Abstract
Background:
Mutations in β-globin gene may result in β-thalassemia major, which is one of the most common genetic disorders in Iran and some other countries. Knowing the beta-globin mutation spectrum improves the efficiency of prenatal diagnosis in the affected fetuses (major β-thalassemia) of heterozygote couples.
Methods:
Couples with high hemoglobin A2 and low mean corpuscular volume were studied as suspicious of β-thalassemia carriers in Genetic Laboratory of Afzalipour Hospital, Kerman, Iran. We used amplification refractory mutation system, reverse hybridization, and DNA sequencing to determine the spectrum of β-globin gene mutation in the people who involved with β-thalassemia minor in this province.
Results:
Among the 266 subjects, 17 different types of mutation in β-globin gene were identified. Three of the mutations account for 77.1% of the studied cases. IVSI-5(G> C) was the most frequent mutation (66.2%) followed by IVSII-I (G> A) (6%) and Fr 8–9 (+G) (4.9%). The less frequent mutations include: IVSI-6(T> C), codon 15 (G>A), codon 44 (-C), codon 39 (C>T), codon 8 (-AA), codon30 (G> C), IVSI-110 (G > A), codon 36–37 (-T), 619bp deletion, codon 5 (-CT), IVSI-25bp del, codon 41–42(-TTCT), IVSI-I (G> A), and βnt30 (T>A) were accounted for 19.5%. Unknown alleles comprised 3.4% of the mutations.
Conclusion:
However, the frequencies of different mutations reported here are significantly different from those found in other part of the world and even other Iranian provinces. Reporting a number of these mutations in the neighboring countries such as Pakistan can be explained by gene flow phenomenon.
Keywords: β-globin gene, Mutations, β-thalassemia, Iran
Introduction
Mutations in β-globin gene may result in β-thalassemia major, which is one of the most common genetic disorders in Iran and some other countries. This disease causes in moderate to severe anemia; depend on the genetic defects underlying the disease, whether both β-alleles carry a mutation, or potential interactions with genetic modifiers.
Beta-thalassemia inherits typically as autosomal recessives and its alleles are frequent in regions where malaria is endemic. Inheritance of a single allele results in beta-thalassemia minor (carriers), while two β-globin mutations, one from each parent, cause clinical severity of the β-thalassemia major or Cooley’s anemia. The severe form leads to dependency on blood transfusions for long-life along with iron chelating therapy to combat iron overload. Expensive treatment imposes a significant burden on the available resources for health care and the disease may have dramatically psychological effects on families. However, β-thalassemia major can lead to decreased life expectancy, if left untreated. Therefore, β-thalassemia mutations detection, genetic counseling, and prenatal diagnosis programs play an important role in the prevention of the disease.
Beta-globin gene mutations show heterogeneity at molecular level and usually have a geographic and ethnic distribution in the world. About more than 700 hemoglobin variants (1) and 200 beta-thalassemia alleles have been recognized, but population studies indicate that 40 mutations account for 90% or more of the beta-thalassemia worldwide (2). An updated list can be accessed on the internet at http://globin.cse.psu.edu/.
More than 90% of β-thalassemia mutations in the Middle East region are IVSI-5 (G> C), IVSI-110 (G> A), IVSI-1(G> A), codon 39 (C > T), IVSI-6 (T> C), IVSII-1(G> A), and codon 5(-CT) (3, 4).
There are more than two million carriers of beta-thalassemia and over 25,000 people affected with beta-thalassemia major who live in Iran (5, 6). Previous studies show the most prevalent mutations in Iran are IVSI-5 (G> C), IVSII-I (G> A), Fr 8–9 (+G), and IVS-I-110 (G> A) (7). Different mutations in ethnic groups reflect the heterogeneity and gene flow phenomenon between these populations.
Kerman Province is located in the southeastern of Iran; with an area of 11% of the whole country and more than a 2.5 million population. (Fig. 1 and Table 2). Beta-thalassemia is a common genetic disorder in the province and the incidence rate of carriers varies in its different regions (Table 2).
Fig. 1:
Map of Iran
Table 2:
Incidence rate of thalassemia minor/100000 persons who referred to Afzalipour genetic lab during 2005–2007
| City | Population | Incidence | City | Population | Incidence |
|---|---|---|---|---|---|
| Bam | 292,341 | 26.68 | Rafsanjan | 301,373 | 2.98 |
| Jiroft | 313,986 | 25.16 | Sirjan | 251,107 | 1.19 |
| Kahnooj | 344,640 | 12.85 | Zarand | 146,828 | 0 |
| Baft | 147,425 | 9.5 | Shahrbabak | 107,888 | 0 |
| Bardsir | 92,037 | 6.51 | Ravar | 39,676 | 0 |
| Kerman (Capital) | 693,119 | 4.9 | - | - | - |
Although the disorder is common in Iran, there is limited published information about the pattern of mutations. Hence, the aim of this study was to find this spectrum in Kerman Province and check its spatial variations.
Materials and Methods
By law, red cell indices are checked in marriage registrars in Iran through public health system. Couples who had low mean corpuscular volume (MCV< 82 fl) and high HbA2 (> 3%) were selected as suspicious of β-thalassemia carriers or iron deficiency. These red blood cell indices may were along with other low values: hemoglobin (Hb<12 g/dl in females and Hb< 14 g/dl in males), mean corpuscular hemoglobin (MCH< 26 pg), and mean cell hemoglobin concentration (MCHC< 31 g/dl).
After ruled out iron deficiency in the selected females by two months iron therapy, all of eligible subjects based on the above explanation who were referred to the central lab between July 2006 and December 2007 were assessed sequentially. In total, we managed to collect blood samples from 200 couples and identified 266 carries. This sample size was big enough to detect as a minimum of one positive for any types of mutations with at least 2.2% prevalence and 95% confidence. In the first session, we explained the objectives of our study and obtained informed consent for in the beginning. Then we collected 8 ml whole blood from their brachial vein in tubes containing 200 μl EDTA (Ethylene Diamine Tetra-acetic Acid).
Genomic DNA was isolated from leukocytes of the whole blood using salt-saturation method as previously prescribed (8). Briefly in this technique, the whole blood sample is washed three times with cold water in order to lyses and discards the red blood cells. Then, the remaining white blood cells are incubated at 37 °C at the presence of proteinase K that denatures proteins. Then, saturated NaCl is added to separate unwanted parts. Finally, the upper phase is centrifuged at the presence of isopropanol and the remaining sediment DNA is dissolved in elusion buffer or deionized water.
The isolated DNA was applied to detect mutations of β-globin gene. The 15 most common β-globin gene defects were examined by amplification refractory mutations system-polymerase chain reaction (ARMS-PCR) technique (9) in our laboratory. The Vienna-lab β-globin strip assay kit (viennalab labor-diagnostika GmbH, Vienna, Austria) was used to identify those mutations that not covered by the above method.
Rare and unknown mutations were studied using DNA sequencing. To accomplish this, a 1830 bp DNA fragment of the β-globin gene including exon 1, exon 2, exon 3 and contiguous introns was amplified using a forward primer (5′-AAC TCC TAA GCC AGT GCC AGA AGA G -3′) and a reverse primer (5′-CAC TGA CCT CCC ACA TTC CCT TTT-3′). Ingredients and condition of the PCR reaction was according to ARMS-PCR method (9). PCR products were checked on 1% agarose gel and taken through an automated DNA sequencer.
Results
Among 200 couples studied in our laboratory during July 2006 to December 2007, a total of 266 β-thalassemia carriers with 17 types of β-globin gene mutation were detected.
Nearly, most of the studied couples had no children and they were at adulthood stage (between 20 to 30 yrs old). However, a number of subjects had one or more children who affected or not with major β-thalassemia. None of them had a history of genetic disorder except β-thalassemia. MCV in the most detected β-thalassemia carriers was less than 70fl and their A2 was more than 3. However, A2 level of a few β-thalassemia carriers was at normal range (less than 3) and/or their MCV were more than 70fl. Other blood cell indices such as Hb, MCH and MCHC were lower than normal range in these people. These indices can be found in silent β-thalassemia carriers. All the hematological data were collected at our laboratory.
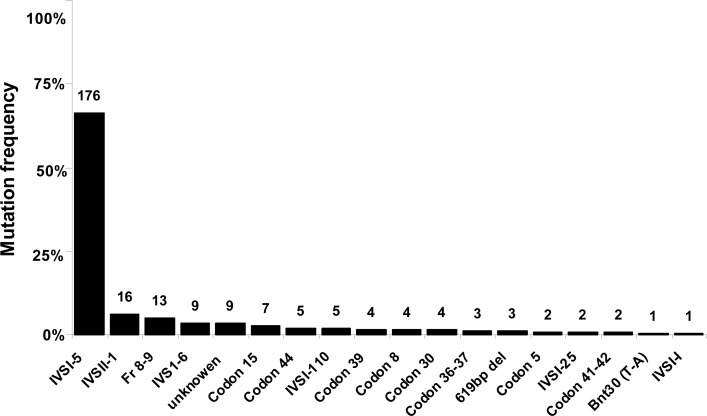
We could not detect the type of mutation in 9 patients with minor thalassemia phenotype. These unknown alleles comprised 3.4% of the mutations. The most prevalent mutation in overall samples analyzed in Kerman Province was IVSI-5 (G> C), with the frequency of 66.2% (Fig. 2 and Table 1). The IVSII-I (G> A) (6%) and Fr 8–9 (+G) (4.9%) were the second and third common mutations, respectively. Other mutations were included: IVSI-6 (T> C) (3.4%), codon 15 (G>A) (2.6%), codon 44 (1.9%), IVSI-110 (G > A) (1.9%), codon 39 (C>T) (1.5%), codon 8 (-AA) (1.5%), codon 30 (G> C) (1.5%), codon 36–37 (-T) (1.1%), 619 bp deletion (1.1%), codon 5 (-CT) (0.8%), IVSI-25bp deletion (0.8%), codon 41–42 (-TTCT) (0.8%), βnt30 (T>A) (0.3%), and IVSI-I (G > A) (0.3%) (Fig. 2 and Table1).
Fig. 2:
β-thalassemia mutation spectrum in Kerman province
Table 1:
Distribution of β-globin gene mutations in Kerman Province
| Mutation type | Bam (%) | Jiroft (%) | Kahnooj (%) | Kerman (%) | Baft (%) | Rafsanjan (%) | Bardsir (%) | Sirjan (%) | Province Total (%) |
|---|---|---|---|---|---|---|---|---|---|
| IVSI-5 | 59(75.6) | 56(70.9) | 34(79.1) | 17(50) | 6(42.8) | 4(44.5) | − | − | 176(66.2) |
| IVSII-I | 2(2.6) | 8(10) | 1(2.3) | 2(5.9) | 1(7.2) | − | 2(33.3) | − | 16(6) |
| Fr 8–9 | 2(2.6) | 2(2.5) | − | 7(20.6) | 1(7.2) | 1(11.1) | − | − | 13(4.9) |
| IVSI-6 | 1(1.3) | − | − | 4(11.9) | 3(21.4) | 1(11.1) | − | − | 9(3.4) |
| Cd15 | 2(2.6) | 1(1.3) | − | 1(2.9) | − | − | − | 3(100) | 7(2.6) |
| Cd 44 | 1(1.3) | 1(1.3) | 2(4.6) | 1(2.9) | − | − | − | − | 5(1.9) |
| IVSI-110 | 1(1.3) | 1(1.3) | − | 1(2.9) | 1(7.2) | 1(11.1) | − | − | 5(1.9) |
| Cd 39 | − | 1(1.3) | 1(2.3) | − | − | − | 2(33.3) | − | 4(1.5) |
| Cd 8 | − | 3(3.8) | 1(2.3) | − | − | − | − | − | 4(1.5) |
| Cd 30 | 1(1.3) | 1(1.3) | 2(4.7) | − | − | − | − | − | 4(1.5) |
| Cd 36–37 | 2(2.6) | − | 1(2.3) | − | − | − | − | − | 3(1.1) |
| 619 bp del* | − | 2(2.5) | 1(2.3) | − | − | − | − | − | 3(1.1) |
| Cd 5 | 2(2.6) | − | − | − | − | − | − | − | 2(0.8) |
| IVSI-25 | 2(2.6) | − | − | − | − | − | − | − | 2(0.8) |
| Cd 41–42 | − | − | − | − | 2(14.2) | − | − | − | 2(0.8) |
| Bnt30(T-A) | − | − | − | − | − | 1(11.1) | − | − | 1(0.3) |
| IVSI-I | 1(1.3) | − | − | − | − | − | − | − | 1(0.3) |
| Unknown | 2(2.6) | 3(3.8) | − | 1(2.9) | − | 1(11.1) | 2(33.3) | − | 9(3.4) |
| Total | 78(29.3) | 79(29.7) | 43(16.2) | 34(12.8) | 14(5.3) | 9(3.4) | 6(2.2) | 3(1.1) | 266(100) |
Del; Deletion. Cd; Codon
Although a few thalassemia major patients live in Zarand, Ravar, and Shahrbabak, but no suspicious couple with β-globin gene defect was referred to the laboratory from these cities during our study. We found three cases of 619 bp deletion and one case of Bnt (30) (T> A) that were not previously reported from any regions of Iran (Table 1).
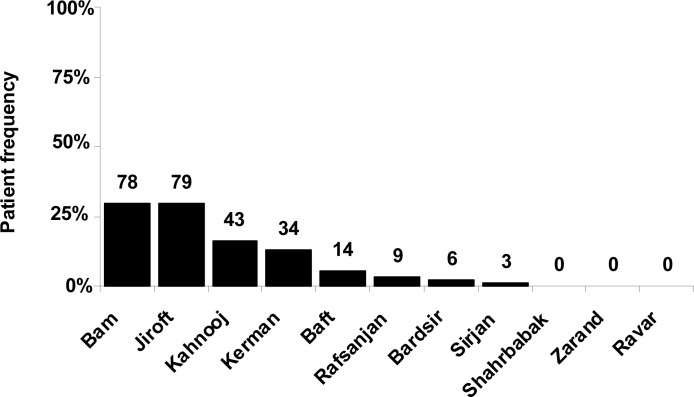
The most frequent patients were from southeast of Kerman territory (Fig. 3), at border of Sistan-va-Baluchestan Province, where Bam, Jiroft and Kahnooj are located (Fig. 1). These cities had a unique mutation pattern, since more than 70% of patients had IVSI-5 (G> C) and the remaining had other mutations (Table 1). Although IVSI-5 (G> C) was the most frequent mutation, IVSII-I (G> A) had the highest frequency in Jiroft among all of these regions (Table 1).
Fig. 3:
Relative frequency of β-globin gene mutations detected in Kerman Province cities.
Bam and Baft had an exclusive mutation spectrum. Since, the rare IVSI-I (G> A), codon 5 (-CT) and IVSI (25 bp deletion) gene defects were only identified in Bam and the two detected cases of codon 41–42 (-TTCT) were from Baft city (Table 1).
The only case of Bnt30 (T>A) mutation was found in Rafsanjan at the north part of the province (Fig.1 and Table 1).
We found only 6 types of β-globin gene mutation in Baft city. After IVSI-5 (G> C) (42.8%), the IVSI-6 (T> C) (21.4%) was the most prevalent mutation in this area. This β-globin gene mutation pattern in Baft is unique among the cities of Kerman province (Table 1).
The incidence rate value shows the ratio of β-thalassemia carriers to population. Jiroft and Bam nearly have the same relative frequency of β-globin gene mutations, while the incidence rate of gene defects in Bam is more than Jiroft (Fig. 3 and Table 2).
Although β-thalassemia carriers are more frequent in Kerman City (Center of the province) than Bard-sir and baft (Fig. 3), but their incidence rate in Kerman is less than these two cities (Table 2).
This value was zero for Zarand, Ravar, and Shahrbabak, the northern cities of Kerman Province (Fig. 1 and Table 2). These regions are located far from Bam, Jiroft, and Kahnooj, where the highest prevalence of β-globin gene mutations was found (Fig. 3).
Discussion
Our result showed IVSI-5 (G> C) as the most frequent mutation (68.5%) in Kerman Province (Table 1). This type of gene defect was previously reported at high prevalence in Hormozgan (10) and Sistan-va-Balochistan (11). These provinces are located in south and southeast of Kerman territory (Fig.1). Also, IVSI-5 (G> C) was reported as the most frequent mutation in south of Iran (7), Baluchistan and Sindh provinces of Pakistan (12), and widespread in the neighboring countries: Western province of Saudi Arabia, Bahrain, Kuwait, UAE, and Oman have 22.5%, 16.2%, 18%, 55%, and 62% of IVSI-5 (G> C), respectively (13). These data show that IVSI-5 is the predominant mutation in the countries around Persian Gulf, and the southern regions of Iran. The β-globin gene mutation spectrum in this area can be explained by gene flow phenomenon.
The type of mutation was not identified in nine cases (3.4%) by ARMS, strip assay or even β-globin gene sequencing. Since our research only covers β-thalassemia, these unknown cases may be caused by other anemia disorders.
Table 3 shows distribution of β-globin gene mutations in Kerman and some other provinces of Iran. As expected, spectrum of mutations is unique for each province. It is interesting that IVSI-5 (G> C), IVSII-I (G> A), and Fr 8–9 (+G) mutations existed in all of the provinces. The three common mutations are frequent in southern and could be spread in the other regions of Iran.
Table 3:
Distribution of β-thalassemia mutations in some Iran Provinces. Del; Deletion. Cd; Codon
| Mutation type | Kurdestan (%) (Ref: 14) | Bushehr (%) (Ref:15) | Lorestan (%) (Ref:16) | Eastern Azerbaijan (%)(Ref:17) | Western Azerbaijan (%) (Ref:18) | Sistan-Baluchistan (%) (Ref:11) | Sari (%) (Ref:19) | Kerman (%) |
|---|---|---|---|---|---|---|---|---|
| IVSI-5 | 1(1.52) | 16(7.69) | 6(4.67) | 4(2.6) | 0.8 | 33.3(76.5) | 6(7.5) | 172(66.2) |
| IVSII-I | 21(31.81) | 27(12.98) | 36(27.69) | 48(32) | 50.7 | 6(1.4) | 55(68.7) | 16(6) |
| Fr 8–9 | 9(13.62) | 17(8.17) | 14(10.76) | 28(18.67) | 16 | 16(3.7) | 5(6.3) | 14(4.9) |
| IVSI-6 | 1(1.52) | 3(1.44) | − | − | 0.8 | − | − | 9(3.4) |
| Cd 15 | − | − | − | − | − | − | − | 7(2.6) |
| Cd 44 | 3(4.55) | − | 1(0.76) | − | 9.3 | 3(0.7) | − | 5(1.9) |
| IVSI-110 | 1(1.52) | 12(5.77) | 15(11.53) | 24(16) | 5.3 | − | − | 5(1.9) |
| Cd39 | 3(4.55) | 7(3.36) | − | − | − | 6(1.4) | 1(1.2) | 4(1.5) |
| Cd 8 | 4(6.06) | − | − | − | − | − | 2(2.5) | 4(1.5) |
| Cd30 | − | 7(3.36) | − | 8(5.34) | 2.7 | − | 3(3.8) | 4(1.5) |
| Cd36/37 | 1(1.52) | − | 44(33.84) | − | − | 1(0.2) | − | 3(1.1) |
| 619 bp del | − | − | − | − | − | − | − | 3(1.1) |
| Cd5 | − | 5(2.4) | 1(0.76) | − | − | 2(0.5) | 2(2.5) | 2(0.8) |
| Cd 41–42 | − | − | − | − | − | − | − | 2(0.8) |
| Bnt30 (T-A) | − | − | − | − | − | − | − | 1(0.3) |
| IVSI-I | 8(12.1) | 19(9.13) | − | 34(22.67) | − | 3(0.7) | 1(1.2) | 1(0.3) |
| IVSII-745 | − | 7(3.36) | 2(4.67) | − | − | 1(0.2) | 2(2.5) | − |
| IVSI(25bp del) | − | 50(24.04) | 1(0.76) | − | − | 3(0.7) | 3(3.8) | 2(0.8) |
| −88(C>T) | − | − | − | − | − | 3(0.7) | − | − |
| Unknown | 14(21.12) | 38(18.3) | 10(7.63) | − | − | 58(13.3) | − | 9(3.4) |
| Total | 66 | 208 | 130 | 144 | 150 | 435 | 80 | 266 |
The β-globin gene mutation spectrum in the other Iranian regions was found different as compared to the data obtained from Kerman Province (Table 3). Interestingly, prevalence of the IVS1-5 (G> C) mutation in Sistan-VA-Bluchistan province was the highest among all of the provinces. Two common mutations, namely IVSII-745 (C> G) and −88 (C>T) were completely absent in the population of Kerman province. Also, codon 15 (-CT), 619 bp deletion, codon 41–42 (-TTCT), and Bnt30 (T>A) were identified just in Kerman Province (Table 3). These β-globin gene mutation spectrum discrepancies could be explained by the fact that we used three methods to detect the rare β-globin gene defects, while only ARMS technique applied in the other studies. In addition, a significant immigration happened into Kerman province following recent earthquake in Bam. This occurrence may be acted as an interference factor in our survey.
It is interesting that three cases of 619 bp deletion, found in our study, (one case from Kahnooj and two cases from Jiroft), was not previously reported in any regions of Iran. This type of mutation may have been diffused from Pakistan into Kerman province, since it was found frequent (46%) in Gujratis and Memons residing in the province Sindh of Pakistan (12).
The highest incidence rate of thalassemia minor was found in Bam, Jiroft and Kahnooj (Table 2). We can speculate that the majority of β-thalassemia major patients must live in these three cities. The highest gene frequency in these cities confirms our finding. This index is 0.0004 in Kerman province for β-thalassemia major and it differs markedly among different populations of Kerman cities. No thalassemia carriers were referred us from Zarand, Shahrbabak, and Ravar, while 25 thalassemia major patients are living in these regions. Therefore, we can deduce that no marriage was happened between thalassemia minor populations of these cities during our study.
About 3.4% of the β-globin gene mutations, including the only case of Bnt30 (T>A), was found in Rafsanjan (Table 1). Bnt30 (T>A) mutation was not previously reported from any regions of Iran. Rafsanjan is one of the northern cities of Kerman province; therefore, we did not expect to find the high prevalence of mutation in this area. Since, 11 afghan refugees who affected with thalassemia major are living in this city, finding these extraordinary results may be due to transmission of the β-thalassemia alleles from this country to Rafsanjan.
In conclusion, the IVSII-745(C> G), −88 (T>C), and some other rare and unknown mutations were not found in our survey. Study the spectrum of β-globin gene mutations in Kerman Province was done during a limited time. Extension of the research period in future studies may reveal more kinds of β-gene defects.
Ethical Consideration
All Ethical issues (such as informed consent, conflict of interest, plagiarism, misconduct, co-authorship, double submission, etc) have been considered carefully.
Acknowledgments
We thank Fareshteh Bang-Tavakoli, Arezo Khosravi-Mashizi, Faegheh Hafezi, and Mahla Hoseini-Mehrabi for helping us during this survey. We are also indebted to Dr Ali-Akbar Haghdoost for his brilliant statistical comments and Afzalipour Hospital for supporting financially this work.
References
- 1.Carver MF, Hushiman TH. International Hemoglobin Information Center variant list. Hemoglobin. 1996;20(3):213. doi: 10.3109/03630269609027930. [DOI] [PubMed] [Google Scholar]
- 2.Flint J, Harding RM, Boyce AG, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1998;11(1):1–51. doi: 10.1016/s0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 3.Cao A, Gossens M, Pirastu M. β-thalassaemia mutations in Mediterranean populations. Br J Haematol. 1989;71(3):309–12. doi: 10.1111/j.1365-2141.1989.tb04285.x. [DOI] [PubMed] [Google Scholar]
- 4.Zahed L. The spectrum of β-thalassaemia mutations in the Arab populations. J Biomed Biotechnol. 2001;1(3):129–32. doi: 10.1155/S1110724301000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahimi Z, Vaisi Raygani A, Merat A, Haghshenass M, Gerard N, Nagel RL, et al. Thalassemic mutation in southern Iran. IJMS. 2006;31(2):71–3. [Google Scholar]
- 6.Derakhshandeh-Peykar P, Akhavan-Niaki H, Tamaddoni A, Ghawidel-Parsa S, Naieni Kh, Rahmani M, et al. Distribution of beta-thalassemia mutations in the northern provinces of Iran. Hemoglobin. 2007;31(3):351–56. doi: 10.1080/03630260701462030. [DOI] [PubMed] [Google Scholar]
- 7.Najmabadi H, Karimi-Nejad R, Sahebjam S, Pourfarzad F, Teimourian S, Sahebjam F, et al. The beta-thalassemia mutation spectrum in the Iranian population. Hemoglobin. 2001;25(3):285–96. doi: 10.1081/hem-100105221. [DOI] [PubMed] [Google Scholar]
- 8.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215–16. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton CR, Graham A, Heptinstall LE, Powell SJ, Summeres C, Kalsheker N, et al. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17(7):2503–16. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yavarian M, Harteveled CL, Batelaan D, Bernini LF, Giordano PC. Molecular spectrum of beta-thalassemia in the Iranian Province of Hormozgan. Hemoglobin. 2001;25(1):35–43. doi: 10.1081/hem-100103068. [DOI] [PubMed] [Google Scholar]
- 11.Miri Moghadam E, Narooie Nejad M, Eshgi P, Zeinali S, Savad koohi F. Molecular basis and prenatal diagnosis of β-Thalassemia in southeast of Iran. Jornal of Mazanderan University of Medical Sciences. 2005;48(15):105–11. [Google Scholar]
- 12.Khan SN, Riazuddin S. Molecular characterization of beta-thalassemia in Pakistan. Hemoglobin. 1998;22(4):333–45. doi: 10.3109/03630269809071528. [DOI] [PubMed] [Google Scholar]
- 13.Al-Ali AK, Al-Ateeq S, Imamwerdi BW, Al-sowayan S, Al-Madan M, Al-Muhanna F, Bashaweri L, Qaw F. Molecular Bases of beta-Thalassemia in the Eastern Province of Saudi Arabia. J Biomed Biotechnol. 2005;(4):322–25. doi: 10.1155/JBB.2005.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousefi MH. Beta Thalassemia trait in Sanandaj. Scientific Journal of Kurdestan University of Medical Sciences. 1997;4(1):11–3. [Google Scholar]
- 15.Khodaei H, Zeinali C, Delmaghani S. Molecular studies on Beta-Thalassemia mutations in Bushehr province. Iranian South Medical Journal. 2001;2(3):89–3. [Google Scholar]
- 16.Kiani AA, Mortazavi Y, Zainali S, Shirkhani Y. The molecular analysis of beta-thalassemia mutations in Lorestan Province, Iran. Hemoglobin. 2007;31(3):343–49. doi: 10.1080/03630260701459382. [DOI] [PubMed] [Google Scholar]
- 17.Mohaddess Ardabili SM, Jabbarzadeh Tabrizi S, Nikan Far AR, Rahbani Nobar M. Prevalent mutations of Beta-globin gene at the central part of east Azerbaijan, Iran. Medical Journal of Tabriz University of Medical Sciences & health Services. 2004;62:72–67. [Google Scholar]
- 18.Omrani MD. Finding the most prevalent?-Thalassemia mutations in west Azerbaijan province using ARMS/PCR method. Urmia Medical Journal. 2003;2(14):126–17. [Google Scholar]
- 19.Modjtahed Zadeh F. Beta Thalassemia gene mutations in Thalassemic patients referred to Boo Ali Sina Hospital of Sari the year 1373. Journal of Mazanderan University of Medical Sciences. 1999;(9):23–22. 37–2. [Google Scholar]