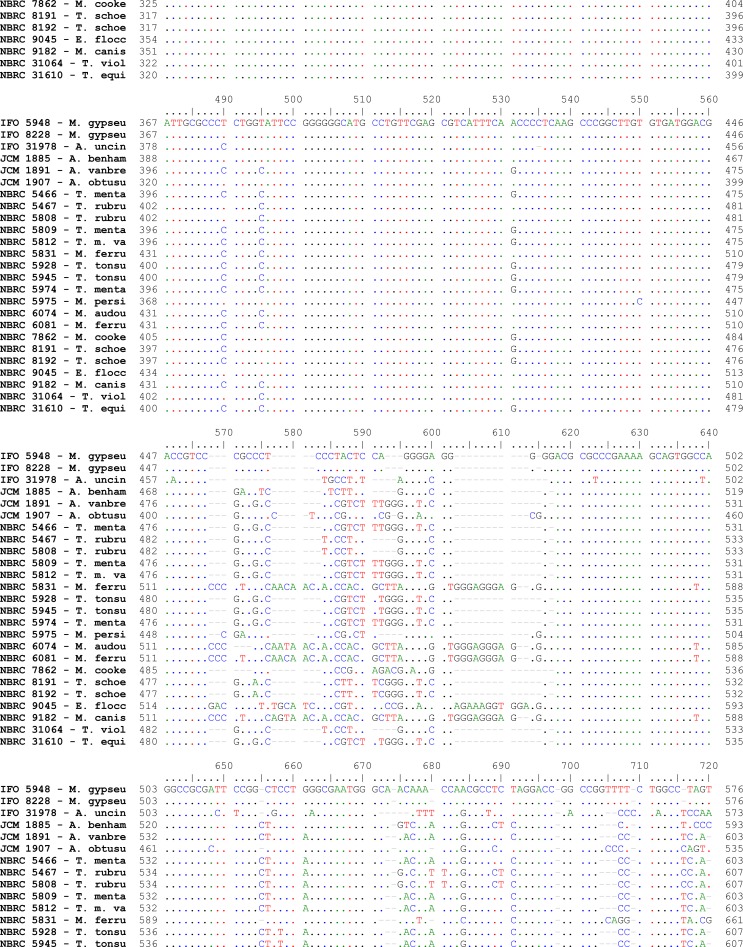
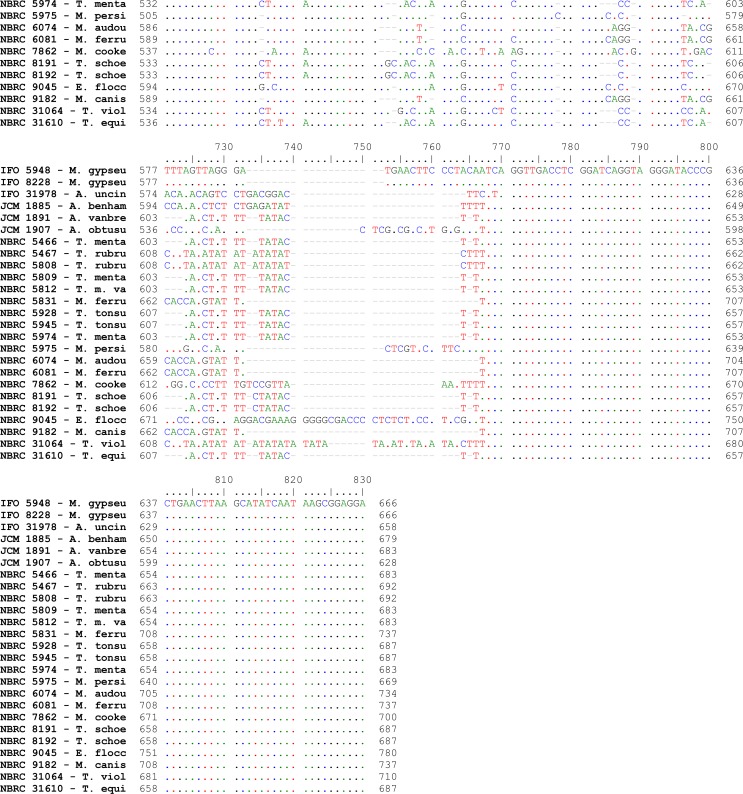
Alignments of consensus sequences by MEGA software showed that almost all dermatophyte species have expectedly similar sequence in 5.8S subunit but are different in ITS1 and ITS2 non-coding regions of rDNA complex. The size of entire ITS1-5.8S-ITS2 fragment (including primers) ranked between 614 bp for M. gallinae to 780 bp for E. floccosum (Table 1). The dissimilarities seemed to be enough to select the enzymes for distinguishing between the species in a PCR-RE system. In silico analysis of the sequences by DNASIS software revealed that many restriction enzymes can digest the intended sequences. Some enzymes had no cutting site in all or some species. Some others had many cutting sites; however, the sites were not sufficiently divergent between different species and could not meet our purpose (data not shown). Finally MvaI was considered as the enzyme with the most discriminatory power for differentiation of many species. Table 1 shows the cutting sites and produced fragments from ITS regions after digestion with MvaI. The ITS1 region was successfully amplified in all tested strains using the ITS1/ITS4 primers. The obtained bands were variable in size among different species as the biggest size for E. floccosum and the smallest one for A. obtusum (data not shown). In actual restriction digestion of the amplified products by MvaI all achieved electrophoretic patterns were congruent with those findings in in-silico analysis (Fig. 1). Some species including T. interdigitale, T. rubrum, T. violaceum, M. persicolor, M. audouinii, M. nanum (A. obtusum) and E. floccosum produced specific profile in both virtual and actual ITS-RFLP with MvaI (Table 1 and 2, Fig. 1), however some closely related species like T. equinum / T. tonsurans, M. canis / M. ferrugineum and M. cookei / M. racemosum had the same profiles. We did not use T. simii, T. verrucosum, M. fulvum and M. gallinae in our experiment, however, T. simii produced two patterns and three other species also produced unique pattern in computational PCR-RFLP (Table 1). In RFLP analysis, two different patterns were distinguished for M. gypseum and T. ajelloi (A. uncinatum) (Table 1); nonetheless, it was observed only one electrophoretic profile for each species (Table 2, Fig. 1; lanes 20, 24–25). The in silico MvaI-restriction profiles for T. gourvilii, T. soudanense and T. yaoundei resembled to the T. violaceum. Comparing the obtained sequences of all reference strains with the open access validated CBS-database confirmed their species identification by established RFLP system. The comparison of sequencing data and PCR-RFLP profiles for identification of the tested strains were outlined in Table 2. The overall alignment of ITS1-5.8S-ITS2 sequences for all 25 standard strains was illustrated in Figure 2. As it observed, the sequence of 5.8S subunit is relatively similar among all type strains species but both ITS1 and ITS2 regions have variable sequence between all species.
Table 1:
Fragment size of the ITS1-5.8S-ITS2 regions in tested species of dermatophytes before and after in silico digestion with MvaI
| Species of dermatophyte | GenBank accession no. for ITS1-5.8S-ITS2 regions | Size of the ITS1-5.8S-ITS2 (bp) | Size of fragments after digestion with MvaI |
|---|---|---|---|
| A. vanbreuseghemii, T. interdigitale | AF170465, AJ270790 | 683 | 247, 159, 124, 89, 50, 14 (1st pattern) |
| A. vanbreuseghemii (T. interdigitale) | AB246678 | 683 | 406, 124, 89, 50, 14 (2nd pattern) |
| A. benhamiae | Z98015 | 679 | 360, 158, 141, 20 |
| T. erinacei | Z97997 | 679 | 360, 157, 142, 20 |
| T. simii | Z98017 | 685 | 372, 159, 90, 50, 14 (1st pattern) |
| T. simii | AJ000605 | 685 | 372, 159, 104, 50 (2nd pattern) |
| T. mentagrophytes (T. m. var quinckeanum) | Z97995 | 683 | 406, 124, 103, 50 |
| T. schoenleinii | Z98011 | 685 | 405, 124, 104, 52 |
| T. verrucosum | Z98003 | 678 | 517, 141, 20 |
| M. persicolor | EU181457 | 670 | 323, 148, 112, 68, 19 |
| T. rubrum | AF170471 | 692 | 368, 164, 95, 65 |
| T. violaceum | AJ270796, EU590656 | 701, 711 | 369, 161(164), 106(114), 45, 20 |
| T. gourvilii | EU181448 | 710 | 368, 164, 113, 45, 20 |
| T. yaoundei | AJ270811, FJ479792 | 700, 710 | 368, 164, 103 (113), 45, 20 |
| T. soudanense | EF631621, AF170473 | 690 | 368, 164, 93, 45, 20 |
| T. tonsurans | EF043270 | 688 | 251, 124, 103, 90, 56, 50, 14 |
| T. equinum | EF043274 | 688 | 251, 124, 103, 90, 56, 50, 14 |
| E. floccosum | AY213646 | 780 | 361, 231, 169, 20 |
| M. canis | EU200371 | 737 | 441, 165, 103, 28 |
| M. ferrugineum | EF581133 | 737 | 441, 165, 103, 28 |
| M. audouinii | AJ000625 | 734 | 441, 162, 131 |
| M. nanum | AJ970149 | 628 | 459, 150, 19 |
| M. gallinae (M. vanbreuseghemii) | AJ000620 (AJ970147) | 614 (617) | 319 (323), 150 (149), 126, 19 |
| M. fulvum | AM850135 | 652 | 322, 147, 112, 52, 19 |
| A. uncinatum (T. ajelloi) | EF568086 | 658 | 271, 195, 192 (1st pattern) |
| A. uncinatum (T. ajelloi) | AJ000608 | 657 | 465, 192 (2nd pattern) |
| A. gypseum (M. gypseum) | EF568061 | 666 | 289,179, 146, 33, 19 (1st pattern) |
| A. incurvatum (M. gypseum) | AJ000621 | 619 | 389, 147, 64, 19 (2nd pattern) |
| M. racemosum | AJ970146 | 701 | 225, 165, 144, 120, 47 |
| M. cookei | AB193713 | 701 | 225, 166, 143, 120, 47 |
Fig. 1:
Electrophoretic patterns of ITS-RFLP with MvaI for reference dermatophyte species.
Lanes 1, 3 and 5: T. mentagrophytes (NBRC 5809, NBRC 5974 and NBRC 5466), lane 2: A. vanbreuseghemii (JCM 1891), lane 4: T. mentagrophytes var. interdigitale (NBRC 5812), lane 6: A. benhamiae (JCM 1885), lane 7: M. persicolor (NBRC 5975), lane 8–9: T. tonsurans (NBRC 5928, NBRC 5945), lane 10: T. equinum (NBRC 31610), lane 11–12: T. rubrum (NBRC 5808, NBRC 5467), lane 13: T. violaceum (NBRC 31064), lane 14: E. floccosum (NBRC 9045), lane 15: M. canis (NBRC 9182), lane 16 and 17: M. ferrugineum (NBRC 6081, NBRC 5831), lane 18: M. audouinii (NBRC 6074), lane 19: A. obtusum (JCM 1907), lane 20: A. uncinatum (NBRC 31978), lane 21 and 22: T. schoenleinii (NBRC 8192, NBRC 8191), lane 23: M. cookei (NBRC 7862), lane 24 and 25: M. gypseum (NBRC 8228, NBRC 5948), lanes M: 100 bp Ladder
Table 2:
Results of ITS-RFLP with MvaI and Sequencing for identification of reference dermatophyte strains
| Strain (upon receipt) | Size of the ITS amplicon (bp)1 | ITS-RFLP profile after digestion with MvaI (bp)1 | Species identification by ITS-RFLP profile | Species identification by ITS sequencing |
|---|---|---|---|---|
| T. mentagrophytes2 (NBRC 5974, NBRC 5466) | 683 | 247, 159, 124, 89, 50, 14 | T. interdigitale3 | T. interdigitale3 |
| T. mentagrophytes var. Interdigitale2 (NBRC 5812) | 683 | 247, 159, 124, 89, 50, 14 | T. interdigitale3 | T. interdigitale3 |
| T. mentagrophytes2 (NBRC 5809) | 683 | 406, 124, 89, 50,14 | T. interdigitale3 | T. interdigitale3 |
| A. vanbreuseghemii (JCM 1891) | 683 | 406, 124, 89, 50, 14 | T. interdigitale | T. interdigitale |
| A. benhamiae (JCM 1885) | 679 | 360, 158, 142, 20 | A. benhamiae | A. benhamiae |
| T. schoenleinii (NBRC 8192, NBRC 8191) | 687 | 407, 124, 104, 52 | T. schoenleinii & T. mentagrophytes (T. m. var quinckeanum) | T. schoenleinii |
| T. rubrum (NBRC 5808, NBRC 5467) | 692 | 368, 164, 95, 65 | T. rubrum | T. rubrum |
| T. violaceum (NBRC 31064) | 710 | 368, 164, 114, 45, 20 | T. violaceum | T. violaceum |
| E. floccosum (NBRC 9045) | 780 | 361, 231, 168, 20 | E. floccosum | E. floccosum |
| T. tonsurans (NBRC 5928, NBRC 5945) | 686 | 251, 124, 103, 89, 56, 50, 14 | T. tonsurans & T. equinum | T. tonsurans |
| T. equinum (NBRC 31610) | 686 | 251, 124, 103, 89, 56, 50, 14 | T. tonsurans & T. equinum | T. equinum |
| M. canis (NBRC 9182) | 737 | 441, 165, 103, 28 | M. canis & M. ferrugineum | M. canis |
| M. ferrugineum (NBRC 6081, NBRC 5831) | 737 | 441, 165, 103, 28 | M. ferrugineum & M. canis | M. ferrugineum |
| M. audouinii (NBRC 6074) | 734 | 441, 162, 131 | M. audouinii | M. audouinii |
| A. obtusum (JCM 1907) | 628 | 459, 150, 19 | A. obtusum (M. nanum) | A. obtusum (M. nanum) |
| M. persicolor (NBRC 5975) | 669 | 323, 147, 112, 68, 19 | M. persicolor | M. persicolor |
| M. cookei (NBRC 7862) | 700 | 225, 165, 143, 120, 47 | M. cookei & M. racemosum | M. cookei |
| M. gypseum (NBRC 8228, NBRC 5948) | 666 | 289, 179, 146, 33, 19 | M. gypseum | A. gypseum (M. gypseum) |
| A. uncinatum (NBRC 31978) | 658 | 270, 195, 193 | A. uncinatum (T. ajelloi) | A. uncinatum (T. ajelloi) |
The exact size of the amplicons and restriction fragments was respectively determined after sequencing and in-silico RFLP of obtained sequences.
The former name of the species (upon receipt from collection)
The current name for the species
Fig. 2:
Pair wise alignment of ITS1-5.8S-ITS2 sequence in reference strains of drmatophytes used in the study
Discussion
Identification of dermatophytes at the species level is essential because of the therapeutic and epidemiological importance. Identification process for this closely related group of fungi classically is based on phenotypic and physiological criteria (1, 10, 13). Therefore, due to the high degree of phenotypic similarity between these relative species identification problems are unavoidable. Furthermore, traditional methods are time-consuming, laborious and many isolates reveal unusual characteristics (2, 10). To overcome these limitations, recently PCR-based appliances relying on genetic makeup have been developed. At present, sequencing of ITS rDNA region is the golden standard for delineation of dermatophyte species (13–18). In this study we presented a virtual and practical PCR-RFLP assay, targeting the ITS-rDNA complex, for identification/differentiation of common pathogenic dermatophyte species. For the first time Jackson et al. (11) introduced a PCR-RFLP assay targeting the ITS regions for identification of 17 dermatophyte species and after that this method was used by some researchers (8, 10, 19). However, our study is the first quest that completely was performed based on sequence analysis and outlined the details of RFLP pattern representative for nearly all pathogenic dermatophytes by both computational and experimental digestion of the ITS regions. Likewise, our PCR-RE findings were compatible with the latest suggested changes in the classification of dermatophytes (13). For instance, based on the ITS sequence phylogeny, recently four new species have been created in the species formerly known as Trichophyton mentagrophytes complex (13, 20): the zoophilic T. mentagrophytes sensu stricto that previously was known as T. mentagrophytes var. quinckeanum (its teleomorph is related to A. simii), the zoophilic and anthropophilic T. interdigitale sensu stricto (related to A. vanbreuseghemii teleomorph), the zoophilic T. erinacei (related to A. benhamiae) and the zoophilic T. anamorph of A. benhamiae. All of these new species had ITS restriction profiles related to their teleomorphs in in silico ITS-RFLP analysis (Table 1). We found two ITS-RFLP profiles by MvaI, specific for T. interdigitale (A. vanbreuseghemii), in both in silico and experimental practice (Tables 1 and 2, Fig. 1; lanes 1–5) while, Jackson et al. described only one profile for this species. Although, we did not use any standard strain of T. mentagrophytes sensu stricto, however like the study of Jackson et al. the in silico restriction profile obtained for this species was the same as T. schoenleinii. T. erinacei and T. anamorph of A. benhamiae, both related to A. benhamiae teleomorphic stage, had similar restriction pattern in virtual and practical restriction analysis (Table 1 and 2). The only type strain of A. benhamiae (JCM 1885) produced expected restriction pattern in RFLP (Table 2 and Fig. 1). As mentioned in the results, T. interdigitale, T. rubrum, T. violaceum, M. persicolor, M. audouinii, M. nanum (A. obtusum) and E. floccosum were distinctively identifiable in both virtual and actual digestion of the ITS regions with MvaI (Table 1 and 2, Fig. 1). The ITS restriction profiles for T. gourvilii, T. soudanense and T. yaoundei were similar to those of T. violaceum (Table 1) and this is congruent with the recent conclusion of Graser et al. (21) that reduced T. gourvilii, T. soudanense and T. yaoundei as synonyms for T. violaceum based on the ITS sequence, PCR fingerprinting, and AFLP analysis. Comparison with previous study (11) that found no cutting site for MvaI in ITS-RFLP of M. audouinii, our exploration showed not only this enzyme has cutting site for this Microsporum species (Table 1) but also the obtained restriction profile is characteristic (Table 2, Fig. 1; lane 18). Jackson et al. (11) did not included the species of M. ferrugineum, M. nanum, M. fulvum, M. gallinae, T. ajelloi, M. racemosum, M. cookei and T. simii in their study, while we computationally depicted the exact ITS-RFLP diagram by MvaI for the mentioned species (Table 1) as well as the actual profiles at least for M. ferrugineum, M. nanum, T. ajelloi and M. cookei in electrophoretic assessment (Table 2, Fig. 1). We observed no pattern differences in the in silico RFLP between T. equinum / T. tonsurans, M. canis / M. ferrugineum and M. cookei / M. racemosum species (Table 1). We also observed such a result in experimental ITS-RFLP of T. equinum / T. tonsurans and M. canis / M. ferrugineum reference strains (Table 2 and Figure 1). T. equinum and T. tonsurans are two closely related Trichophyton species that differ just in a single base pair on ITS1 region (15, 22, 23) and in the study of Jackson and Mochizuki et al. (11, 19) also both species had the same RFLP pattern, however these species ecologically are different because T. tonsurans is an anthropophilic species that isolated only from human infections while T. equinum is a zoophilic (horse associated) Trichophyton that rarely causes infection in human and almost all infections acquired by direct contact with a horse or its fomites (24). M. ferrugineum and M. canis, two members of the A. otae cmplex, vary entirely in two base pair in ITS2 region (13) that is not the cutting site for MvaI therefore differentiation among them may need to more restriction analysis. At present study we included a type strain of M. cookei but not any of M. racemosum and the electrophoretic restriction profile that we attained for the cookei species was as the same as in silico estimated one (Table 2, Fig. 1; lane 23). M. cookei and M. racemosum are two geophilic species that have more than 97% similarity in ITS regions sequence (25). Our sequence analysis indicated that two species differed in 6 bp of entire ITS sequence (data not shown) and these differences were not placed in MvaI cutting sites. That's why both species had the same restriction profile, however as two species rarely isolated from human infections this similarity is insignificant. All of our practical PCR-RE findings were confirmed by sequencing and comparison of the obtained sequences by the Dermatophytes ITS DNA barcode database. As there are many wrong sequences in GenBank, the reliable identification of dermatophytes cannot be performed by BLAST analysis (22). Contrariwise, there are many reliable ITS sequences from all species of dermatophyte in CBS-database (www.cbs.knaw.nl/dermatophytes) that some belong to the CBS collection strains while the remaining sequences have been selected from GenBank in view of covering the extant biodiversity in this database. We even compared the sequences used in virtual analysis with this database and retrieved the reliable sequences for our study. Alignment of the obtained sequences for standard stains used in this project (Fig. 2) plus the reliable GenBank sequences confirmed this fact that almost all dermatophyte species have different ITS1 and ITS2 sequences, make the ITS region as good targets for post PCR maneuvers such our PCR-RFLP assay.
In conclusion, it should be emphasized even though using of MvaI for differentiation of dermatophytes species in a PCR-RFLP system was not new, however the data of such PCR-RE schema in this study had novelty about many species. Despite the similarity of restriction profile obtained for some closely related species, ITS-RFLP by MvaI is a powerful tool for both identification and preliminary screening of many dermatophyte species, especially in large scale epidemiological studies.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
This work was financially supported by both Tehran University of Medical Sciences (TUMS) Tehran, Iran, project No. 87-01-27-6962; and Teikyo University Institute of Medical Mycology (TIMM), Tokyo, Japan. We thank all personnel in Molecular Biology laboratory in TIMM and in Medical Mycology laboratory in TUMS. The authors declare that there is no conflict of interests.
References
- 1.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8(2):240–59. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Baere T, Summerbell R, Theelen B, Boekhout T, Vaneechoutte M. Evaluation of internal transcribed spacer 2-RFLP analysis for the identification of dermatophytes. J Med Microbiol. 2010;59:48–54. doi: 10.1099/jmm.0.013870-0. [DOI] [PubMed] [Google Scholar]
- 3.Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;67(1):101–8. [PubMed] [Google Scholar]
- 4.Ninet B, Jan I, Bontems O, et al. Identification of dermatophyte species by 28S ribosomal DNA sequencing with a commercial kit. J Clin Microbiol. 2003;41(2):826–30. doi: 10.1128/JCM.41.2.826-830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anosike JC, Keke IR, Uwaezuoke JC, Anozie JC, Obiukwu CE, Nwoke BEB, Amajuoyi OU. Prevalence and distribution of ringworm infection in primary school children in parts of Eastern, Nigeria. J Appl Sci Environ Manage. 2005;9(3):21–25. [Google Scholar]
- 6.Panasiti V, Devirgiliis V, Borroni RG, Mancini M, Curzio M, Rossi M, Bottoni U, Calvieri S. Epidemiology of dermatophytic infections in Rome, Italy: a retrospective study from 2002 to 2004. Med Mycol. 2007;45(1):57–60. doi: 10.1080/13693780601028683. [DOI] [PubMed] [Google Scholar]
- 7.Aghamirian MR, Ghiasian SA. Dermatophytoses in outpatients attending the Dermatology Center of Avicenna Hospital in Qazvin, Iran. Mycoses. 2007;51(2):155–60. doi: 10.1111/j.1439-0507.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- 8.Shin JH, Sung JH, Park SJ, Kim JA, Lee JH, Lee DY, Lee ES, Yang JM. Species identification and strain differentiation of dermatophyte fungi using polymerase chain reaction amplification and restriction enzyme analysis. J Am Acad Dermatol. 2003;48(6):857–865. doi: 10.1067/mjd.2003.491. [DOI] [PubMed] [Google Scholar]
- 9.Kanbe T. Molecular Approaches in the Diagnosis of Dermatophytosis. Myc-opathologia. 2008;166:307–317. doi: 10.1007/s11046-008-9107-2. [DOI] [PubMed] [Google Scholar]
- 10.Shehata AS, Mukherjee PK, Aboulatta HN, el-Akhras AI, Abbadi SH, Ghannoum MA. Single-step PCR using (GACA)4 primer: utility for rapid identification of dermatophyte species and strains. J Clin Microbiol. 2008;46(8):2641–5. doi: 10.1128/JCM.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson CJ, Barton RC, Evans EG. Species Identification and Strain Differentiation of Dermatophyte Fungi by Analysis of Ribosomal-DNA Intergenic Spacer Regions. J Clin Microbiol. 1999;37(4):931–936. doi: 10.1128/jcm.37.4.931-936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makimura K, Tamura Y, Mochizuki T, et al. Phylogenetic Classification and Species Identification of Dermatophyte Strains Based on DNA Sequences of Nuclear Ribosomal Internal Transcribed Spacer 1 Regions. J Clin Microbiol. 1999;37(4):920–924. doi: 10.1128/jcm.37.4.920-924.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graser Y, Scott J, Summerbell R. The New Species Concept in Dermatophyts_a Polphasic Approach. Mycopathologia. 2008;166:239–256. doi: 10.1007/s11046-008-9099-y. [DOI] [PubMed] [Google Scholar]
- 14.Rezaei-Matehkolaei A, Makimura K, de Hoog GS, Shidfar MR, Satoh K, Najafzadeh MJ, Mirhendi H. Multilocus differentiation of the relative dermatophytes Microsporum canis, M. ferrugineum and M. audouinii. J Med Microbiol. 2012;61:57–63. doi: 10.1099/jmm.0.036541-0. [DOI] [PubMed] [Google Scholar]
- 15.Rezaei-Matehkolaei A, Makimura K, De Hoog GS, Shidfar MR, Satoh K, Najafzadeh MJ, Mirhendi H. Discrimination of Trichophyton tonsurans and Trichophyton equinum by PCR-RFLP and by β-tubulin and Translation Elongation Factor 1-α sequencing. Med Mycol. 2012 doi: 10.3109/13693786.2012.661885. Early Online: 1–5. [DOI] [PubMed] [Google Scholar]
- 16.Bergmans AM, van der Ent M, Klaassen A, Böhm N, Andriesse GI, Wintermans RG. Evaluation of a single-tube real-time PCR for detection and identification of 11 dermatophyte species in clinical material. Clin Microbiol Infect. 2010;16(6):704–10. doi: 10.1111/j.1469-0691.2009.02991.x. [DOI] [PubMed] [Google Scholar]
- 17.Li HC, Bouchara JP, Hsu MM, Barton R, Su S, Chang TC. Identification of dermatophytes by sequence analysis of the rRNA gene internal transcribed spacer regions. J Med Microbiol. 2008;57:592–600. doi: 10.1099/jmm.0.47607-0. [DOI] [PubMed] [Google Scholar]
- 18.Nenoff P, Hermann J, Graser Y. Trichophyton mentagrophytes sive interdigitale? A dermatophyte in the course of time. J Dtsch Dermatol Ges. 2007;5:198–202. doi: 10.1111/j.1610-0387.2007.06180.x. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki T, Tanabe H, Kawasaki M, Ishizaki H, Jackson CJ. Rapid identification of Trichophyton tonsurans by PCR-RFLP analysis of ribosomal DNA regions. J Dermatol Sci. 2003;32(1):25–32. doi: 10.1016/s0923-1811(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 20.Heidemann S, Monod M, Gräser Y. Signature polymorphisms in the internal transcribed spacer region relevant for the differentiation of zoophilic and anthropophilic strains of Trichophyton interdigitale and other species of T. mentagrophytes sensu lato. Br J Dermatol. 2010;162(2):282–95. doi: 10.1111/j.1365-2133.2009.09494.x. [DOI] [PubMed] [Google Scholar]
- 21.Graser Y, Kuijpers AF, Presber W, de Hoog GS. Molecular taxonomy of the Trichophyton rubrum complex. J Clin Microbiol. 2000;38(9):3329–36. doi: 10.1128/jcm.38.9.3329-3336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summerbell RC, Moore MK, Starink-Willemse M, Van Iperen A. ITS barcodes for Trichophyton tonsurans and T. equinum. Med Mycol. 2007;45(3):193–200. doi: 10.1080/13693780601087614. [DOI] [PubMed] [Google Scholar]
- 23.Kong F, Tong Z, Chen X, et al. Rapid identification and differentiation of Trichophyton species, based on sequence polymorphisms of the ribosomal internal transcribed spacer regions, by rolling-circle amplification. J Clin Microbiol. 2008;46(4):1192–9. doi: 10.1128/JCM.02235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasch J, Logering B, Graser Y. Tinea capitis caused by Trichophyton equinum. Acta Derm Venereol. 2009;89(2):204–5. doi: 10.2340/00015555-0596. [DOI] [PubMed] [Google Scholar]
- 25.Sharma R, Rajak RC, Pandey AK, Graser Y. Internal Transcribed Spacer (ITS) of rDNA of appendaged and non-appendaged strains of Microsporum gypseum reveals Microsporum appendiculatum as its synonym. Antonie Van Leeuwenhoek. 2006;89(1):197–202. doi: 10.1007/s10482-005-9018-x. [DOI] [PubMed] [Google Scholar]