Abstract
Objective
Endothelial cell activation is an important mediator of monocyte recruitment to sites of vascular inflammation. We hypothesised that high-affinity dual-ligand microparticles of iron oxide, targeted to P-selectin and vascular cell adhesion molecule-1 (VCAM-1) (PV-MPIO), would identify activated endothelial cells during atherosclerosis progression.
Methods and Results
In vivo MRI in apolipoprotein E-deficient mice showed rapid binding of PV-MPIO to the aortic root, which was maximal 30 minutes post-MPIO injection and maintained at 60 minutes. Minimal binding was observed for control IgG-MPIO. Intensely low MR signal areas, corresponding to PV-MPIO binding, were detected early (14 weeks), during foam cell formation. Contrast effects increased at 20 weeks during fibrofatty lesion development (P < 0.05), but reduced by 30 weeks (P < 0.01). Across all lesion severities, MRI contrast effects correlated with lesion macrophage area quantified by immunohistochemistry (R = 0.53; P < 0.01). Near-infrared fluorescently labeled PV-MPIO were shown, by flow cytometry, to bind only activated endothelial cells, and not to macrophages. Using en face immunofluorescence, we further demonstrate selective PV-MPIO accumulation at atherosclerosis-susceptible sites, with minimal binding to atherosclerosis-spared regions.
Conclusions
This high affinity leukocyte mimetic MRI agent reveals endothelial activation. PV-MPIO demonstrate exceptionally rapid in vivo steady state accumulation, providing conspicuous MR contrast effects that can be objectively quantified. In atherosclerosis progression, PV-MPIO tracked closely with the burden and distribution of plaque macrophages, not merely plaque size. On a biocompatible platform, this approach has potential for quantitative MRI of inflammatory disease activity.
Keywords: adhesion molecules, atherosclerosis, iron oxide contrast agent, leukocyte, magnetic resonance imaging
Correspondence: Professor Robin Choudhury Department of Cardiovascular Medicine, Level 6 West Wing, John Radcliffe Hospital, Oxford. OX3 9DU United Kingdom Telephone: +44-1865-234663 Fax: +44-1865-234667 robin.choudhury@cardiov.ox.ac.uk
Introduction
Inflammation, notably macrophage infiltration, is an important determinant in the pathogenesis of atherosclerosis.1, 2 Macrophages are involved in all stages of atherosclerotic lesion development and may trigger clinical events such as myocardial infarction or stroke by promoting fibrous cap degradation and plaque disruption.1,3,4-6 Conversely, interventions that regress atherosclerosis and stabilize plaques have been associated with reduced inflammation and a diminution in plaque macrophage content.7, 8 There is increasing evidence that the lesion macrophage population is not static, but is involved in ongoing influx and excursion.9 Despite the critical role played by macrophages, non-invasive MRI techniques for their accurate quantification are still imperfect.10
Monocyte recruitment to the vascular wall is promoted by upregulation of endothelial adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1; CD106) and P-selectin (CD62P) at atherosclerosis-prone sites.11-13 Initial monocyte-endothelial interactions are mediated by P-selectin, which stimulates monocyte rolling along the activated endothelium,13 whereas firm adhesion of monocytes depends on the engagement of integrin α4β1 (also termed very late antigen-4, VLA-4) with endothelial VCAM-1, preceding their transmigration to the nascent lesion.14, 15 Appreciation of these mechanisms and their relevance to atherogenesis lays a foundation for the design of molecular imaging probes that can determine, non-invasively, in vivo, the propensity of regional arterial endothelial activation to attract monocytes.16-18
To image vascular endothelial cell activation with magnetic resonance imaging (MRI), we previously developed prototypic 4.5 μm diameter microparticles of iron oxide (MPIO) conjugated to antibodies against both VCAM-1 and P-selectin.19 Although this approach proved promising in feasibility experiments, demonstrated by ex vivo MRI of explanted mouse aortas (with advanced atherosclerosis), retention of MPIO was insufficient at acceptable iron doses for reliable in vivo molecular MRI. To overcome this limitation, we have developed a second-generation of smaller (1.0 μm) MPIO that have higher surface area to volume ratio for polyvalent ligand conjugation and which, we hypothesized, would be less buoyant under conditions of flow and high shear stress. These micron size-range particles should be distinguished from the targeted20 or untargeted21, 22 nano-scale particles that have more commonly been used for atherosclerosis imaging. Compared to nano-scale particles, MPIO offer distinct advantages: (a) the payload of iron and, therefore, sensitivity is high;23, 24 (b) the clearance of MPIO from circulation is very rapid so “background” blood phase contrast is minimal;25 (c) the obligate intravascular MPIO are much more tractable for endothelial molecular imaging than nanoparticles, which are susceptible to passive accumulation26, 27 and (d) they are readily functionalized allowing conjugation of one or more high valency targeting ligands.28-30
Accordingly, we have developed a leukocyte mimetic contrast agent, based on size and surface ligands, which targets both VCAM-1 and P-selectin. We test the extent to which dual-ligand leukocyte mimetic MPIO home to activated endothelium and reflect inflammatory cell content in vivo across a range of atherosclerotic lesion complexities in apolipoprotein E−/− mice. We further determine cellular binding patterns of dual-ligand MPIO in regions of the aorta that are susceptible to atherosclerotic lesion development. In so doing, we have sought to use the leukocyte mimetic properties of MPIO to map vascular inflammation in atherosclerosis.
Methods
A detailed Supplemental Methods section is available online at http://atvb.ahajournals.org.
Preparation of MPIO
Rat anti-mouse monoclonal VCAM-1 (clone M/K2) (Cambridge Bioscience Ltd, UK) and P-selectin antibodies (CD62P clone RB40.34) (BD Biosciences, UK) were covalently conjugated to the surface of tosyl activated MPIO (1 μm diameter) (Invitrogen, UK) in a 50:50 ratio to produce dual-ligand MPIO (PV-MPIO) as previously described.26, 31 Isotype control rat IgG-1 antibody (clone Lo-DNP-1) (Serotec, UK) conjugated MPIO (IgG-MPIO) were also prepared. For fluorescence imaging using en face immunofluorescence and flow cytometry, near infrared fluorescently labeled dual-ligand MPIO were developed. P-selectin and VCAM-1 monoclonal antibodies (50:50 mix) were combined with a near infra-red Alexa Fluor 750 dye for 60 minutes at RT according to the Small Animal In Vivo Imaging (SAIVI™) Alexa Fluor 750 antibody labeling kit (Invitrogen), which is azide-free and thus suitable for in vivo applications. Approximately two Alexa Fluor 750 molecules were coupled to each antibody molecule, according to the manufacturers protocol. The SAIVI™ 750 labeled P-selectin and VCAM-1 antibody mix was purified by gel column filtration and then conjugated to the surface of tosyl MPIO, as above. The SAIVI™ 750 dual-ligand MPIO were stored in the dark at 4°C. For in vitro flow chamber experiments, MPIO conjugated to mouse anti-human P-selectin and VCAM-1 antibodies or their counter-ligands, P-selectin glycoprotein ligand-1 (PSGL-1) or very late antigen 4 (VLA-4; α4β1) were developed (Supplemental Methods).
Mouse atherosclerosis
Female apolipoprotein knockout (apoE−/−) mice on a C57BL/6 background were weaned at 3 weeks of age and fed a standard rodent chow diet ad libitum. Mice underwent MR imaging after 8, 14, 20 and 30 weeks on chow diet to determine the ability of dual-ligand MPIO to track endothelial adhesion molecule expression from early foam cell formation to more advanced atherosclerotic lesions. Animal procedures were performed in accordance with UK Home Office Animals (Scientific Procedures) Act 1986.
In vivo MRI
Mice underwent in vivo MR imaging of the aortic root using a 9.4 Tesla horizontal magnet interfaced to a VNMRS DirectDrive MR system (Varian Inc. USA). Animals were anesthetized with 4% isofluorane in O2 gas mixture and maintained with 1.5-1.8% isofluorane in 100% O2 (3 L/minute) delivered through a nosecone and positioned in a cradle in a 33 mm birdcage coil (Rapid Biomedical, Rimpar, Germany). Cardiac and respiratory signals were continuously monitored using an in-house developed ECG- and respiratory gating device.32 The aortic root was identified in a coronal section on a localizing sequence. T2* weighted images were acquired in end-diastole using a double-gated, flow-compensated, segmented 3D gradient-echo sequence (TE / TR = 2.4 / 4.0 ms, repetition time per segment 120 ms to 150 ms depending on heart rate, 2 k-space lines per cardiac cycle; matrix size 256 × 256 × 48; number of signal averages 1, slice thickness 8 mm, field of view 30 × 30 ×12 mm; flip angle 15°; reconstructed resolution 59 × 59 × 125 μm3). After pre-contrast imaging, the mice were administered either dual-ligand PV-MPIO (n = 6 - 7 per group) or control IgG-MPIO (n = 2-3 per group) (3.3 mg Fe / kg body weight) via tail vein injection. Post-contrast images were acquired at ~30 minutes and ~60 minutes after MPIO injection. Mice were terminally anesthetized with isofluorane and perfusion fixed with 4% paraformaldehyde (PFA). Histopathological examination of aortic roots was performed (Supplemental Methods).
MR image analysis
Aortic root low signal intensity areas were measured by drawing an area of interest around the outer wall of the aortic root using ImagePro Plus version 6.1. The threshold of low signal, corresponding to MPIO binding, was defined to be 3 standard deviations below the mean signal intensity of the pre-contrast root on signal intensity histograms. This threshold was applied to sequential MR slices through the aortic root, before and after MPIO contrast, to determine the mean area of MPIO low signal contrast. Pseudo-colored maps of bound MPIO were generated using the same greyscale thresholds, to visualise the distribution and magnitude of MPIO signal intensities in the aortic root wall, using a pseudocolor range from 0-255 colours. The contrast to noise ratios (CNR) of identified MPIO-positive lesion areas were compared to the CNR of equivalent areas on the pre-contrast images as CNR = blood pool signal – lesion signal/ standard deviation of the noise as previously described.33
Flow cytometry
To assess the cellular distribution of dual-ligand MPIO binding, flow cytometry experiments were performed on cell suspensions from disaggregated whole aortas (Supplemental Methods). Whole blood from apoE−/− mice was also assessed to establish whether there was binding of dual-ligand MPIO to circulating platelets. Apo E−/− mice (n = 3), 20 wks on chow diet, were injected i.v. with near-infrared SAIVI™ 750 PV-MPIO (3.3 mg/kg). After 60 minutes circulation, mice were terminally anesthetized and perfused with ice-cold PBS. Aortas from apo E−/− mice (n = 3) without MPIO injection served as controls.
En Face Immunofluorescence
ApoE−/− mice, 20 weeks on chow diet, were injected with near-infrared SAIVI™ 750 labelled PV-MPIO (3.3 mg / kg). After 60 minutes, mice were terminally anesthetised and aortas perfused in situ with PBS and then 4% PFA via the left ventricle. Aortas were further fixed in 4% PFA for 30 minutes. Mouse aortas were examined using en face immunofluorescence (Supplemental Methods) to assess the binding patterns of dual-ligand MPIO binding in regions of the proximal and descending aorta with high and low probability (HP and LP) for atherosclerotic lesion development.34, 35
Statistical analysis
Results are expressed as mean ± S.D. GraphPad Prism 5 (GraphPad Software, Inc., San Diego, USA) was used for statistical analysis. For imaging experiments with multiple groups, differences were evaluated using two-way analysis of variance (ANOVA). Unpaired 2-tailed Student’s t-tests were applied to determine differences between two groups and paired 2-tailed Student’s t-tests to compare differences in MRI data within a group before and after MPIO administration. Spearman rank non-parametric correlations were performed to correlate MRI and histological quantification of aortic root dual-ligand MPIO binding and the relationship between aortic root lesion macrophage content and dual-ligand MPIO binding. The significance level in all tests was P < 0.05.
Results
Antibody conjugated MPIO show high binding affinity to stimulated endothelial cells in vitro
To test surface orientation of the cognate ligands VLA-4 and PSGL-1 conjugated to MPIO, the particles were incubated with human recombinant VCAM-1-Fc or human recombinant P-selectin-Fc chimeras. Figure 1A shows green fluorescent MPIO confirming binding of VLA-4 MPIO to recombinant VCAM-1 and PSGL-1 MPIO to recombinant P-selectin. There was no binding of PSGL-1-MPIO to recombinant VCAM-1-Fc but VLA-4 MPIO showed some cross reactivity to recombinant P-selectin-Fc. In cellular binding experiments, quantification of MPIO adherent to stimulated HUVEC after 5 min of flow showed that antibody conjugated MPIO were retained significantly more than either VLA-4 MPIO (18-fold; P < 0.0001) or PSGL-1 MPIO (185-fold; P < 0.01) (Figure 1C). Antibody conjugated MPIO were therefore used for in vivo experiments in mice.
Figure 1. Antibody-conjugated MPIO bind with greater affinity in vitro than cognate ligand MPIO.
(A) VLA-4 MPIO and PSGL-1 MPIO were incubated with either Fc-VCAM or Fc-P-selectin. Green fluorescence indicates binding of Fc-VCAM to VLA-4 MPIO and Fc-P-selectin to PSGL-1 MPIO, with no MPIO binding to secondary antibody only or to non-his-tagged protein. (B) Immunofluorescent staining of VCAM-1 and P-selectin expression using cognate ligands and antibodies. VLA-4 binding was detected using an anti-human his tagged FITC while anti-mouse Alexa Fluor 594 was used to detect primary anti-human VCAM-1. PSGL-1 binding was detected using an anti-human FITC antibody and anti-P-selectin detected with anti-mouse Alexa Fluor 488. (C) Representative images of MPIO binding to stimulated HUVEC after 5 min of flow (flow rate 1 dyne cm−2). Antibody conjugated MPIO binding was significantly greater than VLA-4 MPIO (P < 0.0001) or PSGL-1 MPIO (P < 0.01).
In vivo imaging of adhesion molecules in atherosclerotic mice using dual-ligand MPIO
To determine whether dual-ligand MPIO can detect endothelial activation in vivo, MRI was performed at 8, 14, 20 and 30 weeks during atherosclerotic lesion development. Representative MR images of aortic root atherosclerotic lesions pre- and post-injection of either dual-ligand MPIO or negative control IgG-MPIO are shown in Supplemental Figure 1. MR images showed highly conspicuous areas of low signal after dual-ligand MPIO injection, with minimal MR signal effects observed in pre-contrast images and in aortic root images of mice injected with control IgG-MPIO. Sequential MR images through the root showed strong low signal areas 30 minutes post-MPIO injection, which persisted at similar levels at 60 minutes (Figure 2A). Pseudo-color maps illustrated the localisation and magnitude of the T2* signal intensity enhancement in the aortic root wall (Figure 2B). Contrast effects were confined to regions of the root affected by atherosclerosis and not to lesion-free areas, as confirmed by histology (Figure 2C). The contrast-to-noise ratios (CNR) of MPIO-positive lesion areas and adjacent blood pool increased by 87% (P<0.05), 100% (P<0.01) and 96% (P<0.01) at 14, 20 and 30 weeks respectively compared to equivalent pre-contrast aortic lesion areas. No difference in lesion CNR was observed between time-points.
Figure 2. In vivo MR images of aortic root post dual-ligand MPIO injection.
(A) Consecutive MR images show low signal areas in the aortic root in apoE−/− mouse (14 weeks on chow diet) at 30 minutes post PV-MPIO injection, which are maintained at 60 minutes. (B) Pseudo-colored maps (0-255 range) show the localisation and magnitude of the T2* signal intensity in the aortic root, with red indicating the greatest and blue the least signal intensities. (C) The distribution of MR signal intensity is confined to atherosclerotic foam cell lesions and not to lesion-free endothelium, as confirmed by histology (arrows) using combined Masson’s trichrome and Elastin stain. (D) Contrast-to-noise ratio (CNR) of MPIO-positive lesion areas was significantly increased (P<0.05 at 14 weeks; P<0.01 at 20 and 30 weeks) after injection of PV-MPIO compared to equivalent lesion areas on pre-contrast images (mean ± SD), with no significant difference in post-MPIO contrast CNR between time-points. Scale bars = 1 mm.
Semi-quantitative analysis of the area of PV-MPIO contrast effects in the aortic roots showed minimal detectable MR contrast effects at 8 weeks, with a 3.9-fold increase in the area of low MR contrast effects at 14 weeks (P < 0.05), and a further 1.8-fold increase by 20 weeks (P < 0.05). However, at 30 weeks, there was a 1.8-fold reduction in MR contrast effects compared to 20 weeks (P < 0.01) (Figure 3A).
Figure 3. MRI and histological quantification of MPIO binding to aortic roots.
(A) Mean area (± SD) of low MR signal areas in aortic roots at baseline and 30 and 60 minutes after injection of PV-MPIO. Dynamic MRI identified rapid PV-MPIO binding to aortic roots of apoE−/− mice by 30 minutes post-injection, which persisted at 60 minutes (*P < 0.05, **P < 0.01; n = 6-7 apoE−/− mice per group injected with PV-MPIO; n = 2-3 apoE−/− mice per group injected with IgG-MPIO; n=2 C57Bl/6 wild type mice injected with PV-MPIO. (B) Histological quantification found a significant increase in the number of bound PV-MPIO between 8 and 20 weeks, and a significant reduction between 20 and 30 weeks. No MPIO binding was observed to plaque-free areas of the endothelium in aortic roots of apoE−/− mice or control wild type mice. (C) Low MR signal areas significantly correlated with the number of bound PV-MPIO by histology. (D) Immunohistochemistry confirmed aortic root VCAM-1 expression.
To test whether MR contrast effects reflected MPIO retention, the number of bound PV-MPIO were quantified by histology (Figure 3B). Significant increases in the number of PV-MPIO binding were observed at 14 and 20 weeks, with a reduction at 30 weeks, reflecting the MR contrast effects. The number of bound PV-MPIO quantified by histology correlated with MR signal intensity areas corresponding to aortic root PV-MPIO binding (P < 0.001) (Figure 3C). Irrespective of age, dual-ligand MPIO localized only to endothelium overlying atherosclerotic lesions, with no MPIO seen within lesions or bound to lesion-free endothelium. Immunohistochemistry for VCAM-1 confirmed endothelial activation on atherosclerotic lesions (Figure 3D).
Dual-ligand MPIO binding tracks quantitatively with lesion macrophage content
To investigate whether dual-ligand MPIO accumulation tracked with macrophage content within the lesion over time, aortic root sections were immunostained for Gal-3 (Figure 4A). Lesion macrophage area increased between 8 and 14 weeks, with a further increase by 20 weeks. However, between 20 and 30 weeks, there was a significant reduction in total macrophage area, which reflected the pattern of dual-ligand MPIO binding to the overlying endothelium (Figure 3B). Total lesion size also increased up to 20 weeks, but between 20 and 30 weeks, plaque size remained constant (Fig 4B). Lesion macrophage area correlated with dual-ligand MPIO binding to the aortic root assessed by in vivo MRI (R = 0.53; P < 0.01) (Figure 4C).
Figure 4. Dual-ligand MPIO binding quantitatively reflects lesion macrophage content.
(A) Representative images of aortic roots with lesion macrophage immunostained using Gal-3 antibody. Scale bar = 0.5 mm. Quantification of total lesion macrophage area (mm2) showed significant increases in lesion macrophage content from 8 to 20 wks and a significant reduction between 20 and 30 weeks. Magnification x 5. (B) Bound dual-ligand MPIO (yellow spheres) localized to endothelium overlying atherosclerotic lesions, with no binding observed to macrophages within the lesion. Magnification x 100. (C) Lesion size also increased from 8 to 20 weeks, but there was no change between 20 to 30 weeks. (D) Lesion macrophage content correlated significantly with dual-ligand MPIO MRI signal area.
Dual-ligand MPIO bind to activated endothelial cells at atherosclerosis-susceptible sites
In order to test the cellular specificity of MPIO binding in this context, we developed dual-ligand MPIO, fluorescently labelled with a near-infrared SAIVI™ alexa fluor 750 fluorophore. Flow cytometry was performed on cellular suspensions from digested aortas and whole blood of mice 60 minutes after intravenous injection of near-infrared SAIVI™ 750 PV-MPIO. Binding was predominantly to VCAM-1 positive (CD31+ CD11b−) aortic endothelial cells, with a minority bound to non-activated VCAM-1 negative endothelial cells (P < 0.001). There was no association of MPIO with VCAM-1+ CD31− cells and only minimal MPIO uptake observed for macrophages (CD11b+ F4./80+) (Figure 5). Analyses of whole blood found that 22% of cells were CD41+ CD11b− platelets, of which 1% were CD41+ CD11b− CD62P+. No MPIO binding to CD41+ CD11b− or to CD41+ CD11b− CD62P+ circulating platelets was detected (Supplemental Figure 2).
Figure 5. Flow cytometry shows predominant binding of near-infrared fluorescently labeled dual-ligand MPIO to activated aortic endothelial cells in apoE−/− mice.
(A) Overlay of fluorescence signal intensity histograms in the Alexa Fluor 750 PV-MPIO channel for each cell type. (B) Mean fluorescence intensity identified activated endothelial cells (CD31+ VCAM+ CD11b−) as the dominant cellular signal source with minimal signal in macrophages (CD11b+ F4/80+) or other cell types.
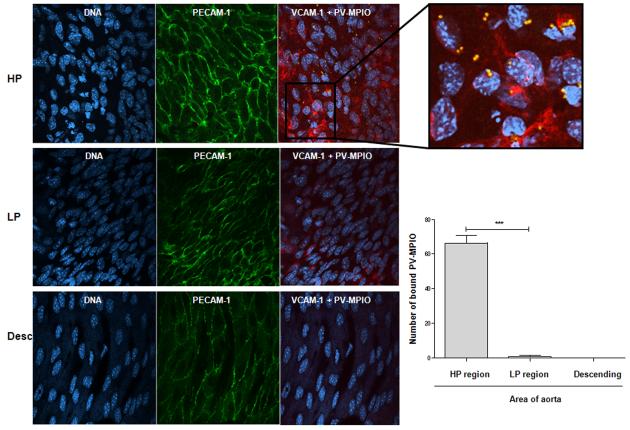
Predisposition to atherosclerosis is asymmetric in the proximal aorta with VCAM-1 up-regulation in the atherosclerosis-prone lesser curvature and is relatively sparing in the greater curvature of the arch and descending tubular aorta. We next investigated the spatial localisation of near-infrared SAIVI™ 750 dual-ligand MPIO binding in the aorta using en face immunofluorescence microscopy. Endothelial cells in the atherosclerosis-susceptible (HP region) inner curvature of the aortic arch displayed irregular cuboidal shapes, which did not align longitudinally and showed upregulation of VCAM-1 (Figure 6A). PV-MPIO were selectively retained by the endothelial in this area compared to the atherosclerosis-resistant outer curvature (LP region) of the aortic arch and descending aorta, which displayed endothelial cells of normal shape and alignment, with minimal levels of VCAM-1 expression (Figure 6B).
Figure 6. Dual-ligand MPIO binding localises to activated endothelial cells in atherosclerosis-susceptible sites of the aortic arch.
Representative images of the spatial distribution of PV-MPIO binding to the inner curvature (high probability [HP] region) or outer curvature (low probability [LP] region) of the aortic arch and the descending aorta assessed by en face immunofluorescence. The blue colour represents endothelial cell nuclei, counterstained with DAPI. The green fluorescent staining indicates endothelial PECAM-1 expression, the red fluorescent staining indicates endothelial VCAM-1 expression and the yellow spheres represent endothelial-bound PV-MPIO (fluorescently labelled with near-infrared alexa fluorophore 750 nm). The HP area had high levels of endothelial VCAM-1 expression, with cell nuclei that are not aligned in the same direction while the LP area and the descending aorta has low levels of VCAM-1 expression and cell nuclei that are aligned in a unilateral direction. Quantification of PV-MPIO binding in 4 images per area showed co-localisation of PV-MPIO to endothelial cells expressing VCAM-1 in the HP region, with minimal PV-MPIO binding in the LP region of the aortic arch or the descending aorta.
Discussion
Quantitative assessment of local vascular inflammation is a key goal in the diagnosis and characterisation of vascular disease and is likely to become more important as drugs directly targeting inflammatory pathways are developed for the treatment of high-risk patients with atherosclerosis.16, 18, 36 The vascular endothelium is the principal regulatory interface that governs the recruitment of mononuclear cells to sites of injury and inflammation, through the up-regulation of adhesion molecules, and is therefore an important target for this purpose.13, 37 Here, we demonstrate specific in vivo molecular imaging of endothelial cell activation during atherosclerotic lesion development in apoE−/− mice using leukocyte-mimetic MPIO, targeting both VCAM-1 and P-selectin. These particles rapidly achieve steady state accumulation at target and provide exceptionally conspicuous, objectively quantifiable contrast effects. The MR contrast effects generated by dual-ligand MPIO binding also tracked quantitatively with lesion macrophage content across a range of lesion complexities. However, we show through both microscopy and flow cytometry that MPIO binding is confined to activated endothelial cells, and they are not bound to, or taken up by, macrophages within plaque. Using en face techniques to evaluate anatomically distinct areas of aorta, we further demonstrate selective binding of PV-MPIO in atherosclerosis-prone segments of the aortic arch that were associated with VCAM-1 up regulation and loss of normal endothelial cell architecture.
Adhesion molecule up-regulation was identified during early foam cell lesion, at 14 weeks on chow diet, with a further increase detected at 20 weeks, in fibrofatty lesions. However, at 30 weeks, in vivo MRI quantification detected a significant, apparently paradoxical, reduction in adhesion molecule expression. However, histological examination showed that PV-MPIO accumulation faithfully reflected actual changes in macrophage content. This is consistent with regulation of monocyte influx at the level of the vascular endothelium, with overall lesion macrophage content reflecting the net effects of recruitment vs. egress, apoptosis and necrosis.9 In addition, the distribution of PV-MPIO was spatially related to the areas of the root showing foam cell formation, while non-lesional areas did not retain MPIO. This agrees with reports that VCAM-1 is most strongly up-regulated in vascular endothelium overlying plaque.38
Molecular imaging approaches benefit from rapid accumulation and attainment of steady state at target, with clearance of unbound (blood phase) contrast. Serial imaging revealed that PV-MPIO accumulated by 30 minutes on activated vascular endothelium and was maintained at 60 minutes, thus providing a practical imaging time frame. In an alternative strategy, peptides generated by phage display have been selected to bind specifically to activated endothelium and conjugated to nanoparticles.20 After binding to VCAM-1, the nanoparticles are internalized by endothelial cells and macrophages over 24-48 hours, allowing progressive concentration sufficient for noninvasive imaging applications. Our work builds on these previous approaches to imaging activated endothelium by providing exceptionally conspicuous contrast within 30 minutes (rather that 24-48 hours), without the need for intracellular accumulation and thereby maintaining specificity for the original molecular targets. Furthermore, flow cytometry of single-cell suspensions from digested aortas showed that near-infrared SAIVI™ 750 dual-ligand MPIO bound specifically to activated endothelial cells and were not taken up by macrophages or other cell types.
In common with earlier studies19, 33 we imaged the aortic root in its short axis, since atherosclerotic lesions develop here early in apoE−/− mice and progress in a semi-predictable fashion.39, 40 Secondly, the aortic valve position in relation to the chambers of the heart provides an excellent fiducial reference to align both the imaging plane and circumferential orientation. As shown in Figure 3, an eccentric lesion identified with in vivo contrast MRI is readily confirmed in tissue section.
Endothelial activation also occurs early in regions of endothelium prone to the development of atherosclerosis. Regions of low shear stress are associated with loss of the normal endothelial cell shape, disorganised endothelial cells that do not align in the direction of blood flow and increased expression of cell adhesion molecules. Using en face immunofluorescence, we show that the atherosclerosis-prone region of the aortic arch retains dual-ligand MPIO in association with VCAM-1 up-regulation while the relatively athero-resistant portions of aorta maintained normal EC morphology; aligned in the direction of flow, low level cell adhesion molecule expression and absent MPIO binding. Previously, microbubbles targeted to either P-selectin or VCAM-1 have been applied for molecular imaging of early atherosclerosis progression, which showed preferential binding to lesion prone areas in the aortic arch, with similar increases in endothelial P-selectin and VCAM-1 expression in atherosclerotic lesions with age.41
In seeking the optimal leukocyte mimetic MPIO, we compared the interactions of the cognate ligands VLA-4 and PSGL-1 with the antibody-targeting approach. After confirming that these natural ligands were conjugated and orientated on MPIO, we found that their binding to activated endothelial cells under low shear stress conditions in vitro had neither the specificity nor affinity of antibodies. We had reasoned that the cognate ligands may show greater affinity and would be less immunogenic in any future translational application. However, even on leukocytes the mere expression of ligands is not sufficient to attain binding, which is dependent on conformational change / clustering on the cell membrane, orchestrated by the cytoskeleton.42 We therefore pursued an antibody-based approach, utilizing our earlier observations in a flow chamber model43 and in vivo19 that a dual targeting strategy directed at both cell adhesion molecules and selectins is more effective than either alone.
The MPIO that we have used are not suitable for human use due to their non-biodegradable polyurethane coat. However, surface-functionalized biodegradable, dextran-coated MPIO have recently been developed that are actively taken up by macrophages in the liver and spleen and rapidly dispersed through the specific action of macrophage cathepsins.44, 45 Combined with humanized antibodies, to minimize immunogenicity, these particles are potentially suitable for human testing. The dose of MPIO administered in our study was 3.3 mg Fe / kg body weight, which is similar to that used clinically for non-targeted iron contrast agents in oncological imaging (2.6 mg / kg)46 and significantly lower than some USPIO doses used experimentally for imaging larger animal models such as rabbits (11 to 56 mg / kg).21, 47
Atherosclerotic plaque macrophage content and activity are important determinants of plaque instability and the ability to image these may also be important in predicting risk.48 Monocytes have been shown to be actively recruited in a progressive manner that is proportional to the extent of atherosclerotic disease.49 Treatments that reduce lesion macrophage content have also been shown to reduce VCAM-1 expression.50, 51 The correlation between endothelial bound dual-ligand MPIO and lesion macrophage content, and thus putatively that dual-ligand MPIO behave as a monocyte mimetic, may provide a novel imaging probe to assess the localization and extent of inflammatory atherosclerotic disease. Considerable effort has been directed towards the use of 2-[18F]fluoro-2-deoxy-d-glucose (18F-FDG-PET) for positron emission tomography (PET) assessment of atherosclerotic plaque inflammation, with success in identifying ‘acute’ lesions36 and in monitoring response to therapy.36, 52 However, whilst 18F-FDG appears to be preferentially taken up by inflammatory macrophages, a recent in vitro study suggests that this technique may be sensitive to hypoxia in plaque macrophages rather than measuring inflammation per se.53
MPIO, due to their relatively large size, do not penetrate the vessel wall and therefore remain highly specific for targeting endothelial molecular markers. This is unlike untargeted USPIO, which are subject to vascular egress and passive accumulation within atherosclerotic lesions or uptake by macrophages.21, 22 The rapid binding kinetics of dual-ligand MPIO, coupled with the immediate19 accessibility of endothelial cells (without the need to permeate the plaque), distinguish targeted MPIO as a powerful molecular imaging platform. The expression of endothelial cell adhesion molecules is not specific to any given stage or size of lesion. Therefore, if used in clinical practice, it would be anticipated that molecular imaging of endothelial inflammation would be combined in an integrated examination of vessel characterisation with soft tissue arterial wall imaging and MR angiography.
Conclusions
We have developed a leukocyte mimetic contrast agent, for in vivo molecular MRI, that rapidly homes to activated vascular endothelium during atherosclerotic lesion development. Although confined to the intravascular compartment and bound to endothelium, in the context of atherosclerosis in the aortic root, the accumulation of MPIO contrast agent quantitatively tracked with atherosclerotic lesion macrophage content. This approach provides new and significant benefits: (i) exceptionally rapid steady state accumulation; (ii) unusually conspicuous MR contrast effects that can be objectively quantified using semi-automated techniques and (iii) faithful reflection of endothelial activation associated with leukocyte accumulation. As degradable biocompatible particles become available this potent platform may find utility in both imaging and, potentially, drug delivery.
Supplementary Material
Acknowledgments
We acknowledge Hannah Barnes and Rachel Hagen for technical assistance in the BHF Experimental MR Unit (BMRU). We thank Dr Eileen McNeill and Dr Jyoti Patel for technical advice on flow cytometry experiments, Dr Paul Evans and Ms Narges Amini, Imperial College London for helpful advice on en face techniques and John M. Saunders, University of Virginia, for advice on VCAM-1 immunohistochemistry. Phil Townsend and James Brown are gratefully acknowledged for general laboratory management.
Sources of Funding This research was funded by The Wellcome Trust (RPC #088291). JES is a BHF Senior Basic Science Research Fellow.
Footnotes
Disclosures R.P.C. holds equity in Oxford Contrast Molecular Diagnostics. The other authors have no conflicting interests to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 2.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–2184. doi: 10.1161/01.cir.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 3.Moreno PR. Pathophysiology of plaque disruption and thrombosis in acute ischemic syndromes. J Stroke Cerebrovasc Dis. 2001;10:2–9. doi: 10.1053/jscd.2001.24785. [DOI] [PubMed] [Google Scholar]
- 4.Falk E. Why do plaques rupture? Circulation. 1992;86(6 suppl):III30–42. [PubMed] [Google Scholar]
- 5.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 6.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- 7.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein e-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 9.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayad ZA, Razzouk L, Briley-Saebo KC, Mani V. Iron oxide magnetic resonance imaging for atherosclerosis therapeutic evaluation: Still “rusty?”. J Am Coll Cardiol. 2009;53:2051–2052. doi: 10.1016/j.jacc.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of vcam-1 and icam-1 at atherosclerosis-prone sites on the endothelium in the apoe-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 12.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules icam-1, vcam-1, pecam, and e-selectin in human atherosclerosis. J Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 13.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K. Direct demonstration of p-selectin- and vcam-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein e-deficient mice. Circ Res. 1999;84:1237–1244. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 14.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 15.May AE, Neumann FJ, Schomig A, Preissner KT. Vla-4 (alpha(4)beta(1)) engagement defines a novel activation pathway for beta(2) integrin-dependent leukocyte adhesion involving the urokinase receptor. Blood. 2000;96:506–513. [PubMed] [Google Scholar]
- 16.Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009;29:983–991. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- 18.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 19.McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 21.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi RA, Mallawarachi C, JM UK-I, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Identifying inflamed carotid plaques using in vivo uspio-enhanced mr imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: Cellular mri using micron-sized iron oxide particles. Magn Reson Med. 2005;53:329–338. doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]
- 24.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In situ labeling of immune cells with iron oxide particles: An approach to detect organ rejection by cellular mri. Proc Natl Acad Sci U S A. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Q, Wu YL, Foley LM, Hitchens TK, Eytan DF, Shirwan H, Ho C. Longitudinal tracking of recipient macrophages in a rat chronic cardiac allograft rejection model with noninvasive magnetic resonance imaging using micrometer-sized paramagnetic iron oxide particles. Circulation. 2008;118:149–156. doi: 10.1161/CIRCULATIONAHA.107.746354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAteer MA, Sibson NR, von Zur Muhlen C, Schneider JE, Lowe AS, Warrick N, Channon KM, Anthony DC, Choudhury RP. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med. 2007;13:1253–1258. doi: 10.1038/nm1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis. 2010;209:18–27. doi: 10.1016/j.atherosclerosis.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhtar AM, Schneider JE, Chapman SJ, Jefferson A, Digby JE, Mankia K, Chen Y, McAteer MA, Wood KJ, Choudhury RP. In vivo quantification of vcam-1 expression in renal ischemia reperfusion injury using non-invasive magnetic resonance molecular imaging. PLoS One. 5:e12800. doi: 10.1371/journal.pone.0012800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von zur Muhlen C, Peter K, Ali ZA, Schneider JE, McAteer MA, Neubauer S, Channon KM, Bode C, Choudhury RP. Visualization of activated platelets by targeted magnetic resonance imaging utilizing conformation-specific antibodies against glycoprotein iib/iiia. J Vasc Res. 2009;46:6–14. doi: 10.1159/000135660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG. Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc Natl Acad Sci U S A. 2009;106:18–23. doi: 10.1073/pnas.0806787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAteer MA, von Zur Muhlen C, Anthony DC, Sibson NR, Choudhury RP. Magnetic resonance imaging of brain inflammation using microparticles of iron oxide. Methods Mol Biol. 2011;680:103–115. doi: 10.1007/978-1-60761-901-7_7. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy PJ, Schneider JE, Grieve SM, Lygate C, Neubauer S, Clarke K. Assessment of motion gating strategies for mouse magnetic resonance at high magnetic fields. Journal of Magnetic Resonance Imaging. 2004;19:229–237. doi: 10.1002/jmri.10454. [DOI] [PubMed] [Google Scholar]
- 33.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 34.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The nf-kappa b signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1009–1016. doi: 10.1161/ATVBAHA.108.165563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- 38.Broisat A, Riou LM, Ardisson V, Boturyn D, Dumy P, Fagret D, Ghezzi C. Molecular imaging of vascular cell adhesion molecule-1 expression in experimental atherosclerotic plaques with radiolabelled b2702-p. Eur J Nucl Med Mol Imaging. 2007;34:830–840. doi: 10.1007/s00259-006-0310-4. [DOI] [PubMed] [Google Scholar]
- 39.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. Apoe-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, Caplan ES, Khangura J, Bullens S, Bunting S, Lindner JR. Molecular imaging of the initial inflammatory response in atherosclerosis: Implications for early detection of disease. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:54–59. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chigaev A, Waller A, Zwartz GJ, Buranda T, Sklar LA. Regulation of cell adhesion by affinity and conformational unbending of alpha4beta1 integrin. J Immunol. 2007;178:6828–6839. doi: 10.4049/jimmunol.178.11.6828. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson A, Wijesurendra RS, McAteer MA, Digby JE, Douglas G, Bannister T, Perez-Balderas F, Bagi Z, Lindsay AC, Choudhury RP. Molecular imaging with optical coherence tomography using ligand-conjugated microparticles that detect activated endothelial cells: Rational design through target quantification. Atherosclerosis. 2011;219:579–587. doi: 10.1016/j.atherosclerosis.2011.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Balderas F, Davis BG, Van Kasteren SI, Khrapichev A, Anthony DC, Sibson NR. Multimeric iron oxide micro particles: Novel high sensitivity and biodegradable mri contrast agents. Intl. Soc. Mag. Reson. Med. 2010;18:1899. [Google Scholar]
- 45.Perez-Balderas F, Davis BG, van Kasteren SI, Khrapichev A, Jefferson A, Bristow C, Serres S, Choudhury RP, Anthony DC, Sibson NR. New biodegradable multimeric mpio contrast agent shows rapid in vitro and in vivo degradation and high sensitivity contrast. Proc. Intl. Soc. Mag. Reson. Med. 2011;19:1689. [Google Scholar]
- 46.Will O, Purkayastha S, Chan C, Athanasiou T, Darzi AW, Gedroyc W, Tekkis PP. Diagnostic precision of nanoparticle-enhanced mri for lymph-node metastases: A meta-analysis. Lancet Oncol. 2006;7:52–60. doi: 10.1016/S1470-2045(05)70537-4. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz SA, Coupland SE, Gust R, Winterhalter S, Wagner S, Kresse M, Semmler W, Wolf KJ. Superparamagnetic iron oxide-enhanced mri of atherosclerotic plaques in watanabe hereditable hyperlipidemic rabbits. Invest Radiol. 2000;35:460–471. doi: 10.1097/00004424-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 48.de Boer OJ, van der Wal AC, Teeling P, Becker AE. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: A prominent role for neovascularization? Cardiovasc Res. 1999;41:443–449. doi: 10.1016/s0008-6363(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 49.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- 51.Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 52.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. Journal of the American College of Cardiology. 2006;48:1825. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 53.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages implications for imaging atherosclerosis with (18)fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.