Abstract
Background:
Long-term use of topical and systemic steroids produces secondary open-angle glaucoma similar to chronic simple glaucoma. The increased intraocular pressure [IOP] caused by prolonged steroid therapy is reversible but the damage produced by it is irreversible.
Materials and Methods:
In this study, we analyzed 200 patients with steroid-induced glaucoma, who were on systemic and topical corticosteroids for various dermatological conditions. The variation in IOP caused by different steroid preparations was studied.
Results:
Two hundred patients who were on systemic steroids for more than 8 weeks developed raised IOP. Three of these patients also developed bilateral posterior subcapsular cataract.
Conclusion:
We conclude that systemic steroids can induce rise of IOP and cataract formation. If it is not detected and treated in time, rise in IOP can lead to irreversible damage to the eyes.
Keywords: Cataract, glaucoma, steroids
INTRODUCTION
Prolonged use of topical as well as systemic steroids produces a type of glaucoma that is very similar to chronic simple glaucoma. While elevated intraocular pressure (IOP) is reversible, glaucomatous cupping and field defects are irreversible. The aim of this study was to analyze the variations in IOP and the changes in the lens in cases of steroid-induced glaucoma. We wish to draw attention to the disastrous complications that steroids can produce if these patients are not closely followed up.
MATERIALS AND METHODS
This was a prospective study carried out by the Department of Ophthalmology of the Kamineni Institute of Medical Sciences, Narketpally, Andhra Pradesh. During a period of 1 year, from September 2009 to September 2010, patients who were advised systemic steroids for various dermatological conditions were referred to the Department of Ophthalmology as part of pre-treatment workup. These patients had been advised steroids for periods ranging from 6 to 52 weeks; patients who were prescribed steroids for a period of less than 6 weeks were not considered for the study. A detailed clinical history was taken for all patients and the indications for prescribing steroids were recorded. The patients were divided into three groups based on the mode of steroid intake: group I included patients on systemic steroids, group II comprised patients on systemic steroids plus topical steroids, and group III included those on systemic pulse steroids. All patients underwent ophthalmological examination and those with preexisting cataract and glaucoma were excluded from the study. Patients were followed up every 2 weeks. At each visit they underwent slit-lamp examination to look for any cataractous changes in the lens and Goldmann applanation tonometry to evaluate IOP changes. IOP was measured on the first visit and after 8 weeks. Increase in IOP of more than 30% of the initial recording was considered significant. The t-test was done using SigmaStat® software to look for statistical significance of difference between the median values of IOP before start of steroid therapy and after 8 weeks of treatment.
Observations
Two hundred patients who were on systemic steroids for more than 8 weeks developed raised IOP. Three of these patients also developed bilateral posterior subcapsular cataract. Of the 200 patients, 137 were males and 63 were females. The age of the patients ranged from 24 years to 46 years. Indications for steroid therapy were as follows: pemphigus in 21 patients, lepra reaction in 18 patients, generalized vitiligo in 54 patients, and parthenium dermatitis in 107 patients [Table 1]. Systemic prednisolone in tablet form, at a dose of 0.5–1.5 mg/kg body weight, was used by 107 patients for 8 weeks and by 18 patients for 12 weeks; these 125 patients constituted group I. In addition to oral prednisolone, 21 patients concurrently used topical clobetasol cream and these patients were designated as group II. Pulse therapy with tablet methyl prednisolone, 64 mg/week, was used by 54 patients for 52 weeks; these patients were designated as group III [Table 2]. All the patients, except those on pulse therapy, had increase of IOP by 30%–50% after 6 weeks of therapy. Patients on systemic prednisolone plus topical clobetasol (group II) showed 40% rise in IOP, while patients on systemic prednisolone alone (group I) showed a rise of 30%. Patients on pulse therapy did not show any rise in IOP. The mean IOP rise in group I was 5.56 and the mean increase in group II was 6.14; in both cases the change was statistically significant (P<0.0001). The mean increase in IOP in group III was 0.18, which was not a statistically significant change (P=0.6057) [Table 3]. A total of seven patients (three from the group III, two from the group II, and two from group I) showed a 50% increase in IOP; these patients were labeled as high steroid responders. The steroid dose was rapidly tapered off in these patients and they were advised steroid-sparing drugs [Figure 1]. Three patients on pulse methyl prednisolone developed subcapsular cataract; they were advised to undergo cataract surgery with intraocular lens implantation.
Table 1.
Indications for steroid therapy
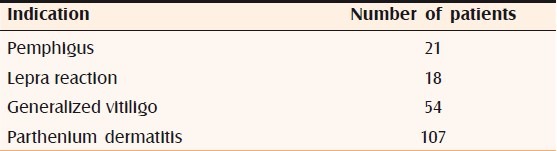
Table 2.
Drugs used for therapy
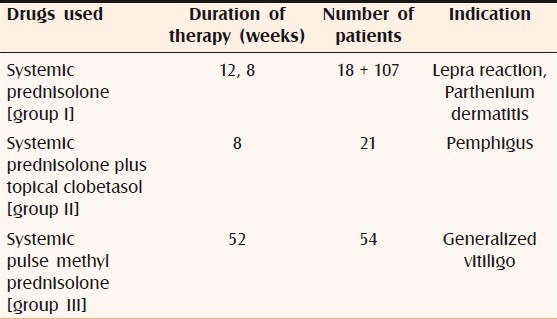
Table 3.
Statistical analysis
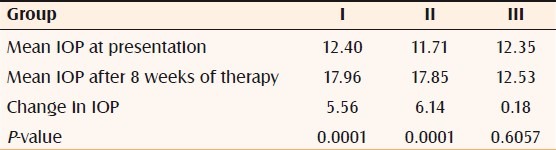
Figure 1.
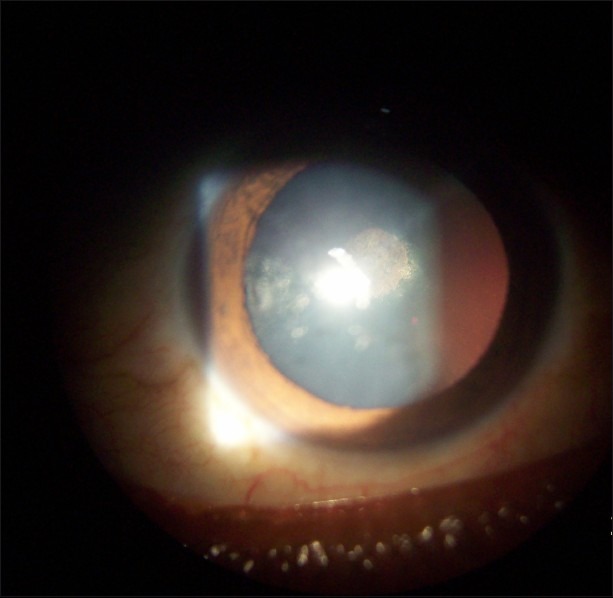
Posterior subcapsular cataract
IOP was controlled using topical timolol maleate eye drops. Once the dermatological condition had resolved, steroids were discontinued and the IOP returned to normal within 2–4 weeks. In no case were optic disc cupping or visual field changes noted.
DISCUSSION
Topical and systemic steroids have proven to be invaluable agents in the treatment of a wide range of disorders, but their use is not without potential complications. Irrespective of the route of administration, steroid use has been associated with both glaucoma and cataract formation.[1] Numerous studies have shown that topical and systemic steroids cause glaucoma and posterior subcapsular cataracts.[2–4] It has been reported that the elevation of IOP by systemic steroids is much less than that with topical steroids. Topical steroid applied around lesions over periorbital area can cause ocular complications on long term usage.[5,6] However, it has also been reported that topical as well as systemic steroids are equally potent in producing glaucoma.[7] Although all patients may respond with some change in pressure after prolonged corticosteroid use, studies have shown that 30%–40% of the population may be ‘intermediate responders,’ showing a pressure increase of at least 6 mm Hg, and 5%–6% of individuals may be ‘high responders,’ showing pressure rises in excess of 16 mmHg.[8,9]
We conclude that systemic steroids taken for more than 8 weeks leads to rise in IOP. However, the IOP will return to previous levels on discontinuation of the drug. A combination of a systemic steroid along with a topical preparation is more potent in producing raised IOP than the use of a systemic steroid alone. Pulse therapy with steroids does not lead to any rise in IOP. Development of cataract appears to be dependent on the duration of steroid therapy: the longer the duration the greater the possibility of developing cataract.
CONCLUSION
Corticosteroids are widely used for various dermatological conditions and it is the duty of the prescribing physician to keep a watchful eye on the adverse effects these drugs may cause when used for prolonged periods. Glaucoma is a sight-threatening condition. The dictum is ‘vision once lost is lost forever’ and the dermatologist should be careful when advising steroids and should not delay referral to an ophthalmologist when indicated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Urban RC, Jr, Dreyer EB. Corticosteroid-induced glaucoma. Int Ophthalmol Clin. 1993;33:135–39. doi: 10.1097/00004397-199303320-00013. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz B. Physiological effects of corticosteroids on the eye. Int Ophthalmol Clin. 1966;6:753–97. [PubMed] [Google Scholar]
- 3.Sihota R, Konkal VL, Dada T, Agarwal HC, Singh R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye (Lond) 2008;22:26–30. doi: 10.1038/sj.eye.6702474. [DOI] [PubMed] [Google Scholar]
- 4.Urban RC, Jr, Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1986;31:102–10. doi: 10.1016/0039-6257(86)90077-9. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwa RK, Potamitis T, Chong NHV, et al. Extensive visual loss with topical facial steroids. Eye. 1993;7:664–6. doi: 10.1038/eye.1993.152. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzenberg GWS, Buys Y. Glaucoma secondary to topical use of steroid cream. Can J Ophthalmol. 1993;34:222–5. [PubMed] [Google Scholar]
- 7.Mohan R, Muralidharan AR. Steroid induced glaucoma and cataract. Indian J Ophthalmol. 1989;37:13–6. [PubMed] [Google Scholar]
- 8.Armaly MF. Statistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of response. Invest Ophthalmol. 1965;4:187–97. [PubMed] [Google Scholar]
- 9.Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol. 1965;4:198–205. [PubMed] [Google Scholar]


