Abstract
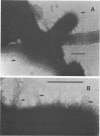
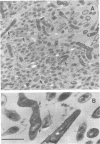
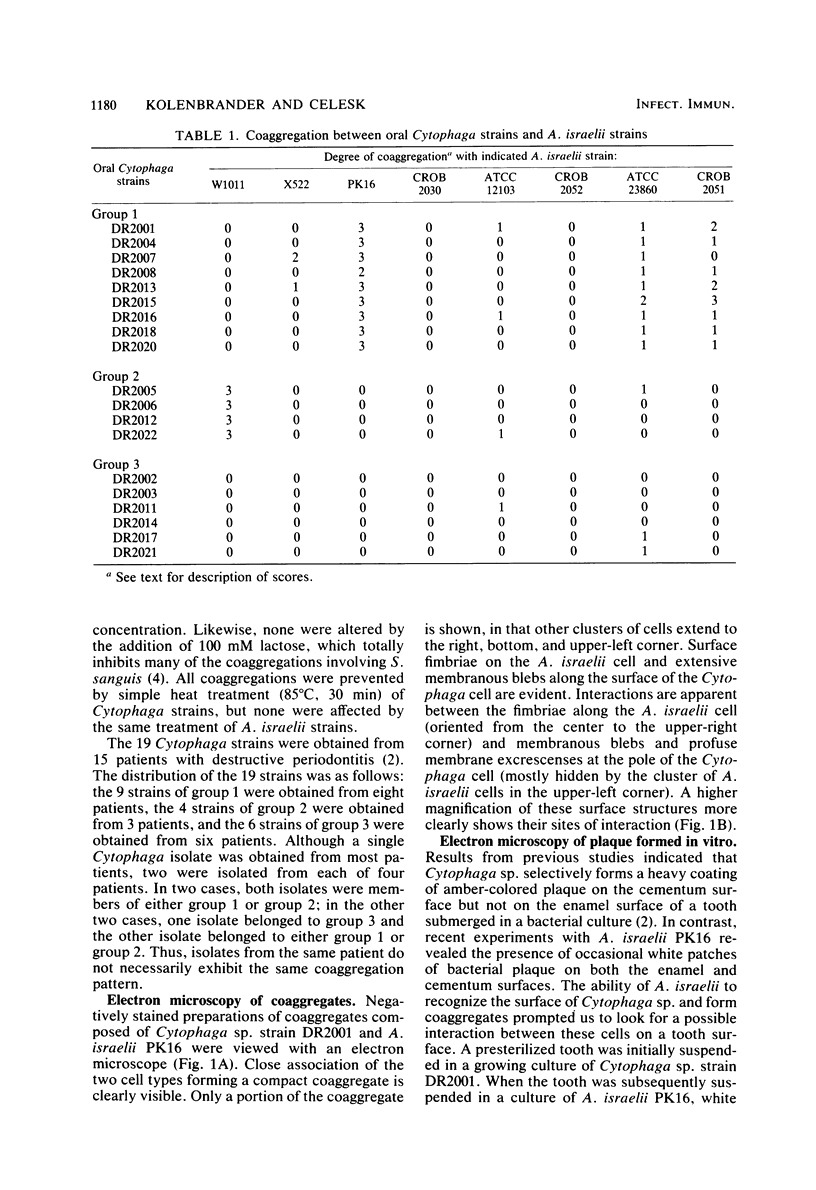
A total of 19 strains of oral Cytophaga sp. obtained from subgingival plaque deposits were tested for their ability to coaggregate with strains of Actinomyces israelii, A. viscosus, A. naeslundii, Streptococcus sanguis, S. mutans, S. salivarius, and S. mitis. Coaggregation was observed only with A. israelii. Based on their coaggregation patterns with eight A. israelii strains, the Cytophaga strains were distributed among three distinct groups: those that coaggregated with A. israelii PK16 but not with A. israelii W1011 (ATCC 29322), those that coaggregated with A. israelii ATCC 29322 but not with A. israelii PK16, and those that coaggregated with none of the eight A. israelii strains. In each of the coaggregations, prior heat treatment (85 degrees C, 30 min) of the Cytophaga cells prevented coaggregation, whereas identical treatment of the A. israelii cells had no effect. The ability of A. israelii PK16 to form adherent plaque on a tooth surface previously coated with Cytophaga plaque was tested with one of the coaggregating Cytophaga strains. White patches of A. israelii plaque were found covering both the amber-colored Cytophaga plaque on the cementum surface as well as the enamel surface to which Cytophaga strains do not adhere. Electron micrographs of thin-sectioned mixed-plaque material revealed both cell types in close proximity. In addition, electron micrographs of negatively stained coaggregated cells showed interbacterial adherence between surface fimbrae on A. israelii and outer membrane blebs on the gram-negative Cytophaga sp. The kinetics of binding of A. israelii to spheroidal hydroxyapatite and to root powder were indicative of a high-affinity binding system with comparatively large numbers of available binding sites on both substrata. These results indicate the highly specific nature of Cytophaga sp.--A. israelii recognition. The contribution of such recognition toward the mechanisms that are responsible for the indigenous nature of these oral bacteria is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celesk R. A., London J. Attachment of oral Cytophaga species to hydroxyapatite-containing surfaces. Infect Immun. 1980 Aug;29(2):768–777. doi: 10.1128/iai.29.2.768-777.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesk R. A., McCabe R. M., London J. Colonization of the cementum surface of teeth by oral Gram-negative bacteria. Infect Immun. 1979 Oct;26(1):15–18. doi: 10.1128/iai.26.1.15-18.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Webb E. L., Wheeler T. T., Fischlschweiger W., Birdsell D. C., Mansheim B. J. Role of surface fimbriae (fibrils) in the adsorption of Actinomyces species to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Sep;33(3):908–917. doi: 10.1128/iai.33.3.908-917.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter E. R., Holt S. C., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. I. General characteristics, taxonomic considerations and significance. Arch Microbiol. 1979 Jul;122(1):9–16. doi: 10.1007/BF00408040. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Holt S. C., Leadbetter E. R., Tanner A. C., Savitt E., Hammond B. F. Capnocytophaga: new genus of gram-negative gliding bacteria. III. Physiological characterization. Arch Microbiol. 1979 Jul;122(1):29–33. doi: 10.1007/BF00408042. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]