Abstract
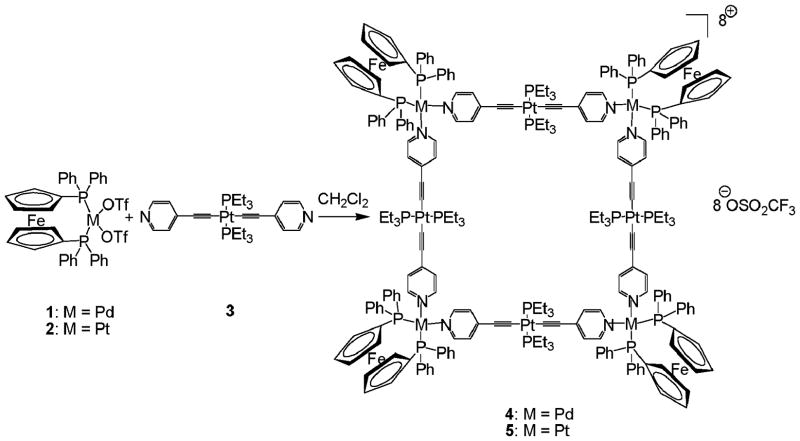
Self-assemblies between a linear Pt-based donor and ferrocene-chelated metallic acceptors produce novel heterometallic squares 4 and 5, which show fluorescence quenching upon addition of nitro-aromatics.
Coordination-driven self-assembly is one of the most useful protocols in contemporary supramolecular research due to its simple, rapid and efficient approach for the construction of complex molecular topologies with tailor-made properties like molecular reactors and host-guest chemistry.1,2 Metal-based acceptors are widely employed to provide an accessible range of angles and shapes for the ligand-metal-ligand subunits of supramolecular assemblies.3–5 Although various combinations between organic donors and metal-based acceptors have been used to design self-assembled metallomacrocycles6,7, little attention has been paid on the preparation of heterometallic supramolecules assembled from metal-based donors.8
Subsequent to the early developments, we aimed to design novel heterometallic electron-rich molecular squares using a metal-based donor in combination with cis-blocked square planar metallic acceptors. As a tactics to accomplish this purpose, ferrocene-chelated metallic acceptors were chosen since they preferred to provide the sufficient rigidity at corners which help in producing discrete molecular squares. Also, the linear donor trans-[(4-pyridylethynyl)2Pt(PEt3)2] (3) containing Pt-ethynyl groups was selected to introduce another metallic centers on the sides of the squares, and to make the final assemblies π electron-rich. This electron-rich system can be anticipated for the detection of electron-poor guests like nitro-aromatics, the common chemical component for many explosives.9 Interestingly, the presence of Pt-ethynyl functionality endows the supramolecules with photo-luminescent properties10 which have been exploited for sensing applications.11 Herein, we report the first examples of [4 + 4] self-assembled heterometallic molecular squares 4 and 5 from 90° metallic acceptors cis-(dppf)M(OTf)2 [where M = Pd (1) or Pt (2)] and the Pt-based donor 3. These new macrocycles can be utilized as a molecular sensor for the detection of nitro-aromatic explosives.
The molecular square 4 (Scheme 1) was obtained upon equimolar reaction of cis-(dppf)Pd(OTf)2 (1) with 3 in CH2Cl2 with continuous stirring for 3 h at room temperature. The final product was isolated as a dark-red solid in a high yield (95%) by adding diethyl ether. The molecular square 4 has been fully characterized by multi-nuclear NMR, ESI-MS and X-ray diffraction study.
Scheme 1.
Self-assembly of the heterometallic squares 4 and 5.
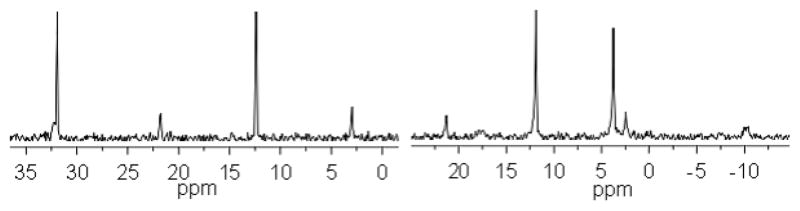
The 31P{1H} NMR spectrum (Figure 1; left) of 4 shows two singlets at δ 32.9 and 12.3 ppm corresponding to the phosphorous peak of metallic acceptor and metallic donor, respectively.
Fig. 1.
31P NMR spectra of the complexes 4 (left) and 5 (right).
The downfield shift of the latter peak compared to 3 indicates the coordination of 3 to Pd(II). Consistently, 1H NMR spectrum also shows the expected downfield shifts of the pyridyl protons of 3 due to coordination (Supporting Information).
Likewise, the reaction of the acceptor 2 with the metal-based donor 3 in a 1:1 molar ratio in CH2Cl2 led to the molecular square 5 (97%). The 31P NMR spectra of 5 exhibited two sharp singlets at δ 11.85 ppm and 3.74 ppm originated from Pt donor and acceptor, respectively, (Figure 1; right) with a significant shift and decrease in coupling of the 195Pt satellites as compared to the starting precursors. Additionally, the proton resonances of the pyridine rings exhibited downfield shifts resulting from the loss of electron density upon coordination of the pyridyl N atom to the Pt(II) metal center. Electrospray ionization mass spectrometry (ESI-MS) provided further evidence for the formation of molecular squares. For example, the ESI mass spectrum of 5 showed peaks at m/z 2094, 1533, and 1197 corresponding to the consecutive loss of triflate anions, [M-3CF3SO3]3+, [M-4CF3SO3]4+ and [M-5CF3SO3]5+. The [M-3CF3SO3]3+ and [M-5CF3SO3]5+ peaks were isotopically resolved and well matched with the corresponding theoretical distribution patterns (Supporting Information).
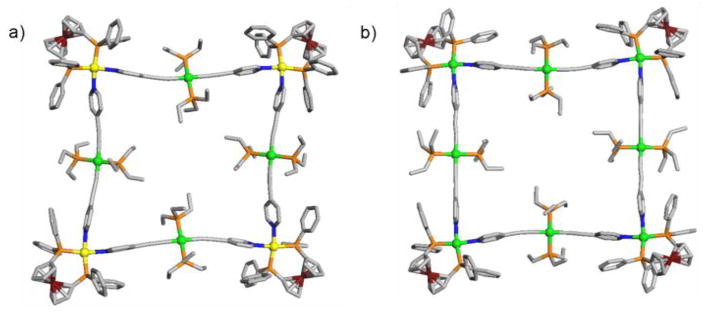
Finally, crystal structures of 4 and 5 were determined by X-ray crystallography. Needle-shaped single crystals of 4 and 5 suitable for X-ray analysis by a synchrotron radiation were obtained by vapor diffusion of ether into acetone and CH2Cl2 solution, respectively. The structural analysis provides an unequivocal evidence of the molecular squares (Figure 2). Interstitial sites of the crystal structures for 4 and 5 are occupied by triflate counter anions and solvent molecules. Pd(II) and Pt(II) corners in 4 and 5 adopt square planar geometry. The bite angles of bidentate phosphine (P–Pd–P and P–Pt–P) in 4 and 5 are 99.9° and 100.5° with a bond distance of 2.30 Å and 2.29 Å on average, respectively. The ethyl groups of the four PEt3 moieties in 4 and 5 are oriented towards the interior of the macrocycle, which makes the hydrophobic pocket of the macrocycle. Interestingly, the complex 4 and 5 have two different sizes of molecular cavities; the complex 4 with a bent geometry of 3 has a square cavity with 5.5 Å × 5.8 Å, while the complex 5 with a straight geometry of 3 shows an elongated cavity with 20 Å × 4 Å.
Fig. 2.
X-ray crystal structure of 4 (left) and 5 (right). Color codes: yellow = Pd, green = Pt, brown = Fe, orange = P, gray = C. H-atoms are omitted for clarity and pertinent bond distances and angles are given in the text.
Next, the optical properties of complexes 4 and 5 were investigated with absorption and emission studies. The UV-vis absorption spectra of 4, 5 and the linker 3 were recorded in THF at ambient temperature. The spectra of 4 and 5 show absorption bands centered at 342 nm mainly due to metal to ligand or intramolecular charge transfer (Supporting Information).12 Upon excitation at 342 nm, the THF solutions (5×10−6 M) of 4 and 5 exhibited intense photo-luminescence at around 380 nm and 396 nm for 4 and 5 (Supporting Information). On comparing the emission spectra of 3, 4 and 5 we can tentatively assign that the emissive property of these squares is originated from the electron-rich and photoluminescent Pt-ethynyl donor center.
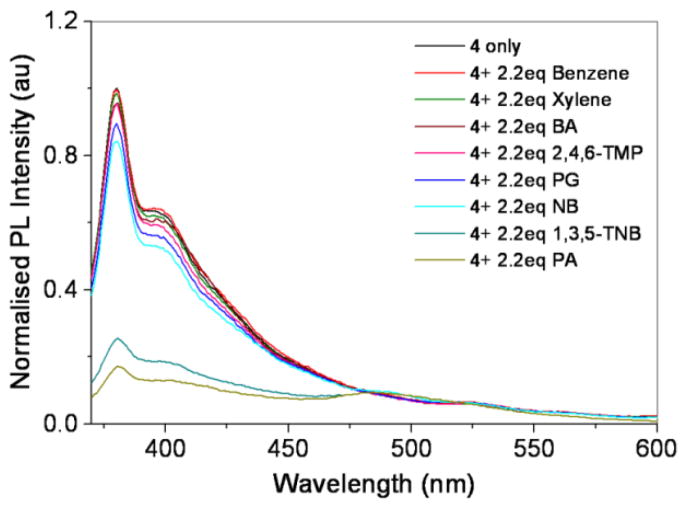
Since the observed intense emission could be served as a basis for the sensing of guest molecules, we finally tested the complex 4 for the detection of small molecules, particularly aromatic compounds which can potentially induce π–π interactions with the π electron system of the metallic donor. Although it was reported that conjugated aromatic compounds, self-assembled cages and polymers can be used as efficient fluorescent sensing materials,11,13 however, 2-D metallomacrocycles have not been explored much for this purpose. We have studied a variety of analytes ranging from electron-rich to electron-poor derivatives. As shown in Figure 3, no significant quenching was noticed with electron-rich molecules like benzene, xylene and 2,4,6-TMP even at a high quencher concentration (Supporting Information). The similar quenching pattern was observed with polar aromatics like benzoic acid and phloroglucinol. The weak quenching responce towards carboxylic acid and phenols also ruled out the possibility of simple acid-base reaction of the analytes with molecular square 4. Further extending the investigation successively to nitro-aromatics known as electron-poor molecules like 4-NB and 1,3,5-TNB, apparent quenching of photoluminiscence is noticed. Particularly, high quenching reponse is observed for 1,3,5-TNB guest. This result indicates that the increased electron-withdrawing nature of nitro-aromatics may induce the enhanced π–π interactions with the π electron-rich molecular square 4. Since the UV-vis and NMR studies did not provide any evidence for the direct binding between the molecular square 4 and electron-deficient analytes, the observed emission quenching could be ascribed to weak interactions such as π–π interactions.
Fig. 3.
Emission quenching of 4 upon addition of the various guest molecules (1 × 10−4 M in THF). BA = benzoic acid, 2,4,6-TMP = 2,4,6-trimethylphenol, PG = phloroglucinol, 4-NB = 4-nitrobenzene, 1,3,5-TNB = 1,3,5-trinitrobenzene, PA = picric acid.
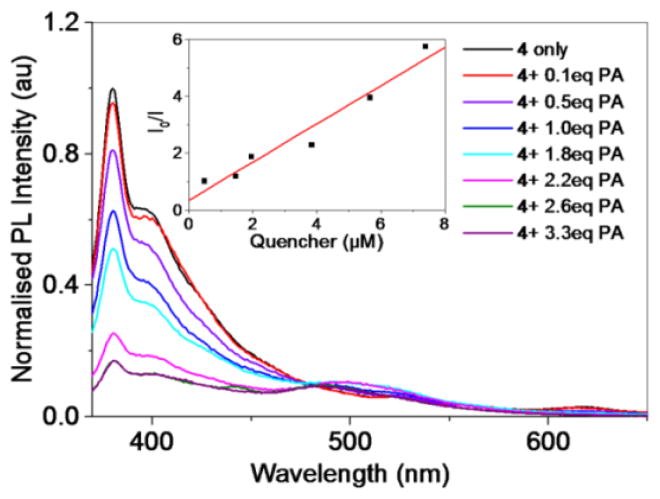
Since the electron-deficient nitro-aromatic molecules are the core constituents for the explosive materials, we have carried out detailed investigations by using picric acid (known as explosive) as a quencher and established the high sensitivity of 4 (Figure 4) and 5 (Supporting Information) toward picric acid. As shown in Figure 3, picric acid exhibits the higher quenching response than 1,3,5-TNB. The high polarizability of the picric acid could be another factor along with π–π interactions contributing to the high sensitivity. According to the emission quenching titrations with picric acid (Figure 4), the quenching constants (Ksv) which describe the quenching efficiency of the receptor were estimated to be ca. 6.72 × 105 M−1 for 4 and 7.00 × 105 M−1 for 5, pointing to the high sensitivity of molecular squares toward picric acid. As we have stated earlier that photoluminescence in molecular squares is originated from π electron-rich donor center 3, to defend this statement we have also checked the quenching phenomena with donor 3 and metal acceptor 1 alone. As expected, some emission quenching in the presence of picric acid was noticed (Supporting Information) with 3 and no quenching was observed in case of 1 respectively. This result validates the involvement of donor center in the quenching process and also supports the enhanced response of the these squares for the sensing of picric acid. These results signify that the strong π–π interactions facilitate energy transfer from the electron-rich donor of these squares to the electron-deficient nitro-aromatics and thereby the enhanced emission quenching is noticed. The similar Ksv values for 4 and 5 also support that the metallic donor 3 is mainly associated to the π–π interactions leading to emission quenching.
Fig.4.
Emission quenching of complex 4 by picric acid and Stern-Volmer plot (inset).
In summary, we have synthesized and characterized a new class of heterometallic squares possesing a metal-based donor via the self-assembly approach. These new, electron-rich and photoluminescent molecular squares have been examined for emission quenching effects with various aromatic compounds and they show the high quenching selectivity and sensitivity towards nitro-aromatics particularly picric acid. Thus we demonstrate that our unique self-assembled squares can be used as a molecular sensor for the detection of picric acid. The host–guest chemisty and detailed photophysical studies with the relavent self-assembled metallomacrocycles are in progress.
Supplementary Material
Acknowledgments
V.V., P.J.S., M.H.L. and K.W.C. appreciate the financial support of WCU program (R33-2008-000-10003) of National Research Foundation of Korea, and the Pohang Accelerator Labolatory (PAL) for X-ray structual analysis. P.S.M acknowledges the DST, India for financial support and Johnson Mathey Pvt. Ltd. U.K. for supplying K2PtCl4 as loan.
Footnotes
Electronic Supplementary Information (ESI) available:[Experimental procedure, NMR spectra, ESI-MSspectra, important bold lengths and bond angles for compounds 4 and 5 and additional fluorescence spectra]. See DOI: 10.1039/b000000x/
Crystal data for 4: C128.52H147.02F12Fe2N4O14.99P8Pd2Pt2S4, M = 3306.28, triclinic, P-1 (No. 2), a = 13.682(3) Å, b = 20.077(4) Å, c = 26.280(5) Å, α = 94.76(3) °, β = 93.90(3) °, γ = 94.29(3) °, V = 7154(2) Å3, Z = 2, T = 100 K, μ(synchrotron) = 4.228 mm−1, ρcalc = 1.535 g·cm−3, 29184 reflections measured, 15082 unique (Rint = 0.0195), R1 = 0.0800, wR2 = 0.2329 for 12073 reflections (I > 2σ(I)), R1 = 0.0927, wR2 = 0.2499 (all data), GoF = 1.104, 1651 parameters and 498 restraints. Crystal data for 5: C127H157Cl6F12Fe2N4O21.50P8Pt4S4, M = 3786.28, monoclinic, P21/c (No. 14), a = 13.851(3) Å, b = 22.464(5) Å, c = 51.056(10) Å, β = 96.35(3) °, V = 15789(5) Å3, Z = 4, T = 100 K, μ(synchrotron) = 6.479 mm−1, ρcalc = 1.595 g·cm−3, 61729 reflections measured, 19153 unique (Rint = 0.0660), R1 = 0.1220, wR2 = 0.3434 for 13674 reflections (I > 2σ(I)), R1 = 0.1474, wR2 = 0.3743 (all data), GoF = 1.567, 1722 parameters and 1000 restraints. The aromatic rings and PEt3 groups of complex 4 with a dynamic motion were refined using SADI restraints. In addition, ISOR and SIMU restraints were adapted to restrain the anisotropic displacement parameters (ADPs) of H2O, and PEt3, OSO2CF3− and diethyl ether, respectively. In the case of complex 5, OSO2CF3−, PEt3, and CH2Cl2 were refined using the SADI, DFIX, DELU and SIMU restraints because of their dynamic motions. Moreover, the aromatic rings of complex 5 were adapted to their regular hexagonal geometries by FLAT restraint. CCDC 777385(4) and 777386(5) contain the supplementary crystallographic data for these complexes. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Contributor Information
Peter J. Stang, Email: stang@chem.utah.edu.
Ki-Whan Chi, Email: kwchi@ulsan.ac.kr.
Notes and references
- 1.(a) Lehn J-M. Supramolecular Chemistry, concepts and perspectives. VCH; New York: 1995. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Cotton FA, Lin C, Murillo CA. Acc Chem Res. 2001;34:759. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (d) Schalley CA, Lutzen A, Albrecht M. Chem-Eur J. 2004;10:1072. doi: 10.1002/chem.200305150. [DOI] [PubMed] [Google Scholar]; (e) Northrop BH, Yang HB, Stang PJ. Chem Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Lehn JM. Angew Chem, Int Ed Engl. 1990;29:1304. [Google Scholar]; (b) Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]; (c) Bar AK, Chakrabarty R, Mostafa G, Mukherjee PS. Angew Chem Int Ed. 2008;47:8455. doi: 10.1002/anie.200803543. [DOI] [PubMed] [Google Scholar]; (d) Ghosh K, Hu J, White HS, Stang PJ. J Am Chem Soc. 2009;131:6695. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sawada T, Yoshizawa M, Sato S, Fujita M. Nature Chemistry. 2009;1:53. doi: 10.1038/nchem.100. [DOI] [PubMed] [Google Scholar]
- 3.(a) Stahl J, Mohr W, de Quadras L, Peters TB, Bohling JC, Martin-Alvarez JM, Owen GR, Hampel Gladysz A. J Am Chem Soc. 2007;129:8282. doi: 10.1021/ja0716103. [DOI] [PubMed] [Google Scholar]; (b) Hamilton TD, Buĉar D-K, MacGillivray LR. Chem Commun. 2007:1603. doi: 10.1039/b700748e. [DOI] [PubMed] [Google Scholar]; (c) Ghosh S, Mukherjee PS. Organometallics. 2007;26:3362. [Google Scholar]
- 4.(a) Holliday B, Mirkin CA. Angew Chem, Int Ed. 2001;40:2022. [PubMed] [Google Scholar]; (b) Heo J, Jeon YM, Mirkin CA. J Am Chem Soc. 2007;129:7712. doi: 10.1021/ja0716812. [DOI] [PubMed] [Google Scholar]; (c) Chi KW, Addicott C, Arif AM, Das N, Stang PJ. J Org Chem. 2003;68:9798–9801. doi: 10.1021/jo0353785. [DOI] [PubMed] [Google Scholar]; (d) Peinador C, Pía E, Blanco V, García MD, Quintela JM. Org Lett. 2010;12:1380. doi: 10.1021/ol1004577. [DOI] [PubMed] [Google Scholar]; (e) Sakata Y, Hiraoka S, Shionoya M. Chem Eur J. 2010;16:3318. doi: 10.1002/chem.200903509. [DOI] [PubMed] [Google Scholar]
- 5.(a) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem Commun. 2001:509. [Google Scholar]; (b) Yang HB, Northrop BH, Zheng YR, Ghosh K, Stang PJ. J Org Chem. 2009;74:7067. doi: 10.1021/jo9013589. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yang HB, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J Am Chem Soc. 2007;129:2120. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]
- 6.(a) Rang A, Nieger M, Engeser M, Lützen A, Schalley CA. Chem Commun. 2008:4789. doi: 10.1039/b806916f. [DOI] [PubMed] [Google Scholar]; (b) Baytekin HT, Sahre M, Rang A, Engeser M, Schulz A, Schalley CA. Small. 2008;4:1823. doi: 10.1002/smll.200800135. [DOI] [PubMed] [Google Scholar]; (c) Tzeng B-C, Chen Y-F, Wu C-C, Hu C-C, Chang Y-T, Chen C-K. New J Chem. 2007;31:202. [Google Scholar]; (d) Fujita M, Sasaki O, Mitsuhashi T, Fujita T, Yazaki J, Yamaguchi K, Ogura K. J Chem Soc, Chem Commun. 1996:1535. [Google Scholar]; (e) Fujita M, Yazaki J, Ogura K. J Am Chem Soc. 1990;112:5645. [Google Scholar]; (f) Zhang L, Niu YH, Jen AKY, Lin W. Chem Commun. 2005:1002. doi: 10.1039/b413708f. [DOI] [PubMed] [Google Scholar]
- 7.(a) You CC, Würthner F. J Am Chem Soc. 2003;125:9716. doi: 10.1021/ja029648x. [DOI] [PubMed] [Google Scholar]; (b) Slone RV, Hupp JT. Inorg Chem. 1997;36:5422. [Google Scholar]; (c) Slone RV, Yoon DI, Calhoun RM, Hupp JT. J Am Chem Soc. 1995;117:11813. [Google Scholar]; (d) Drain CM, Lehn JM. J Chem Soc Chem Commun. 1994;19:2313. [Google Scholar]; (e) Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502. [Google Scholar]
- 8.(a) Sun SS, Lees AJ. Inorg Chem. 2001;40:3154. doi: 10.1021/ic0101681. [DOI] [PubMed] [Google Scholar]; (b) Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J Am Chem Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- 9.Sohn H, Calhoun RM, Sailor MJ. Angew Chem Int Ed. 2001;40:2104. doi: 10.1002/1521-3773(20010601)40:11<2104::AID-ANIE2104>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zheng L, Niu YH, Len AKY, Lin W. Chem Commun. 2005:1002. doi: 10.1039/b413708f. [DOI] [PubMed] [Google Scholar]; (b) Jiang H, Lin W. J Am Chem Soc. 2003;125:8084. doi: 10.1021/ja034402t. [DOI] [PubMed] [Google Scholar]
- 11.(a) Ghosh S, Mukherjee PS. Organometallics. 2008;27:316. [Google Scholar]; (b) Ghosh S, Gole B, Bar AK, Mukherjee PS. Organometallics. 2009;28:4288. doi: 10.1039/b911622m. [DOI] [PubMed] [Google Scholar]
- 12.Flynn DC, Ramakrishna G, Yang HB, Northrop BH, Stang PJ, Goodson T., III J Am Chem Soc. 2010;132:1348. doi: 10.1021/ja9082655. [DOI] [PubMed] [Google Scholar]
- 13.(a) Yang JS, Swager TM. J Am Chem Soc. 1998;120:5321. [Google Scholar]; (b) Naddo T, Che Y, Zhang W, Balakrishnan K, Yang X, Yen M, Zhao J, Moore JS, Zang L. J Am Chem Soc. 2007;129:6978. doi: 10.1021/ja070747q. [DOI] [PubMed] [Google Scholar]; A. Name, B. Name and C. Name, Journal Title, 2000, 35, 3523; A. Name, B. Name and C. Name, Journal Title, 2000, 35, 3523.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.