Abstract
Objective
We examined the prognostic significance of elevated albuminuria in youth with type 2 diabetes.
Patients and Methods
Cross-sectional and prospective studies were conducted in Pima Indian youth aged 5-19 years at baseline who were examined between July 1, 1982 and December 31, 2007. Prevalence and sequential changes in the level of microalbuminuria (30≤ albumin-to-creatinine ratio <300 mg/g) and macroalbuminuria (albumin-to-creatinine ratio ≥300 mg/g) and incidence of macroalbuminuria were computed according to the presence or absence of type 2 diabetes.
Results
The prevalence of micro- and macroalbuminuria was 6.5% and 0.6% in the 3,856 nondiabetic youth and 18.5% and 2.9% in the 103 youth with diabetes. One-hundred-forty-one (75.4%) of 187 nondiabetic youth, but only one (7.1%) of 14 diabetic youth with elevated albumin-to-creatinine ratio (≥30 mg/g) regressed to undetectable or normal albumin-to-creatinine ratio (<30 mg/g) on subsequent examination. In a subset of 2,666 youth with a median follow-up of 8.1 years, 36 nondiabetic and 30 diabetic youth with baseline albumin-to-creatinine ratio <300 mg/g developed macroalbuminuria. For a given albumin-to-creatinine ratio level, the incidence of macroalbuminuria was 15.9-fold (95% CI = 11.1 to 22.6) higher in the diabetic than in the nondiabetic youth.
Conclusions
Elevated albuminuria is infrequent and largely transient in nondiabetic youth, but is relatively frequent and largely persistent in those with diabetes. Microalbuminuria in youth with type 2 diabetes strongly predicts progression to macroalbuminuria, supporting annual screening for albuminuria.
Keywords: diabetic nephropathy, epidemiology, incidence, longitudinal, prevalence, risk factors
Introduction
Type 2 diabetes is emerging among youth and accounts for up to 45% of new cases of diabetes in persons less than 20 years old [1, 2]. Elevated urinary albumin excretion is an early marker of kidney disease in youth and adults with type 1 diabetes [3-7], but the prognostic significance of elevated albuminuria in youth with type 2 diabetes or in nondiabetic youth is not clear. Most studies of the prevalence and risk factors for elevated albuminuria in youth with type 2 diabetes are clinic-based [8-11] and may therefore suffer from referral bias and not accurately reflect the long-term implications of elevated albuminuria in a diabetic population.
Among Pima Indian youth from the Gila River Indian Community, the prevalence of type 2 diabetes has increased two-fold since 1967 [12]. Diabetes is entirely type 2 [13, 14], and kidney disease is a major complication of diabetes in this population [15]. We describe sequential changes in urinary albumin-to-creatinine ratio (ACR) and estimate prevalence of micro- and macroalbuminuria in Pima Indians aged 5-19 years with or without type 2 diabetes. In a prospective analysis, we compute incidence of macroalbuminuria according to ACR level.
Patients and Methods
Since 1965, members of the Gila River Indian Community who were ≥5 years old were invited to have a research examination approximately every 2 years. This study was approved by the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant gave informed consent.
Examinations were conducted after an overnight fast and included measurement of venous plasma glucose, obtained 2h after a 75g oral glucose load, and assessment for complications of diabetes. Diabetes was diagnosed by 1985 World Health Organization criteria [16] when the 2h post-load plasma glucose (2hPG) was ≥11.1 mmol/l (200 mg/dl), impaired glucose tolerance (IGT) when the 2hPG was ≥7.8 mmol/l (140 mg/dl) and <11.1 mmol/l (200 mg/dl), and normal glucose tolerance (NGT) when the 2hPG was <7.8 mmol/l (140 mg/dl). Fasting glucose was not measured routinely, so for consistency only the 2hPG was used for diagnosis. The date of diagnosis of diabetes was determined from these examinations or from review of clinical records if diabetes was diagnosed during routine care.
Albumin and creatinine concentrations were measured in urine specimens collected at the end of the glucose tolerance test. Urinary albumin was measured at all examinations on or after July 1, 1982 by nephelometric immunoassay [17] and urinary creatinine by a modification of the Jaffe reaction [18]. Microalbuminuria was defined by an ACR ≥30 and <300 mg albumin/g creatinine and macroalbuminuria by an ACR ≥300 mg/g. Urinary albumin concentrations below the threshold detected by the assay (6.8 mg/l or lower) were shown as undetectable in the frequency distribution, but were combined with normal ACR for other analyses. ACR ≥30 mg/g, i.e., microalbuminuria or macroalbuminuria, was considered “elevated”.
Glomerular filtration rate (eGFR) was estimated using the Schwartz equation [19] in subjects <18 years old and by the 4-variable Modification of Diet in Renal Disease (MDRD) study equation [20] in those ≥18 years old and the level of eGFR was staged according to National Kidney Foundation guidelines [21]. Mean arterial pressure (MAP) was calculated as (systolic blood pressure + 2*diastolic blood pressure)/3. Body mass index (BMI) was defined as weight divided by the square of height (kg/m2). BMI standard deviation scores (SDS) were derived from the Centers for Disease Control and Prevention standards [22]. HbA1 was measured by electrophoresis [23] through December 1989, and HbA1c by high-performance liquid chromatography [24] thereafter. The correlation between the two measures was 0.92, and a linear regression formula HbA1c = 0.99 × HbA1 – 1.535 was used to estimate HbA1c in the 710 subjects in whom HbA1 was measured [25].
The study population included persons of at least half Pima or Tohono O'odham heritage who were ≥5 years of age and had one or more research examinations between July 1, 1982 and December 31, 2007 that included ACR measurements. Subjects 5-19 years old were classified as youth and those ≥20 years old as adults.
Statistical Analysis
Cross-sectional analysis used data from the first research examination during the study period. Clinical features at baseline were compared between groups by analysis of covariance adjusted for age and sex. Differences in ACR between groups were analyzed with the Kruskal–Wallis test. Mean glycemia levels in the newly-diagnosed and known diabetic youth and mean BMI SDS between diabetic and nondiabetic youth were compared by t-tests. Relative frequency distributions of ACR in youth were examined on a logarithmic scale according to the presence or absence of diabetes, and compared with those in adults. All other cross-sectional and longitudinal analyses included only those who were youth at the first research examination; follow-up for the sequential assessment of albuminuria or for the incidence of macroalbuminuria could extend into adulthood. The associations of micro- and macroalbuminuria with glucose tolerance were analyzed by the Mantel-Haenszel Chi-square test adjusted for age and sex [26]. The 50th, 75th, and 95th percentiles of ACR were computed by age. Sequential changes in ACR category were assessed in those who remained in the same nondiabetic or diabetic category at a second research examination.
Incidence of macroalbuminuria was computed as the number of new cases per 1,000 person-years at risk. New cases were identified at the first appearance of macroalbuminuria in subjects who had at least one previous examination before 20 years of age with an ACR <300 mg/g. In nondiabetic subjects, the period at risk extended from the date of the first nondiabetic research examination to the date of the first nondiabetic examination at which macroalbuminuria was diagnosed or, in those who did not develop macroalbuminuria, to the date of the last nondiabetic examination within the study period. In diabetic subjects, the period at risk extended from the date of the first diabetic examination to the date of the first examination at which macroalbuminuria was diagnosed or, in those who did not develop macroalbuminuria, to the date of the last examination within the study period. Subjects could be counted as both nondiabetic and diabetic if they developed diabetes during follow-up and had at least two nondiabetic and two diabetic examinations with ACR measurements. Time-dependent Cox regression models were used to estimate the risk of macroalbuminuria associated with ACR level adjusted for age, sex, BMI, blood pressure and duration of diabetes (in the diabetic subjects). The final model in the diabetic subjects was stratified by blood pressure because this variable violated proportionality assumptions and by sex because of its significant interaction with ACR.
Tests for linear association, controlled for age and sex, were computed by the Mantel extension test [27] modified for person-time denominators. Poisson regression was used to examine the incidence of macroalbuminuria in diabetic youth relative to nondiabetic youth adjusted for ACR [28]. Analyses were performed using SAS software version 8 (SAS Institute, Cary, NC).
Results
The cross-sectional study included 3,959 Pima Indian youth (3,597 with NGT, 259 with IGT, and 103 with diabetes). The prevalence of elevated albuminuria was virtually identical in youth with NGT (7.2%) and IGT (6.6%), but was significantly higher in those with type 2 diabetes (21.4%; p<0.0001). Accordingly, NGT and IGT were combined in subsequent analyses. Characteristics of the youth are presented in Table 1. Youth with diabetes had higher BMI, BMI SDS, blood pressure, eGFR, and median ACR, and lower serum creatinine concentration than those without diabetes (p<0.001). By definition, measures of glycemia were also higher in the youth with diabetes. Among the diabetic youth, those known to have diabetes at the baseline examination had, on average, higher HbA1c (9.5%±3.3 vs. 7.1%±2.4; p<0.001) and slightly higher 2hPG (18.3±7.8 mmol/L vs. 16.3±5.0 mmol/L; p=0.23) than those with newly-diagnosed diabetes. Median ACR level was undetectable in the nondiabetic youth, and the youngest subjects had the highest 75th and 95th percentiles of ACR. By contrast, the proportion of diabetic youth with detectable ACR was far higher. At baseline, one nondiabetic youth with chronic glomerulonephritis had an eGFR <60 ml/min/1.73m2 and 15 had eGFR of 60-89 ml/min/1.73m2; no diabetic youth had eGFR <90 ml/min/1.73m2. Only four nondiabetic youth and three diabetic youth reported taking antihypertensive medicines at baseline.
Table 1.
Demographic and clinical characteristics of Pima Indian youth according to diabetes.
| No Diabetes | Diabetes | |
|---|---|---|
| N | 3,856 | 103 |
| Male | 1,876 | 40 |
| Female | 1,980 | 63 |
| Age (years) | 13.0 ± 3.8 | 14.5 ± 3.0 |
| Duration of diabetes (years)* | - | 1.3(0-2.1) |
| BMI (kg/m2) | 25.7 ± 7.3 | 34.9 ± 7.4 |
| BMI SDS | 1.34±1.01 | 2.25±0.50 |
| HbA1c (%) | 5.0 ± 0.5 | 8.1 ± 3.0 |
| 2-hour plasma glucose (mmol/L) | 5.7 ± 1.3 | 16.9 ± 6.0 |
| MAP (mmHg) | 75.9 ± 10.1 | 83.6 ± 9.9 |
| Serum creatinine (μmol/L) | 56 ± 15 | 55 ± 11 |
| Estimated GFR† | 141±21 | 154±27 |
| ACR (mg/g)‡ | UD (UD-8.9) | 6.6 (UD-21.9) |
| Percentiles of ACR (mg/g) by age group | ||
| Age 5-9 years | N=982 | N=4§ |
| 50th | UD | |
| 75th | 11.4 | |
| 95th | 51.3 | |
| Age 10-14 years | N=1,469 | N=48 |
| 50th | UD | 8.5 |
| 75th | 9.1 | 23.6 |
| 95th | 48.3 | 190.2 |
| Age 15-19 years | N=1,405 | N=51 |
| 50th | UD | 5.2 |
| 75th | 7.4 | 20.9 |
| 95th | 27.7 | 210.0 |
Abbreviations: ACR, urinary albumin-to-creatinine ratio; BMI, body mass index; BMI SDS, body mass index standard deviation score; GFR, glomerular filtration rate; MAP, mean arterial pressure; UD, undetectable albuminuria.
Statistics are mean and standard deviation
%median (25th-75th percentile).
Glomerular filtration rate was estimated using the Schwartz equation [19] in subjects less than 18 years of age and by the 4-variable Modification of Diet in Renal Disease (MDRD) study equation [20] in those 18 years and older.
Percentiles not reported due to the small number of subjects.
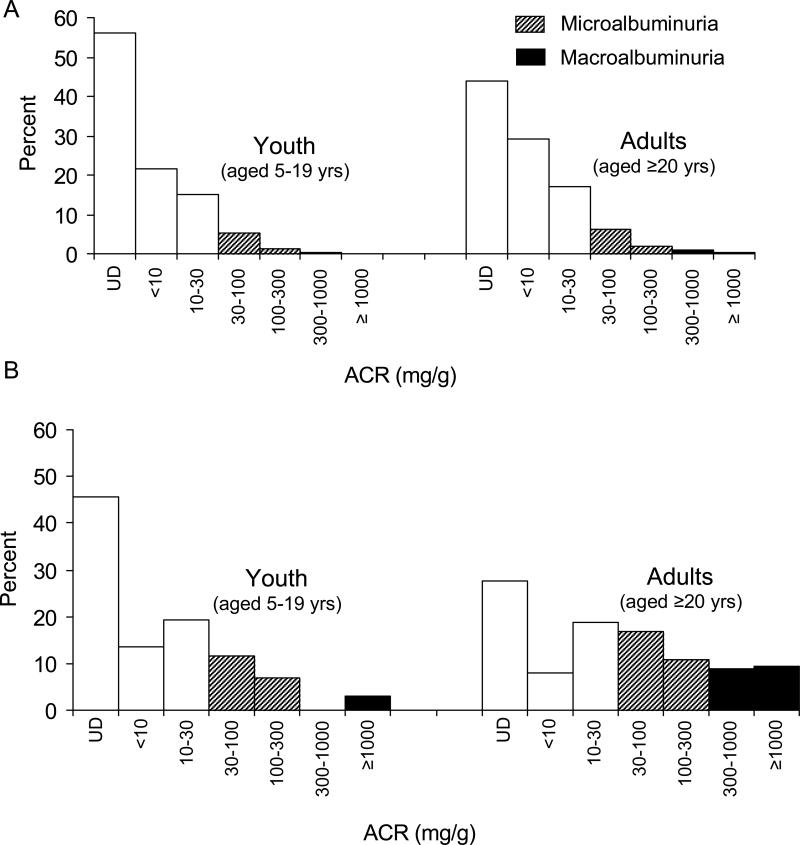
Relative frequency distributions of ACR in youth and adults are compared in Figure 1. In nondiabetic subjects, 56.0% of the youth and 44.0% of the adults had undetectable albuminuria. The prevalence of micro- and macroalbuminuria in nondiabetic subjects was 6.5% and 0.6% in the youth and 8.1% and 1.5% in the adults, respectively (Figure 1A). In diabetic subjects, 45.6% of the youth and 27.6% of the adults had undetectable albuminuria. The prevalence of micro- and macroalbuminuria in diabetic subjects was 18.5% and 2.9% in the youth and 27.5% and 18.2% in the adults, respectively (Figure 1B).
Figure 1.
Frequency distribution of several ACR categories in youth and adults without (A) or with type 2 diabetes (B). The hatched and black bars represent micro- and macroalbuminuria, respectively. UD represents undetectable albuminuria, in which case ACR cannot be calculated.
Sequential changes in ACR category were examined in the 2,549 nondiabetic and 76 diabetic youth who remained in the nondiabetic or diabetic category for two successive examinations (Table 2). The median time between the two ACR measurements was 2.8 years (range = 1.1-24.0 years) in nondiabetic subjects and 3.0 years (range = 1.1-17.8 years) in diabetic subjects. Among those with normal ACR at the first measurement, 94.1% of the nondiabetic subjects and 53.2% of the diabetic subjects remained in this category at the second measurement. Among those with micro- or macroalbuminuria at the first measurement, 75.4% of the nondiabetic subjects regressed to normal or undetectable ACR, whereas only one (7.1%) of the diabetic subjects regressed. Among those with microalbuminuria at the first measurement, two (1.2%) nondiabetic subjects progressed to macroalbuminuria, whereas three (27.3%) diabetic subjects progressed at the subsequent examination.
Table 2.
Changes in ACR categories between two ACR measurements in Pima Indian youth with and without type 2 diabetes. Cells represent the number of subjects in each category (percent of row). Bolded numbers represent subjects with stable ACR.
| No Diabetes | Diabetes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACR2 |
ACR2 |
||||||||||
| Normo | Micro | Macro | N | Normo | Micro | Macro | N | ||||
| Normo | 2,223 (94.1%) | 132 (5.6%) | 7 (0.3%) | 2,362 | Normo | 33 (53.2%) | 25 (40.3%) | 4 (6.5%) | 62 | ||
| ACR1 | Micro | 133 (76.9%) | 38 (22.0%) | 2 (1.2%) | 173 | ACR1 | Micro | 1 (9.1%) | 7 (63.6%) | 3 (27.3%) | 11 |
| Macro | 8 (57.1%) | 3 (21.4%) | 3 (21.4%) | 14 | Macro | 0 | 0 | 3 (100%) | 3 | ||
| N | 2363 | 173 | 12 | 2,549 | N | 34 | 32 | 10 | 76 | ||
Abbreviations: ACR, urinary albumin-to-creatinine ratio; Normo, normoalbuminuria; Micro, microalbuminuria; Macro, macroalbuminuria.
ACR1: ACR category at the first examination, ACR2: ACR category at the second examination
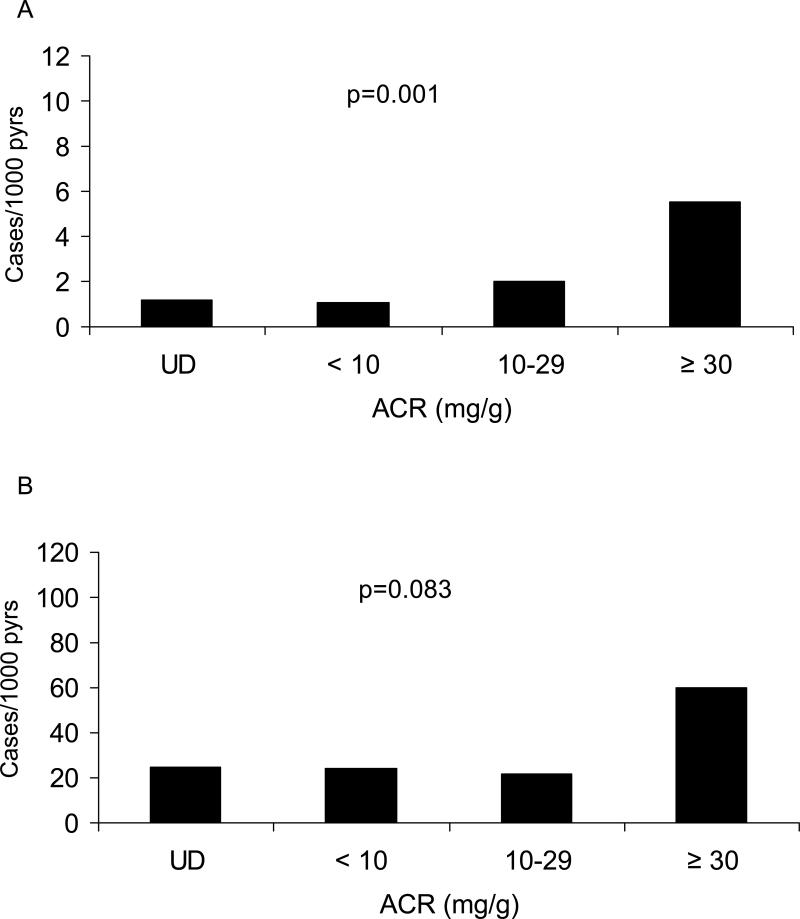
Incidence of macroalbuminuria was computed in 2,666 youth who had follow-up ACR measurements. Thirty-six of the 2,534 nondiabetic and 30 of the 132 diabetic subjects developed macroalbuminuria during a follow-up of up to 25.2 years. Unadjusted incidence of macroalbuminuria in nondiabetic and diabetic subjects according to the presence or absence of microalbuminuria at baseline is shown in Table 3. The incidence of macroalbuminuria was positively associated with baseline ACR in the nondiabetic (ptrend=0.001) and more modestly in the diabetic (ptrend=0.083) youth. The incidence of macroalbuminuria was 15.9-fold (95% CI = 11.1 to 22.6) higher in the diabetic youth when adjusted for ACR (Figure 2). The incidence of macroalbuminuria in subjects with undetectable ACR and in those with ACR <10 mg/g was similar in both the diabetic and nondiabetic groups. In nondiabetic subjects, the age-sex-adjusted incidence of macroalbuminuria in those with microalbuminuria was 8.2 (95% CI, 2.1-32.4) times as high as in those with normoalbuminuria; in diabetic subjects the incidence was 7.6 (95% CI, 1.8-32.8) times as high (Table 3). After further adjustment for age, sex, BMI, MAP and duration of diabetes (in those with diabetes), the incidence rate ratios were 4.3 (95% CI, 1.9-9.8) and 4.2 (95% CI, 1.6-11.3) in the nondiabetic and diabetic subjects, respectively. These results were unchanged if systolic blood pressure was substituted for MAP in the proportional-hazards models.
Table 3.
Unadjusted incidence (cases/1,000 person-years) of macroalbuminuria in nondiabetic and diabetic Pima Indians aged 5-19 years at baseline according to the presence or absence of microalbuminuria and age-sex-adjusted incidence rate ratios (IRR; microalbuminuria/normoalbuminuria and 95% confidence intervals (CI).
| Pearson-Years | Cases | Incidence | |
|---|---|---|---|
| No Diabetes | |||
| Normoalbuminuria | 21,830.0 | 28 | 1.3 |
| Microalbuminuria | 1,450.0 | 8 | 5.5 |
| IRR=8.2 (95% CI, 2.1- 32.4) | |||
| Diabetes | |||
| Normoalbuminuria | 800.5 | 19 | 23.7 |
| Microalbuminuria | 183.4 | 11 | 60.0 |
| IRR=7.6 (95% CI, 1.8- 32.8) |
Figure 2.
Unadjusted incidence rates of macroalbuminuria according to ACR categories in youth aged 5-19 years without diabetes (A) or with type 2 diabetes (B). UD represents undetectable albuminuria. Note the 10-fold difference in the y-axis scales between panels.
Follow-up data after the first appearance of macroalbuminuria were available for 21 of 36 nondiabetic subjects (58.3%) and for 22 of 30 diabetic subjects (73.3%). The 21 nondiabetic subjects had a median follow-up of 6.7 years (range=1.4-13.2 years) after the first onset of macroalbuminuria; the 22 diabetic subjects had a median follow-up of 5.0 years (range=1.0-17.5 years). Only 42.9% of the nondiabetic subjects, whereas 90.9% of the diabetic subjects, remained macroalbuminuric after its first appearance.
Discussion
The prevalence of elevated albuminuria is significantly higher in Pima Indian youth with type 2 diabetes than in those without. In those with diabetes, elevated albuminuria is more likely to persist and is highly predictive of progression to persistent macroalbuminuria, whereas in those without diabetes it is largely transient. The persistence of elevated ACR in diabetic youth is consistent with previous findings in diabetic Pima adults [29]. The frequency of persistent albuminuria is higher than in youth with type 1 in the Steno Diabetes Study [6] or the Oxford Regional Prospective Study [7].
The prevalence of micro- and macroalbuminuria in diabetic Pima youth was lower than in diabetic adults, perhaps because of the youths’ shorter average duration of diabetes—more than half of the ACR measurements in the youth were made at the diagnosis of diabetes. Nevertheless, the prevalence of elevated albuminuria was similar to that reported among youth with type 2 diabetes in the SEARCH for Diabetes in Youth Study (SEARCH), but much higher than the 9% reported among those with type 1 diabetes in the same study [30]. Youth with type 2 diabetes from other populations are also reported to have a significantly higher prevalence of elevated albuminuria than those with type 1 diabetes, despite shorter duration of diabetes and lower HbA1c; however long-term follow-up was not available in these previous studies [8].
At baseline, only one nondiabetic subject had eGFR <60 ml/min/1.73m2 and 15 had eGFR of 60-89 ml/min/1.73m2; no diabetic youth had eGFR <90 ml/min/1.73m2. Because only one diabetic subject developed eGFR <60 ml/min/1.73m2 and four developed eGFR of 60-89 ml/min/1.73m2 during follow-up, progression of diabetic kidney disease was assessed only by the incidence of macroalbuminuria. The present study indicates that microalbuminuria strongly predicts progression to macroalbuminuria in diabetic youth. A similar probability of progression to macroalbuminuria was reported in the Oxford Regional Prospective Study [7] in youth and adults with type 1 diabetes. Moreover, previous findings indicate that an equivalent duration of type 2 diabetes in a young person is as damaging to the kidneys as it is in an older person [31, 32], reflecting the importance of diabetes duration, regardless of age, in the development of kidney disease in both types of diabetes.
Although the relationship between ACR and kidney disease progression is continuous, for clinical purposes a cut point is useful for identifying those at substantially increased risk. In adults, we previously arbitrarily selected an ACR cut point of ≥30 mg/g as abnormal, since this level was approximately the 95th percentile of ACR in a “healthy” subset of Pima Indians aged ≥15 years who had NGT, took no medicines, had no known renal or cardiovascular diseases, normal blood pressure, and normal serum creatinine [15]. This cut point is equivalent to ≥20 μg/min or ≥30 mg/24 hr from timed urine collections [21]. In diabetic youth with microalbuminuria by this ACR definition, the age-sex-adjusted incidence of macroalbuminuria was 7.6 times (95% CI, 1.8-32.8) as high as in those with normal ACR, suggesting that the adult cut points are useful for identifying increased risk of more advanced kidney disease in diabetic youth. In nondiabetic youth, the incidence of macroalbuminuria was also much higher in those with ACR≥30 mg/g, but the risk of developing macroalbuminuria was much lower than in those with diabetes and macroalbuminuria was more often transient. Furthermore, most nondiabetic individuals with ACR ≥30 mg/g regressed to normoalbuminuria. Thus, the clinical utility of measuring albuminuria is less certain in nondiabetic youth.
The prevalence of microalbuminuria in nondiabetic Pima youth was 6.5%; lower than 9.5% prevalence in nondiabetic youth in NHANES III [33]. The higher prevalence in NHANES may be due, in part, to the higher probability of undiagnosed diabetes, since subjects in that study were not tested for diabetes with an oral glucose tolerance test, as in the present study. Urinary albumin excretion in youth may also be influenced by a number of other factors, as in adults. Lower creatinine excretion for a given level of albumin excretion will increase the prevalence of microalbuminuria, particularly in younger children, due to their lower muscle mass. This effect is illustrated in Table 1 by the higher 75th and 95th percentiles of ACR in the younger nondiabetic children. Likewise, in adults, low muscle mass is an important confounder of ACR as a marker of microalbuminuria [34].
Precision of albuminuria estimates is enhanced by obtaining multiple specimens, so guidelines from the American Diabetes Association [35] and the National Kidney Foundation [21] recommend repeated measurements when screening diabetic patients. Nevertheless, single measures of albuminuria, as used in the present study, are highly predictive of progressive kidney disease in both children and adults with type 2 diabetes [36]. Moreover, a recent study in the Pima Indians [29] finds that preceding ACR measures add minimal predictive value beyond the latest measurement. Accordingly, we do not believe a single measurement of albuminuria represents a serious limitation. Furthermore, we propose that in screening situations in which multiple measurements are not feasible, a single screening test is useful for identifying persons with diabetic kidney disease.
Observations in the Pima Indians are relevant to other populations. The bimodality of plasma glucose, first described in this population [37], combined with the presence of retinopathy and nephropathy among those in the upper component of the distribution [38] led to standardized criteria for the diagnosis of diabetes adopted by the National Diabetes Data Group [39], the World Health Organization [16, 40], and the American Diabetes Association [41]. In the Diabetes Prevention Program [42], a clinical trial of diabetes prevention, Southwestern American Indians (many of whom were Pima), had the same risk of type 2 diabetes and the same reductions in risk from the interventions as members of other race/ethnic groups. Each of the major determinants of diabetic kidney disease found in the Pima Indians has been reported in other populations. Of note, type 2 diabetes in children was first reported in the Pima Indians [43]. Decades of follow-up of this population-based cohort permits us to examine the impact of elevated albuminuria in children with type 2 diabetes before it can be adequately assessed in other populations. Findings from the Pima Indians may therefore provide clinicians with potentially useful information as they encounter children with type 2 diabetes and elevated albuminuria in their practice.
Conclusion
Elevated ACR is infrequent and largely transient in youth without diabetes. On the other hand, elevated albuminuria is relatively frequent, largely persistent, and highly predictive of progression to more severe kidney disease in those with type 2 diabetes, supporting annual ACR screening in diabetic youth.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. We are indebted to the members of the Gila River Indian Community for participating in this investigation and to the staff of the Diabetes Epidemiology and Clinical Research Section for conducting the examinations.
There are no financial arrangements between any author and any company whose product or competing product may have been mentioned in this manuscript.
Abbreviations
- 2hPG
two-hour post-load plasma glucose concentration
- ACR
urinary albumin-to-creatinine ratio
- BMI
body mass index
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- HbA1
glycosylated hemoglobin A1
- HbA1c
glycosylated hemoglobin A1c
- IGT
impaired glucose tolerance
- MAP
mean arterial pressure
- MDRD
Modification of Diet in Renal Disease
- NGT
normal glucose tolerance
Footnotes
Financial Disclosures: none
Conflicts of Interest: none
References
- 1.Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Shaw J. Epidemiology of childhood type 2 diabetes and obesity. Pediatr Diabetes. 2007;8(Suppl 9):7–15. doi: 10.1111/j.1399-5448.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 3.Cowell CT, Rogers S, Silink M. First morning urinary albumin concentration is a good predictor of 24-hour urinary albumin excretion in children with type 1 (insulin-dependent) diabetes. Diabetologia. 1986;29:97–99. doi: 10.1007/BF00456117. [DOI] [PubMed] [Google Scholar]
- 4.Gorman D, Sochett E, Daneman D. The natural history of microalbuminuria in adolescents with type 1 diabetes. J Pediatr. 1999;134:333–337. doi: 10.1016/s0022-3476(99)70459-2. [DOI] [PubMed] [Google Scholar]
- 5.Perkins BA, Krolewski AS. Early nephropathy in type 1 diabetes: a new perspective on who will and who will not progress. Curr Diab Rep. 2005;5:455–463. doi: 10.1007/s11892-005-0055-7. [DOI] [PubMed] [Google Scholar]
- 6.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105–1109. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. 2008;336:697–701. doi: 10.1136/bmj.39478.378241.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 9.Eppens MC, Craig ME, Jones TW, et al. Type 2 diabetes in youth from the Western Pacific region: glycaemic control, diabetes care and complications. Curr Med Res Opin. 2006;22:1013–1020. doi: 10.1185/030079906X104795. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama H, Okudaira M, Otani T, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–311. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoo EG, Choi IK, Kim DH. Prevalence of microalbuminuria in young patients with type 1 and type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1423–1427. doi: 10.1515/jpem.2004.17.10.1423. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Hanson RL, Bennett PH, et al. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia. 1988;41:904–910. doi: 10.1007/s001250051006. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Bennett PH, Bottazzo GF, Doniach D. Islet cell antibodies and diabetes mellitus in Pima Indians. Diabetologia. 1979;17:161–164. doi: 10.1007/BF01219743. [DOI] [PubMed] [Google Scholar]
- 14.Dabelea D, Palmer JP, Bennett PH, Pettitt DJ, Knowler WC. Absence of glutamic acid decarboxylase antibodies in Pima Indian children with diabetes mellitus. Diabetologia. 1999;42:1265–1266. doi: 10.1007/s001250051303. [DOI] [PubMed] [Google Scholar]
- 15.Nelson RG, Kunzelman CL, Pettitt DJ, et al. Albuminuria in type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in Pima Indians. Diabetologia. 1989;32:870–876. doi: 10.1007/BF00297452. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization: Diabetes mellitus . Technical Report Series No 727. Geneva: 1985. [PubMed] [Google Scholar]
- 17.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycaemia. Diabetologia. 1984;26:127–133. doi: 10.1007/BF00281119. [DOI] [PubMed] [Google Scholar]
- 18.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30:207–212. [PubMed] [Google Scholar]
- 19.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984;104:849–854. doi: 10.1016/s0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Menard L, Dempsey ME, Blankstein LA, Aleyassine H, Wacks M, Soeldner JS. Quantitiative determination of glycosylated hemoglobin A1 by agar gel electrophoresis. Clin Chem. 1980;26:1598–1602. [PubMed] [Google Scholar]
- 24.Ellis G, Diamandis EP, Giesbrecht EE, Daneman D, Allen LC. An automated “high-pressure” liquid-chromatographic assay for hemoglobin A1c. Clin Chem. 1984;30:1746–1752. [PubMed] [Google Scholar]
- 25.Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol. 1998;148:650–656. doi: 10.1093/aje/148.7.650. [DOI] [PubMed] [Google Scholar]
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 27.Mantel N. Chi-square tests with one degree of freedom: extension of the Mantel-Haenszel procedure. J Am Stat Assoc. 1963;59:690–700. [Google Scholar]
- 28.Vine MF, Schoenbach VJ, Hulka BS, Koch GG, Samsa G. Atypical metaplasia and incidence of bronchogenic carcinoma. Am J Epidemiol. 1990;131:781–793. doi: 10.1093/oxfordjournals.aje.a115569. [DOI] [PubMed] [Google Scholar]
- 29.Pavkov ME, Knowler WC, Hanson RL, Bennett PH, Nelson RG. Predictive power of sequential measures of albuminuria for progression to end-stage renal disease or death in Pima Indians with type 2 diabetes. Am J Kidney Dis. 2008;51:759–766. doi: 10.1053/j.ajkd.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30:2593–2598. doi: 10.2337/dc07-0450. [DOI] [PubMed] [Google Scholar]
- 31.Krakoff J, Lindsay RS, Looker HC, et al. Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care. 2003;26:76–81. doi: 10.2337/diacare.26.1.76. [DOI] [PubMed] [Google Scholar]
- 32.Pavkov ME, Bennett PH, Knowler WC, et al. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 33.Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 34.Cirillo M, Laurenzi M, Mancini M, Zanchetti A, De Santo NG. Low muscular mass and overestimation of microalbuminuria by urinary albumin/creatinine ratio. Hypertension. 2006;47:56–61. doi: 10.1161/01.HYP.0000197953.91461.95. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson RG, Knowler WC, Pettitt DJ, et al. Assessment of risk of overt nephropathy in diabetic patients from albumin excretion in untimed urine specimens. Arch Intern Med. 1991;151:1761–1765. [PubMed] [Google Scholar]
- 37.Rushforth NB, Bennett PH, Steinberg AG, Burch TA, Miller M. Diabetes in the Pima Indians: evidence of biomodality in glucose tolerance distributions. Diabetes. 1971;20:756–765. doi: 10.2337/diab.20.11.756. [DOI] [PubMed] [Google Scholar]
- 38.Rushforth NB, Miller M, Bennett PH. Fasting and two-hour post-load glucose levels for the diagnosis of diabetes: the relationship between glucose levels and complications of diabetes in the Pima Indians. Diabetologia. 1979;16:373–379. doi: 10.1007/BF01223157. [DOI] [PubMed] [Google Scholar]
- 39.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose tolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 40.WHO Expert Committee on Diabetes Mellitus Second report. WHO Tech Rep Ser. 1980;646:9–14. [PubMed] [Google Scholar]
- 41.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 42.The Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savage PJ, Bennett PH, Senter RG, Miller M. High prevalence of diabetes in young Pima Indians: evidence of phenotypic variation in a genetically isolated population. Diabetes. 1979;28:937–942. doi: 10.2337/diab.28.10.937. [DOI] [PubMed] [Google Scholar]