Abstract
Fumaric acid esters (FAE) are substances of interest in dermatology. FAE exert various activities on cutaneous cells and cytokine networks. So far only a mixture of dimethylfumarate (DMF) and three salts of monoethylfumarate (MEF) have gained approval for the oral treatment of moderate-to-severe plaque-type psoriasis in Germany. DMF seems to be the major active component. There is evidence that FAE are not only effective and safe in psoriasis but granulomatous non-infectious diseases like granuloma annulare, necrobiosis lipoidica and sarcoidosis. In vitro and animal studies suggest some activity in malignant melanoma as well.
Keywords: Fumaric acid esters, pharmacology, psoriasis, granulomatous skin diseases, melanoma
INTRODUCTION
Fumaric acid esters (FAE) have been used individually in patients with psoriasis since the late 1950s.[1] In the 1990s, Fumaderm® has become a registered drug in Germany for the systemic treatment of psoriasis. The drug consists of dimethylfumarate (DMF) and three salts of monoethylfumarate (MEF).
A homologous series of mono- and diesters of fumaric acid has been studied with respect to the sites and kinetics of presystemic ester degradation, using pancreas extract, intestinal perfusate, intestinal homogenate, and liver S9 fraction. In addition, intestinal permeability has been determined using isolated intestinal mucosa as well as Caco-2 cell monolayer in order to obtain estimates of the fraction of the dose absorbed for these compounds.
The uncharged diester DMF displayed a high presystemic metabolic lability in all the metabolism models. It also showed the highest permeability in the Caco-2 cell model. However, in permeation experiments with intestinal mucosa in the Ussing-type chambers, no undegraded DMF was found on the receiver side, indicating complete metabolism in the intestinal tissue. The intestinal permeability of the monoesters methyl hydrogen fumarate, ethyl hydrogen fumarate, n-propylhydrogen fumarate, and n-pentyl hydrogen fumarate increased with an increase in their lipophilicity, however, their presystemic metabolism rates increased with increasing ester chain length. It is concluded that for fumarates, an increase in intestinal permeability of the more lipophilic derivatives is counterbalanced by an increase in first-pass extraction.[2]
Dimethylfumarate is an active antipsoriatic compound, but only its hydrolysis product monomethylfumarate (MMF) has been detected in the plasma after oral intake.[3,4] Interestingly, DMF is hardly hydrolyzed to MMF in a buffer of pH 7.4, but rapidly hydrolyzed in human serum having the same pH. Moreover, in whole blood the half-life of DMF is dramatically reduced as compared to that in the serum. The concentrations of MMF and MEF in the serum and whole blood decrease with increasing time. These data indicate that the majority of FAE in the circulation are metabolized by one or more types of blood cells.[4] In vitro, DMF exerts pharmacodynamic effects at low concentrations, whereas MMF needs much higher concentrations.[5]
In vivo, MMF and MEF were detected after t (lag) = 120 minutes. The T (max) and c (max) of MMF were 210 minutes and 11.2 μM, and for MEF they were 210 minutes and 5.2 μM The half-life was 38.7 minutes (MMF) and 25.4 minutes (MEF). The AUC(0-infinity) of MMF was 172 minutes μg / ml and that of MEF was 63.6 minutes μg / ml. No plasma levels of DMF or fumaric acid (FA) were detected. The data of this study provide evidence that DMF is most likely absorbed out of the duodenum into the presystemic circulation and is not completely hydrolyzed to MMF before uptake.[6]
The paradox of the high activity of DMF, but low if there are any detection rates in the circulation, has been solved recently. DMF reacts fast and completely with glutathione at the physiological pH. MEF also reacts with glutathione, but slower and to a lesser extent, leading to a mixture of S-(1-carboxy-2-methoxycarbonylethyl)glutathione and S-(2-carboxy-1-methoxycarbonylethyl)glutathione. These compounds are subject to a sequence of enzymatically catalyzed reactions leading to mercapturic acid, which can be detected in urine. In urine, N-acetyl-S-(1,2-dimethoxycarbonylmethyl)cysteine can be identified. This gives further support to the concept that DMF treatment is glutathione consumptive. DMF penetrates into the blood cells and decreases the intracellular concentrations of glutathione. By this action a considerable part of DMF is not hydrolyzed, but escapes detection in the plasma or serum samples.[7]
The immunosuppressive effects of FAE are accompanied by a strong induction of the anti-inflammatory stress protein, heme oxygenase 1 (HO-1). Supplementation with exogenous glutathione (GSH), which is known to bind DMF, prevents both HO-1 induction as well as the anti-inflammatory effects of DMF. Inhibition of the HO-1 activity restores the diminished interleukin-12 and interferon (IFN)-gamma production after FAE treatment. These findings suggest that DMF at least partially mediates its immunomodulatory activity by the induction of the anti-inflammatory stress protein, HO-1, ascribed to the functional depletion of reduced GSH.[8]
Topical application of FAE has no antipsoriatic activity. On the other hand, diethyl fumarate caused non-immunologic contact urticaria in both human and guinea pig skin (Lahti and Maibach, 1985).[9] Topical application of MEF to healthy human volunteers caused a spontaneous persistent erythema, probably due to mast cell degranulation.[10]
Of late, unusual cases of contact dermatitis to chairs or sofas in Scandinavia, the UK, and other European countries had been attributed to DMF in furniture from a Chinese company. As a consequence, the European Union had banned the use of DMF in consumer products.[11]
PHARMACODYNAMIC EFFECTS OF FUMARIC ACID ESTERS
Fumaric acid esters have effects on various blood and tissue cells that might contribute to the clinical activity. FAE are thought to improve psoriasis by altering leukocyte, keratinocyte, and/or endothelial functions.
Epidermal keratinocytes
Fumaric acid (FA) and FAE were studied in the mouse tail test to evaluate their effects on epidermal cell differentiation. FA did not induce orthokeratotic differentiation, FAE had only a slight effect.[12]
In a study on human skin biopsies from psoriatic patients during systemic treatment with FAE, FAE reduced the degree of acanthosis with a rapid initial response, but was slowing down subsequently. FAE decreased the rate of proliferation and thereby decrease the number of cells per rete peg, as well as the size of the individual keratinocytes.[13]
In vitro studies with human HaCaT keratinocytes demonstrated a significant reduction of interferon (IFN)-gamma-induced keratin K17 expression by incubation with a subtotoxic and not antiproliferative concentration of 3 μM DMF.[14]
The p38 mitogen-activated protein kinase (MAPK) signaling pathway which regulates the activity of different transcriptions is activated in lesional psoriatic skin. Keratinocyte cell cultures were incubated with DMF, MHF, or FA and then stimulated with interleukin-1beta, before the kinase activation was determined by Western blotting. A significant inhibition of both MSK1 and 2 activations was seen after preincubation with DMF and stimulation with IL-1beta, whereas MHF and FA had no effect. DMF also decreased the phosphorylation of NF-kappaB / p65 (Ser276), which is known to be transactivated by MSK1. Furthermore, incubation with DMF before stimulation with IL-1beta resulted in a significant decrease in NF-kappaB binding to interleukin-8 kappaB and interleukin-20 kappaB-binding sites. This resulted in a subsequent decrease in interleukin-8 and interleukin-20 mRNA expression. These data suggest that DMF specifically inhibits MSK1 and 2 activations and subsequently inhibits NF-kappaB-induced gene transcriptions, which are believed to be important in the pathogenesis of psoriasis.[15]
G protein-coupled receptor 109A (GPR109A) is involved in flushing — a side effect of FAE, including MEF. Interestingly, both Langerhans cells and keratinocytes express GPR109A in mice. Using cell ablation approaches and transgenic cell type-specific GPR109A expression in Gpr109a- / - mice, it could be shown that the early phase of flushing depends on the GPR109A expressed on the Langerhans cells, whereas the late phase is mediated by GPR109A expressed on keratinocytes. The first phase of flushing was blocked by a selective cyclooxygenase-1 (COX-1) inhibitor, and the late phase was sensitive to a selective COX-2 inhibitor. MEF induced PGE2 formation in isolated keratinocytes through activation of GPR109A and COX-2. Thus, the early and late phases of the GPR109A-mediated cutaneous flushing reaction involves different epidermal cell types and prostanoid-forming enzymes.[16,17]
Granulocytes
Dimethylfumarate and MMF stimulate polarization and elastase release and enhance the intracellular killing of bacteria by granulocytes. Furthermore, MMF is able to suppress the formyl-Met-Nle-Phe (FMLP)-stimulated respiratory burst in these cells. MMF induces an increase in the intracellular Ca++ and cyclic adenosine monophosphate concentration.[18]
T-lymphocytes
Histological studies of psoriatic lesions during treatment with FAE demonstrate effects on the inflammatory infiltrate. The very first effect is the disappearance of the CD 15-positive cells in the upper dermis, accompanied by a significant reduction in CD 4-positive T-helper cells, pointing to an immunosuppressive activity.[19]
Interactions between infiltrating T cells and keratinocytes via the secretion of the TH1 have been investigated in vitro using keratinocytes from psoriatic patients as well as from healthy volunteers. Cells were grown both in mono- and co-cultures with HUT 78 T cells with / without the addition of FAE. Only DMF diminished Interleukin-6 and tumor growth factor-alpha secretion in the psoriatic co-cultures, but not in co-cultures from control subjects or in monocultures. DMF suppressed EGF-induced TGF-alpha mRNA induction in psoriatic keratinocytes. DMF inhibited INF-gamma secretion in all the cultures, but stimulated interleukin-10 secretion.[20]
The effects of DMF and its main metabolite, methylhydrogenfumarate (MHF), were assessed on the nuclear binding of NF-kappaB, nuclear factor of activated T cells (NF-AT), and CCAAT / enhancer binding protein beta (C / EBPbeta) in purified human T cells. DMF inhibited nuclear binding of NF-kappaB1, but not of NF-AT or C / EBPbeta. No effect of MHF on any of these transcription factors could be seen. These results provide evidence for a specific effect of DMF on NF-kappaB.[21]
FAE reduced the leukocyte rolling on endothelial surfaces due to the decreased expression of CD 25, HLA-DR, and cutaneous lymphocyte antigens on CD 3-positive T lymphocytes. The binding to E-selectin, P-selectin, and vascular adhesion molecule VCAM-1 are markedly diminished.[22]
Dimethylfumarate (10 to 20 μg / ml) and not MHF is capable of inducing apotosis in vitro in interleukin-2 activated T lymphocytes as shown by the increased expression of Apo2.7 and decreased expression of anti-apoptotic molecule bcl-2.[23]
Natural killer cells
In an immunohistochemical study six patients with moderate-to-severe psoriasis treated with DMF 720 mg daily for 16 weeks were analyzed. Biopsies were taken from the lesional skin at the baseline and after 16 weeks of treatment. At week 16 in both the lesional psoriatic dermis and epidermis, cells expressing NK receptors (CD94 and CD161) persisted.[24]
Endothelial cells
Dimethylfumarate inhibited the intracellular adhesion molecule ICAM-1, vascular adhesion molecule VCAM-1, and E-selectin expression, and reduced the adhesion of U937 cells to stimulated human vascular endothelial cells (HUVEC). In contrast, MEF and FA had no effect. Similar inhibitory effects of DMF on VCAM-1 expression were observed after stimulation of HUVEC with LPS, PMA, IL-4, and IL-1 alpha or in combination with TNF alpha. The inhibitory effect on cytokine-induced endothelial adhesion molecule expression may represent another target of DMF in psoriasis.[25]
CD62E and CD54 expression is considered as an activation marker in endothelial cells. In the lesional skin of psoriatic patients, oral FAE treatment results in a marked reduction of CD62E, but not CD54 expression on dermal microvessels. Using human umbilical vein endothelial cells, DMF almost completely inhibits the tumor-necrosis-factor-induced CD62E, but not CD54 expression, at concentrations less or equal to 70 μM. Preincubation with DMF for 60 minutes is sufficient to block tumor-necrosis-factor-induced CD62E expression for up to 24 hours. In contrast, equimolar concentrations of MHF, a hydrolysis product of DMF, or other FAE, do not suppress tumor-necrosis-factor-induced CD62E expression. Using CD62E, the nuclear factor (NF)-kappa B or the AP-1-responsive promoter constructs, DMF inhibits tumor-necrosis-factor-induced activation of the CD62E and the NF-kappa B, but not the AP-1 promoter construct. DMF appears to be a specific inhibitor of CD62E expression in an NF-kappa B-dependent manner.[26]
Dermal fibroblasts
Dimethylfumarate exerts inhibitory effects on tumor-necrosis-factor-alpha-induced or interleukin-1 alpha-induced ICAM-1 expression in normal human dermal fibroblasts. Western blot analysis of normal human dermal fibroblast cytoplasmic extracts shows that DMF has only minor effects on the ICAM kappa B alpha, beta, and epsilon proteins: their cytokine-induced degradation and resynthesis slows down. No inhibitory effect of DMF is observed on cytokine-induced RelA / p65 or c-Rel accumulation in the nuclear extracts of cytokine-treated normal human dermal fibroblast cells. In contrast, the cytokine-induced nuclear factor kappa B1 / p50 nuclear accumulation is specifically inhibited by DMF. Cytokine-induced activation of a pNF kappa B::luciferase reporter construct in transiently transfected normal human dermal fibroblasts is inhibited by DMF. The data suggest that the DMF reducing nuclear factor kappa B1 leads to changes in the nuclear factor kappa B1-RelA nuclear balance and inhibition of cytokine-induced adhesion molecule expression in normal human dermal fibroblasts.[27]
Dendritic cells
Dendritic cells (DC) regulate the differentiation of the T helper (Th) cells. MMF-incubated, lipopolysaccharide-stimulated DC (MMF-DC), produced dramatically (p < 0.05) reduced levels of interleukin-12p70 and interleukin-10 (8 ± 4% and 20 ± 4%, respectively) compared to control DC. MMF-DCs are mature cells. MMF affects polarization of DC, irrespective of the polarization factor(s) and ligands for the various Toll-like receptors used. Co-culture of MMF-DC with naive and primed allogenous Th cells result in the lymphocytes producing less IFN-gamma, that is, 59 and 54% of that by the respective Th cells co-cultured with control DC. IL-4 production by primed, but not naive Th cells co-cultured with MMF-DC is decreased as compared to co-cultures with control DC. IL-10 production by naive and primed Th cells co-cultured with MMF-DC and control DC do not differ. In addition, MMF inhibits LPS-induced NF-kappaB activation in DC. Together, the beneficial effects of FAE in psoriasis involve the modulation of DC polarization by MMF, such that these cells downregulate the IFN-gamma production by Th cells.[28]
The presence of MMF during the differentiation of monocytes into immature DCs results in cells that retain low levels of CD14 and hardly express CD1a. During maturation, immature MMF-DCs upregulate CD83, costimulatory molecules, and HLA-DR on their surface indicating that these cells respond to lipopolysaccharide (LPS), albeit in a lesser degree than the control. In response to LPS, immature MMF-DCs do not decrease the capacity to capture antigens when compared with controls. MMF-DCs hardly produce IL-12p70 and IL-10 and low levels of TNF-alpha, whereas interleukin-8 produced by the MMF-DCs and control DCs do not differ. MMF-DCs are less likely to induce IFN-gamma production by naive Th lymphocytes compared to controls. MMF inhibits monocyte-derived DC differentiation, resulting in cells that cannot appropriately mature to DCs. Consequently, these MMF-DCs are less effective in stimulating the type 1 cytokine, but not type 2 cytokine production in Th lymphocytes.[29]
FUMARIC ACID ESTERS FOR PSORIASIS
The first randomized, double-blind, placebo-controlled study included 39 adult patients with psoriasis. During 16 weeks, the patients were treated with tablets containing a combination of DMF and different salts of MEF, with octylhydrogen fumarate or placebo tablets. All patients were treated with an identical indifferent topical therapy and followed an elimination diet (avoidance of spices, wine, and nuts). Thirty-four patients completed the study and five patients dropped out because of side effects or aggravation of psoriasis. The patients treated with a combination of MEF and DMF showed a significantly better therapeutic response compared to those who were treated with placebo or octylhydrogen fumarate.[30]
A randomized double-blind study was carried out on 100 patients with psoriasis, comparing oral FAE with placebo. The results indicated statistically significant superiority of the FA derivatives over placebo.[31] This was followed by a German prospective multicenter study which investigated the efficacy and safety of FAE therapy in 101 patients. Seventy patients completed the treatment period of four months. Discontinuation was due to adverse events in seven, lack of efficacy in two, and other reasons, such as non-attendance for scheduled visits, in 22 patients. Evaluation of the overall efficacy showed a decrease in psoriasis area and severity index (PASI) of 80% after four months of FAE therapy. Gastrointestinal complaints in five patients and flushing in two patients led to their withdrawal from the study. Taken together, the results of this multicenter study showed that systemic FAE treatment was effective in severe psoriasis vulgaris in a large number of patients.[32]
In a cohort study, 12 adult patients with psoriasis vulgaris were followed during 24 months of FAE treatment. Disease activity in the patients was determined by the Psoriasis Area and Severity Index (PASI) score. Within six months of fumarate treatment, the mean ± standard deviation (SD) PASI score had decreased to 22 ± 9% of its initial value. A common adverse effect was mild lymphopenia.[33]
Carboni et al. performed an open trial with 40 psoriasis patients. DMF was orally administered at a daily dose of 30 mg up to 360 mg for a minimum of six months. Patients were followed-up with PASI score assessment and clinical and photographic documentation. Thirty-three (82.5%) patients achieved complete clinical remission: eight after three months and twenty-five after six months. Adverse events, such as intolerable abdominal cramps and incoercible diarrhea, occurred in four patients who interrupted the treatment.[34]
A retrospective study from Ireland was carried out on patients treated with FAEs over 21 months. In total, 31 patients were included. The mean age was 46.8 years. All patients had been treated with other modalities and over 60% had received other systemic treatments earlier. There was good-to-excellent response in 58.6% of the FAE-treated patients.[35]
In Germany, FAE is considered as one of the first line systemic treatments for moderate-to-severe psoriasis. In a retrospective German study on the long-term treatment of psoriasis with FAE (Fumaderm®), 984 patients were included, with a mean duration of 44 months of continuous treatment. The percentage of patients documented as markedly improved or clear was 67% after six months, 78% after 24 months, and 82% after 36 months of therapy. Improvement was similar in patients with moderate and severe psoriasis. Changes of laboratory parameters were usually insignificant and did not require a modification of FAE treatment in more than 90% of the cases. The authors concluded that in the long-term treatment of patients with moderate and severe psoriasis, FAE showed a good and sustained clinical efficacy combined with a favorable safety profile.[36]
A single-center, open, non-randomized, prospective study was performed in a regional referral center for patients with severe psoriasis in London / UK. Eighty patients were recruited. Fifty-nine percent were taking a concomitant oral antipsoriatic agent. Twenty percent achieved a PASI of 50 or higher on an intention-to-treat analysis, at three months, with an overall reduction in PASI from 13.9 ± 9.0 to 11.3 ± 9.2 (P < 0.0001).[37]
Kokelj et al. reported an open trial including four patients with mild psoriasis. Oral therapy was realized with Psocaps® containing 73 mg of DMF and 42 mg of MEF. Dose escalation was done weekly starting with one tablet / day with a final dosage of three tablets / day. After four months improvement was observed in 19 patients, stable disease in 10, and worsening in six patients. The mean PASI decreased from 5.9 to 3.0 after four months. Psocaps® was chemically different from Fumaderm® approved in Germany.[38]
Although several clinical trials demonstrate the efficacy of plaque-type, moderate-to-severe psoriasis with significant reduction of PASI after three months, data for other types of psoriasis are sparse. Case reports demonstrate a beneficial effect of FAE on nail psoriasis or pustular psoriasis in some patients.[32,39–41]
There is a single, double-blind, placebo-controlled study on FAE in psoriatic arthritis, demonstrating some effect.[42] Today, FAE is not recommended for the treatment of psoriatic arthritis because of lack of significant activity in arthritis, dactylitis, and enthesitis.[43]
COMPARISION OF FAE WITH OTHER DISEASE MODIFYING DRUGS IN PSORIASIS
A randomized, controlled trial compared the effectiveness and safety of FAE with methotrexate. A total of 60 patients with moderate-to-severe psoriasis vulgaris were randomly assigned to treatment for 16 weeks with either methotrexate (30 patients; 15 mg per week) or FAE (30 patients; 30 mg, followed by 120 mg orally according to a standard progressive dosage regimen) and were followed-up for four weeks. Six patients were excluded because five were not eligible and one withdrew the informed consent. Two patients in the methotrexate group and one in the FAE group dropped out during the 12 weeks of treatment because of non-appearance at the outpatient clinic. After 12 weeks of treatment, the mean (±SD) PASI decreased from 14.5 ± 3.0 at baseline to 6.7 ± 4.5 in the 25 patients treated with methotrexate, whereas it decreased from 18.1 ± 7 to 10.5 ± 6.7 in the 26 patients treated with FAE. In this randomized trial methotrexate and fumarates were found to be equally effective in the treatment of patients with psoriasis. No serious or irreversible adverse events were observed in any of the patients.[44]
GRANULOMATOUS DISORDERS
Necrobiosis lipoidica is an uncommon granulomatous skin disease, with a possible association with diabetes mellitus. Eighteen patients with histopathologically proven necrobiosis lipoidica were consecutively recruited into a prospective uncontrolled study. The dosage of FAE was given according to the standard therapy regimen for psoriasis. FAE were administered for at least six months. The treatment outcome was evaluated by means of clinical and histological scoring and 20-MHz ultrasound assessments. Three patients discontinued therapy with FAE, while the remaining 15 patients finished the study. After a mean ± SD treatment period of 7.7 ± 2.9 months, a significant decrease in the clinical score was observed. Clinical improvement was accompanied by a significant increase in dermal density as assessed by means of 20-MHz ultrasound and significant reduction of the histological score. Adverse effects were moderate and consisted mainly of gastrointestinal complaints and flushing. During follow-up of at least six months, the clinical outcome remained stable in all patients.[45]
Granuloma annulare is another granulomatous disease of unknown etiology. The disseminated type is difficult to treat; corticosteroids, dapsone, and phototherapy have been used with variable success. In an open trial, eight patients with disseminated granuloma annulare were treated with oral FAE according to the standard therapy regimen used in psoriasis. The color and elevation of the skin lesions were assessed by a visual analog scale before and after therapy. Systemic therapy with FAE induced a significant clinical improvement in the elevation and color of skin lesions, with remission in three and partial remission in four patients. One patient remained unchanged. Side-effects associated with the therapy were seen in six patients.[46]
Weber et al. treated eight adult patients with a low-dose of FAE for 1–18 months. One patient showed complete clearance, four showed marked improvement, one showed slight-to-moderate improvement, and one no response. One patient discontinued treatment due to nausea after one month and another stopped it after 18 months. Five out of eight patients tolerated the treatment well. Six patients developed transient, mild leukopenia, and one eosinophilia. None of these blood abnormalities necessitated discontinuation of the therapy. Low-dose FAE significantly improved disseminated granuloma annulare in approximately 63% of the patients.[47]
Uncontrolled observations suggest that a combination of oral FAE with PUVA may induce a more rapid response compared to FAE monotherapy [Figure 1].[48]
Figure 1.
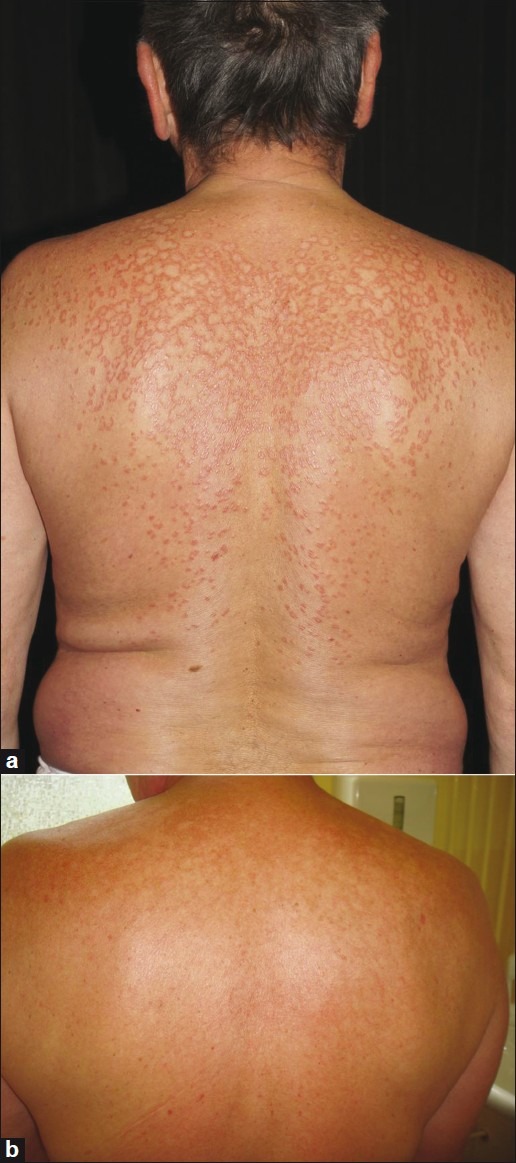
Disseminated granuloma annulare in a 64-year old male, resistant to topical corticosteroids. (a) Before treatment (b) After two weeks of Fumaderm® and bath-PUVA, complete remission of infiltrates and redness. Some residues of hyperpigmented skin remained
Sarcoidosis is a multisystem disease of unknown origin characterized by the formation of noncaseating granulomas, in particular in the lungs, lymph nodes, eyes, and skin. Nowak et al. reported three patients with recalcitrant cutaneous sarcoidosis, who were treated with oral FAE. The dosage of FAE was performed according to the standard therapy regimen for psoriasis patients. After treatment with FAE for 4 – 12 months a complete clearance of skin lesions was achieved in all the patients. The side effects included flush, minor gastrointestinal complaints, and lymphopenia.[49]
In a retrospective trial, the therapeutic efficacy and side-effects of FAE were analyzed in 32 patients with various non-infectious granulomatous diseases: disseminated granuloma annulare (n = 13), annular elastolytic giant cell granuloma (n = 3), sarcoidosis (n = 11), necrobiosis lipoidica (n = 4), and granulomatous cheilitis (n = 1). Three patients discontinued treatment within four weeks because of side-effects. Of the remaining 29 patients, 18 patients responded to oral treatment with FAE. Marked improvement or complete clearance was seen in seven patients, slight-to-moderate improvement in 11 patients, and 11 patients did not respond. In patients showing a complete remission, the maximum effect was observed after 8.5 months. In two patients with systemic sarcoidosis, the pulmonary changes improved in parallel with the skin. The side-effects were usually mild and resolved spontaneously upon dose reduction or discontinuation of the therapy.[50]
MALIGNANT MELANOMA
Dimethylfumarate is a substance of potential interest in malignant melanoma. Thus far no human in vivo data are available. On the other hand, cell culture data and animal models argue for anti-tumor effects.
Dimethylfumarate inhibited the proliferation of human melanoma cells A375 and M24met in vitro. The cell cycle was arrested at the G2-M boundary. Moreover, DMF was proapoptotic, as shown by cell cycle analysis and by Annexin V and Apo2.7 staining. These results were confirmed in vivo. DMF reduced the proliferation rates of tumor cells as assessed by Ki-67 immunostaining and increased apoptosis as assessed by terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling staining. In conclusion, DMF is antiproliferative and proapoptotic and reduces melanoma growth and metastasis in animal models.[51]
The inhibition of tumor invasion and metastasis by DMF was studied in the melanoma cell line B16BL6. DMF inhibited B16BL6 cell invasion and metastasis by suppressing the expression and activities of matrix metalloproteinases. DMF also inhibited the nuclear entry of NF-kappaB / p65, thus inhibiting B16BL6 cell invasion and metastasis.[52]
Using the severe combined immunodeficiency (SCID) mouse model, in which xenografted human melanoma cells metastasized from primary skin sites to sentinel nodes, dacarbazine (DTIC) and DMF, alone or in combination, reduced tumor growth at the primary sites. The main finding of this study was that metastasis to the sentinel nodes was significantly delayed only in mice treated with a combination of DTIC and DMF. Subsequent experiments were able to show that a combination of DTIC / DMF significantly reduced lymph vessel density in primary tumors, as examined by real-time PCR and immunohistochemistry. In addition, DTIC / DMF treatment significantly impaired melanoma cell migration in vitro. In vivo, DTIC / DMF therapy significantly reduced mRNA expression and protein concentration of the promigratory chemokines CXCL2 and CXCL11.[53]
OTHER POSSIBLE INDICATIONS
Intensive investigations are performed with FAE for relapsing / remittent multiple sclerosis. Acute exposure to FAE deprives the astrocytes of their GSH, most likely by the reaction of the reactive alpha, beta-unsaturated diesters with GSH.[54] It was shown that DMF had a significant inhibitory effect on the lipopolysaccharide (LPS)-induced nitric oxide burst in microglia.[55] The other potential effects are the inhibition of neurogenic inflammation by DMF. DMF pretreatment decreased the synthesis of proinflammatory mediators iNOS, TNF-alpha, IL-1beta, and IL-6, at the RNA level in activated microglia and astrocytes in vitro, associated with a decrease in ERK phosphorylation in the microglia.[56]
A randomized, double-blind, prospective controlled multicenter IIb study was published recently using oral fumarate BG00012. Two hundred and fifty-seven adult patients with relapsing / remitting multiple sclerosis were randomly assigned to receive 120 mg once daily (n = 64), 120 mg thrice daily (n = 64), or 240 mg thrice daily (n = 64) BG00012, or placebo (n = 65) for 24 weeks. During an extension period of 24 weeks for safety assessment, the patients treated with placebo received BG00012, 240 mg thrice daily. Treatment with BG00012, 240 mg thrice daily reduced the mean total number of new gadolinium enhancing (GdE) brain lesions by 69% from week 12 to 24 compared to placebo (1.4 vs. 4.5, P < 0.0001). BG00012 reduced the annualized relapse rate by 32%. Adverse events more common in patients given BG00012 than in those given placebo included abdominal pain, flushing, and hot flush. Dose-related adverse events in patients on BG00012 were headache, fatigue, and feeling hot.[57]
Dimethylfumarate has been use in experimental malaria. The interaction of DMF with glutathione has led to a more rapid clearance of infected erythrocytes.[58]
ADVERSE EFFECTS
Adverse events were reported in 69 – 87% of the patients, mainly consisting of gastrointestinal complaints (56%) and flushing (31%).[32,35,37] Other typical side effects of the FAE containing tablets were reversible elevation of transaminases, lymphocytopenia, and eosinophilia. These adverse effects were usually mild. The laboratory parameters return to normal after dose reduction or discontinuation of therapy. Disturbances of kidney function were reported from one in 39 to none in 101 patients.[30,32] Kidney damage caused either by acute renal injury or the Fanconi syndrome was reported. Control of urinary excretion of β2-microglobulin, a marker of renal proximal tubular dysfunction, allowed early detection of kidney damage, before an increase in serum creatinine or significant proteinuria occurred.[59] Personal experience of 15 years did not argue for a high risk of kidney damage when moderate dosages (i.e., 3 to 4 tablets of Fumaderm® per day) were used. A very rare, possible adverse effect of long-term FAE therapy might be pneumonia, unresponsive to antibiotics, but responsive to prednisolone.[60]
There is no known drug interaction that would demand dose reduction or would forbid the use of FAE. Nevertheless, concomitant use of drugs with possible adverse effects on kidney function should be avoided. There is no data arguing for either teratogenicity or mutagenicity of FAE. FAE should be avoided during pregnancy or lactation because of lack of data.[61]
The German guideline for psoriasis therapy recommends laboratory controls before initiation of FAE therapy (blood count, differential blood count, transaminases, gamma-glutamyl transferase, serum creatinine, and urine analysis). In the first six months the control should be performed every month, thereafter every two months. Lymphopenia with less than 500 per ml needs dose reduction or discontinuation of the FAE therapy.[62]
FAE should not be combined with systemic antipsoriatic drugs like methotrexate or cyclosporine A. A controlled trial demonstrated a faster response when FAE were used with topical calcipotriol.[63] From uncontrolled clinical experience, FAE can be combined with topical anthralin or corticosteroids and PUVA. This will lead to a faster response in psoriasis and granulomatous skin diseases (for corticosteroids and PUVA only).[61]
DOSING
Fumaric acid ester tablets are available in two types, Fumaderm initial® and Fumaderm®. One tablet of Fumaderm initial® is the usual starting dose with a dose-escalation every week. When three tablets of Fumaderm initial® are well tolerated the treatment is switched to one tablet of Fumaderm®. Dose-escalation is done weekly with a final maximum dosage of six tablets. Most patients need lower dosages, that is, 1 tablet/3×4 times a day. Dose reduction is possible for maintenance treatment.[62]
Footnotes
Source of Support: Nil
Conflict of Interest: The author declares no conflict of interest or financial involvement with the company/companies manufacturing the drug. This is a review of a drug that has not yet entered the Indian market but will do so in the future as most other drugs.
REFERENCES
- 1.Schweckendiek W. Cure of psoriasis [German] Med Msch. 1959;13:103–4. [PubMed] [Google Scholar]
- 2.Werdenberg D, Joshi R, Wolffram S, Merkle HP, Langguth P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm Drug Dispos. 2003;24:259–73. doi: 10.1002/bdd.364. [DOI] [PubMed] [Google Scholar]
- 3.Nieboer C, de Hoop D, Langendijk PN, van Loenen AC, Gubbels J. Fumaric acid therapy in psoriasis: A double-blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica. 1990;181:33–7. doi: 10.1159/000247856. [DOI] [PubMed] [Google Scholar]
- 4.Litjens NH, van Strijen E, van Gulpen C, Mattie H, van Dissel JT, Thio HB, et al. In vitro pharmacokinetics of anti-psoriatic fumaric acid esters. BMC Pharmacol. 2004;4:22. doi: 10.1186/1471-2210-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu K, Mrowietz U. Inhibition of dendritic cell differentiation by fumaric acid esters. J Invest Dermatol. 2001;116:203–8. doi: 10.1046/j.1523-1747.2001.01159.x. [DOI] [PubMed] [Google Scholar]
- 6.Rostami-Yazdi M, Clement B, Mrowietz U. Pharmacokinetics of anti-psoriatic fumaric acid esters in psoriasis patients. Arch Dermatol Res. 2010;302:531–8. doi: 10.1007/s00403-010-1061-4. [DOI] [PubMed] [Google Scholar]
- 7.Rostami-Yazdi M, Clement B, Schmidt TJ, Schinor D, Mrowietz U. Detection of metabolites of fumaric acid esters in human urine: implications for their mode of action. J Invest Dermatol. 2009;129:231–4. doi: 10.1038/jid.2008.197. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann JC, Listopad JJ, Rentzsch CU, Igney FH, von Bonin A, Hennekes HH, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol. 2007;127:835–45. doi: 10.1038/sj.jid.5700686. [DOI] [PubMed] [Google Scholar]
- 9.Lahti A, Maibach HI. Contact urticaria from diethyl fumarate. Contact Dermatitis. 1985;12:139–40. doi: 10.1111/j.1600-0536.1985.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 10.Gehring W, Gloor M. Persistent spontaneous erythema caused by topical use of fumaric acid monoethyl ester--an obligate mast cell degranulation? [German] Dermatol Monatsschr. 1990;176:123–8. [PubMed] [Google Scholar]
- 11.Susitaival P, Winhoven SM, Williams J, Lammintausta K, Hasan T, Beck MH, et al. An outbreak of furniture related dermatitis (‘sofa dermatitis’) in Finland and the UK: History and clinical cases. J Eur Acad Dermatol Venereol. 2010;24:486–9. doi: 10.1111/j.1468-3083.2009.03429.x. [DOI] [PubMed] [Google Scholar]
- 12.Sebök B, Szabados T, Kerényi M, Schneider I, Mahrle G. Effect of fumaric acid, its dimethylester, and topical antipsoriatic drugs on epidermal differentiation in the mouse tail model. Skin Pharmacol. 1996;9:99–103. doi: 10.1159/000211404. [DOI] [PubMed] [Google Scholar]
- 13.Bacharach-Buhles M, Röchling A, el Gammal S, Altmeyer P. The effect of fumaric acid esters and dithranol on acanthosis and hyperproliferation in psoriasis vulgaris. Acta Derm Venereol. 1996;76:190–3. doi: 10.2340/0001555576190193. [DOI] [PubMed] [Google Scholar]
- 14.Bonnekoh B, Böckelmann R, Ambach A, Gollnick H. Dithranol and dimethylfumarate suppress the interferon-gamma-induced up-regulation of cytokeratin 17 as a putative psoriasis autoantigen in vitro. Skin Pharmacol Appl Skin Physiol. 2001;14:217–25. doi: 10.1159/000056350. [DOI] [PubMed] [Google Scholar]
- 15.Gesser B, Johansen C, Rasmussen MK, Funding AT, Otkjaer K, Kjellerup RB, et al. Dimethylfumarate specifically inhibits the mitogen and stress-activated kinases 1 and 2 (MSK1/2): Possible role for its anti-psoriatic effect. J Invest Dermatol. 2007;127:2129–37. doi: 10.1038/sj.jid.5700859. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar RL, Gelfand JM. Seeing red: Flushing out instigators of niacin-associated skin toxicity. J Clin Invest. 2010;120:2651–5. doi: 10.1172/JCI44098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120:2910–9. doi: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nibbering PH, Thio B, Zomerdijk TP, Bezemer AC, Beijersbergen RL, van Furth R. Effects of monomethylfumarate on human granulocytes. J Invest Dermatol. 1993;101:37–42. doi: 10.1111/1523-1747.ep12358715. [DOI] [PubMed] [Google Scholar]
- 19.Ockenfels HM, Schultewolter T, Ockenfels G, Funk R, Goos M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br J Dermatol. 1998;139:390–5. doi: 10.1046/j.1365-2133.1998.02400.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerdes S, Shakery K, Mrowietz U. Dimethylfumarate inhibits nuclear binding of nuclear factor kappaB but not of nuclear factor of activated T cells and CCAAT/enhancer binding protein beta in activated human T cells. Br J Dermatol. 2007;156:838–42. doi: 10.1111/j.1365-2133.2007.07779.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubant SA, Ludwig RJ, Diehl S, Hardt K, Kaufmann R, Pfeilschifter JM, et al. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J Invest Dermatol. 2008;128:326–31. doi: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- 22.Treumer F, Zhu K, Gläser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121:1383–8. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 23.Bovenschen HJ, Langewouters AM, van de Kerkhof PC. Dimethylfumarate for psoriasis: Pronounced effects on lesional T-cell subsets, epidermal proliferation and differentiation, but not on natural killer T cells in immunohistochemical study. Am J Clin Dermatol. 2010;11:343–50. doi: 10.2165/11533240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Bacharach-Buhles M, Pawlak FM, Matthes U, Joshi RK, Altmeyer P. Fumaric acid esters (FAEs) suppress CD 15- and ODP 4-positive cells in psoriasis. Acta Derm Venereol Suppl (Stockh) 1994;186:79–82. [PubMed] [Google Scholar]
- 25.Vandermeeren M, Janssens S, Borgers M, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun. 1997;234:19–23. doi: 10.1006/bbrc.1997.6570. [DOI] [PubMed] [Google Scholar]
- 26.Loewe R, Pillinger M, de Martin R, Mrowietz U, Gröger M, Holnthoner W, et al. Dimethylfumarate inhibits tumor-necrosis-factor-induced CD62E expression in an NF-kappa B-dependent manner. J Invest Dermatol. 2001;117:1363–8. doi: 10.1046/j.0022-202x.2001.01576.x. [DOI] [PubMed] [Google Scholar]
- 27.Vandermeeren M, Janssens S, Wouters H, Borghmans I, Borgers M, Beyaert R, et al. Dimethylfumarate is an inhibitor of cytokine-induced nuclear translocation of NF-kappa B1, but not RelA in normal human dermal fibroblast cells. J Invest Dermatol. 2001;116:124–30. doi: 10.1046/j.1523-1747.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 28.Litjens NH, Rademaker M, Ravensbergen B, Rea D, van der Plas MJ, Thio B, et al. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol. 2004;34:565–75. doi: 10.1002/eji.200324174. [DOI] [PubMed] [Google Scholar]
- 29.Litjens NH, Rademaker M, Ravensbergen B, Thio HB, van Dissel JT, Nibbering PH. Effects of monomethylfumarate on dendritic cell differentiation. Br J Dermatol. 2006;154:211–7. doi: 10.1111/j.1365-2133.2005.07002.x. [DOI] [PubMed] [Google Scholar]
- 30.Nugteren-Huying WM, van der Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy in psoriasis; A double-blind, placebo-controlled study [Dutch] Ned Tijdschr Geneeskd. 1990;134:2387–91. [PubMed] [Google Scholar]
- 31.Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, et al. Antipsoriatic effect of fumaric acid derivatives.Results of a multicenter double-blind study in 100 patients. Am Acad Dermatol. 1994;30:977–81. doi: 10.1016/s0190-9622(94)70121-0. [DOI] [PubMed] [Google Scholar]
- 32.Mrowietz U, Christophers E, Altmeyer P. Treatment of psoriasis with fumaric acid esters: Results of a prospective multicentre study.German Multicentre Study. Br J Dermatol. 1998;138:456–60. doi: 10.1046/j.1365-2133.1998.02124.x. [DOI] [PubMed] [Google Scholar]
- 33.Wain EM, Darling MI, Pleass RD, Barker JN, Smith CH. Treatment of severe, recalcitrant, chronic plaque psoriasis with fumaric acid esters: A prospective study. Br J Dermatol. 2010;162:427–34. doi: 10.1111/j.1365-2133.2009.09267.x. [DOI] [PubMed] [Google Scholar]
- 34.Kokelj F, Plozzer C, Avian A, Trevisan G. Fumaric acid and its derivates in the treatment of psoriasis vulgaris: Our experience in forty-one patients. Acta Dermatovenereol Croat. 2009;17:170–5. [PubMed] [Google Scholar]
- 35.Litjens NH, Nibbering PH, Barrois AJ, Zomerdijk TP, Van Den Oudenrijn AC, Noz KC, et al. Beneficial effects of fumarate therapy in psoriasis vulgaris patients coincide with downregulation of type 1 cytokines. Br J Dermatol. 2003;148:444–51. doi: 10.1046/j.1365-2133.2003.05153.x. [DOI] [PubMed] [Google Scholar]
- 36.Carboni I, De Felice C, De Simoni I, Soda R, Chimenti S. Fumaric acid esters in the treatment of psoriasis: An Italian experience. J Dermatolog Treat. 2004;15:23–6. doi: 10.1080/09546630310019346. [DOI] [PubMed] [Google Scholar]
- 37.Brewer L, Rogers S. Fumaric acid esters in the management of severe psoriasis. Clin Exp Dermatol. 2007;32:246–9. doi: 10.1111/j.1365-2230.2007.02389.x. [DOI] [PubMed] [Google Scholar]
- 38.Reich K, Thaci D, Mrowietz U, Kamps A, Neureither M, Luger T. Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis--a retrospective study (FUTURE) J Dtsch Dermatol Ges. 2009;7:603–11. doi: 10.1111/j.1610-0387.2009.07120.x. [DOI] [PubMed] [Google Scholar]
- 39.Vlachou C, Berth-Jones J. Nail psoriasis improvement in a patient treated with fumaric acid esters. J Dermatolog Treat. 2007;18:175–7. doi: 10.1080/09546630701264331. [DOI] [PubMed] [Google Scholar]
- 40.Pawlak FM, Matthes U, Bacharach-Buhles M, Altmeyer P. Treatment of psoriasis pustulosa generalisata resistant to therapy by fumaric acid ester monotherapy [German] Akt Dermatol. 1993;19:6–8. [Google Scholar]
- 41.Ständer H, Stadelmann A, Luger T, Traupe H. Efficacy of fumaric acid ester monotherapy in psoriasis pustulosa palmoplantaris. Br J Dermatol. 2003;149:220–2. doi: 10.1046/j.1365-2133.2003.05424.x. [DOI] [PubMed] [Google Scholar]
- 42.Peeters AJ, Dijkmans JK, van der Schroeff JG. Fumaric acid therapy for psoriatic arthritis.A randomized, double-blind, placebo-controlled study. Br J Rheumatol. 1992;31:502–4. doi: 10.1093/rheumatology/31.7.502. [DOI] [PubMed] [Google Scholar]
- 43.Wollina U, Unger L, Heinig B, Kittner T. Psoriatic arthritis. Dermatol Ther. 2010;23:123–36. doi: 10.1111/j.1529-8019.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 44.Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates versus methotrexate in moderate to severe chronic plaque psoriasis: A multi-centre prospective randomized controlled clinical trial. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.10195.x. doi: 10.1111/j.1365-2133.2010.10195.x. [DOI] [PubMed] [Google Scholar]
- 45.Kreuter A, Knierim C, Stücker M, Pawlak F, Rotterdam S, Altmeyer P, et al. Fumaric acid esters in necrobiosis lipoidica: Results of a prospective noncontrolled study. Br J Dermatol. 2005;153:802–7. doi: 10.1111/j.1365-2133.2005.06762.x. [DOI] [PubMed] [Google Scholar]
- 46.Eberlein-König B, Mempel M, Stahlecker J, Forer I, Ring J, Abeck D. Disseminated granuloma annulare - treatment with fumaric acid esters. Dermatology. 2005;210:223–6. doi: 10.1159/000083514. [DOI] [PubMed] [Google Scholar]
- 47.Weber HO, Borelli C, Röcken M, Schaller M. Treatment of disseminated granuloma annulare with low-dose fumaric acid. Acta Derm Venereol. 2009;89:295–8. doi: 10.2340/00015555-0647. [DOI] [PubMed] [Google Scholar]
- 48.Wollina U. Granuloma annulare disseminatum responding to fumaric acid esters. Dermatol Online J. 2008;14:12. [PubMed] [Google Scholar]
- 49.Nowack U, Gambichler T, Hanefeld C, Kastner U, Altmeyer P. Successful treatment of recalcitrant cutaneous sarcoidosis with fumaric acid esters. BMC Dermatol. 2002;2:15. doi: 10.1186/1471-5945-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breuer K, Gutzmer R, Völker B, Kapp A, Werfel T. Therapy of noninfectious granulomatous skin diseases with fumaric acid esters. Br J Dermatol. 2005;152:1290–5. doi: 10.1111/j.1365-2133.2005.06585.x. [DOI] [PubMed] [Google Scholar]
- 51.Loewe R, Valero T, Kremling S, Pratscher B, Kunstfeld R, Pehamberger H, et al. Dimethylfumarate impairs melanoma growth and metastasis. Cancer Res. 2006;66:11888–96. doi: 10.1158/0008-5472.CAN-06-2397. [DOI] [PubMed] [Google Scholar]
- 52.Yamazoe Y, Tsubaki M, Matsuoka H, Satou T, Itoh T, Kusunoki T, et al. Dimethylfumarate inhibits tumor cell invasion and metastasis by suppressing the expression and activities of matrix metalloproteinases in melanoma cells. Cell Biol Int. 2009;33:1087–94. doi: 10.1016/j.cellbi.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 53.Valero T, Steele S, Neumüller K, Bracher A, Niederleithner H, Pehamberger H, et al. Combination of dacarbazine and dimethylfumarate efficiently reduces melanoma lymph node metastasis. J Invest Dermatol. 2010;130:1087–94. doi: 10.1038/jid.2009.368. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt MM, Dringen R. Fumaric acid diesters deprive cultured primary astrocytes rapidly of glutathione. Neurochem Int. 2010;57:460–7. doi: 10.1016/j.neuint.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Moharregh-Khiabani D, Blank A, Skripuletz T, Miller E, Kotsiari A, Gudi V, et al. Effects of fumaric acids on cuprizone induced central nervous system de- and remyelination in the mouse. PLoS One. 2010;5:e11769. doi: 10.1371/journal.pone.0011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: A multicentre, randomized, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–72. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 58.Ghashghaeinia M, Bobbala D, Wieder T, Koka S, Brück J, Fehrenbacher B, et al. Targeting glutathione by dimethylfumarate protects against experimental malaria by enhancing erythrocyte cell membrane scrambling. Am J Physiol Cell Physiol. 2010;299:C791–804. doi: 10.1152/ajpcell.00014.2010. [DOI] [PubMed] [Google Scholar]
- 59.Häring N, Sprenger Mähr H, Mündle M, Strohal R, Lhotta K. Early detection of renal damage caused by fumaric acid ester therapy by determination of urinary β2-microglobulin. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.10171.x. doi: 10.1111/j.1365-2133.2010.10171.x. [DOI] [PubMed] [Google Scholar]
- 60.Deegan AP, Kirby B, Rogers S, Crotty TB, McDonnell TJ. Organising pneumonia associated with fumaric acid ester treatment for psoriasis. Clin Respir J. 2010;4:248–51. doi: 10.1111/j.1752-699X.2009.00180.x. [DOI] [PubMed] [Google Scholar]
- 61.Mrowietz U, Rostami-Yazdi M, Neureither M, Reich K. 15 years of Fumaderm®: Fumaric acid ester for systemic treatment of moderate to severe psoriasis vulgaris [German] J Dtsch Dermatol Ges. 2009;7:S3–16. doi: 10.1111/j.1610-0387.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- 62.Nast A, Kopp IB, Augustin M, Banditt KB, Boehncke W-H, Follmann M, et al. S3-guideline for psoriasis therapy [German] J Dtsch Dermatol Ges. 2006;4:S51–4. [Google Scholar]
- 63.Gollnick H, Altmeyer P, Kaufmann R, Ring J, Christophers E, Pavel S, et al. Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology. 2002;205:46–53. doi: 10.1159/000063148. [DOI] [PubMed] [Google Scholar]


