Abstract
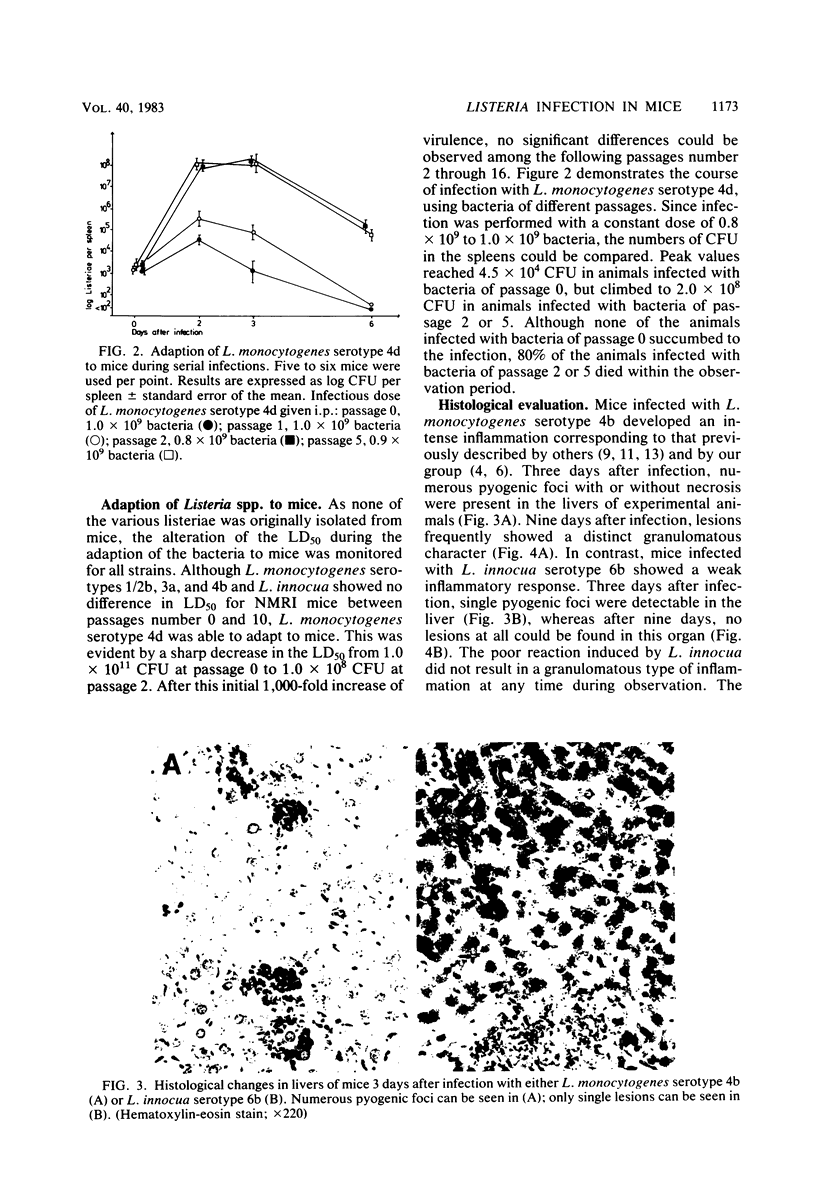
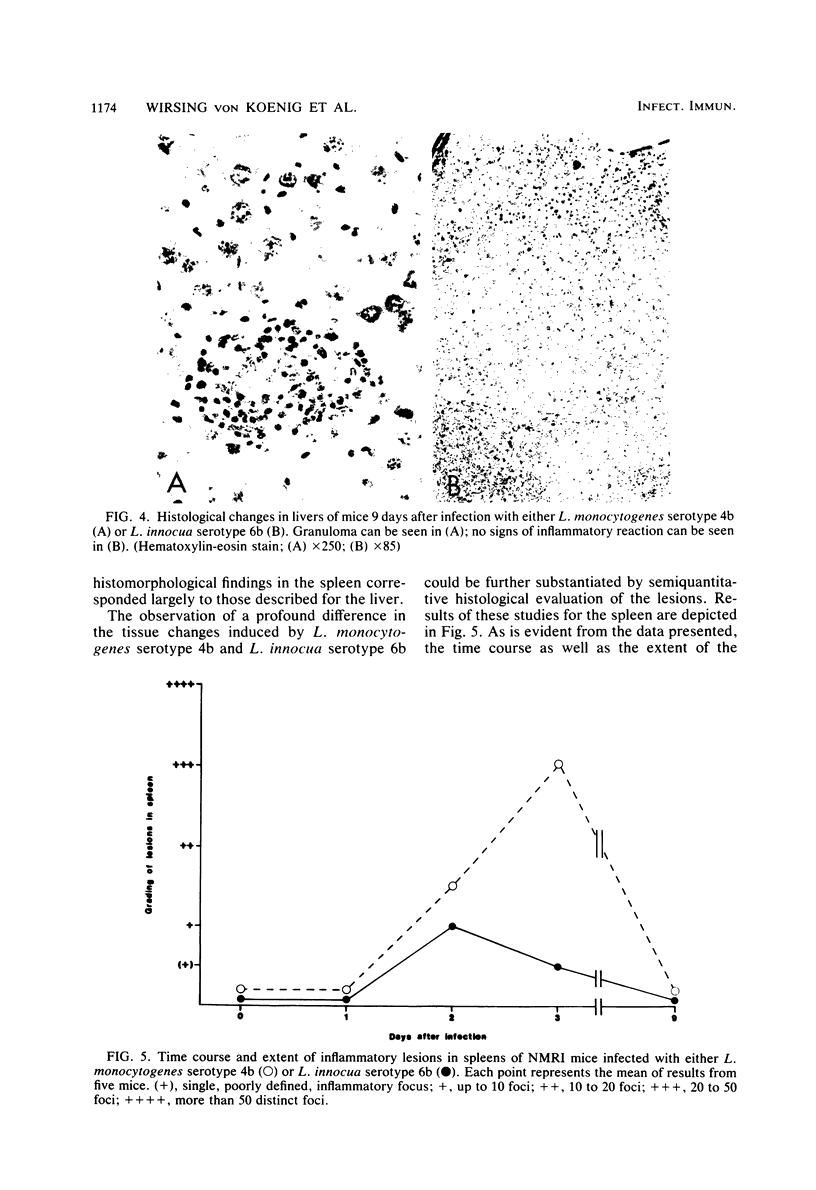
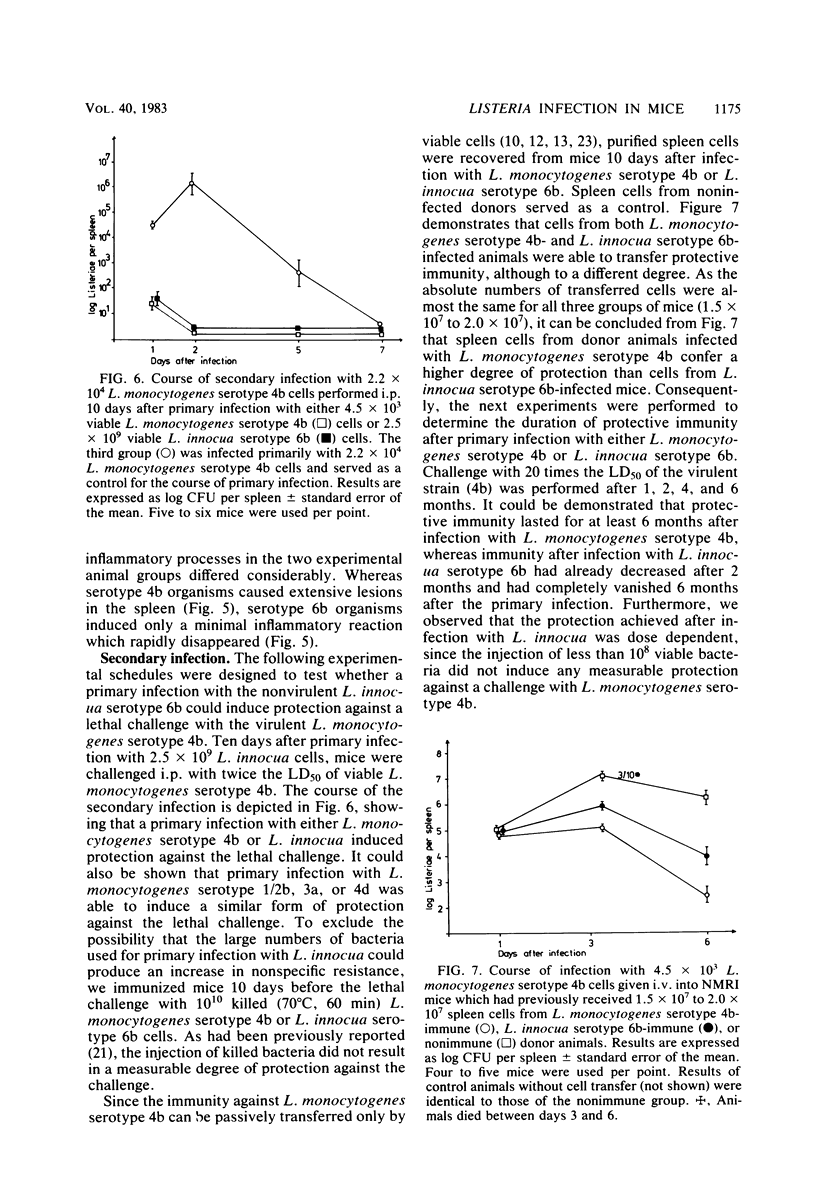
NMRI mice were experimentally infected with Listeria monocytogenes serotypes 1/2b, 3a, 4b, and 4d and Listeria innocua serotype 6b by different means. The course of infection was monitored, using bacteriological and histological methods. The following typical features of experimental infection with the various L. monocytogenes and L. innocua serotypes were observed. (i) On the basis of the mean lethal dose, L. monocytogenes 4b, 4d, and 1/2b proved to be mouse pathogenic, although to different degrees, L. monocytogenes 3a and L. innocua can be regarded as nonpathogenic for NMRI mice. The virulence of L. monocytogenes serotype 4d was increased 1,000-fold after adaptation to mice. (ii) Primary infection with any serotype of L. monocytogenes or L. innocua resulted in protection against a lethal challenge with the most virulent serotype, 4b. This protective immunity could be transferred by spleen cells. Compared with the duration of immunity achieved by infection with L. monocytogenes serotype 4b, the protection induced by infection with L. innocua was short lived and dose dependent. The data obtained also suggest that immunity after experimental infection with any serotype of L. monocytogenes or L. innocua is produced only when the animal host is filled with bacteria. (iii) The distribution of the germs in the internal organs of the mouse shortly after infection was dependent on the route of infection rather than on the serotype used. (iv) The main difference among the Listeria serotypes tested was their ability to multiply within the host and to induce a granulomatous inflammation. The results indicate that mouse pathogenicity and virulence of Listeria spp. cannot be defined only by the capacity of the bacteria to infect or kill conventional mice. Such a definition should include an analysis of the immune system of the host, a kinetic study of experimental infection, and a histomorphological evaluation of the lesions induced.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audurier A., Pardon P., Marly J., Lantier F. Experimental infection of mice with Listeria monocytogenes and L. innocua. Ann Microbiol (Paris) 1980 Jul-Aug;131B(1):47–57. [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Hof H., Finger H. Age-related defense against infection with intracellular pathogens. Gerontology. 1979;25(6):327–336. doi: 10.1159/000212361. [DOI] [PubMed] [Google Scholar]
- Finger H., Heymer B., Wirsing C. H., Emmerling P., Hof H. Reversion of dextran sulfate-induced loss of antibacterial resistance by Bordetella pertussis. Infect Immun. 1978 Mar;19(3):950–960. doi: 10.1128/iai.19.3.950-960.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymer B., Hobik H. P., Schäfer H., Bültmann B., Spanel R., Haferkamp O. Animal experimental studies on chronic granulomatous inflammation and T-lymphocyte-system. Beitr Pathol. 1975 Nov;156(2):128–144. doi: 10.1016/s0005-8165(75)80146-6. [DOI] [PubMed] [Google Scholar]
- Heymer B., Hof H., Emmerling P., Finger H. Morphology and time course of experimental listeriosis in nude mice. Infect Immun. 1976 Sep;14(3):832–835. doi: 10.1128/iai.14.3.832-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns R. J., Hinrichs D. J. Kinetics and maintenance of acquired resistance in mice to Listeria monocytogenes. Infect Immun. 1977 Jun;16(3):923–927. doi: 10.1128/iai.16.3.923-927.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Seaman A., Woodbine M. Proceedings: The pathogenicity of Listeria monocytogenes. Acta Microbiol Acad Sci Hung. 1972;19(4):421–426. [PubMed] [Google Scholar]
- Lannigan R., Skamene E., Kongshavn P. A., Duguid W. P. Morphology of liver lesions in experimental listeriosis is splenectomized mice. J Reticuloendothel Soc. 1979 May;25(5):457–461. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patocka F., Mencíková E., Seeliger H. P., Jirásek A. Neurotropic activity of a strain of Listeria innocua in suckling mice. Zentralbl Bakteriol Orig A. 1979 Jun;243(4):490–498. [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Weis J., Seeliger H. P. Incidence of Listeria monocytogenes in nature. Appl Microbiol. 1975 Jul;30(1):29–32. doi: 10.1128/am.30.1.29-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsing von König C. H., Finger H., Hof H., Emmerling P. Postnatal development of resistance against infection in an experimental model. Zentralbl Bakteriol Orig A. 1978 Dec;242(4):547–554. [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]
- von Koenig C. H., Finger H., Hof H. Failure of killed Listeria monocytogenes vaccine to produce protective immunity. Nature. 1982 May 20;297(5863):233–234. doi: 10.1038/297233a0. [DOI] [PubMed] [Google Scholar]





