Abstract
Objective To determine the feasibility and acceptability of an interdisciplinary intervention for mothers of children newly diagnosed with cancer and to estimate effect sizes for the intervention in reducing distress. Management of illness uncertainty was a key framework for the intervention. Methods Mothers (N = 52) were randomly assigned to the intervention or a treatment as usual group, completing measures at baseline and follow-up time points. Results Mothers’ satisfaction ratings were consistently high, and intervention implementation appeared feasible. Significant mean effects or trends in favor of the intervention group were found for pre-to-post change on measures of distress. Evidence of a preventative effect was also observed; mothers in the intervention group tended to improve or remain stable in their adjustment, whereas many parents in the treatment as usual group showed worsening outcomes. Conclusions An interdisciplinary intervention targeting maternal illness uncertainty has clinical value within this sample.
Keywords: clinical intervention, psychosocial outcomes, uncertainty
Cure rates for pediatric cancer have improved (American Cancer Society, 2010); however, the aggressive nature of cancer treatment exerts a significant toll on the child and family, placing each member at risk for compromised psychosocial adjustment (Kazak, Simms, et al., 2005; Sahler et al., 1997). Parents of children with cancer face considerable demands as they adjust to their child’s diagnosis. They must quickly learn complicated and often confusing medical terminology and treatment protocols, communicate with a multitude of health professionals, monitor medications and side effects, and provide comfort and support to a child who is often in distress and coping with treatment demands. Thus, caring for a child with cancer is a role accompanied by considerable stress, as well as heightened uncertainty.
Although many parents cope relatively well in the long term, many experience significant distress when their child is first diagnosed, and a small subgroup of parents cope poorly in the long term (Kazak, 1994). For example, 50% of mothers and 40% of fathers of children newly diagnosed with cancer meet criteria for acute stress disorder (Patiño-Fernández et al., 2008), and many will experience symptoms of distress for years beyond their child’s diagnosis (Pai, Drotar, Zebracki, Moore, & Youngstrom, 2006). A recent meta-analysis also documents that parents of children diagnosed with cancer experience an array of both clinical and subclinical psychological symptoms that compromise their quality of life (Pai et al., 2006). Thus, pediatric cancer researchers have been challenged to develop new innovative intervention approaches to improve the long-term adjustment of parents of children with cancer (Kazak, Boeving, Alderer, Hwang, & Reilly, 2005).
Interventions designed specifically to enhance parental adjustment to pediatric cancer are still relatively few (Kazak, Simms, et al., 2005; Sahler et al., 2002; Sahler et al., 2005; Stehl et al., 2009), and only one has demonstrated significant reduction of psychological adjustment problems and distress among parents (Sahler et al., 2002; Sahler et al., 2005), suggesting that there is significant room for advancement in this area. Kazak and colleagues (Kazak, Simms, et al., 2005; Stehl et al., 2009) conducted a three-session manualized family-based intervention for caregivers of children newly diagnosed with cancer (Surviving Cancer Competently Intervention Program [SCCIP-ND]). Their pilot work suggested that caregivers in the intervention group (IG) showed reductions in anxiety and posttraumatic stress symptoms at a 2-month follow-up (Kazak, Simms, et al., 2005), but no significant differences were observed between the intervention and treatment as usual (TAU) groups in their larger randomized clinical trial (Stehl et al., 2009). Sahler and colleagues (2002, 2005) have documented the efficacy of problem-solving skills training (PSST) for mothers of children recently diagnosed with cancer. In their initial work, they evaluated a manualized eight-session PSST program (Sahler et al., 2002). Compared with the TAU group, mothers receiving the intervention showed significant improvements in problem-solving skills and decreases in negative affect at both posttreatment and 3-month follow-up. A large-scale extension of this study yielded similar results (Sahler et al., 2005).
We have sought to develop an intervention for mothers of children newly diagnosed with cancer that builds on the strengths of previous interventions, including their focus on acquisition of cognitive behavioral skills and problem-solving skills, and use of social support. Specifically, although the SCCIP-ND trial teaches parents to identify and change cognitions about their child’s cancer, the current intervention teaches mothers to identify and cope with specific appraisals of their child’s illness and its treatment that are frequently out of parents’ control, specifically, within the context of managing illness uncertainty. The PSST intervention teaches mothers a single approach (i.e., problem solving) during the course of 8 weeks, and therefore it may not be as beneficial to mothers who already have good problem-solving skills or for specific situations where outcomes cannot be improved by problem-solving (e.g., waiting for a child’s test results). The current intervention teaches skills for managing maladaptive cognitions, including not only problem-solving skills but also cognitive reframing, communication skills, and social support. Finally, the current intervention took place during the child’s regularly scheduled outpatient visits at the pediatric oncology clinic to decrease burden on already overburdened and distressed parents.
Importantly, the current intervention used an illness uncertainty framework as its overarching theme. Illness uncertainty is a cognitive construct that has been consistently associated with psychological distress among multiple illness groups. Illness uncertainty is defined as an experience elicited in situations where the meaning of illness-related events is unclear and outcomes are unpredictable because sufficient information or cues are lacking (Mishel, 1990). Sources of uncertainty include ambiguity of the illness, complexity of the treatment, lack of information regarding the severity of illness and prognosis, and unpredictability of the illness course (Mishel, 1984). Illness uncertainty has been shown to be a salient predictor of distress among parents of children with a chronic illness and is associated with symptoms of anxiety and depression (Stewart & Mishel, 2000), as well as general measures of psychological distress and posttraumatic stress disorder symptoms (Fuemmeler, Mullins, & Marx, 2001). Importantly, we have previously used uncertainty as a target for intervention in a sample of parents of children newly diagnosed with diabetes, demonstrating significant improvements in maternal and paternal distress and maternal ratings of child internalizing problems for the IG (Hoff et al., 2005). Specifically, parents of children with diabetes were provided with psychoeducation regarding uncertainty in the context of diabetes, as well as a variety of coping skills for preventing and managing uncertainty regarding their child’s illness. Further, treatments targeting uncertainty among adults with cancer have clearly demonstrated significant improvements in adaptation to the illness postintervention (Mishel et al., 2003, 2005). Such documented success with adult cancer patients and parents of children newly diagnosed with diabetes would certainly argue for further investigation of uncertainty management interventions targeting parents of children newly diagnosed with cancer. In this manner, we argue that effective management of uncertainty has the potential to significantly reduce various forms of psychological distress, including general distress, as well as posttraumatic stress symptoms and perceived burden of care.
Illness uncertainty, ultimately, is a function of the interaction between objective illness events (e.g., blood tests, biopsy, bone marrow transplant) and a parent’s subjective appraisal of the potential outcomes (e.g., blood cell counts, presence of residual cancerous cells, engraftment) of the illness event. This interaction between medical information and parent cognitions lends itself to an interdisciplinary treatment model. In addition, the Institute of Medicine (2003) stresses the importance of delivering health services via coordinated interdisciplinary treatment teams to improve the quality and safety of patient care. Thus, the current study aimed to build on the existing parent intervention literature using Mishel’s model of illness uncertainty (1990) and an interdisciplinary approach to target maternal adjustment in the context of pediatric cancer. Interdisciplinary efforts have typically been defined as two or more complementary disciplines working on mutual goals with overlapping roles (Mullins, Keller, & Chaney, 1994). The distinguishing feature of the interdisciplinary (as opposed to multidisciplinary) approach is the emphasis on both communication and coordinated care. In this manner, functional roles and responsibilities overlap to provide input from multiple vantage points, culminating in comprehensive patient-centered care (Mullins, Balderson, & Chaney, 1999). This approach has great relevance to pediatric oncology, as families must deal with treatment teams consisting of physicians, nurses, physician assistants, social workers, and psychologists, among others.
We would further argue that the nursing and psychology disciplines have unique potentially synergistic role relationships, as they concern facilitating psychosocial outcomes for families facing cancer. Each discipline has overlapping and complementary roles of providing emotional care and support and facilitating communication with other medical personnel. At the same time, each discipline offers unique skills in terms of psychosocial interventions (i.e., psychology) and expertise in medical treatment (i.e., nursing). Jointly delivered by psychology and nursing personnel, the intervention, which involved education about illness uncertainty, teaching of cognitive reframing and problem-solving skills, communication training, and the establishment and maintenance of social support, was designed to attenuate the impact of the diagnosis and its associated uncertainty on adjustment outcomes among mothers. Our primary aims for this pilot study were as follows: (1) to determine the feasibility and acceptability of implementing a 12-session clinic-based interdisciplinary intervention for mothers of children newly diagnosed with cancer, (2) to examine whether the clinic-based interdisciplinary intervention is effective in reducing maternal distress (i.e., general psychological distress, posttraumatic stress symptoms, and caregiver burden) in pediatric cancer, and (3) to explore a hypothesized change model for the intervention, specifically, whether reductions in distress attributable to the treatment are associated with better management of uncertainty.
Methods
Participants and Procedure
Participants in the current study were recruited from a large children’s hospital in the midwestern United States. Eligible participants were mothers of children between the ages of 2 and 18 years, who were newly diagnosed with (1) leukemia or lymphoma, (2) solid tumor, or (3) brain tumor within the past 4–16 weeks. Other inclusion criteria were as follows: (1) English as their primary language, (2) a working land line or cell phone in the home, and (3) the child’s treatment protocol included chemotherapy +/− radiation. Exclusion criteria were as follows: (1) the child was experiencing an imminent medical crisis necessitating significant medical intervention; (2) the child was determined to be in the terminal phase and/or receiving palliative care; (3) the diagnosis was determined to be a relapse or a second malignancy; (4) the parent was currently being treated for a serious psychiatric disorder or evidenced mental retardation; and (5) the parent was <18 years of age.
The current study was approved by the institutional review board. Before randomization, all mothers completed baseline measures. Eligible families were identified through physician consultation. All eligible families were approached by a graduate assistant and were given information about the current study. On receiving informed consent, participants were asked to complete the measures during their clinic visit. If a parent was unable to complete the measures at that time, they were asked to return the measures during the next clinic visit. All participants were asked to complete measures at three different time points, before the intervention (pre), 1 month after the conclusion of the intervention (post), and 3 months after the conclusion of the intervention (follow-up). As the intervention was designed to be completed in 12 weeks, participants in the TAU group were asked to complete the post and follow-up measures at 16 and 24 weeks, respectively, from the date they completed the initial packet. Completion of questionnaires took approximately 1hr.
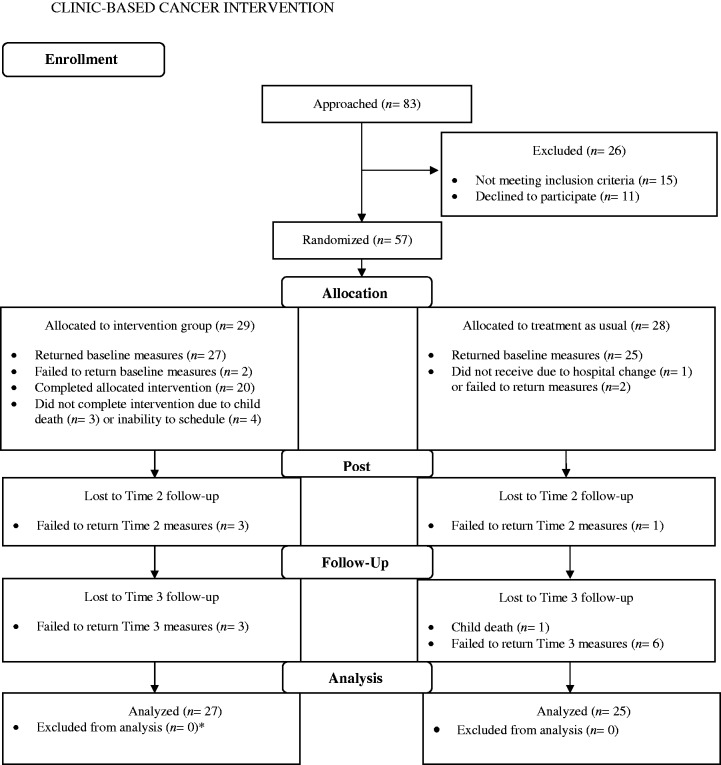
Following Zelen (1974) blocked randomization was used for participant assignment to condition. Participant flow through the study is outlined in Figure 1. Participants were compensated $30 at the completion of each of the three assessment time points, thus receiving a total of $90 for completion of the entire study. Demographic and illness-related variables for all participants are presented in Table I. The overall consent rate for the study was 84%.
Figure 1.
Participant flow through the intervention.
Note. All cases were analyzed via growth curve modeling if participants provided any data. *Owing to missing data, the analyzed intervention group sample was slightly smaller (n = 26) for the Care of My Child With Cancer Scale, Impact of Events Scale-Revised, and Symptom Checklist 90-Revised outcomes.
Table I.
Demographic and Illness Characteristics of Sample at Baseline
| Variablea | Treatment as usual (n = 25) | Intervention (n = 27) | ||||
|---|---|---|---|---|---|---|
| Range | M (SD) | N (%) | Range | M (SD) | N (%) | |
| Child’s age (years) | 2–17 | 7.32 (4.23) | 2–16 | 9.06 (4.85) | ||
| Child sex | ||||||
| Male | 13 (52) | 15 (55.6) | ||||
| Female | 12 (48) | 12 (44.4) | ||||
| Mother’s age (years) | 22–46 | 33.36 (5.66) | 24–55 | 36.81 (8.19) | ||
| Mother’s education | ||||||
| <High school | N/A | 6 (22.2%) | ||||
| High school diploma | 6 (24%) | 4 (14.8%) | ||||
| Partial college/technical school | 9 (36%) | 7 (25.9%) | ||||
| College/university graduate | 9 (36%) | 9 (33.3%) | ||||
| Graduate/professional degree | 1 (4%) | 1 (3.7%) | ||||
| Annual family income | ||||||
| <$29,999 | 10 (40%) | 11 (40.7%) | ||||
| $30,000–$59,999 | 2 (8%) | 8 (29.6%) | ||||
| $60,000–$89,999 | 8 (32%) | 3 (11.1%) | ||||
| >$90,000 | 3 (12%) | 3 (11/1%) | ||||
| Self-identified race/ethnicity | ||||||
| Caucasian | 14 (56%) | 18 (66.7%) | ||||
| African American | 3 (12%) | 3 (11.1%) | ||||
| Native American | 1 (4%) | 4 (14.8%) | ||||
| Hispanic | 5 (20%) | 2 (7.4%) | ||||
| Other | 2 (8%) | N/A | ||||
| Illness duration at consent (months) | 1–4 | 2.64 (.99) | 1–4 | 2.22 (.97) | ||
Note. Percentages may not sum to 100% because of missing data. SD = standard deviation; N/A = not applicable.
aGroups compared using χ2 for categorical variables (gender and race), ordinal logistic models for ordinal categorical variables (income and education categories) and ANOVA for continuous variables (ages and illness duration). No comparisons were statistically significant.
TAU Group
Consistent with previous randomized clinical trials with parents of children with cancer, a TAU control group was used (Sahler et al., 2005). Participants randomized to the TAU group received standard psychosocial care. Standard psychosocial care involved each family receiving ad hoc services from the oncology team, which included a psychologist who was available on an as-needed basis during both inpatient hospitalization and outpatient clinic visits. In addition, each family received nursing support, as well as consultation from a social worker and child life services. On completion of data collection for the IG, mothers in the TAU group were given the opportunity to receive the intervention.
IG
Participants in the IG received an individually administered interdisciplinary intervention from psychology and nurse interventionists. The interventionists consisted of two dyads, each with one psychologist interventionist (an advanced doctoral student in a doctoral clinical psychology training program) and one nursing interventionist (master’s level registered nurse with pediatrics specialization). The intervention alternated weeks, with the in-clinic intervention provided by psychology interventionists being delivered on the odd numbered weeks, and the phone intervention conducted by nurse interventionists on the even numbered weeks. The intervention was manualized in an effort to ensure treatment fidelity across interventionists. Each session included specific treatment goals that were discussed with participants. All interventionists were trained by the corresponding author, and regular meetings were held with interventionists to discuss implementation issues and correct any protocol deviations.
Six distinct modules were covered in the intervention, including the nature of uncertainty, communication with medical staff, cognitive coping, problem solving, social support, and consolidation of all skills. The choice of these six modules followed from the demonstrated effectiveness of our previous intervention of youth with diabetes (Hoff et al., 2005). The sequencing of the intervention allowed for the presentation of core content first by the psychology interventionist in the clinic. The nurse interventionist was then able to follow up on this core content to reinforce the acquisition of important knowledge and skills while also being able to answer emergent medical questions about side effects and treatment protocols. The alternating of psychologists and nurses resulted in the 12-week format. Although lengthy, efforts were made to minimize burden to families by having nursing sessions conducted by phone. Each session was designed to be highly interactive, with solicitation of feedback about personal experiences coping and dealing with uncertainty, use of Socratic questioning, and review of homework. Families also received written handouts and cognitive-behavioral homework assignments specific to the core content of each module in a program binder. In-clinic sessions took approximately 45 min to 1 hr, whereas telephone interventions took approximately 15–30 min. Copies of all intervention materials can be requested from the corresponding author.
Measures
Demographic and Medical Information
A self-report questionnaire was used to obtain the following demographic information: child’s sex, age, race, and grade; parent’s age, marital status, and education level; and annual family income. Child medical information was also provided by mothers (i.e., type of cancer and treatment, date of diagnosis, number of hospitalizations, co-occurring illnesses, and side effects of therapy). Following consent, medical information was corroborated by medical chart review and physician consultation.
Intensity of Treatment Rating
The Intensity of Treatment Rating (ITR) categorizes cancer treatment protocols into three groups based on intensity: mild, moderate, and severe. This rating was determined by an attending oncology physician, blind to participant group status, and was based on patient medical chart review (Hobbie et al., 2000). The ITR has been successfully used by other researchers in pediatric oncology (Hobbie et al., 2000) as a measure of treatment intensity, given that severity of protocols may vary considerably across diagnoses. The ITR was collected for descriptive purposes.
Parent Perception of Uncertainty Scale
The Parent Perception of Uncertainty Scale (PPUS) is a 31-item self-report measure of perceived uncertainty in reference to a child’s illness. Mothers respond on a 5-point scale ranging from “strongly disagree” to “strongly agree” (Mishel, 1983). The scale yields a total uncertainty score, with higher scores indicating greater uncertainty. Cronbach’s α for the current study ranged from .74 to .79 across the three time points.
Symptom Checklist 90-Revised
The Symptom Checklist 90-Revised (SCL-90-R) is a widely used 90-item self-report inventory that yields a composite index of general psychological distress (i.e., Global Severity Index), which was used to assess overall parental distress in the current study (Derogatis, 1994). The SCL-90-R has been frequently used in other studies of mothers of chronically ill children (Rao, Pradhan, & Shah, 2004). Cronbach’s α for the current study was .98 for all three time points.
Impact of Events Scale-Revised
The Impact of Events Scale-Revised (IES-R) is a 22-item self-report instrument designed to assess one’s reaction to a stressful life event (i.e., child’s cancer diagnosis) (Weiss & Marmar, 1997). Each item is rated for frequency of occurrence on a 4-point scale, ranging from “not at all” to “often.” The IES-R total score was used as a measure of posttraumatic stress symptoms. Cronbach’s α for the current study ranged from .94 to .96 across the three time points.
Care of My Child With Cancer Scale
The Care of My Child With Cancer Scale (CMCC) is a 28-item scale that assesses the burden of caregiving tasks related to the child’s cancer. Burden scores are calculated using a combination of the raw time and raw effort scores (Wells et al., 2002). Higher scores indicate more time and effort. Cronbach’s α for the current study ranged from .94 to .96 across the three time points.
Participant Satisfaction Surveys
Following Dwyer (2006) a series of comprehensive questionnaires was created for use in the current study to assess the acceptability of the intervention for participants in the IG condition. At the beginning of each in-clinic session (starting with session 2), mothers were asked to complete a brief survey that assessed their opinions of the material presented in the previous module. This timing was chosen based on the fact that the mother had received both components of the module (i.e., psychology and nursing) before rating her satisfaction with the module. This survey included 13 questions with Likert-style response options in addition to 5 open-ended questions about the intervention. Further, at the completion of the intervention, participants were asked to complete a survey that assessed their satisfaction with the intervention as a whole. This survey consists of 23 Likert-style questions, as well as 5 open-ended questions. The Likert-style questions were on a 5-point scale, with higher scores indicating greater satisfaction.
Overview of Analyses
Preliminary analyses were conducted to determine whether the intervention and TAU groups differed at baseline on treatment intensity or dependent variables. Additionally, a series of independent t-tests was conducted to compare participants who completed the intervention with noncompleters on all demographic variables. For the purpose of the current study, completion of the intervention was defined as completing three or more (50%) face-to-face intervention sessions with the clinical interventionist, consistent with other randomized controlled trials involving parents of children with cancer (Salher et al., 2005). Analyses were also conducted to determine whether the TAU and IG differed on the length of time the participants were involved in the study. Because we were interested primarily in pre-to-post change and post-to-follow-up change units, rather than rates of change over time, growth models were coded in terms of wave number.
To address aim 1, indices of the feasibility of the intervention were calculated. Specifically, consent and completion rates were tabulated, as well as the average number of completed intervention sessions. Qualitative information obtained from the participant’s satisfaction surveys was also examined. With regard to acceptability of the intervention, descriptive statistics were conducted on the 5-point Likert scale of the satisfaction surveys for each session, and a one-way ANOVA was conducted to test whether participants favored a particular session.
For aim 2, change over time was modeled using latent growth models for pre, post, and follow-up time points. A maximum likelihood estimator was used, under the covariate dependent assumption for missing data, and final models used bootstrapped standard errors (SEs) across 2,000 bootstrap draws, executed in MPlus 6.11 software. Because of nonlinearity of observed slope plots, we opted to use a piecewise coding strategy for the growth models, as described by Raudenbush and Bryk (2002). Under this coding approach, separate pre-to-post and post-to-follow-up slopes are estimated. Pre-to-post slopes and intercepts were treated as random variables, were allowed to covary, and then were regressed along with the fixed variance post-to-follow-up slope on the treatment condition (0 = TAU; 1 = IG). Effect size estimates for the slopes were calculated in two ways, first using model-generated slope variability as the denominator, analogous to Cohen’s d of the “true score” changes between groups, and then using observed baseline measure variability as the denominator, analogous to a difference of Becker’s g effect sizes of raw score changes between groups (Feingold, 2009; Raudenbush & Liu, 2001). Separate models were run for the three maternal outcome measures (i.e., CMCC, SCL-90-R, and IES-R). Scatter plots were also generated to display mean estimated slopes by group, as well as the distribution of individual slope values by group.
The change model outlined in aim 3 hypothesizes that reduced maternal distress (SCL-90-R and IES-R) results from how well participants managed uncertainty (PPUS). Because sample size was limited, the exploratory analyses relied on simple contrasts among regression coefficients. The change model makes three predictions: (1) uncertainty might be positively associated with distress at pretreatment, although for a preventative treatment effect this might not be required; (2) the correlation between uncertainty and distress will become lower in the IG over time relative to TAU; and (3) treatment might or might not reduce the stressor itself but would reduce the impact of the stressor (i.e., its association with symptoms). Two models were constructed to examine the predictions about the correlation between uncertainty and symptoms, whether this correlation changed over time, and whether changes in association over time differed between groups. Each model used a multigroup approach for predicting the outcome at pre, post, and follow-up from the PPUS score at the corresponding time point. Coefficients for the outcome regressed on the corresponding PPUS score were set as group specific between the IG and TAU conditions. Between-condition coefficient contrasts were then tested at each time point, along with a difference-in-differences contrast for pre-to-post change in coefficient values. Variance equality constraints were imposed to make the regression coefficients comparable.
Results
Preliminary Analyses
The TAU group and IG did not differ with respect to baseline treatment intensity [χ2(2, N = 52) = 1.30, p = .552]. Additionally, there were no significant pretreatment differences between groups on any of the outcome variables—CMCC [F(1, 45) = .74, p = .395], PPUS [F(1, 49) = .34, p = .56], SCL-90-R [F(1, 47) = .80, p = .38], and IES-R [F(1, 48) = 1.02, p = .32]. There were no significant differences on any of the assessed demographic variables between participants who completed (n = 20 or 74%) and did not complete (n = 7 or 26%) the intervention.
Time from diagnosis to pretest and from posttest to follow-up did not significantly differ between the groups, but there were significant differences in the pre-to-post interval time [20 vs. 33 weeks for the IG and TAU group, respectively, F(1, 40) = 17.2, p < 0.001], and therefore tests were planned to examine timing effects on pre-to-post change. Three complete or partial data collection points were observed for 31 (60%) participants, two waves for 10 (19%), and one wave for 11 (21%), with the TAU group having a higher number of data collection points [2.6 vs. 2.1, F(1, 50) = 5.04, p = .03].
Feasibility of the Intervention (Aim 1)
Several pieces of data suggest that the current interdisciplinary intervention is feasible within a clinical setting for the majority of participants. Specifically, the overall consent rate for the current study was 84%. Within the IG, 74% of participants were classified as completing the intervention. Furthermore, the average number of completed face-to-face sessions with the clinical interventionist [M = 5.55, standard deviation (SD) = 1.0] and nurse interventionist (M = 4.35, SD = 1.90) was relatively high. Examination of the qualitative information in the satisfaction surveys also revealed several positive statements about the intervention, including “I wish this could be in place permanently for all new families with cancer,” “It helped me learn how to deal with everything better,” and “(I) learned some good coping skills and problem solving skills.” It is important to note, however, that the time requirements of the intervention may have been problematic for some participants as reflected by 4 of the 27 participants who were randomized to the intervention condition not being able to schedule sessions. Furthermore, although the intervention was designed to take 12 weeks, participants in the IG often took several more weeks owing to being unable to complete face-to-face sessions in the clinic. Common reasons included appointment cancellations or needing to be rescheduled, an alternative caregiver bringing the child in for their appointment, and their child having an abbreviated clinic visit (e.g., low blood counts). These observations may point toward steps to further refine feasibility.
Acceptability of the Intervention (Aim 1)
Descriptive analyses revealed that, overall, participants appeared to be satisfied with each intervention session: nature of uncertainty (M = 4.05, SD = .67, N = 22), communication with medical staff (M = 4.21, SD = .60, N = 21), uncertainty and coping (M = 4.45, SD = .80, N = 19), problem solving (M = 4.04, SD = .72, N = 15), social support (M = 4.12, SD = .55, N = 14), and consolidation of skills (M = 4.37, SD = .55, N = 13). Participants also appeared to be satisfied with the overall intervention (M = 4.51, SD = .64, N = 13). Further, an Analysis of Variation (ANOVA) comparing satisfaction across sessions revealed that participants appeared similarly satisfied with each session, F(5, 103) = 1.18, p = .326.
Effectiveness of Intervention to Reduce Maternal Distress (Aim 2)
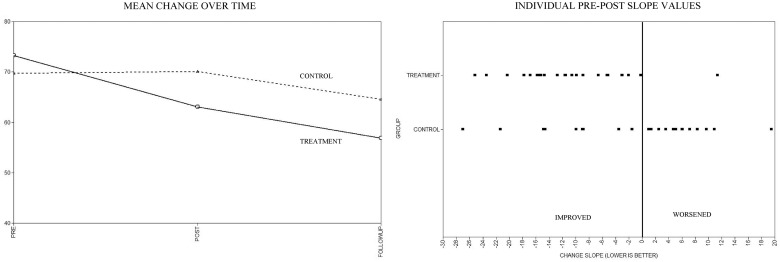
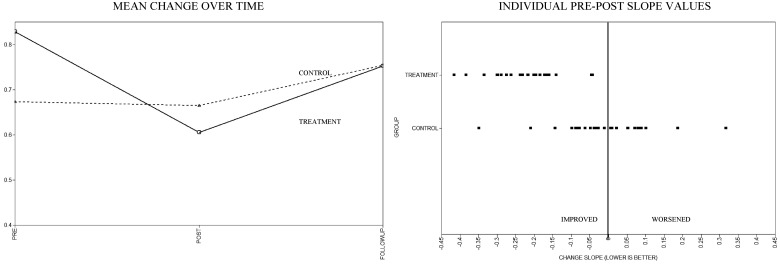
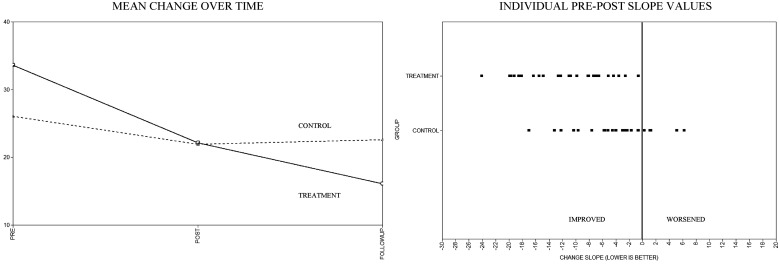
All final growth models fit the data well (as demonstrated by root mean square error of approximation [RMSEA] < .001). Both the participant distress outcomes (SCL-90-R and IES-R) demonstrated significant decreases, favoring the IG for the pre-to-post slopes. Significant intervention effects in favor of the IG also were found for caregiver burden (CMCC). No treatment differences in intercepts or post-to-follow-up slopes approached significance. Pre-to-post effect estimates, significance levels, effect sizes, and confidence intervals for the maternal outcome measures are shown in Table II.
Table II.
Pre-Post Change Slope Estimates
| Outcome Measure | Pre-post treatment effect estimate | Standard error | p (two-tailed)) | Effect sizea | Effect sizea 90% confidence interval | Effect sizeb | Effect sizeb 90% confidence interval |
|---|---|---|---|---|---|---|---|
| p (one-tailed | |||||||
| CMCC | −10.6 | 5.57 | 0.06 | −.78 | −.10 to −1.46 | −.60 | −.08 to −1.12 |
| 0.03 | |||||||
| SCL-90-R | −.214 | 0.11 | 0.05 | −1.03 | −1.9 to −.15 | −.32 | −.61 to −.05 |
| 0.03 | |||||||
| IES-R | −7.33 | 4.45 | 0.10 | −.87 | −1.7 to −.01 | −.34 | −.69 to −.01 |
| 0.05 |
Note. CMCC = Care of My Child With Cancer Scale; SCL-90-R = Symptom Checklist 90-Revised; IES-R = Impact of Events Scale-Revised.
aTreatment slope—control slope/slope variance½.
bTreatment slope—control slope/pre-treatment measure variance½.
Scatter plots of mean estimated slopes by group, as well as the distribution of individual slope values by group, are displayed in Figures 2–4. As noted in the aforementioned quantitative analyses, visual inspection of these figures reveals reductions for all outcomes in the pre-to-post slopes. Notably, these initial treatment gains appear to continue for some outcomes (IES-R and CMCC) but reverse for others (SCL-90-R) during the post-to-follow-up period. When examining the individual pre-to-post slope values, across the SCL-90-R, IES-R, and CMCC, a number of participants in the TAU group showed worsening scores over time compared with the IG, where worsening was either rare or nonexistent.
Figure 2.
Care of My Child With Cancer Scale mean and individual change estimates.
Figure 3.
Symptom Checklist 90-Revised mean and individual change estimates.
Figure 4.
Impact of Events Scale-Revised mean and individual change estimates.
To test whether the difference in pre-post measurement intervals may have impacted slopes (i.e., that greater improvement in the IG was a function of the longer time interval rather than the treatment), we first examined raw time distributions for both treatment conditions, confirming that there was substantial overlap. A series of models was then tested regressing the pre-to-post slopes on the pre-to-post time interval. None of the time interval effects approached significance in any model, either alone or in combination with treatment condition. Next, we also conducted an additional set of growth models for each outcome variable that included times from baseline as predictor along with treatment group. These models with time were then compared with a corresponding nested pre-post-follow-up model that did not include raw time values. Log-likelihood difference tests for the nested models were conducted to determine whether raw time values significantly added prediction to the models. None of the tests remotely approached significance for any outcome variable.
Observed power in the study was estimated to contextualize interpretation of findings. Estimates were calculated using Optimal Design 3.01 software for a simple pre-/post-/follow-up linear growth model with n = 52, α = .05 (one-tailed), and estimating the power to detect a medium standardized effect size of 0.50. Level 1 residual and coefficient variability values were taken from the observed growth models for each respective outcome. Power was estimated to be 0.62, 0.49, 0.70, and 0.68 for the SCL-90R, IES, CMCC, and PPUS, respectively. Low power is not surprising given that, as a preliminary study, the trial was powered to detect only larger effect sizes.
Exploratory Analyses Testing an Intervention Change Model (Aim 3)
For the SCL-90-R, baseline PPUS scores modestly but significantly predicted baseline SCL-90-R scores in both the IG and TAU group (r = .23, SE = .08, p = .006; r = .22, SE = .11, p = .04). This correlation decreased in size from pre-to-post under the IG (estimate = −.02, SE = .01, p = .01), with a trend in the TAU group (estimate = −.01, SE = .01, p = .10). The difference-in-differences test contrasting these two changes in coefficients did not approach significance.
For the IES-R outcome, there was no significant baseline correlation with PPUS for either group. The regression coefficient decreased in size in the IG (estimate = −.71, SE = .36, p = .05) vs. no change in the TAU group. There was a trend for the difference-in-differences contrast in favor of the IG showing greater reduction over time in the correlation between the PPUS and the IES-R (estimate = .71, SE = .45, p = .11). Differences in associations at follow-up did not approach significance for either outcome.
Discussion
To date, few empirically supported psychoeducational interventions exist for parents of children with cancer. In the current pilot study, we examined the feasibility and outcomes of a clinic-based interdisciplinary intervention for mothers of children newly diagnosed with cancer. Most mothers accepted enrollment, successfully completed the intervention, and rated the intervention as highly beneficial, supporting its feasibility. Additionally, mothers did not appear to favor a particular session, rating each session favorably.
For general psychological distress, posttraumatic stress symptoms, and caregiver burden, consistent significant effects or trends in favor of the IG were found for pre-to-post change. These results suggest that the intervention did indeed reduce maternal general psychological distress, posttraumatic stress symptoms, and burden. To further investigate the nature of this change, scatter plots were generated, which revealed that several participants in the TAU group showed worsening scores over time; this pattern was not observed in the IG. Thus, it may be that the intervention served a preventive, protective, or buffering function for those mothers who were at risk for experiencing worsening symptoms over time. It is also important to note that the intervention was conducted in a clinic that provides routine ad hoc psychological services to all children and families. Thus, the benefits observed from the IG can be interpreted as incremental. Effects may have been larger with a no-services control comparison.
Intervention effects tended not to extend beyond the intervention period, and all post-to-follow-up between-group comparisons failed to approach significance. Visual examination of graphs suggested that gains tended to continue for some outcomes (IES-R and CMCC) but reversed for others (SCL-90-R). It is possible that distress may reemerge after services end or may change in type (i.e., from traumatic types of symptoms to other types) as the course of the child’s illness and treatment unfolds. This could suggest a need for monitoring, booster services for reemergent types of distress, or different services in response to different types of distress. How courses of service delivery might be matched to courses of distress or symptoms remains an open topic for future investigation.
Exploratory analyses examining the change model for the intervention offered partial support for the hypothesis that the intervention may have prevented or reduced distress by lowering the association between uncertainty and symptoms or by preventing an association from developing. Certainly, larger sample studies are needed to confirm these exploratory trends. The small size of this effect suggests that the benefits of the IG may derive from other pathways in addition to a pathway involving improved coping with uncertainty.
The current study is limited by the small sample size, and future studies with much larger samples across multiple sites are warranted. The use of a TAU control group, as opposed to an attention or placebo control group, is also a limiting factor. It must be acknowledged that the sheer amount of time spent with supportive individuals may have resulted in an improvement in adjustment. In addition, the multidimensional and multicomponent nature of this study precludes any conjecture as to the precise elements that are operative in the intervention itself. Although speculative, it may well be that uncertainty is best conceptualized as a stressor in and of itself to which one attempts to cope and adapt. Notably, other researchers have demonstrated the efficacy of problem-solving therapy (Sahler et al., 2005) for parents of children with cancer, and comparisons of these approaches might help identify common and unique contributions to the management of distress. It should also be noted that the methodology used in the current study does not allow for examination of the relative contribution of the psychology vs. nursing intervention components. However, parents viewed the intervention as facilitating their understanding of their child’s condition and learned coping strategies as shown by the data reported earlier. It may also be helpful to consolidate the intervention into a shorter format, as our feasibility data suggested that it could be challenging to deliver the entire program in a 12-week time period. Future interventions should attempt to also include fathers.
In summary, our results indicate that an interdisciplinary intervention targeting parental illness uncertainty has value for parents of children diagnosed with cancer and that such an intervention is feasible. Such an intervention also has the potential to indirectly impact child adjustment, as demonstrated by Hoff et al. (2005) in children newly diagnosed with type 1 diabetes. Targeting parents for intervention is also particularly relevant in the pediatric cancer context, given the young age of many newly diagnosed patients whose developmental level may preclude intervention. Developing clinic-based interventions, such as this one, is also particularly important for pediatric cancer centers because families often travel great distances to receive treatment and an additional visit for psychosocial services could be considered a burden.
Furthermore, manualized interventions such as this have the potential to be readily translated into electronic forms, deliverable by a variety of mechanisms (e.g., web-based, DVD) to provide greater reach in a much more cost-effective manner. Development of such electronic tools might result in greater reach for the intervention and also help with potential problems with attrition (Palermo, Wilson, Peters, Lewandowski, & Somhegyi, 2009).
Funding
This work was supported by a grant (R21NR010103) to L. L. Mullins, PhD. (Principal Investigator) from the National Institutes of Health (NIH/NINR).
Conflicts of interest: None declared.
Acknowledgments
The authors thank the families who participated in this research project, as well as Kathy Kirk, Mark Fisher, and Philip Rambo, all of whom were key personnel on this project.
References
- American Cancer Society. Cancer facts & figures 2010. 2010. Retrieved from http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- Derogatis LR. Symptom checklist-90-revised: Administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems, Inc; 1994. [Google Scholar]
- Dwyer K. Beyond cancer: Fostering transitions post-treatment. Bethesda, MD: National Institutes of Health; 2006. Grant #3R21CA100120-02S1. [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14:43–53. doi: 10.1037/a0014699. doi:10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuemmeler B F, Mullins L L, Marx B P. Posttraumatic stress and general distress among parents of children surviving a brain tumor. Children’s Health Care. 2001;30:169–182. doi:10.1207/S15326888CHC3003_1. [Google Scholar]
- Hobbie W L, Stuber M, Meeske K, Wissler K, Rourke M T, Ruccione K, Hinkle A, Kazak AE. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. Journal of Clinical Oncology. 2000;18:4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- Hoff A L, Mullins L L, Gillaspy S R, Page M C, Van Pelt J C, Chaney J M. An intervention to decrease uncertainty and distress among parents of children newly diagnosed with diabetes: A pilot study. Families, Systems, & Health. 2005;23:329–342. doi:10.1037/1091-7527.23.3.329. [Google Scholar]
- Institute of Medicine. Health professions education: A bridge to quality. Washington DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Kazak A E. Implications of survival: Pediatric oncology patients and their families. In: Bearison D J, Mulhern R K, editors. Pediatric psycho-oncology: Psychological perspectives on children with cancer. New York: Oxford University Press; 1994. pp. 171–192. [Google Scholar]
- Kazak A E, Boeving C A, Alderer M A, Hwang W T, Reilly A. Posttraumatic stress symptoms during treatment in parents of children with cancer. Journal of Clinical Oncology. 2005;23:7405–7410. doi: 10.1200/JCO.2005.09.110. doi:10.1200/JCO.2005.09.110. [DOI] [PubMed] [Google Scholar]
- Kazak A E, Simms S, Alderfer M A, Rourke M T, Crump T, McClure K, Jones P, Rodriguez A, Boeving A, Hwang W T, Reilly A. Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnosed with cancer. Journal of Pediatric Psychology. 2005;30:644–655. doi: 10.1093/jpepsy/jsi051. doi:10.1093/jpepsy/jsi051. [DOI] [PubMed] [Google Scholar]
- Mishel M H. Parents’ perception of uncertainty concerning their hospitalized child. Nursing Research. 1983;32:324–330. doi:10.1097/00006199-198311000-00002. [PubMed] [Google Scholar]
- Mishel M H. Perceived uncertainty and stress in illness. Research in Nursing and Health. 1984;7:163–171. doi: 10.1002/nur.4770070304. doi:10.1002/nur.4770070304. [DOI] [PubMed] [Google Scholar]
- Mishel M H. Reconceptualization of the uncertainty in illness theory. Image: Journal of Nursing Scholarship. 1990;22:256–262. doi: 10.1111/j.1547-5069.1990.tb00225.x. doi:10.1111/j.1547-5069.1990.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Mishel M H, Germino B B, Belyea M, Stewart J L, Bailey D E, Mohler J, Robertson C. Moderators of an uncertainty management intervention: For men with localized prostate cancer. Nursing Research. 2003;52:89–97. doi: 10.1097/00006199-200303000-00005. doi:10.1097/00006199-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Mishel M H, Germino B B, Gil K M, Belyea M, Laney I C, Stewart J, Porter L, Clayton M. Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psycho-Oncology. 2005;14:962–978. doi: 10.1002/pon.909. doi:10.1002/pon.909. [DOI] [PubMed] [Google Scholar]
- Mullins L L, Balderson B H K, Chaney J M. Implementing team approaches in primary and tertiary care settings: Applications from the rehabilitation context. Families, Systems, and Health. 1999;17:487–500. [Google Scholar]
- Mullins L L, Keller J, Chaney J M. A systems and social cognitive approach to team functioning in physical rehabilitation settings. Rehabilitation Psychology. 1994;39:161–178. [Google Scholar]
- Pai A L H, Drotar D, Zebracki K, Moore M, Youngstrom E. A meta-analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. Journal of Pediatric Psychology. 2006;31:978–988. doi: 10.1093/jpepsy/jsj109. doi:10.1093/jpepsy/jsj109. [DOI] [PubMed] [Google Scholar]
- Palermo T M, Wilson A C, Peters M, Lewandowski A, Somhegyi H. Randomized control trial of an internet delivered family cognitive behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146:205–213. doi: 10.1016/j.pain.2009.07.034. doi:10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patiño-Fernández A M, Pai A, Alderfer M, Hwang W T, Reilly A, Kazak A E. Acute stress in parents of children newly diagnosed with cancer. Pediatric Blood & Cancer. 2008;50:289–292. doi: 10.1002/pbc.21262. doi:10.1002/pbc.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Pradhan P V, Shah H. Psychopathology and coping in parents of chronically ill children. Indian Journal of Pediatrics. 2004;71:695–699. doi: 10.1007/BF02730656. doi:10.1007/BF02730656. [DOI] [PubMed] [Google Scholar]
- Raudenbush S W, Bryk A S. Hierarchical linear models. Second ed. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush S W, Liu X. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychological Methods. 2001;6:387–401. doi:10.1037/1082-989X.6.4.387. [PubMed] [Google Scholar]
- Sahler O J, Fairclough D L, Phipps S, Mulhern R K, Dolgin M J, Noll R B, Katz E R, Varni J W, Copeland D R, Butler R W. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: Report of a multisite randomized trial. Journal of Consulting and Clinical Psychology. 2005;73:272–283. doi: 10.1037/0022-006X.73.2.272. doi:10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- Sahler O J, Roghmann K J, Mulhern R K, Carpenter P J, Sargent J R, Copeland D R, Barbarin O A, Zeltzer L, Dolgin M J. Sibling adaptation to childhood cancer collaborative study: The association of sibling adaptation with maternal well-being, physical health, and resource use. Journal of Development and Behavioral Pediatrics. 1997;18:233–243. [PubMed] [Google Scholar]
- Sahler O J, Varni J W, Fairclough D L, Butler R W, Noll R B, Dolgin M J, Phipps S, Copeland D R, Katz E R, Mulhern R K. Problem-solving skills training for mothers of children with newly diagnosed cancer: A randomized trial. Journal of Developmental and Behavioral Pediatrics. 2002;23:77–86. doi: 10.1097/00004703-200204000-00003. doi:10.1097/00004703-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Stehl M L, Kazak A E, Alderfer M A, Rodriguez A, Hwang W, Pai A L H, Boeving A, Reilly A. Conducting a randomized clinical trial of a psychological intervention for parents/caregivers of children with cancer shortly after diagnosis. Journal of Pediatric Psychology. 2009;34:803–816. doi: 10.1093/jpepsy/jsn130. doi:10.1093/jpepsy/jsn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J S, Mishel M H. Uncertainty in childhood illness: A synthesis of the parent and child literature. Scholarly Inquiry for Nursing Practice: An International Journal. 2000;14:299–319. [PubMed] [Google Scholar]
- Weiss D, Marmar C. The impact of event scale—Revised. In: Wilson J, Keane T, editors. Assessing psychological trauma and PTSD. New York: Guildford Press; 1997. pp. 399–411. [Google Scholar]
- Wells D K, James K, Stewart J L, Moore I M, Kelly K P, Moore B, Bond D, Diamond J, Hall B, Mahan R, Roll L, Speckhart B. The care of my child with cancer: A new instrument to measure caregiving demand in parents of children with cancer. Journal of Pediatric Nursing. 2002;17:201–210. doi: 10.1053/jpdn.2002.124113. doi:10.1053/jpdn.2002.124113. [DOI] [PubMed] [Google Scholar]
- Zelen M. The randomization and stratification of patients to clinical trials. Journal of Chronic Diseases. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. doi:10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]