Abstract
Objective
Microparticles of iron oxide (MPIO) distort magnetic field creating marked contrast effects far exceeding their physical size. We hypothesized that antibody-conjugated MPIO would enable magnetic resonance imaging (MRI) of endothelial cell adhesion molecules in mouse atherosclerosis.
Methods and Results
MPIO (4.5 μm) were conjugated to monoclonal antibodies against vascular cell adhesion molecule-1 (VCAM–MPIO) or P-selectin (P-selectin–MPIO). In vitro, VCAM–MPIO bound, in dose-dependent manner, to tumor necrosis factor (TNF)-α stimulated sEND-1 endothelial cells, as quantified by light microscopy (R2=0.94, P=0.03) and by MRI (R2=0.98, P=0.01). VCAM–MPIO binding was blocked by preincubation with soluble VCAM-1. To mimic leukocyte binding, MPIO targeting both VCAM-1 and P-selectin were administered in apolipoprotein E–/– mice. By light microscopy, dual-targeted MPIO binding to endothelium overlying aortic root atherosclerosis was 5- to 7-fold more than P-selectin–MPIO (P<0.05) or VCAM–MPIO (P<0.01) alone. Dual-targeted MPIO, injected intravenously in vivo bound aortic root endothelium and were quantifiable by MRI ex vivo (3.5-fold increase versus control; P<0.01). MPIO were well-tolerated in vivo, with sequestration in the spleen after 24 hours.
Conclusions
Dual-ligand MPIO bound to endothelium over atherosclerosis in vivo, under flow conditions. MPIO may provide a functional MRI probe for detecting endothelial-specific markers in a range of vascular pathologies.
Introduction
Magnetic resonance imaging (MRI) has demonstrated substantial utility in phenotyping vascular disease. Using inherent physico-chemical properties that confer particular tissue relaxivities, it has been possible to characterize the vessel wall in atherosclerosis at a submillimeter level.1,2 However, to capitalize fully on the diagnostic potential of MRI requires imaging at molecular and cellular levels.3–9 To achieve this, purpose-built contrast agents are needed that can identify molecules of interest with high specificity, while conveying sufficient contrast to be easily distinguished from unenhanced tissue.
Specificity can be achieved through conjugation of contrast agent with monoclonal antibodies or their immunospecific fragments F(ab), peptides, or peptide-mimetics. Previous approaches have included integrin-conjugated gadolinium-rich perfluorocarbon nanoparticles,10 peptide-conjugated nanoparticles of iron oxide,11 and fibrin-specific cyclic peptide labeled with gadolinium.12
Gadolinium-based contrast agents shorten T1 providing positive contrast on T1-weighted images. However, for the quantities that can be delivered to an endothelial monolayer, the contrast effects are relatively modest. By comparison, iron oxide nanoparticles provide greater contrast effects, but require many particles to be delivered to a given voxel. Another potential drawback is that contrast effects are manifest in T2*-weighted images as dark signal areas that can be difficult to distinguish from surrounding tissue.
Recently, larger microparticles of iron oxide (MPIO) have been used for cellular imaging and tracking.13,14 For some applications the size of these particles would preclude delivery to the site of interest. However, for imaging endovascular targets, MPIO possess several positive attributes. Firstly, MPIO convey a payload of iron that is orders of magnitude greater than ultrasmall particles of iron oxide (USPIO). Secondly, the effects of MPIO on local magnetic field homogeneity, and therefore detectable contrast, extend a distance at least 50 times their physical diameter.13 Thirdly, once bound to endothelium, MPIO remain intravascular thereby allowing bound MPIO to be readily distinguished from the vessel wall. Finally, conjugated MPIO may offer a generic tool for imaging endothelial-specific markers across a range of vascular pathologies such as inflammation, atherosclerosis, and angiogenesis.
In animal models of atherosclerosis, vascular cell adhesion molecule-1 (VCAM-1; CD106) mediates adhesion, rolling, and tethering of mononuclear leukocytes and facilitates their transmigration to the developing atherosclerotic plaque.15–17 VCAM-1 expression is not constitutive, but is present at atherosclerosis-prone sites, even before macroscopic disease is apparent, with persistent expression in more advanced lesions.16,18 VCAM-1 is therefore a potentially useful marker for atherosclerosis from early stages. However, in vivo molecular imaging of the vascular endothelium presents particular challenges since the contrast agent has to bind in sufficient density to the target, a two-dimensional monolayer, that is exposed to significant physiological sheer stresses. The dynamics of leukocyte:endothelial binding are also complex and dependent on multiple receptor-ligand interactions. Initial leukocyte rolling is selectin-mediated, whereas firm adhesion is mediated by integrin binding to intercellular adhesion molecule-1 (ICAM-1) and VCAM-1, with the latter more important in initiation of atherosclerosis. “Adhesion dynamic modeling” predicts synergistic roles for selectins and integrins with transition between rolling and firm adhesion dependent on binding affinities and relative concentrations of receptor-ligand interactions.19,20
Accordingly, we adopted a dual antibody-conjugated MPIO approach for targeted MRI and applied this to the detection of adhesion molecules on the arterial endothelium of apolipoprotein E–knockout (apoE–/–) mice.
Materials and Methods
Antibody Conjugation to Iron Oxide Microparticles
MPIO (4.5 μm diameter) with p-toluenesulphonyl (tosyl) reactive surface groups (Invitrogen) were used for antibody conjugation. These tosyl groups react covalently with primary amino and sulfydryl groups predominantly in the Fc-region of antibodies, favoring optimal orientation. Purified monoclonal rat anti-mouse antibodies for VCAM-1 (clone M/K2) (Cambridge Bioscience Ltd), P-selectin (clone RB40.34), (Fitzgerald Industries Intl), or IgG-1 (clone Lo-DNP-1; Serotec) were covalently conjugated to MPIO, by incubation at 37°C for 20 hours, with constant rotation (1×107 MPIO per 5 μg antibody). Dual-targeted MPIO, incubated with VCAM-1 and P-selectin antibodies (2.5 μg of each), were prepared in the same way.
MPIO were then washed twice in phosphate buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) at 4°C and incubated with tris buffer (0.1 mol/L, 0.1% BSA, pH 7.4) for 4 hours at 37°C, to block remaining active tosyl sites. MPIO were rinsed in PBS (0.1% BSA) at 4°C for 5 minutes and stored at 4×108 MPIO per mL PBS (0.1% BSA) at 4°C.
In Vitro Binding of Anti–VCAM-1-MPIO to TNF-α–Stimulated sEND-1 Cells
Cells of a murine endothelial line (sEND-1) were cultured in Dulbecco Modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, and 100 U/mL penicillin and 0.1 mg/mL streptomycin. Cells (8×105 per 35 mm well) were incubated with 0.1, 1, 10, or 50 ng/mL murine recombinant TNF-α (R&D systems) for 20 hours at 37°C to induce endothelial VCAM-1 expression. Stimulated cells were incubated in duplicate with VCAM–MPIO or control IgG-1-MPIO (1.2×106 per 2 mL media) for 30 minutes at RT, with constant mixing. Unbound MPIO were removed by extensive washing with PBS.
MPIO binding to cells was assessed in 4 fields of view per TNF-α dose by light microscopy (Leica DM R; ×20 objective) and quantified using ImagePro plus (Media Cybernetics).
MRI of Cell Phantoms
Resuspended sEND-1 cells were embedded in 2% high grade agarose. MRI was performed using an 11.7 Tesla vertical magnet (Bruker) and 40-mm probe. A 3D gradient echo sequence was used (TR/TE=4/90 ms, field of view [FOV] 30×30×30 mm, matrix size [512],3 2 averages, imaging time ≈13 hour overnight) with final resolution of 29.3×29.3×29.3 μm after image reconstruction. Low signal areas, corresponding to MPIO binding, were quantified in 8 images per sample (at slice intervals of 293 μm) using a histogram based tool in ImagePro Plus.
ApoE–/– Mice
Homozygous apoE–/– mice, bred in a pathogen-free room, with constant temperature and humidity, were weaned at 3 weeks of age and transferred to Western diet (21% milk fat, 0.15% cholesterol: 100244 Dyets Inc) for 25 weeks. Experiments were performed according to UK Scientific Procedures Act (1986).
Left Ventricular Injection of MPIO
ApoE–/– mice were terminally anesthetized by isofluorane inhalation. The chest cavity was exposed and while the heart was beating, mice were injected via the left ventricle with P-selectin-MPIO (n=4 mice), VCAM–MPIO (n=7 mice), control IgG-1–MPIO (n=3 mice), or dual-ligand MPIO targeting P-selectin and VCAM-1 (n=6 mice) (1.2×107 MPIO in 100 μL PBS; ≈6 mg iron/kg body weight). After 5 minutes, the arterial tree was perfusion fixed via the left ventricle with 10 mL paraformaldehyde (4% in PBS).
In Vivo Systemic Administration of Dual-Targeted MPIO
ApoE–/– mice were anesthetized with Hypnorm (25 mg/kg, Bayer) and Hypnoval (25 mg/kg, Bayer) combined, administered subcutaneously. Dual-targeted MPIO or control IgG-1-MPIO (6×107 MPIO/150 μL PBS; ≈30 mg iron per kg body weight) were injected in vivo via the jugular vein (n=4 mice per group) and allowed to circulate for 30 minutes. Mice were terminally anesthetized and the arterial tree perfusion fixed via the left ventricle with 4% PFA, removed en bloc and embedded in a glass MR tube containing 2% agarose.21 The heart was instilled with 4% PFA containing 2 mmol/L gadoteridiol (Prohance, Bracco) via the left ventricle and the tube sealed with 2% agarose containing 2 mmol/L gadoteridiol.
MRI Analysis
High resolution MRI of the arterial tree was performed ex vivo at 11.7 T using a 13 mm 1H birdcage radiofrequency coil (RAPID Biomedical). A 3D gradient echo sequence was used (TE=4 ms/TR=90 ms, FOV 13×13×19.5 mm, matrix size 256×256×384, two averages, imaging time ≈7 hours) with final resolution of 25×25×25 μm after data reconstruction. Segmentation of bound MPIO was performed by an observer blinded to the MR image identities. MPIO binding was defined as a discrete circular low signal area on the luminal surface of aortic root in ≥2 consecutive MR images. MPIO appearing in multiple consecutive images were counted only once. Segmented images were 3D reconstructed using the 3D Constructor plug-in for ImagePro Plus to visualize MPIO contrast throughout the aortic root.
Statistical Methods
Data are mean±SD. Quantification of cell-bound MPIO assessed by MRI and light microscopy were related to the dose of TNF-α using simple linear regression (GraphPad Prism 4, GraphPad Software Inc). Parametric data were compared using Student t tests, with statistical significance attributed at probability values <0.05.
Competitive Inhibition of VCAM–MPIO Binding In Vitro
Please see supplementary methods, available online at http://atvb.ahajournals.org.
Assessment of In Vitro Macrophage and sEND-1 Cell Uptake of MPIO by MRI
Please see supplementary methods.
Histology
Please see supplementary methods.
Assessment of MPIO Uptake by Organs Following In Vivo Administration
Please see supplementary methods.
Results
VCAM–MPIO Bind to TNF-α–Stimulated sEND-1 Cells
Assessed by light microscopy, VAM–MPIO bound to TNF-α–stimulated sEND-1 cells, but did not bind to unstimulated cells. IgG-1-MPIO did not bind to TNF-α–stimulated sEND-1 cells. The number of cell-bound VCAM–MPIO increased with increasing doses of TNF-α (Figure 1A, R2=0.94, P=0.03). High-resolution MRI of cell phantoms showed punctate low signal areas representing VCAM–MPIO binding to cells, which increased in a similar fashion to increasing dose of TNF-α (Figure 1B, R2=0.98, P=0.01). As a demonstration of specificity, preincubation of VCAM–MPIO with soluble Fc-VCAM-1 inhibited anti–VCAM-1-MPIO binding almost entirely. By contrast, preincubation of VCAM–MPIO with soluble Fc-ICAM-1 had no effect (supplemental Figure I).
Figure 1.
Anti-VCAM-1-MPIO binding to TNF-α stimulated sEND-1 cells. (A) Light microscopy images of anti-VCAM-1-MPIO binding to TNF-α stimulated sEND-1 cells (0.1-100 ng / mL) (x 20 magnification). MPIO binding persisted after extensive washing and was restricted to cellular areas. A dose-dependent increase in anti-VCAM-1-MPIO binding in response to increasing TNF-α stimulation was observed. Data are mean ± SD of duplicate experiments (4 fields per sample). (B) High resolution MRI (11.7 T; resolution 25 × 25 × 25 μm) of MPIO-bound sEND-1 cell phantoms confirmed the dose response of anti-VCAM-1-MPIO binding to TNF-α stimulated sEND-1 cells. Data are mean ± SD for 8 MR images per sample, at intervals of 293 μm.
Assessment of MPIO Uptake by Macrophages and sEND-1 Cells
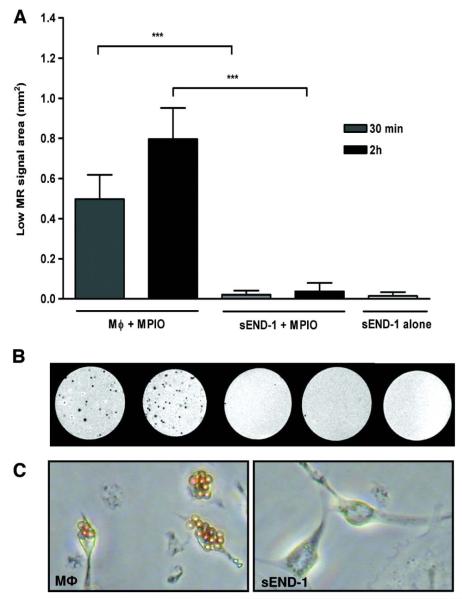
Mouse peritoneal macrophages and sEND-1 cells were incubated with unconjugated MPIO in vitro. By light microscopy, macrophages phagocytosed MPIO after 30 minutes, and more so by 2 hours. By contrast, there was no MPIO uptake by sEND-1 cells (Figure 2C). With high resolution MRI, discrete low signal circular areas were observed for macrophage cell phantoms preincubated with MPIO for 30 minutes which increased further by 2 hours (P=0.004; Figure 2). The equivalent quantification for sEND-1 cells incubated with MPIO for 30 minutes or 2 hours showed no difference from sEND-1 cells alone, indicating absence of uptake.
Figure 2.
Competitive inhibition of anti-VCAM-1-MPIO binding to TNF-α (50 ng / mL) stimulated sEND-1 cells in vitro. (A) Pre-incubation of anti-VCAM-1-MPIO with soluble VCAM-1 protein (Fc-VCAM-1) inhibited MPIO binding almost entirely (P<0.001), while pre-incubation with Fc-ICAM-1 had no effect. Data are mean ± SD for 4 fields per sample (triplicate experiments). (B) Confocal microscopy confirmed cell surface expression of VCAM-1 (green fluorescence) in TNF-α stimulated cells. Fc-ICAM-1 had no effect on anti-VCAM-MPIO binding (autofluorescent green spheres) while Fc-VCAM-1 abolished anti-VCAM-1-MPIO retention, despite demonstrable cell surface VCAM-1 expression. Blue represents DAPI staining for cell nuclei.
Single Versus Dual-Targeted MPIO Binding in Mouse Atherosclerosis
VCAM–MPIO and P-selectin–MPIO bound specifically to endothelium overlying atherosclerotic plaque after left ventricular injection in terminally anesthetized mice. The number of VCAM–MPIO and P-selectin–MPIO bound per aortic root section was similar (16.1±2.7 versus 12.9±4.2) with minimal nonspecific retention of IgG-1–MPIO (1.7±4.2; Figure 3). Dual-targeted MPIO showed significantly enhanced binding efficiency compared with P-selectin–MPIO (6.9 fold increase; P=0.027) and VCAM–MPIO (5.5 fold increase; P=0.005).
Figure 3.
High resolution ex vivo MRI (11.7 T; resolution 29 × 29 × 29 μm) of macrophage or sEND-1 cell phantoms pre-incubated with MPIO for 30 min or 2 h. Data are mean ± SD of low signal areas in 10 MR images per sample at intervals of 293 μm. Low signal areas, corresponding to MPIO uptake by cells, were detected for macrophages pre-incubated with MPIO for 30 min or 2 h but not for sEND-1 cells (***P<0.001). No significant difference was detected for sEND-1 cell phantoms pre-incubated with MPIO for 30 min or 2 h and the negative control sEND-1 ‘cell only’ phantom, not exposed to MPIO.
In Vivo Dual-Targeted MPIO Binding to Atherosclerosis: Quantification With Ex Vivo MRI
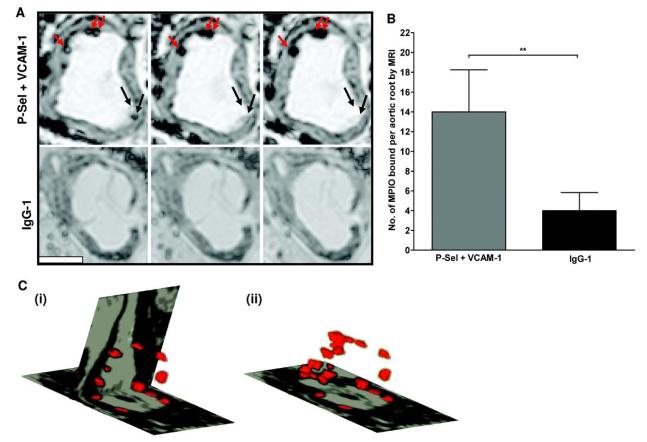
Dual-targeted MPIO or IgG-1–MPIO were injected in vivo via the jugular vein and allowed to circulate for 30 minutes. High-resolution ex vivo MRI detected discrete circular low signal areas on the luminal side of the aortic root, in areas overlying atherosclerotic plaques. The contrast effect extended over ≈8 voxels. The appearance of MPIO changed in a consistent and characteristic fashion, and varied according to their position within a voxel (Figure 4A). The number of dual-targeted MPIO binding, determined by MRI, was 3.5-fold higher compared with IgG-1–MPIO (14±4.2 versus 4±1.8; P<0.01; Figure 4B). 3D reconstruction of segmented images demonstrated that MPIO binding was localized to atherosclerotic plaque endothelium throughout the aortic root. No MPIO binding was observed in atherosclerosis-free areas of the ascending aorta (Figure 4C).
Figure 4.
(A) Histological examination of aortic roots following left ventricular injection of single versus dual-targeted MPIO in terminally anaesthetised apoE-/- mice with beating hearts. (A) Three to four aortic root sections per mouse (15 μm thick) were examined for MPIO binding by light microscopy (x 40 magnification). There was minimal non-specific retention of control IgG-1-MPIO (n = 3 mice). Anti-VCAM-1-MPIO (n = 7 mice) and anti-P-selectin MPIO (n = 4 mice) showed similar levels of binding to aortic root plaque endothelium. However, dual-ligand MPIO (n = 6 mice) targeting VCAM-1 and P-selectin showed synergistically enhanced binding compared to anti-P-selectin MPIO or anti-VCAM-1-MPIO, respectively. (B) Light microscopy image depicting dense dual-targeted MPIO binding to endothelium overlying atherosclerotic plaque in the aortic root (x 40 magnification). The MPIO are clearly distinguished as bright yellow spheres confined to the luminal surface of the vessel. The dark red bodies of irregular shape within the plaque are cell nuclei. Scale bar 20 μm. (*** P < 0.001; ** P < 0.01 and * P < .05).
All mice tolerated MPIO injection and none showed signs of ill health up to 24 hours postinjection. MPIO were identified in lungs, kidneys, liver, and spleen after 30 minutes and 24 hours. Importantly, there was no evidence of infarction, hemorrhage, edema, inflammation, or MPIO extravasation in any of these organs, at either time point. Compared with 30 minutes, at 24 hours postinjection there was significant clearance of MPIO from the lungs (78% decrease; P<0.001) and kidneys (65% decrease; P<0.001) with redistribution of MPIO predominantly to the spleen (305% increase; P<0.001) and to a lesser extent, the liver (69% increase; P<0.01; Figure 5).
Figure 5.
High resolution ex vivo MRI (11.7 T; resolution 25 × 25 × 25 μm) of aortic roots of apoE-/- mice, 30 min after i.v. injection of dual-targeted MPIO or IgG-1-MPIO (A) The number of bound dual-targeted MPIO, quantified by MRI, was 3.5-fold greater than IgG-1 MPIO (P < 0.01; n = 4 mice per group). (B) Dual-targeted MPIO were identified on MR images as distinct circular low signal areas adherent to endothelium overlying atherosclerotic plaque. A ‘halo effect’ was observed for MPIO in some images, reflecting a partial volume effect: As individual MPIO were tracked through adjacent images, a dense low signal area was always present (top panel shows 3 consecutive MR images. Red arrows indicate bound MPIO, black arrows show MPIO passing in and out of the imaging plane). Scale bar 500 μm. (C) 3-D reconstruction of segmented images. Orthogonal slices were taken horizontally through the aortic valve and vertically along the axis of the aorta (panel i). Note the bloom effect of MPIO contrast in lesion areas of the aortic root and the absence of binding in atherosclerosis-free areas of ascending aorta. In panel (ii) the vertical slice was removed to illustrate the volume of MPIO contrast in the aortic root.
Discussion
The activated endothelium displays numerous molecules upregulated in vascular disease providing potential markers for functional molecular imaging and targeted therapeutics. An important challenge for in vivo molecular imaging is to deliver a potent contrast agent in sufficient quantity under conditions of shear stress. Here we combine intense MR contrast effects attainable using microparticles of iron oxide with a dual ligand-targeting strategy modeled on leukocyte interactions with activated endothelium.
Recently, MPIO have been used for imaging inflammatory cells in transplant rejection14 and for tracking single cells during embryogenesis.13 The accessibility of the endothelium to intravascular contrast agents permits the use of MPIO that are orders of magnitude larger than USPIO used previously for similar applications. We report the use of targeted MPIO that specifically bind to adhesion molecules in mouse atherosclerosis after in vivo administration and which are readily identified by ex vivo MRI.
Dual antibody-conjugated MPIO, targeting both P-selectin and VCAM-1, were developed to mimic in vivo dynamics of leukocyte binding to endothelium. Initial leukocyte rolling, which is selectin-mediated, can be simulated under flow conditions using colloidal microspheres coated with sialyl Lewisx or P-selectin glycoprotein ligand-1.22 Firm adhesion is mediated by integrin binding to ICAM-1 and VCAM-1. As predicted by computed models, dual-targeted MPIO showed synergistically enhanced binding compared with either in isolation. A similar approach targeting ultrasound microbubbles to vascular endothelium used a combination of sialyl Lewisx and ICAM-1 antibody, though binding efficiency was increased only marginally, possibly because of stearic limitation from size mismatch between the 2 ligands.23
The role of VCAM-1 in the pathogenesis of atherosclerosis is well established in animal models, where it has been shown to be important in monocyte recruitment.15,18 In humans, Davies et al found VCAM-1 expression in a majority of human coronary atherosclerotic plaques post mortem24 though this is not always consistent.25
High resolution ex vivo MRI showed that, after intravenous injection, dual-targeted MPIO localized rapidly and specifically to aortic root plaque endothelium, a characteristic site for atherosclerosis development in mice. Although MPIO binding was not confluent, the “bloom effect” of magnetic field distortion of MPIO was clearly identifiable as circular low signal areas within the vessel lumen. We did not observe binding to atherosclerosis-free segments of the ascending aorta. Earlier approaches to endothelial molecular MRI have been limited by a lack of sensitivity.26,27 To address this, Nahrendorf et al recently used phage display to generate a specific VCAM-1 ligand that is internalized allowing progressive concentration by endothelial cells. By tagging this ligand with iron it was possible to image VCAM-1 expression in mouse atherosclerosis.28 This is an ingenious approach, but, in comparison to MPIO, also has significant disadvantages. Firstly, cellular uptake is required, with potential for toxicity and ultimately loss of molecular specificity; secondly it was necessary to wait 48 hours after administration before imaging and thirdly, the mode of cellular uptake is so specific to VCAM-1 that the technology is not adaptable to other endovascular molecular targets.
The MPIO reported here are commercially available and can readily be substituted with other ligands of interest. We are currently exploring endogenous receptor ligands and novel peptides as mediators of MPIO binding. Technologies such as phage display can also facilitate identification of novel targets.29 We have purposely used relatively large MPIO (4.5 μm) because of their superiority as contrast vehicles. However, the conjugation technique can also be applied to smaller MPIO. The potential of MPIO for in vivo molecular imaging should also be emphasized. In this respect, Shapiro et al show excellent contrast using 1.63 μm MPIO for cellular imaging,13 whereas 1 μm MPIO, which were cleared rapidly from the blood phase, have been used to monitor graft rejection.14
The MPIO reported here are nonbiodegradeable and not suitable for human use. However, the basic iron contrast mechanism is potentially transferable to humans with suitable adaptation of the carriage particle. For example, biodegradable polymeric microparticles have been used as a vehicle for gadolinium-based contrast in MRI.30 Indeed, clinical grade biodegradable iron-dextran particles are already available for injection into humans (eg, CliniMACS System, Miltenyi Biotec). The saturation magnetization of iron occurs at field strength of ≈2.1 T and would not be expected to increase at the higher field strengths used here.31 For molecular imaging, the contrast effect should increase as the radius of the particle increases, to the third power. The contrast effects observed by us and others are in the region of 100 to 200 μm per particle. Although the case is not proven here, this is of an order that may be detectable clinically.
Conclusions
We have shown that dual-ligand MPIO can be used for functional MRI of endothelial adhesion molecules in mouse atherosclerosis. We have exploited the opportunity of endovascular accessibility to use MPIO of similar size to circulating red blood cells (orders of magnitude larger than USPIO) that convey substantial contrast payload. To optimize MPIO binding under flow conditions in vivo, we have learnt from studies of leukocyte adhesion and computed models of dual-receptor binding to develop MPIO that target both P-selectin and VCAM-1. This work contributes an additional approach to the goal of disease characterization using functional molecular MRI probes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of their colleagues: Phil Townsend, Lynn Clee, and Carol Williams. Dr Colin Clelland is thanked for contributing expertise in histology.
Sources of Funding This work was funded by The Wellcome Trust (Intermediate Clinical Fellowship to R.C.).
Footnotes
Disclosures Dr Choudhury is a named contributor on a patent application for the development of biodegradable imaging particles.
References
- 1.Fayad ZA, Fuster V, Choudhury RP. CMR Atherothrombotic plaque imaging. In: Lardo AC, Fayad ZA, Chronos NA, Fuster V, editors. Cardiovascular Magnetic Resonance. Established and emerging applications. Martin Dunitz; London: 2003. [Google Scholar]
- 2.Wood ML, Wehrli FW. Principles of magnetic resonance imaging. In: Stark DD, Bradley WG Jr, editors. Magnetic Resonance Imaging. Mosby; St Louis: 1999. [Google Scholar]
- 3.Choudhury RP, Fuster V, Badimon JJ, Fisher EA, Fayad ZA. MRI and characterization of atherosclerotic plaque: emerging applications and molecular imaging. Arterioscler Thromb Vasc Biol. 2002;22:1065–1074. doi: 10.1161/01.atv.0000019735.54479.2f. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 4.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–925. doi: 10.1038/nrd1548. CrossRefMedline. [DOI] [PubMed] [Google Scholar]
- 5.Lipinski MJ, Fuster V, Fisher EA, Fayad ZA. Technology insight: targeting of biological molecules for evaluation of high-risk atherosclerotic plaques with magnetic resonance imaging. Nat Clin Pract Cardiovasc Med. 2004;1:48–55. doi: 10.1038/ncpcardio0013. Medline. [DOI] [PubMed] [Google Scholar]
- 6.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47:1328. doi: 10.1016/j.jacc.2006.01.029. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 7.Wickline SA, Lanza GM. Molecular imaging, targeted therapeutics, and nanoscience. J Cell Biochem Suppl. 2002;39:90–97. doi: 10.1002/jcb.10422. Medline. [DOI] [PubMed] [Google Scholar]
- 8.Wickline SA, Neubauer AM, Winter P, Caruthers S, Lanza G. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2006;26:435–441. doi: 10.1161/01.ATV.0000201069.47550.8b. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 9.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 10.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with {alpha}v{beta}3–integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 11.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 12.Botnar RM, Buecker A, Wiethoff AJ, Parsons EC, Jr, Katoh M, Katsimaglis G, Weisskoff RM, Lauffer RB, Graham PB, Gunther RW, Manning WJ, Spuentrup E. In vivo magnetic resonance imaging of coronary thrombosis using a fibrin-binding molecular magnetic resonance contrast agent. Circulation. 2004;110:1463–1466. doi: 10.1161/01.CIR.0000134960.31304.87. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E- deficient mice. Circ Res. 1999;84:1237–1244. doi: 10.1161/01.res.84.11.1237. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 16.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 17.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia SK, King MR, Hammer DA. The State Diagram for Cell Adhesion Mediated by Two Receptors. Biophys J. 2003;84:2671–2690. doi: 10.1016/S0006-3495(03)75073-5. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys J. 2003;85:2720–2731. doi: 10.1016/s0006-3495(03)74695-5. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAteer MA, Schneider JE, Clarke K, Neubauer S, Channon KM, Choudhury RP. Quantification and 3D reconstruction of atherosclerotic plaque components in apolipoprotein E knockout mice using ex vivo high-resolution MRI. Arterioscler Thromb Vasc Biol. 2004;24:2384–2390. doi: 10.1161/01.ATV.0000146811.19029.fb. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 22.Brunk DK, Goetz DJ, Hammer DA. Sialyl Lewis(x)/E-selectin-mediated rolling in a cell-free system. Biophys J. 1996;71:2902–2907. doi: 10.1016/S0006-3495(96)79487-0. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weller GE, Villanueva FS, Tom EM, Wagner WR. Targeted ultrasound contrast agents: in vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol Bioeng. 2005;92:780–788. doi: 10.1002/bit.20625. CrossRefMedline. [DOI] [PubMed] [Google Scholar]
- 24.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. CrossRefMedline. [DOI] [PubMed] [Google Scholar]
- 25.Wood KM, Cadogan MD, Ramshaw AL, Parums DV. The distribution of adhesion molecules in human atherosclerosis. Histopathology. 1993;22:437–444. doi: 10.1111/j.1365-2559.1993.tb00157.x. Medline. [DOI] [PubMed] [Google Scholar]
- 26.Sibson NR, Blamire AM, Bernades-Silva M, Laurent S, Boutry S, Muller RN, Styles P, Anthony DC. MRI detection of early endothelial activation in brain inflammation. Magn Reson Med. 2004;51:248–252. doi: 10.1002/mrm.10723. CrossRefMedline. [DOI] [PubMed] [Google Scholar]
- 27.Sipkins DA, Gijbels K, Tropper FD, Bednarski M, Li KC, Steinman L. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J Neuroimmunol. 2000;104:1–9. doi: 10.1016/s0165-5728(99)00248-9. CrossRefMedline. [DOI] [PubMed] [Google Scholar]
- 28.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive Vascular Cell Adhesion Molecule-1 Imaging Identifies Inflammatory Activation of Cells in Atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. Abstract/FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 29.Valadon P, Garnett JD, Testa JE, Bauerle M, Oh P, Schnitzer JE. Screening phage display libraries for organ-specific vascular immunotargeting in vivo. PNAS. 2006;103:407–412. doi: 10.1073/pnas.0506938103. Abstract/FREE Full Text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen HH, Le Visage C, Qiu B, Du X, Ouwerkerk R, Leong KW, Yang X. MR imaging of biodegradable polymeric microparticles: a potential method of monitoring local drug delivery. Magn Reson Med. 2005;53:614–620. doi: 10.1002/mrm.20395. CrossRefMedline. [DOI] [PubMed] [Google Scholar]
- 31.Reitz J, Milford FJ, Christy R, Claus AC. Foundations of Electormagnetic Theory. Addison-Wesley; Reading, MA: 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.