Abstract
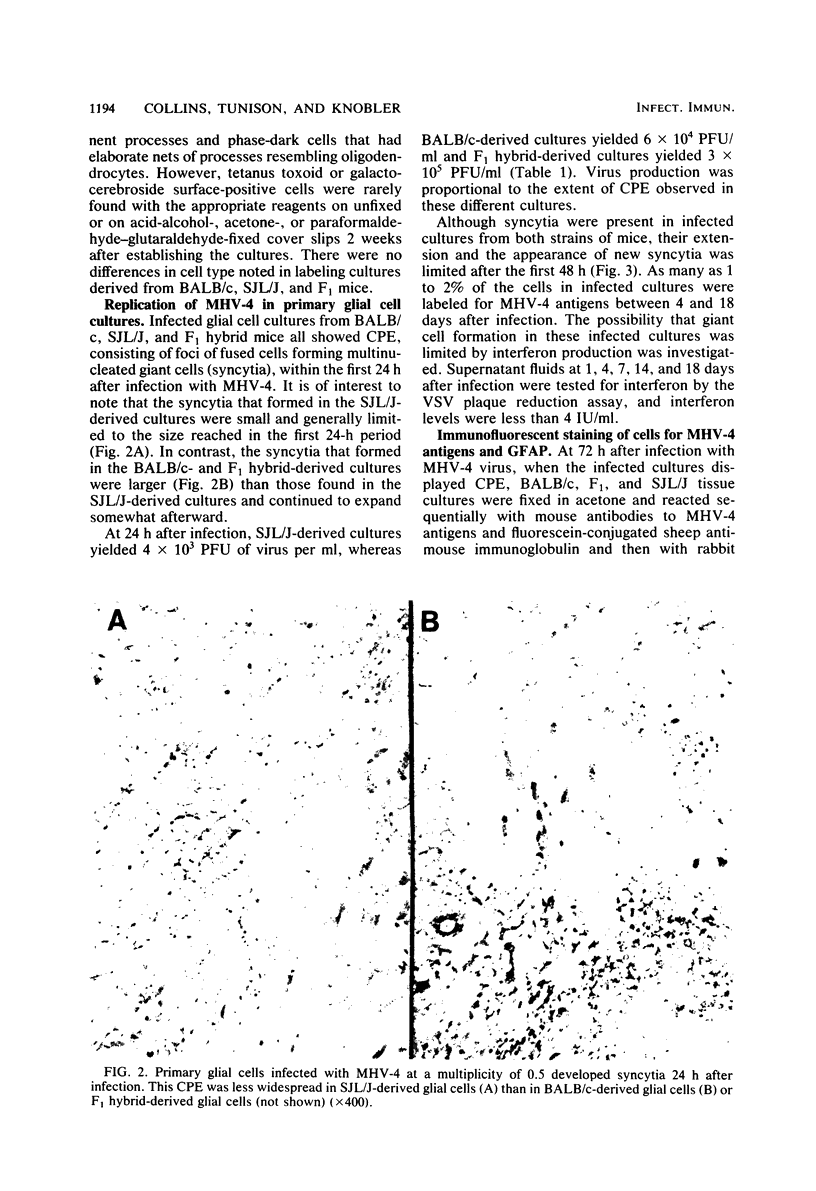
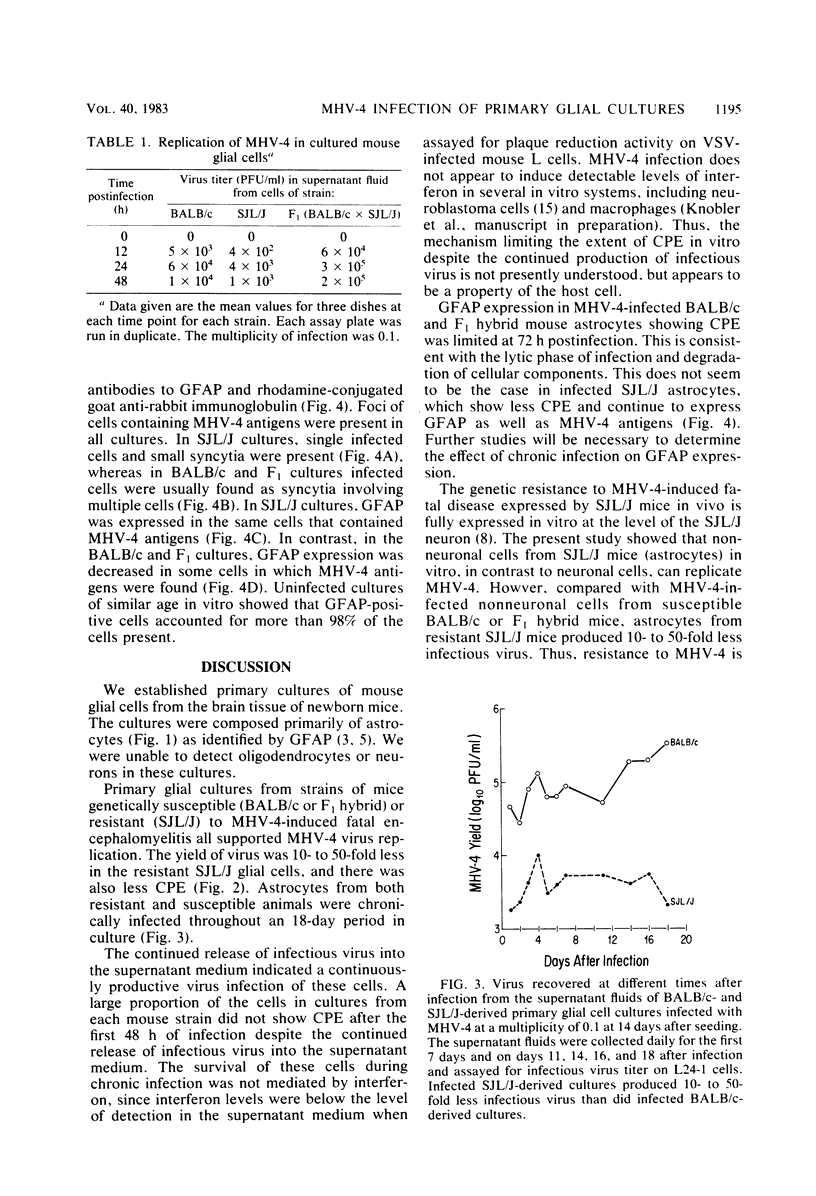
Mouse hepatitis virus type 4 infection of primary glial cultures, which consisted principally of astrocytes (marked by glial fibrillary acidic protein) from encephalitis-susceptible BALB/c or F1 (BALB/c x SJL/J) hybrid mice and resistant SJL/J mice, was studied. Primary neuron cultures from BALB/c and F1 hybrid mice were previously shown to be permissive and were destroyed within 5 days by infection with mouse hepatitis virus type 4, whereas neurons from SJL/J mice were fully resistant. In contrast, in the present study a chronic infection was established and maintained for up to 18 days in glial cultures from all three mouse strains. Infected SJL/J mouse glial cultures produced 10- to 50-fold less infectious virus and showed less cytopathic effect than did cultures from either infected BALB/c or F1 hybrid mice. Cytopathic effect was evident initially in cells from all three strains, and continued virus production occurred in the presence of limited additional cytopathic effect. These results were not due to the production of detectable levels of interferon. This study showed that SJL/J mouse primary glial cultures were permissive for mouse hepatitis virus type 4 infection whereas SJL/J primary neuron cultures were not, and that there was an early lytic phase of infection followed by chronic infection in all three strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Baechi T., Haller O. Adult mouse hepatocytes in primary monolayer culture express genetic resistance to mouse hepatitis virus type 3. J Immunol. 1982 Sep;129(3):1275–1281. [PubMed] [Google Scholar]
- Bignami A., Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974 Jan 1;153(1):27–38. doi: 10.1002/cne.901530104. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Lampert P. W., Oldstone M. B. Temperature-sensitive mutants of mouse hepatitis virus produce a high incidence of demyelination. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4033–4036. doi: 10.1073/pnas.75.8.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Dubois-Dalcq M., Haspel M. V., Claysmith A. P., Lampert P. W., Oldstone M. B. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J Neuroimmunol. 1981 Mar;1(1):81–92. doi: 10.1016/0165-5728(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A., Flintoff W., Anderson R., Percy D., Coulter M., Dales S. In vivo and in vitro models of demyelinating diseases: tropism of the JHM strain of murine hepatitis virus for cells of glial origin. Cell. 1977 Oct;12(2):553–560. doi: 10.1016/0092-8674(77)90131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R., Wendon L. M., Black P., Stolkin C., Bray D. Tetanus toxin: a cell surface marker for neurones in culture. Brain Res. 1978 Jun 9;148(1):251–259. doi: 10.1016/0006-8993(78)90399-2. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Stohlman S. A., Sakaguchi A. Y., Hiti A. Interferon production and activity in mouse neuroblastoma cells. Arch Virol. 1978;57(1):91–96. doi: 10.1007/BF01315640. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., ADAMS R. D. Infectious leukoencephalitis. A critical comparison of certain experimental and naturally-occurring viral leukoencephalitides with experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1962 Oct;21:491–518. [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]