Abstract
Nephrogenic systemic fibrosis (NSF) is a relatively new fibrosing disorder which has caught the attention of various specialities in the past decade. NSF is an extremely disabling and often painful condition, affecting up to 13% of the individuals with chronic kidney disease. The administration of a gadolinium chelate contrast agent has been reported to induce the development of NSF, particularly in patients who have acute or chronic renal disease with a glomerular filtration rate (GFR) lower than 30-mL/min/1.73 m2 and in those with acute renal insufficiency. Mass spectroscopy studies have demonstrated particles of gadolinium in the lesional tissue. The exact pathogenesis of this curious sclerosing condition is unknown. The role of the aberrant targeting of ‘circulating fibrocytes’ to the peripheral tissues and viscera has been hypothesized. NSF has distinct clinicopathological features in the setting of renal failure and needs to be looked upon as a new entity on the block. The condition is characterized by irregular indurated plaques, with amoeba-like projections and islands of sparing, chiefly on the trunk and extremities. Flexion contractures of fingers, knees, and elbow joints are known to occur in advanced cases of NSF. The course is frequently associated with painful episodes and loss of ambulation. Histopathology shows haphazard arrangement of thickened bundles of collagen, varying amount of mucin, and increased population of fibroblast-like cells in the dermis. Immunohistochemistry shows increased deposition of type-I procollagen and CD 34+ cells having fibroblastic activity. The condition is refractory to treatment with corticosteroids and immunosuppressive agents. Various modalities of therapy such as UVA1 phototherapy, imatinib mesylate, photodynamic therapy, plasmapheresis, extracorporeal photochemotherapy, and high-dose intravenous immunoglobulin have shown a moderate degree of improvement in skin thickness scores. A prudent option is restoration of renal function to normalcy via renal transplantation but to date the outcome of renal transplantation is unknown.
Keywords: Gadolinium, nephrogenic fibrosing dermopathy, nephrogenic systemic fibrosis, renal failure
INTRODUCTION
A thorough MEDLINE-based search was performed with MeSH words, such as nephrogenic systemic fibrosis / nephrogenic fibrosing dermopathy, gadolinium, renal failure. Nephrogenic systemic fibrosis (NSF) was originally reported to be a ‘scleromyxedema-like cutaneous disease in renal-dialysis patients’ in 2000.[1] A relationship between exposure to gadolinium-based contrast agents and NSF was suggested in 2006.[2] Clinically, NSF mimics scleromyxedema, but tends to lack paraproteinemia, facial involvement or inflammatory cells, on histology.[3]
The term ‘nephrogenic fibrosing dermopathy’ (NFD) has been replaced by ‘nephrogenic systemic fibrosis,’ after it was shown that the fibrotic process is not only limited to the dermis but can also involve the underlying fascia, muscles, diaphragm, breast, myocardium and rete testis.[4–6] Although rare, NFD should be considered as a differential diagnosis in patients on hemodialysis, with indurated skin lesions along with other conditions like scleromyxedema, scleroderma, eosinophilic fasciitis or toxic syndromes.
The purpose of this article is to give the reader a clear but concise, evidence of NSF relevant to the present level of available information.
ETIOPATHOGENESIS [FIGURE 1]
Figure 1.
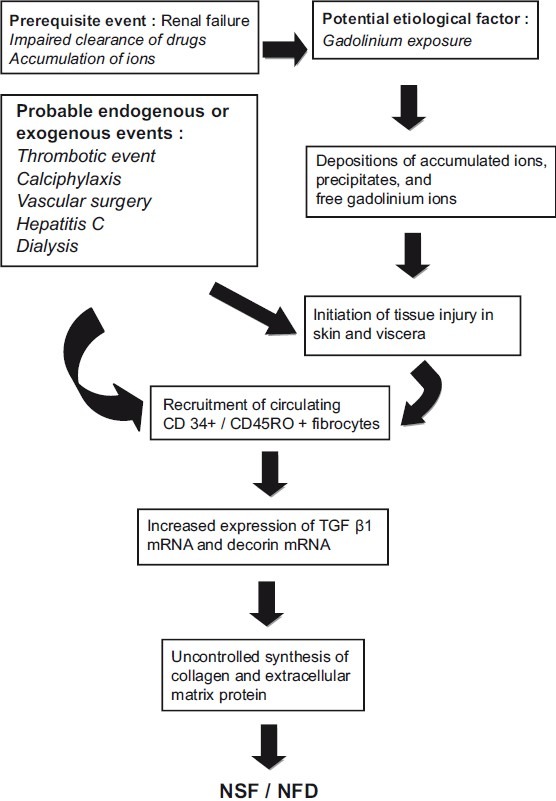
Hypothesized pathway for increased collagen deposition in NSF / NFD[5]
Nephrogenic systemic fibrosis is a disorder primarily affecting middle-aged adults, although there is no racial or sex predilection for this disorder.[5] It is chiefly seen in patients of chronic kidney disease, those on hemodialysis and those who have had a history of exposure to gadolinium-based contrast media.[7] Many endogenous as well as exogenous factors have been implicated in the causation of NSF. Endogenous factors include thrombotic events, hypercoaguable states (antiphospholipid antibody syndrome, anticardiolipin antibody), chronic hepatitis C infection, anasarca, and calciphylaxis.[5,8] Exogenous factors that are hypothesized to initiate the process of this disorder are — erythropoietin, surgical intervention, vascular procedures, peritoneal dialysis, hemodialysis, and the use of gadolinium chelate contrast media during imaging studies.[9] A putative role of endothelial cell damage and toxic substances is proposed, but it is yet to be established.
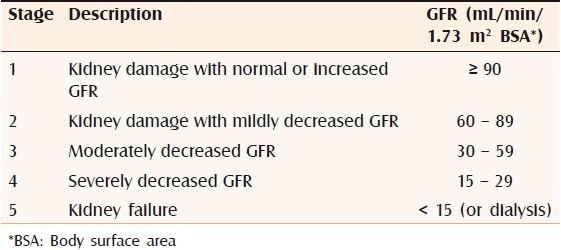
Since NSF occurs chiefly in the setting of chronic renal disease patients on dialysis or in renal transplant patients it is important for us to have a brief look into the pathophysiology of renal failure and its various laboratory parameters. Chronic kidney disease is defined as either kidney damage or a GFR of less than 60 mL/min/1.73m2 for more than three months.[7] Table 1 shows the classification of chronic kidney disease according to glomerular filtration rate.
Table 1.
Classification of chronic kidney disease based on decreasing glomerular filtration rate:[10]
In individuals with normal kidney function gadolinium rapidly distributes between the plasma and the tissue fluids. It has a half-life of approximately two hours and is cleared from the body via the kidney through glomerular filtration with a clearance varying from 1.1 to 1.6 ml/kg/min. More than 95% of the injected dose is eliminated in four to six half-lives and less than 3% is eliminated in the stool. In patients with advanced kidney failure (stage 5), the pharmacokinetics of the gadolinium complexes are altered. Due to their relatively low molecular weight (500 kilodalton), small volume of distribution (0.28 l/kg) and low protein binding, they are easily removed by hemodialysis, but not by peritoneal dialysis.[11] In patients who have renal failure, the half-life of gadolinium is increased substantially (up to 120 hours). Hemodialysis is known to decrease the half-life of gadolinium and can prevent risk of NSF in renal failure patients exposed to contrast agents.[12] Gadolinium is a contrast medication that is used very frequently in magnetic resonance imaging studies like magnetic resonance angiography (MR-angiography). Two agents, namely, gadodiamide and gadopentetate dimeglumine are gadolinium chelate contrast agents routinely used for contrast enhancement. Gadodiamide is a non-ionic chelate of gadolinium (less stable compound) and undergoes transmetallation with endogenous ions like iron, copper or zinc.[13] Transmetallation of gadolinium chelate leads to the release of free gadolinium through the replacement of gadolinium within the chelate molecule by body cations such as zinc or copper. Of the two contrast media used, gadodiamide is more notorious for causing NSF due to the above-mentioned reasons. Free gadolinium ions are less soluble and have a tendency to precipitate into different tissues through direct interaction with the cation-binding sites on the cellular membranes and extracellular matrix (ECM), or through microembolization in an aggregate form.[14] This explains the presence of deposits in the skin and soft tissues of patients who have had prior exposure to this contrast material.[15] Renal insufficiency leads to abnormal accumulation of ions in the body fluids and tissues; these ions can combine with gadolinium and form insoluble conjugates.[16] Deposition of such complexes can lead to the activation of resident dermal fibroblastic cells, ultimately leading to excess accumulation of collagen and extracellular ground matrix (mucin).
The pathogenic mechanism involves CD45RO+ / CD34+ / collagen I – positive circulatory fibrocytes that are recruited by the skin, yielding fibrosis. CD34 is a specific marker for adult hematopoietic stem cells, suggesting that these spindle fibroblast-like cells (called fibrocytes) may be circulating and derived from the bone marrow. These circulating fibrocytes are aberrantly targeted to the dermis, which causes a local release of a profibrotic cytokine-like transforming growth factor (TGF)-β1.[17] The dermis shows infiltration by CD68+ / XIIIa+ dendritic cells, which release profibrotic cytokines. Skin biopsy specimen of patients with NSF shows increased expression of the transforming growth factor (TGF)-β1 messenger RNA and decorin messenger RNA, which plays an important role in collagen metabolism. Decorin has a major role in collagen fibrillogenesis, growth factor modulation, fibrocyte differentiation, and direct regulation of cellular growth.[18] Some authors have noted increased levels of serum procollagen III peptide (PIIIP) in patients of NSF.[19] The serum PIIIP level reflects the degree of tissue fibrosis in various fibrotic diseases and is used routinely to evaluate liver fibrosis in patients with psoriasis treated with methotrexate.[20]
CLINICAL FEATURES
The cutaneous lesions of NSF usually develop over a short period — in most cases the lag time after gadolinium exposure is two weeks — and subsequently assume a chronic, unremitting course. The distribution is often symmetrical, commonly involving the lower extremities, up to the knees, and the upper extremities up to the elbows [Figure 2]. The face is usually spared. The skin is characterized by a lumpy, nodular thickening with a tendency to form indurated irregular plaques with ‘amoeboid’ projections. The affected areas and subcutaneous tissues are extremely hard, woody, and can be slightly warm to the touch. The joints underlying the NSF lesions are usually involved in a deep fibrotic process, causing severe flexion contractures (particularly hands, wrists, ankles, and knees), with substantial loss of range of motion and significant disability. The course is exacerbated by frequent episodes of pain and a burning sensation. The skin and joints involved by the tight fibrotic process are extremely tender. Pruritus and a burning sensation are very common over the affected areas. The condition rapidly follows a downhill trend (even if treated) and the patient becomes wheelchair–bound, with loss of ambulation. Even if no specific diagnostic test is available, the detection in the right clinical context (renal failure) of the characteristic skin changes, with unremarkable laboratory findings, is adequate to prompt a reasonable suspicion for NSF.
Figure 2.
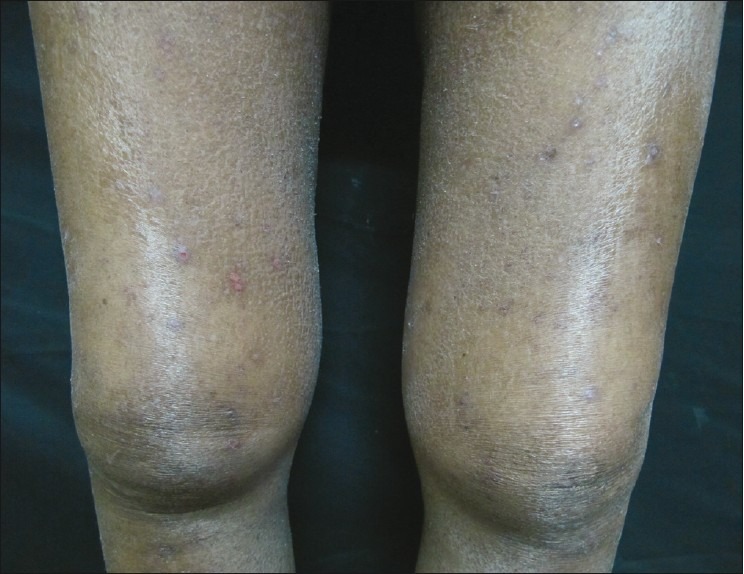
Ill-defined, shiny-pigmented patch on both the thighs
Skin thickness can be measured with the help of the modified Rodnan skin thickness (MRSS) score.[21] Imaging, and in particular MRI (without contrast), can be very helpful to define the extension of the deep fibrosing process and the presence of calcifications. An increased T1 signal is often present within the muscles underlying the affected surfaces, suggesting the presence of atrophy and fat degeneration. In addition, fat suppression protocols (e.g., fat-suppressed fast T2 weighted sequences) can reveal the presence of fascial and muscular edema, particularly during the early phase of the disease. Different from systemic sclerosis (SSc), in NSF, the serological markers for autoimmunity are absent and nail-fold capillary microscopy examination is normal. The body distribution can be similar to systemic sclerosis (i.e., hands can be fully involved), but the face is usually spared. The cobblestone appearance and brawny hyperpigmentation tend to differ from typical systemic sclerosis. There is no history of Raynaud's phenomenon in NSF.[22]
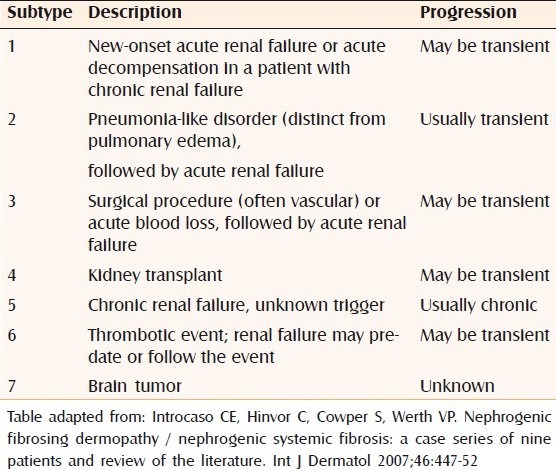
Few authors have proposed a classification system for NSF depending on the clinical setting and prognosis [Table 2].[23,24]
Table 2.
Clinical subtypes of nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy
DIFFERENTIAL DIAGNOSIS
Differential diagnosis of sclerosing / fibrosing skin disorder has been tabulated [Table 3].[22]
Table 3.
Differential diagnosis of nephrogenic systemic fibrosis

LABORATORY TOOLS
There are hardly any diagnostic tests available for detecting NSF. Possibility of NSF should be kept in mind in patients of kidney disease with impaired drug clearance and having sclerotic skin changes. The following findings may aid in the diagnosis of NSF [Table 4].
Table 4.
Investigations that could support the diagnosis of nephrogenic systemic fibrosis in the background of an appropriate clinical setting
HISTOPATHOLOGY
Deep incision biopsy of the involved skin including the muscle is necessary to demonstrate the findings of NSF on histopathology. Dense dermal fibrosis is seen under a normal epidermis. Thickened collagen bundles are deposited haphazardly in the dermis which are separated by large clefts and surrounded by numerous fibroblast-like (spindle) cells [Figure 3a and 3b]. Immunohistochemically these fibroblast-like cells show CD34 and CD45RO positivity. CD34 is a marker for adult hematopoietic stem cells, indicating that these cells are derived from bone marrow and are abnormally targeted to the dermis. Procollagen III peptide reactivity is increased in these circulating fibrocytes, reflecting profibrotic properties. Minimal perivascular inflammatory infiltrate is present. The infiltrative process can extend deep into the subcutaneous adipose interlobular septa. Mass spectroscopy and electron microscopy have demonstrated deposits of gadolinium in the involved tissue.[25]
Figure 3 (a, b).
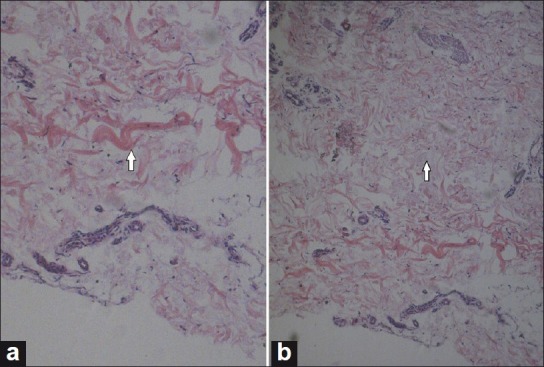
H and E stained section showing thickened collagen bundles in the reticular dermis. Note in a: A bluish staining mucin in the interstitium and the cleft formation between the collagen bundles
MANAGEMENT
Diagnosis of NSF is straightforward in the presence of typical skin changes in the background of renal failure. To date, there are no established treatment guidelines for this devastating fibrosing disorder. Imatinib mesylate was useful in few cases, where it selectively inhibited signaling mediated by c-Abl, c-Kit, and the platelet-derived growth factor receptor [Figure 1].[26,27] The condition is refractory to oral corticosteroids and immunosuppressive agents like cyclophosphamide (Cytoxan), azathioprine, and mycophenolate mofetil. It is very strange to note that NSF develops or progresses rapidly in renal transplant patients despite the fact that they are on potent antigraft rejection regimes (i.e., prednisolone, cyclosporine, tacrolimus or sirolimus).
Long-term treatment with extracorporeal photopheresis in treating NSF is yet not clear pending placebo-controlled randomized studies.[28] In patients with pre-existing end-stage renal (ESRD) disease undergoing hemodialysis and exposed to gadolinium, at least three hemodialysis sessions should be performed to facilitate clearing of the gadolinium; with the first session to be started within three hours of the contrast exposure.[29] High-dose intravenous immunoglobulin was used by a few authors with partial improvement in skin tightness and joint mobility. It is proposed that intravenous immunoglobulin exerts an inhibitory effect on the circulating factor (paraprotein) which may have a stimulatory effect on the fibroblast.[30,31] Few reports have documented success with photodynamic therapy (PDT), with the lipophilic agent methyl aminolaevulinate, in NFD. The PDT with hem-prodrug induces matrix metalloproteinases in fibroblasts and a reduction of collagen synthesis in the dermis.[32] Tran and colleagues have reported moderate success with UV-A1 therapy for NSF in four patients.[33]
CONCLUSION
Nephrogenic systemic fibrosis is a potentially devastating disorder and is associated with suffering and poor quality of life in ill-fated renal failure patients. A thorough knowledge regarding this curious disorder will go a long way in the rational management of patients and will help the physicians to provide palliative treatment until the advent of a breakthrough in the discovery of drugs that will halt the process of aberrant fibrosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxedema-like cutaneous disease in renal-dialysis patients. Lancet. 2000;356:1000–1. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 2.Grobner T. Gadolinium: A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 3.Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383–93. doi: 10.1097/00000372-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/ nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54:31–4. doi: 10.1016/j.jaad.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Raghunath S, Patil A, Inamdar AC, Madkari RM, Yelikar BR. Nephrogenic fibrosing dermopathy. Indian J Dermatol Venereol Leprol. 2009;75:63–7. doi: 10.4103/0378-6323.45224. [DOI] [PubMed] [Google Scholar]
- 6.Ting WW, Stone MS, Madison CK, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903–6. doi: 10.1001/archderm.139.7.903. [DOI] [PubMed] [Google Scholar]
- 7.Wertman R, Altun E, Martin DR, Mitchell DG, Leyendecker JR, O’Malley RB, et al. Risk of Nephrogenic Systemic Fibrosis: Evaluation of Gadolinium Chelate Contrast Agents at Four American Universities. Radiology. 2008;248:799–806. doi: 10.1148/radiol.2483072093. [DOI] [PubMed] [Google Scholar]
- 8.Robinson-Bostom L, DiGiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975–86. doi: 10.1067/mjd.2000.110651. [DOI] [PubMed] [Google Scholar]
- 9.Swaminathan S, Ahmed I, McCarthy JT, Albright RC, Pittelkow MR, Caplice NM, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145:234–5. doi: 10.7326/0003-4819-145-3-200608010-00021. [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 11.Perazella MA, Rodby RA. Gadolinium use in patients with kidney disease: A cause for concern. Semin Dial. 2007;20:179–85. doi: 10.1111/j.1525-139X.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 12.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acta Radiol. 1998;5:491–502. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- 13.Abraham JL, Thakral C, Skov L, Rossen K, Marckmann P. Dermal inorganic gadolinium concentrations: Evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol. 2008;158:273–80. doi: 10.1111/j.1365-2133.2007.08335.x. [DOI] [PubMed] [Google Scholar]
- 14.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260–4. doi: 10.1038/sj.ki.5002338. [DOI] [PubMed] [Google Scholar]
- 15.Barnhart JL, Kuhnert N, Bakan DA, Berk RN. Biodistribution of GdCl3 and Gd-DTPA and their influence on proton magnetic relaxation in rat tissues. Magn Reson Imaging. 1987;5:221–31. doi: 10.1016/0730-725x(87)90023-3. [DOI] [PubMed] [Google Scholar]
- 16.High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissues of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:21–6. doi: 10.1016/j.jaad.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, PieraVelazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238–49. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambichler T, Kreuter A, Skrygan M, Burkert B, Altmeyer P, Schieren G. Decorin Is Significantly Overexpressed in Nephrogenic Systemic Fibrosis. Am J Clin Pathol. 2009;132:139–43. doi: 10.1309/AJCPGB55YDURJXZC. [DOI] [PubMed] [Google Scholar]
- 19.Bangsgaard N, Marckmann P, Kristian Rossen K, Lone Skov L. Nephrogenic Systemic Fibrosis: Late Skin Manifestations. Arch Dermatol. 2009;145:183–7. doi: 10.1001/archdermatol.2008.551. [DOI] [PubMed] [Google Scholar]
- 20.Boffa MJ, Smith A, Chalmers RJ, Mitchell DM, Rowan B, Warnes TW, et al. Serum type III procollagen aminopeptide for assessing liver damage in methotrexate-treated psoriatic patients. Br J Dermatol. 1996;135:538–44. [PubMed] [Google Scholar]
- 21.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: An assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 22.Boin F, Hummers LK. Scleroderma-like Fibrosing Disorders. Rheum Dis Clin N Am. 2008;34:199–220. doi: 10.1016/j.rdc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Introcaso CE, Hinvor C, Cowper S, Werth VP. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: A case series of nine patients and review of the literature. Int J Dermatol. 2007;46:447–52. doi: 10.1111/j.1365-4632.2007.03301.x. [DOI] [PubMed] [Google Scholar]
- 24.Cowper SE. Nephrogenic fibrosing dermopathy: The first 6 years. Curr Opin Rheumatol. 2003;15:785–90. doi: 10.1097/00002281-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 25.White GW, Gibby WA, Tweedle MF. Comparison of Gd (DTPA-BMA) (Omniscan) versus Gd (HP-DO3A) (Pro Hance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41:272–8. doi: 10.1097/01.rli.0000186569.32408.95. [DOI] [PubMed] [Google Scholar]
- 26.Kay H, High WA. Imatinib Mesylate Treatment of Nephrogenic Systemic Fibrosis. Arthritis Rheum. 2008;58:2543–8. doi: 10.1002/art.23696. [DOI] [PubMed] [Google Scholar]
- 27.Chandran S, Petersen J, Jacobs C, Fiorentino D, Doeden K, Lafayette RA. Imatinib in the treatment of nephrogenic systemic fibrosis. Am J Kidney Dis. 2009;53:129–32. doi: 10.1053/j.ajkd.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Richmond H, Zwerner J, Kim Y, Fiorentino D. Nephrogenic systemic fibrosis: Relationship to gadolinium and response to photopheresis. Arch Dermatol. 2007;143:1025–30. doi: 10.1001/archderm.143.8.1025. [DOI] [PubMed] [Google Scholar]
- 29.Mundim JS, Lorena Sde C, Elias RM, Romão Júnior JE. Nephrogenic systemic fibrosis: Mini-review. Clinics (Sao Paulo) 2009;64:482–4. doi: 10.1590/S1807-59322009000500017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung HJ, Chung KY. Nephrogenic fibrosing dermopathy: response to high-dose intravenous immunoglobulin. Br J Dermatol. 2004;150:596–7. doi: 10.1111/j.1365-2133.2003.05795.x. [DOI] [PubMed] [Google Scholar]
- 31.Lister RK, Jolles S, Whittaker S, Black C, Forgacs I, Cramp M, et al. Scleromyxedema: Response to high-dose intravenous immunoglobulin (hdIVIg) J Am Acad Dermatol. 2000;43:403–8. doi: 10.1067/mjd.2000.104001. [DOI] [PubMed] [Google Scholar]
- 32.Schmook T, Budde K, Ulrich C, Neumayer HH, Fritsche L, Stockfleth E. Successful treatment of nephrogenic fibrosing dermopathy in a kidney transplant recipient with photodynamic therapy. Nephrol Dial Transplant. 2005;20:220–2. doi: 10.1093/ndt/gfh473. [DOI] [PubMed] [Google Scholar]
- 33.Tran KT, Prather HB, Cockerell CJ, Jacobe H. UV-A1 therapy for nephrogenic systemic fibrosis. Arch Dermatol. 2009;145:1170–4. doi: 10.1001/archdermatol.2009.245. [DOI] [PubMed] [Google Scholar]


