Abstract
Oral delivery of biopharmaceutical proteins expressed in plant cells should reduce their cost of production, purification, processing, cold storage, transportation, and delivery. However, poor intestinal absorption of intact proteins is a major challenge. To overcome this limitation, we investigate here the concept of receptor-mediated oral delivery of chloroplast-expressed foreign proteins. Therefore, the transmucosal carrier cholera toxin B-subunit and green fluorescent protein (CTB-GFP), separated by a furin cleavage site, was expressed via the tobacco chloroplast genome. Polymerase chain reaction (PCR) and Southern blot analyses confirmed site-specific transgene integration and homoplasmy. Immunoblot analysis and ELISA confirmed expression of monomeric and pentameric forms of CTB-GFP, up to 21.3% of total soluble proteins. An in vitro furin cleavage assay confirmed integrity of the engineered furin cleavage site, and a GM1 binding assay confirmed the functionality of CTB-GFP pentamers. Following oral administration of CTB-GFP expressing leaf material to mice, GFP was observed in the mice intestinal mucosa, liver, and spleen in fluorescence and immunohistochemical studies, while CTB remained in the intestinal cell. This report of receptor-mediated oral delivery of a foreign protein into the circulatory system opens the door for low-cost production and delivery of human therapeutic proteins.
Keywords: transmucosal carrier, furin, ganglioside receptors, genetically modified crops
One of the most challenging problems of human health management is the high cost of prescription drugs in developed countries and their lack of availability in developing countries. For example, interferon (IFN) alpha 2b is used for the treatment of viral diseases such as hepatitis C, as well as for certain cancers. However, IFN treatment for four months costs $26,000 in the United States, where more than forty-five million Americans do not have health insurance (1). Several hundred million people in developing countries are infected with hepatitis, but the daily income of one-third of the world population is less than $2 per day (1). The high cost of prescription drugs is due to a number reasons, including fermentation-based production (each fermenter costs several hundred million dollars to build), expensive purification and in vitro processing methods (such as column chromatography, disulfide bond formation) (2), the need for storage and transportation at low temperature and delivery via sterile injections requiring the involvement of hospitals and highly qualified health professionals (1). Therefore, new approaches to minimize or eliminate most of these expenses are urgently needed. Transgenic plants offer many advantages, including the feasibility of the oral delivery of foreign proteins, low cost of production, storage and transportation, heat stability and protection through bioencapsulation, elimination of the need for expensive purification, in vitro processing, and sterile injections (1–5). The generation of systemic and mucosal immunity (6) or induction of oral tolerance (7), improved safety, and absence of human pathogens (3) are other additional advantages (4, 5).
Chloroplast genetic engineering has recently become an attractive method for production of recombinant proteins (8, 9) because of high concentration of transgene expression [up to 47% of the total soluble protein (10)] due to the presence of 10,000 copies of the transgene per cell, which is uniquely advantageous for oral delivery of therapeutic proteins or vaccine antigens. It is also an environmentally friendly approach due to effective gene containment offered by maternal inheritance of chloroplast genomes in most crops (11, 12) or engineered cytoplasmic male sterility (13). Multigene engineering in a single transformation event (10, 14, 15) should facilitate delivery of polyvalent vaccines or expression of therapeutic proteins with multiple subunits.
Despite these advantages, a major limitation remains in the efficient delivery of plant-expressed therapeutic proteins across the intestinal mucus membrane, primarily because of poor permeability across the intestinal epithelial layer (16). Receptor-mediated oral delivery across the intestine might serve as a possible way to deliver not only vaccines but also biopharmaceutical proteins. Ganglioside M1 (GM1) receptors on the intestinal epithelial cells have been utilized by various pathogens such as V. cholerae to facilitate entry of cholera toxin, into the intestine.
Crystal structures (17–19) of bacterial toxins like cholera toxin, (CT), heat-labile enterotoxin (LT), and shigella toxin show that they belong to AB5 subunit family. In CT, five identical (11.6 kDa) peptides assemble into a highly stable pentameric ring called the B subunit (58 kDa). The nontoxic B subunit (CTB) exhibits specific and high-affinity binding to the oligosaccharide domain of ganglioside GM1 (a lipid-based membrane receptor) and functions to tether the toxin to the plasma membrane of host cells (17, 20, 21). This receptor is present on the intestinal epithelium as well as motoneurons and sympathetic preganglionic neurons (22). GM1 sorts the CT into lipid rafts and a retrograde trafficking pathway to the endoplasmic reticulum, where the enzymatic subunit is transferred to the cytosol, probably by dislocation through the translocon sec61P (20).
To test the concept of receptor-mediated oral delivery of foreign proteins, we have constructed a unique cholera toxin B–green fluorescent protein (CTB–GFP) fusion gene with a furin cleavage site between CTB and GFP and expressed the fusion protein in transgenic chloroplasts. Furin, a member of prohormone-proprotein convertases (23) (PCs), is a ubiquitously expressed protein found in the trans-Golgi network (TGN) (24, 25), endosomes, plasma membrane, and extracellular space (26). Furin cleaves protein precursors with narrow specificity following basic Arg-Xaa-Lys/Arg-Arg-like motifs (27). The furin cleavage site between CTB and GFP would, therefore, facilitate intracellular cleavage of the target protein (GFP).
Transgenic leaves expressing the CTB-GFP or IFN-GFP fusion protein were fed to Balb/c mice to investigate receptor-mediated oral delivery of foreign protein using CTB as a transmucosal carrier across the intestinal epithelium. In this study, we show that CTB-GFP binds to the intestinal mucous membrane, including the lymphoid tissue. Experimental observations suggest that GFP is cleaved from CTB in the intestine through the action of furin and enters the mucosal vasculature. We show that GFP, but not CTB, is delivered to the liver and spleen of the CTB-GFP fed mice. No significant levels of GFP were observed in the liver and spleen of mice fed with IFN-GFP, which suggests that a transmucosal carrier is essential for efficient delivery of proteins across the intestinal lumen. Thus, CTB successfully delivers its fusion protein to the systemic circulation and supports the use of transmucosal carriers in the delivery of therapeutic proteins.
MATERIALS AND METHODS
Construction of chloroplast vector
The pLD-CTB-GFP construct was based on the universal chloroplast vector pLD (Fig. 1) that has been used successfully in our laboratory (28 –31). CTB-GFP construct was engineered with a furin cleavage site, Pro-Arg-Ala-Arg-Arg, in between CTB and GFP. The constitutive 16 s rRNA promoter was used to drive transcription of the aadA and the CTB-GFP genes. The aminoglycoside 3′ adenylyltransferase (aadA) gene conferring spectinomycin resistance was used as a selectable marker. The 5′-UTR from psbA, including its promoter, was engineered to enhance translation of the CTB-GFP because it has several ribosomal binding sites. The 3′ UTR region conferred transcript stability. A GFP-IFN alpha5 fusion construct with a furin cleavage site between the two genes was created and expressed in Nicotiana tabacam chloroplasts, which served as a control molecule for the delivery of GFP without a transmucosal carrier.
Figure 1.
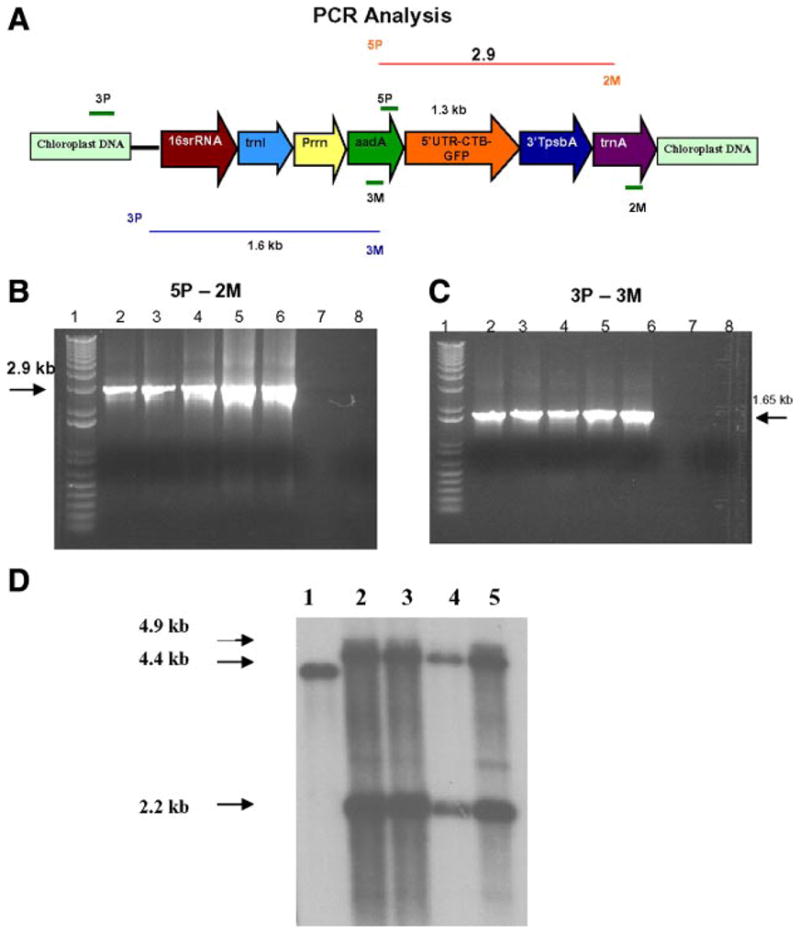
PCR analysis for the confirmation of transgene integration. A) Schematic representation of the transgene cassette. B) 5P/2M—These primers land on the aadA and trnA regions (flanking the CTB-GFP). A 2.9 kb PCR product was obtained from the PCR analysis of trasgenic plants. C) 3P/3M—The 3P primer lands on the native chloroplast genome and the 3M primer lands on the aadA gene. A 1.6 kb PCR product was obtained from the PCR anlysis of the transgenic plants. Lane 1: 1 kb plus ladder. Lanes 2–5: Transgenic lines of CTB-GFP. Lane 6: Positive Control. Lane 7: Empty. Lane 8: Wild-type Plant. D) Southern blot analysis of the plants. Lane 1: WT showing 4.4 kb fragment. Lanes 2–5: Transgenic plants showing 4.9 and 2.2 kb hybridizing fragments. Flanking sequence shown in Figure 1A was used as the probe.
Bombardment and selection of transgenic plants
The Bio-Rad PDS-1000/He biolistic device was used to bombard pLD-CTB-GFP onto sterile Nicotiana tabacum cv. Petit Havana tobacco leaves, on the abaxial side as has been described previously (29, 30, 32). The bombarded leaves were incubated in the dark for 24 h and then placed on shooting media (RMOP) containing 500 μg/ml spectinomycin for two rounds of selection.
PCR analysis to test stable integration
DNA was isolated from the transgenic shoots by using Qiagen DNeasy Plant Mini Kit, and PCR analysis was performed to confirm integration of the transgene in the inverted repeat regions of the chloroplast genome. PCR reactions were performed with two sets of primers, 3P/3M and 5P/2M (28). The samples were denaturated for 5 min at 95°C followed by 30 cycles of the following temperatures: 95°C for 1 min, 65°C for 1 min, and 72°C for 2 min and a 72°C hold for 10 min after all 30 cycles were completed. After confirmation of transgenic plants, the shoots were then transferred to a rooting medium (MSO) with 500 μg/ml spectinomycin as a selective agent.
Southern blot analysis
Total plant DNA was digested with EcoR1, separated on a 0.7% agarose gel at 45V for 8 h, and then transferred to a nitrocellulose membrane. pUC-computed tomography vector DNA was digested with BamHI and BglII to generate a 0.8 kb probe, which was used as a flanking probe (28). After labeling the probe with with P32, hybridization of the membranes was performed by using Stratagene QUICK-HYB hybridization solution and protocol (Stratagene, La Jolla, CA).
Western blot analysis
Approximately 100 mg of leaf tissue was ground in liquid nitrogen and resuspended in 500 μl of plant extraction buffer (0.1% SDS; 100 mM NaCl; 200 mM Tris–HCl, pH 8.0; 0.05% Tween 20; 400 mM sucrose; 2 mM PMSF). After centrifugation at 13,000 rpm for 5 min, the supernatant containing the extracted protein was collected. We boiled 10 μl of the plant extract along with 10 μl of sample loading buffer, which was then run on a 15% SDS-PAGE gel for 40 min at 50 V and then 2 h at 80 V. The protein was then transferred to nitrocellulose membrane for 1 h at 80 V. After blocking the membranes with PTM (1× PBS, 0.05% Tween 20, and 3% dry milk) for 1 h, we added polyclonal rabbit anti-CTB primary antibody (Ab) (Sigma) 1:3000 dilution. Goat anti-rabbit IgG conjugated to alkaline phosphatase (Sigma) at a 1:5000 dilution was used as a secondary Ab.
Furin cleavage assay
Approximately 100 mg of leaf material was powdered in liquid nitrogen and resuspended in 500 μl of plant extraction buffer containing 15 mM Na2CO3, 35 mM NaHCO3, 3 mM NaN3, 5 mM CaCl2, and 0.5% Triton-X, 2-mercaptoethanol at pH 6.0 and 7.0. We added 1 mM PMSF to some of the samples. After centrifugation at 13,000 rpm for 5 min, the supernatant containing the extracted protein was collected. The extract (20 μl) was incubated at 30°C for 4 h with 4 U of furin. A control group was also incubated at 30°C for 4 h without furin. After 4 h, each sample was mixed with 20 μl sample loading buffer, boiled, and run on 12% SDS-PAGE gel for 45 min at 80 V and then 2 h at 100 V. The Western blot analysis was performed as per the procedure outlined above. Chicken anti-GFP Ab (Chemicon) at a 1:3000 dilution was used as the primary Ab, and alkaline phosphatase conjugated rabbit antichicken IgG (Chemicon) at a dilution of 1:5000 was used as a secondary Ab.
ELISA
The CTB-GFP quantification was done using the ELISA (ELISA). The standards and test samples were diluted in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 3 mM NaN3, pH 9.6). The standards, ranging from 50 to 500 ng, were made by diluting recombinant GFP in 1% PBS. The leaf samples were collected from plants exposed to regular lighting pattern (16 h light and 8 h dark), and total protein was extracted using plant protein extraction buffer. Standard GFP dilutions (100 μl) and protein samples were bound to a 96-well plate overnight at 4°C. The background was blocked with fat-free milk in PBST for 1 h at 37°C followed by washing with PBST and water. Primary Ab used was polyclonal chicken anti-GFP Ab (Chemicon) diluted (1:3000) in PBST containing milk powder. Secondary Ab was HRP-conjugated rabbit anti-chicken IgG- secondary Ab (Chemicon) at a 1: 5000 dilution in PBST containing milk powder. For the color reaction, 100 μl of 3,3_,5,5_-tetramethyl benzidine (TMB from American Qualex) substrate was loaded in the wells and incubated for 10 –15 min at room temperature. The reaction was stopped by addition of 50 μl of 2N sulfuric acid per well, and the plate was read on a plate reader (Dynex Technologies) at 450 nM.
GM1 binding assay
To test the functionality of CTB-GFP expressed in chloroplasts, a CTB-GM1 binding assay was performed. We coated 96-well plates with 100 μl of monosialoganglioside- GM1 (Sigma) (3.0 ng/ml in bicarbonate buffer) and incubated them overnight at 4°C. After washing with PBST and water, the standards and samples were incubated for 1 h at 37°C. The plate was blocked with 1% BSA in 1× PBS for 1 h at 37°C. Rabbit anti-CTB primary Ab (Sigma) and alkaline phosphatase (activating protein) conjugated goat anti-rabbit secondary Ab (Sigma) was used to detect the CTB binding to GM1 receptor. The plates were washed with PBST and water, and 200 μl of the substrate p-Nitrophenyl phosphate (PNPP) was added to the wells and incubated in the dark at 37°C for 20 min. The reaction was stopped by adding 50 μl of 3N NaOH, and the plates were read on a plate reader (Dynex Technologies) at 405 nM.
Animal studies
Three groups of 5-week-old female Balb/c mice were fed with CTB-GFP, IFN alpha5-GFP (IFN-GFP), and wild-type (untransformed) plant leaf material. Leaves (350 mg) were powdered in liquid nitrogen, mixed with peanut butter, and fed to the mice, which had been starved overnight prior to this experiment. The mice were then gavaged for two more days, two times a day, with 40 mg of leaf material per gavage that was powdered with liquid nitrogen and mixed with 0.1M PBS (PBS). Five hours after the last gavage, the mice were sacrificed and perfused with 10 ml of PBS followed by 4% paraformaldehyde in PBS. Fresh frozen sections of the liver, spleen, ileum, and jejunum were collected according to Samsam et. al (33). Additional tissue was removed and immersed in Tissue Tec freezing medium (Vector labs) and immediately frozen in nitrogen-cooled isomethylbuthane (Sigma). Fixed tissue was cryoprotected by passing through 10, 20, and 30% sucrose solutions in PBS. Frozen sections (10 μm thick) of various tissues were then made using a cryostat.
Fluorescence microscopy and immunohistochemistry for GFP, CTB, and immune cells
Frozen sections (10 μm thick) of intestine, liver, and spleen were mounted with PBS and observed for GFP fluorescence using a Leica 4500 microscope. Immunohistochemistry was performed in order to show the presence of GFP and/or CTB in various tissues. The slides were first blocked with 10% BSA (BSA) and 0.3% Triton-X 100. Polyclonal chicken anti-GFP (Chemicon) or polyclonal rabbit anti-CTB (Sigma) primary antibodies, at a concentration of 1:500 and 1:300, respectively, in 1% BSA and 0.3% Triton-X, were used for GFP or CTB localization of the tissues. Those sections processed for HRP conjugated secondary antibodies were blocked with a mixture of methanol/hydrogen peroxide 30% (2:1 ratio) to block the endogenous peroxidases. The secondary antibodies were horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG (Chemicon) or HRP-conjugated goat anti-rabbit (Sigma). Tissue-bound peroxidase was developed by using the 3,3′-diaminobenzidine (3,3′-diaminobenzidine) as a substrate to visualize the immunoreaction.
For macrophage localization of the tissues, rat monoclonal F4/80 Ab (Serotec) was used according to Berghoff et al. (34). The secondary Ab was Alexa-555 conjugated Goat anti-rat IgG (Molecular Probes). American hamster anti-CD11c primary Ab and anti-hamster Alexa-546 conjugated secondary Ab (Molecular Probes) were used to visualize dendritic cells in the intestine and other tissues. FITC-labeled anti-chicken IgG was used as a secondary Ab in such immunofluorescence staining to detect GFP in tissues.
RESULTS
Confirmation of transgene integration into chloroplast genome
Nicotiana tabacum cv. Petit Havana leaves were bombarded with the pLD-CTB-GFP vector, and the leaves were grown on selective medium containing 500 mg/l spectinomycin. The resultant shoots were then screened for chloroplast transformants by PCR analysis by using primers 3P/3M, and 5P/2M (Fig. 1A–C). The 3P primer lands on the native chloroplast genome upstream of the site of integration, whereas the 3M primer lands on the aadA transgene producing a 1.65 kb PCR product. This analysis ruled out the nuclear transformants because 3P primer would not anneal and the spontaneous mutants are eliminated because 3M primer would not anneal.
To check for the presence of the transgene in the chloroplast, we performed the 5P-2M PCR analysis. The 5P primer lands on aadA gene and the 2M lands on the trnA coding sequence, which produces a 2.9 kb PCR product with CTB-GFP. This confirmed the site-specific integration of the CTB-GFP fusion gene in the inverted repeat regions of the chloroplast genome.
Southern blot analysis to investigate homoplasmy
To further confirm the integration of the transgene into the chloroplast genome and to determine whether homoplasmy had been achieved, Southern blot analysis was performed. Total plant DNA was digested with the enzyme EcoR1 and hybridized with a chloroplast flanking sequence probe (0.8 kb). Wild-type plants generated a 4.4 kb fragment, and transgenic plants generated 4.9 and a 2.2 kb fragments (Fig. 1D). All of the transgenic lines tested appeared to be homoplasmic (within the levels of detection), which means that all of the chloroplast genomes within plant cells contained the transgene CTB-GFP.
GFP expression and assembly of CTB-GFP pentamers in transgenic lines
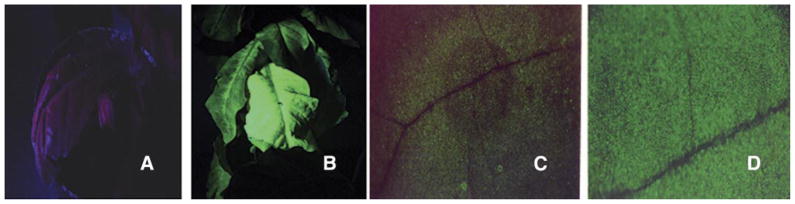
Figure 2 shows the transgenic and wild-type (WT) plants. In Fig. 2B, the GFP expression of the transgenic plants can be seen under the UV light, which is not seen in the wild-type (untransformed) plant (Fig. 2A). Fig. 2C shows WT plant, and Fig. 2D, the CTB-GFP expressing plant under a low-magnification microscope. Expression of GFP is clearly evident in Fig. 2D.
Figure 2.
Visualization of GFP fluorescence in transgenic plants under UV light. A) Wild-type (untransformed) plant seen under UV light. B) CTB- GFP expressing leaf showing fluorescence observed under UV light. C) Wild-type leaf under a low-magnification microscope. D) CTB-GFP expressing leaf showing fluorescence under a low-magnification microscope.
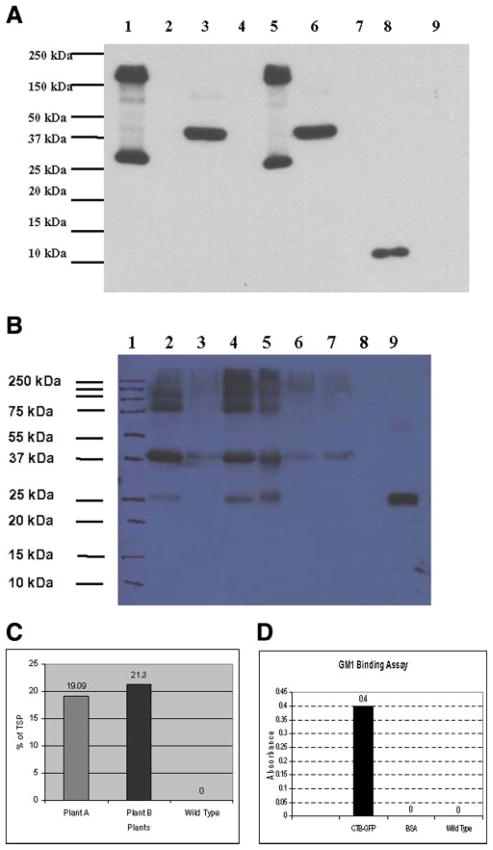
Western blot analysis was performed to investigate the expression of the fusion protein CTB-GFP in transgenic tobacco chloroplasts (Fig. 3A). The pentameric form (188 kDa) was observed in the unboiled samples of the transgenic plants, while predominantly the monomeric form (37.6 kDa) was detected in boiled samples.
Figure 3.
Immunoblot analysis, furin cleavage assay and quantification of CTB-GFP expressed in chloroplasts of transgenic lines. A) Immunoblot demonstrating the expression of CTB-GFP in transgenic plant crude extracts: Lane 1: Un-boiled crude extract of transgenic line A. Lane 3: boiled crude extract of transgenic line A. Lane 5: Unboiled crude extract of transgenic line B. Lane 6: Boiled crude extract of transgenic line A. Lane 8: Purified CTB standard 200 ng. Lane 9: Wild-type plant crude extract. Lanes 2, 4, 7: empty. B) Furin cleavage assay of the plant extract: Lane 1: Marker. Lane 2: CTB-GFP, pH 6.0, with furin, no PMSF. Lane 3: CTB-GFP no incubation, no furin. Lane 4: CTB-GFP pH 6.0 with furin and PMSF. Lane 5: CTB-GFP, pH 7.0, with furin and PMSF. Lane 6: CTB-GFP, pH 6.0, with PMSF, no furin. Lane 7: CTB-GFP, pH 6.0, no PMSF, no furin. Lane 8: Blank. Lane 9: Purified recombinant GFP standard. C) Expression levels in % of CTB-GFP in total soluble protein (TSP) of the CTB-GFP expressing plants. D) GM1 ganglioside binding assayshowing the presence of CTB-GFP functional pentamers.
Furin cleavage assay
The protease furin is present in the constitutive secretory pathway and on the cell surface of virtually all cells (35). An in vitro furin cleavage assay was performed on the CTB-GFP expressing plant extract to show that the engineered cleavage site (Arg-Ala-Arg-Arg) was recognized by furin. As seen in Fig. 3B, a 26 kDa polypeptide that corresponded with the recombinant GFP protein was observed in the samples that were incubated with furin, thus proving that furin could cleave CTB-GFP to release GFP. Furin cleavage occurred at both pH 6.0 and 7.0 in the samples with and without PMSF. Still, some protein did not get cleaved, probably because the amount of enzyme was not sufficient to cleave all the CTB-GFP protein present in the plant extract. The incubation time of 4 h might also have been insufficient. However, the presence of the cleaved GFP product in the samples incubated with furin confirms that the engineered furin cleavage site is functional. The introduction of furin consensus sequences at the B-chain/C-peptide and the C-peptide/A-chain interfaces of human proinsulin has been demonstrated to increase the processing of proinsulin to mature insulin in a wide variety of non-neuroendocrine cells, including fibroblasts, myoblasts, epithelial cells, and lymphocytes (36 – 42). As the furin cleavage site is also recognized by the endopeptidases PC2 and PC3/1, it is likely that CTB-GFP fusion protein is cleaved more efficiently during the process of receptor-mediated delivery.
Quantification of CTB-GFP
To quantify the amount of CTB-GFP fusion protein in transgenic tobacco leaves, ELISA (ELISA) was performed (Fig. 3B). A standard curve was obtained using different concentrations of recombinant GFP. The amount of CTB-GFP in the transgenic plants was compared with the known concentrations of the recombinant GFP (standard curve). Expression levels of CTB-GFP ranged from 19.09 to 21.3% total soluble protein.
GM1 binding assay
The functionality of chloroplast-derived CTB-GFP was determined by its ability to bind to GM1 in an in vitro GM1 binding assay (Fig. 3C). GM1 binding assay showed that pentamers of CTB-GFP were formed. This finding confirms the correct folding and disulfide bond formation of CTB pentamers within transgenic chloroplasts because only the pentameric form of CTB can bind to GM1 (21).
Fluorescent microscopy to detect the presence of GFP in the tissue
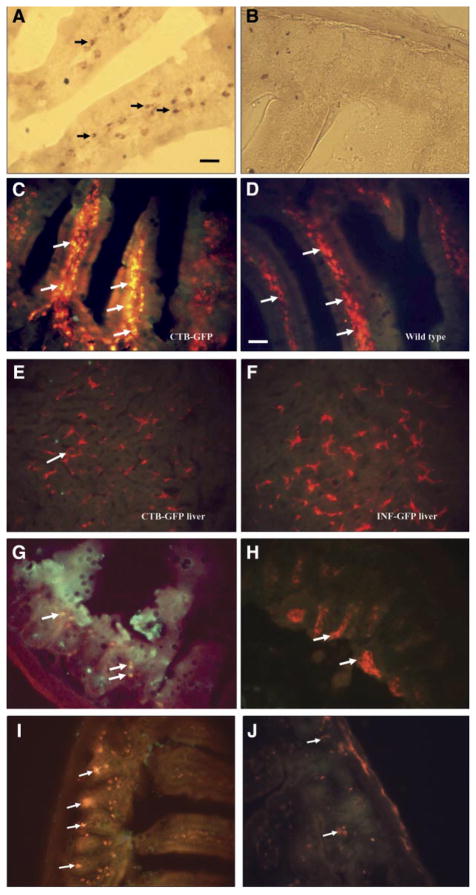
Fixed tissue and fresh frozen sections of the liver, spleen, ileum, and jejunum were made from the three groups of mice fed with plants expressing CTB-GFP, IFN-GFP, and WT plants, respectively. In mice fed with CTB-GFP expressing plant leaf material, fluorescence microscopy showed the presence of GFP in intestinal mucosa and submucosa (Fig. 4A), the hepatocytes of the liver (Fig. 4D) as well as various cells of the spleen (Fig. 4G). In the mice fed with wild-type (untransformed) leaf material, no GFP fluorescence was observed (Fig. 4B, E, and H). In the mice fed with IFN-GFP expressing plant leaf material, no GFP was detected in the liver or spleen (Fig. 4F and I). Detection of GFP in the liver and spleen following oral delivery of CTB-GFP expressing plant leaf material, suggests the successful delivery of the protein across the intestinal lumen into the systemic circulation. Moreover, the lack of detection of a significant amount of GFP in the liver and spleen of mice fed with IFN-GFP expressing plants suggests that a transmucosal carrier such as CTB is required for delivery of an adequate amount of a macromolecule across the intestinal lumen into the systemic circulation.
Figure 4.
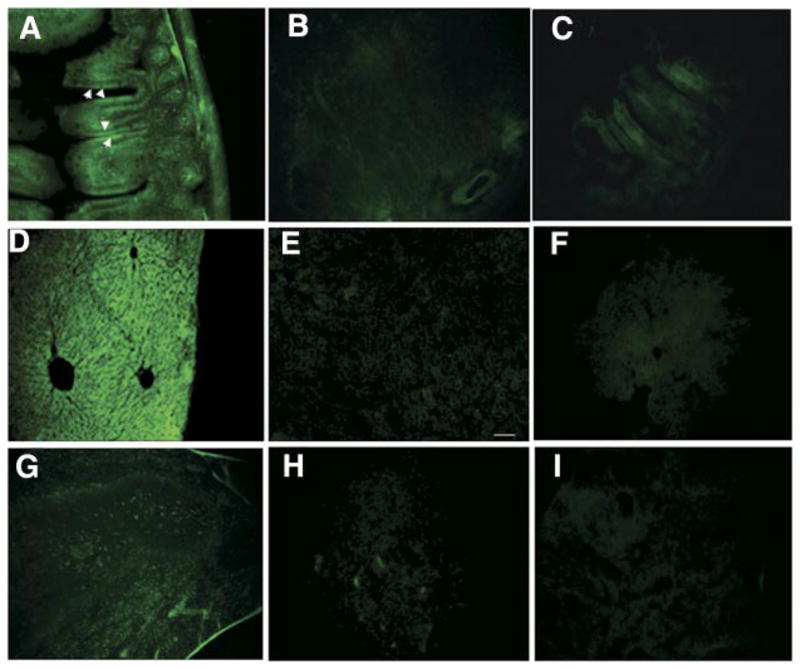
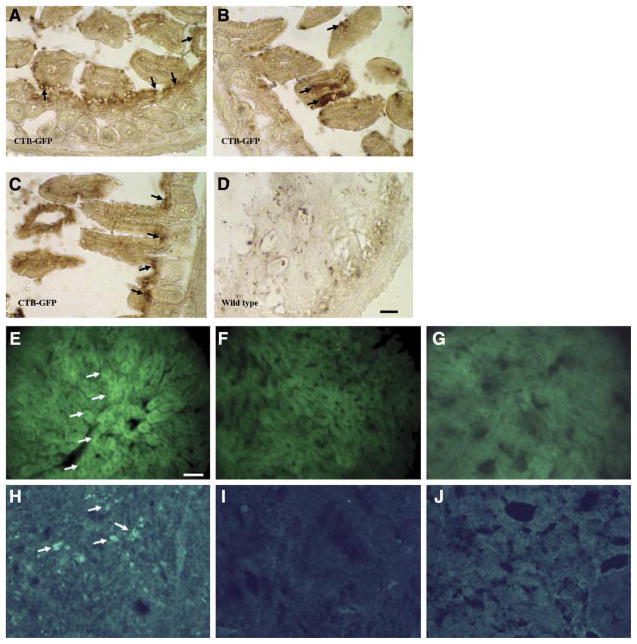
Cryosections of the intestine and liver of the mice fed with CTB-GFP or wild-type plant leaves material. A) GFP in the ileum of a mouse following oral delivery of the CTB-GFP expressing plant leaf material. Arrows show numerous columnar cells of the intestinal mucous membrane, which have up-taken the CTB-GFP. Various cells in the connective tissue beneath the epithelium also show the presence of GFP. B) Section of the ileum of a mouse fed by the wild-type (untransformed) plant leaf material. C) Section of the ileum of a mouse fed by the IFN-GFP leaf material. D) GFP in hepatocytes of a mouse liver following oral delivery of CTB-GFP expressing plant. E) Section of the liver of a mouse fed by the wild-type plant material. F) Section of the liver of a mouse fed by IFN- GFP expressing plant material. G) GFP in the spleen of a mouse following oral delivery of CTB-GFP expressing plant. Arrows show various splenic cells with GFP. H) Section of the spleen of a mouse fed by the wild-type plant material. I) Section of the spleen of a mouse fed by IFN-GFP expressing plant material. Scale bar: 50 μm.
Immunohistochemistry
To confirm the fluorescent microscopy findings, immunostaining was performed with both CTB and GFP antibodies. In the intestine of the mice fed with CTB-GFP, anti-GFP Ab detected GFP inside the epithelial cells of the villi of the intestine, in the crypts, as well as in the submucosal tissue (Fig. 5 A, C), which suggesting GFP uptake by lymphoid cells as well as the circulation. These results confirmed the previous microscopy find-ings (Fig. 4) and showed the presence of GFP in various tissues, confirming that GFP was successfully delivered to blood when transgenic leaf material was orally fed to the mouse. GFP immunoreactivity was detected in the liver and spleen (Fig. 5E and H) in a similar pattern to that seen with fluorescence microscopy of the native tissue (Fig. 4D and G). In the case of the mice fed with wild-type leaf material, no GFP was detected in any of the tissues (Fig. 5F and I). In the mice fed with plants expressing IFN-GFP, GFP was not detected in the liver or spleen cells (Fig. 5G and J).
Figure 5.
Immunohistochemical localization of the GFP in mouse ileum, liver, and spleen. A–C) are sections of the ileum of the mice fed with CTB-GFP expressing plant leaf. Arrows indicate presence of GFP in the intestinal epithelium as well as cells of the crypts. D) Shows a section of the ileum of a mouse fed with wild-type (untransformed) plant leaf materials. E) GFP- immunoreactivity in hepatocytes (arrows) in a mouse fed orally by CTB-GFP expressing plant. F) Section of the liver from a mouse fed by wild-type (untransformed) plant. G) Section of the liver from a mouse fed by IFN-GFP expressing plant. H) GFP-immunoreactivity in the spleen of mouse fed orally by CTB-GFP expressing plant. Arrows indicate various cells with a higher GFP content. I) Section of the spleen from a mouse fed by wild-type (untransformed) plant. J) Section of the spleen from a mouse fed by wild-type (untransformed) plant. Scale bar for A–D = 50 μm, Scale bar for E–J = 25 μm.
To study the route of CTB in the body, we performed immunohistochemistry using anti-CTB antibodies. CTB was detected in the intestinal cells as well as inside the villi (Fig. 6A) in the lamina propia and the submucosa. It was, however, not detected in the liver (Fig. 6E), indicating that GFP is cleaved away from CTB and that, while GFP leaves the cell, CTB probably is translocated to the basolateral membrane of the cell. These results support the feasibility of CTB to act as a transmucosal carrier and orally deliver fused proteins via the intestinal cells.
Figure 6.
Immunohistochemistry of ileum, liver, and spleen tissues of mice fed with CTB-GFP expressing leaves or IFN-GFP expressing leaves or wild-type leaves. A) Shows a section of the intestine of a CTB-GFP treated mouse. The arrows indicate CTB in the submucosa of the intestinal villi. B) Shows a section of mouse ileum fed with wild-type plant, immunostained for CTB. C–F) Double staining for macrophage (red) and CTB (green) in mouse intestine and liver. C) Arrows show macrophages in the sub-mucosa of the intestine containing CTB, in a mouse fed with CTB-GFP expressing plant leaf material. The merged color is yellow. D) Arrows indicate F4/80-positive cells (macrophages, in red) in a merged picture in the intestine of a mouse fed with WT leaf material. E) A merged picture showing double staining for macrophage (Kupffer cells) and CTB in mouse liver. Arrows show macrophages (red) in the liver. No sign of CTB (green) was found in the liver of CTB-GFP fed mouse. F) Liver section of an IFN-GFP fed mouse used as a negative control for CTB. Macrophages are seen in red. G) F4/80 Ab was used as a marker of macrophages in the intestine. Arrows indicate macrophages, which have entrapped GFP (yellow after merging the red and the green). Many of the macrophages are not associated with GFP. H) Many macrophages are seen in the intestine of mouse fed with IFN-GFP expressing plant leaf material, which do not show GFP immunoreactivity. I, J) CD11c (red) and GFP (green) immunoreactivities in the mouse intestine. I) Arrows indicate CD11c (red, presumably dendritic cells, due to having a star shape morphology) with internalized GFP (green), which can be seen in yellow color when the red and green channels were merged. J) Arrows indicate CD11c-positive cells in intestine of mice fed with IFN-GFP expressing plant leaf material. Scale bar for A and B = 25 μm. Scale bar for C–J = 50 μm.
To localize the GFP and/or CTB in the gut associated lymphoid tissue (GALT) and other tissues, double staining for antigen-presenting cells such as macrophages or dendritic cells was performed. A double staining with F4/80 Ab for macrophages showed the presence of CTB inside macrophages (Fig. 6C). Fig. 6G shows macrophages associated with GFP, and Fig. 6I shows dendritic cells taking up the GFP. In either case, associations of GFP with these antigen presenting cells were found. Most of the macrophages were not associated with GFP, which is perhaps due to uptake by the blood and lymph circulation, while the CTB is translocated to the basolateral membrane and is associated with macrophages.
DISCUSSION
In this study, detection of GFP and CTB in the intestinal mucosa (Figs. 5, 6) suggests that CTB–GFP has been taken up by the enterocytes and the gut-associated lymphoid tissue (GALT). The CTB domain of the CTB-GFP forms the pentameric structure within chloroplasts through disulfide bond formation; pentameric form binds to the GM1 receptors on enterocytes and is endocytosed into the intestinal cells as endosomes (20). GM1 functions to concentrate CTB in detergent-insoluble, glycolipid-rich apical membrane microdomains called lipid rafts (43, 44). Binding to lipid rafts is required to couple the lipid-anchored protein with intracellular machinery for protein sorting and vesicular traffic (45, 46). After endocytosis, the CTB-GM1 complex trafficking occurs retrogradely through Golgi cisternae and/or TGN (20, 47) into the lumen of the endoplasmic reticulum (ER; 48). The GM1-CTB-GFP complex in the lipid rafts, targeted to the TGN, loses its endosomal covering. Within the TGN, ubiquitously expressed furin cleaves numerous polypeptide precursors as it gets activated. In eukaryotes, many essential secreted proteins and peptide hormones, enzymes, and neuropeptides are initially synthesized as proproteins (inactive precursors) and are activated by proteolytic cleavage by furin and other members of the prohormone-proprotein convertase (PCs, 23). Abundant experimental evidence indicates that the CTB-GFP protein with furin cleavage site in between the fusion protein gets cleaved and, as a result, the CTB and GFP separate. The CTB is taken into the ER and from there to the basolateral surface of the cell (transcytosis), where it remains membrane bound to GM1 receptor (20). The GFP molecule getting out of the TGN (presumably membrane-bound) is exocytosed through the basolateral membrane and finds its way into extracellular fluid and into the submucosal vessels, including the lymphatic system. Due to the large-size fenestrations of the lymphatic vessels, lymphatics return over 3 L of fluid and ~120 g of protein to the bloodstream every 24 h in an adult human (49).
Besides the entry of CTB-GFP through the GM1 ganglioside receptor, the M cells in intestinal epithelium covering the mucosa-associated lymphoid tissue in the digestive tract also serve as a port of entry of macromolecules and microorganisms by pinocytosis (50). Therefore, a small amount of CTB-GFP could be taken up by the GALT. This is shown in our study by CTB and GFP expression in the antigen presenting cells, including the macrophages as well as the dendritic cells in the intestinal lamina propia and submucosa. Similarly, a small amount of GFP associated with macrophages in the intestine of the INF-GFP fed mice is likely to be taken up by the M cells nonspecifically. The IFN-GFP fusion protein also contains a furin cleavage site but, due to limited uptake by the intestinal epithelial cells, there is not a significant GFP transport to the tissues of the IFN-GFP fed mice. The amount of CTB-GFP reaching the enterocytes via GM1 receptor is very high compared with the entry of IFN-GFP through M cells. This is quite evident due to the GFP detected in various organs of the CTB-GFP fed mice (Figs. 5, 6). Presence of GFP and not CTB in the liver of CTB-GFP treated mice in our study (Figs. 5, 6) suggests the cleavage of the CTB-GFP fusion protein in entero-cytes and uptake of GFP into the vasculature of the lamina propia and the submucosa. CTB, however, might be translocated to the basolateral cell membrane and remain bound to GM1 (20).
The main goal of this study is to develop an efficient oral delivery of protein through GM1 receptor-mediated endocytosis. Moreover, furin cleavage site facilitates the cleavage of the candidate protein in the cell, so that it could be passed into the extracellular space and into the circulation. Internalization of GFP using receptor- mediated endocytosis suggests a possible way of protein delivery across the impermeable intestinal mucous membrane. Because of the rapid turnover of the intestinal epithelial cells (51) in humans (renewal of the intestinal epithelium occurs in every 3– 6 d), repeated feeding of the CTB fused to a therapeutic protein is possible due to the continuous availability of GM1 receptors in the new epithelium. Moreover, Peterson and colleagues suggested a recycling mechanism for GM1 receptor as well (52).
One of the most challenging problems of human health management is the high cost of prescription drugs in developed countries and their lack of availability in developing countries. Such high cost of therapeutic proteins can be attributed to their production in fermentation-based system, expensive purification and processing methods, low-temperature storage, transportation, and sterile delivery using syringes through health professionals. Most of these expenses could be avoided by expressing therapeutic proteins in plant cells and through their oral delivery. This study shows internalization of CTB-GFP by the mouse intestinal mucosal cells as well as the antigen-presenting cells in the intestinal mucosa and submucosa. We also show the presence of GFP but not CTB in the liver of mice following oral delivery of CTB-GFP leaf material. Detection of both CTB and GFP in mouse intestinal cells following oral administration of CTB-GFP expressing leaf material shows that the recombinant protein has been protected from peptidases and/or acids by bioen-capsulation (53) within the plant cells. Several vaccine antigens (28, 54 –57) and human blood proteins (31, 58 – 60) have been expressed in transgenic chloroplasts and shown to be fully functional. The ability to express high levels of foreign proteins in plastids present within edible plant parts (61, 62) and the rapid turnover of intestinal epithelial cells (51) for recycling GM1 receptors make this approach a reality. This study opens the door for low-cost production and delivery of human therapeutic proteins.
Acknowledgments
The authors are grateful to Olga Carmona-Sanchez for generating the CTB-GFP plants, Kim Lorinda Tash for technical assistance, Bob Banks for help with the animal facilities and Dr. Kenneth Teter for critically reading the manuscript. This investigation was supported in part by USDA 3611–21000-017– 00D and NIH R01GM63879 award to H.D.
References
- 1.Daniell H, Carmona-Sanchez O, Burns BE. Chloroplast derived antibodies, biopharmaceuticals and edible vaccines. In: Fischer R, Schillberg S, editors. Molecular Farming. WILEY-VCH Verlag Publishers; Weinheim, Germany: 2004. pp. 113–133. [Google Scholar]
- 2.Petridis D, Sapidou E, Calandranis J. Computer aided process analysis and economic evaluation for biosynthetic human insulin production–a case study. Biotechnol Bioeng. 1995;48:529–541. doi: 10.1002/bit.260480516. [DOI] [PubMed] [Google Scholar]
- 3.Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000;18:1151–1155. doi: 10.1038/81132. [DOI] [PubMed] [Google Scholar]
- 4.Arntzen C, Plotkin S, Dodet B. Plant-derived vaccines and antibodies: potential and limitations. Vaccine. 2005;23:1753–1756. doi: 10.1016/j.vaccine.2005.01.090. [DOI] [PubMed] [Google Scholar]
- 5.Mason HS, Warzecha H, Mor T, Arntzen C. Trends Mol Med. 2002;8:324. doi: 10.1016/s1471-4914(02)02360-2. [DOI] [PubMed] [Google Scholar]
- 6.Mason HS, Haq TA, Clements JD, Arntzen CJ. Vaccine. 1998;16:1336. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 7.Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. Nat Biotechnol. 1998;16:934. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- 8.Grevich JJ, Daniell H. Chloroplast genetic engineering: Recent advances and future perspectives. Criti Rev Plant Sci. 2005;24:83–107. [Google Scholar]
- 9.Daniell H, Kumar S, Dufourmantel N. Breakthrough in chloroplast genetic engineering of agronomically important crops. Trends Biotechnol. 2005;23:238–245. doi: 10.1016/j.tibtech.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Overexpression of the Btcry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniell H. Molecular strategies for gene containment in transgenic crops. Nat Biotech. 2002;20:581–587. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagemann R. The sexual inheritance of plant organelles. In: Daniell H, Chase C, editors. Molecular Biology and Biotechnology of Plant Organelles. Springer; Dordrecht, The Netherlands: 2004. pp. 87–108. [Google Scholar]
- 13.Ruiz O, Daniell H. Engineering Cytoplasmic male sterility via the chloroplast genome by expression of β-ketothiolase. Plant Physio. 2005;138:232–246. doi: 10.1104/pp.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz O, Hussein H, Terry N, Daniell H. Phytore-mediation of organomercurial compounds via chloroplast genetic engineering. Plant Physiol. 2003;132:1–9. doi: 10.1104/pp.103.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quesada-Vargas T, Ruiz O, Daniell H. Characterization of heterologous multigene operons in transgenic chloroplasts. Transcription, processing, and translation. Plant Physiol. 2005;138:1746–1762. doi: 10.1104/pp.105.063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamman JH, Enslin GM, Kotze AF. BioDrugs. 2005;19:165. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, Hol WG. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 18.Merritt EA, Hol WG. AB5 toxins. Curr Opin Struct Biol. 1995;5:165–71. doi: 10.1016/0959-440x(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 19.Ling H, Boodhoo A, Hazes B, Cummings MD, Armstrong GD, Brunton JL, Read RJ. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry. 1998;37:1777–1788. doi: 10.1021/bi971806n. [DOI] [PubMed] [Google Scholar]
- 20.Lencer WI. Microbes and microbial Toxins: paradigms for microbial-mucosal toxins. V Cholera: invasion of the intestinal epithelial barrier by a stably folded protein toxin. Am J Physiol Gastrointest Liver Physiol. 2001;280:G781–786. doi: 10.1152/ajpgi.2001.280.5.G781. [DOI] [PubMed] [Google Scholar]
- 21.Merritt EA, Sarfaty S, van den Akker F, L’Hoir C, Martial JA, Hol WG. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alisky JM, Van de Wetering CI, Davidson BL. Widespread dispersal of cholera toxin subunit b to brain and spinal cord neurons following systemic delivery. Exprimental Neurol. 2002;178:139–146. doi: 10.1006/exnr.2002.8031. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the TGN en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer G, Boileau G, Bendayan M. The proprotein convertase furin colocalizes with caveolin-1 in the Golgi apparatus and endosomes of hepatocytes. Cell Tissue Res. 2004;316:55–63. doi: 10.1007/s00441-004-0866-x. [DOI] [PubMed] [Google Scholar]
- 27.Henrich S, Cameron A, Bourenkov GP, Kiefersauer R, Huber R, Lindberg I, Bode W, Than ME. The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat Struct Biol. 2003;10:520–526. doi: 10.1038/nsb941. [DOI] [PubMed] [Google Scholar]
- 28.Daniell H, Lee SB, Panchal T, Wiebe PO. Expression and assembly of the native cholera toxin B subunit gene as functional oligomers in transgenic tobacco chloroplasts. J Mol Bio. 2001;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniell H, Ruiz ON, Dhingra A. Chloroplast genetic engineering to improve agronomic traits. Methods Mol Biol. 2004;286:111–137. doi: 10.1385/1-59259-827-7:111. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Daniell H. Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens in recombinant protein protocols. Methods Mol Biol. 2004;267:365–383. doi: 10.1385/1-59259-774-2:365. [DOI] [PubMed] [Google Scholar]
- 31.Daniell H, Chebolu S, Kumar S, Singleton M, Falconer R. Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine. 2005;23:1779–1783. doi: 10.1016/j.vaccine.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Daniell H. Transformation and foreign gene expression in plants mediated by microprojectile bombardment. Methods Mol Biol. 1997;62:453–488. doi: 10.1385/0-89603-480-1:463. [DOI] [PubMed] [Google Scholar]
- 33.Samsam M, Mi W, Wessig C, Zielasek J, Toyka KV, Coleman MP, Martini R. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J Neurosci. 2003;23:2833–2838. doi: 10.1523/JNEUROSCI.23-07-02833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berghoff M, Samsam M, Muller M, Kobsar I, Toyka KV, Kiefer R, Maurer M, Martini R. Neuroprotective effect of the immune system in a mouse model of severe dysmyelinating hereditary neuropathy: enhanced axonal degeneration following disruption of the RAG-1 gene. Mol Cell Neurosci. 2005;28:118–127. doi: 10.1016/j.mcn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Taylor NAWJ, Van De Ven J, Creemers W. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–27. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- 36.Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994;269:6241–6245. [PubMed] [Google Scholar]
- 37.Hay CW, Docherty K. Enhanced expression of a furin-cleavable proinsulin. J Mol Endocrinol. 2003;31:597–607. doi: 10.1677/jme.0.0310597. [DOI] [PubMed] [Google Scholar]
- 38.Ito M, Bujo H, Takahashi K, Arai T, Tanaka I, Saito Y. Implantation of primary cultured adipocytes that secrete insulin modifies blood glucose levels in diabetic mice. Diabetologia. 2005;48:1614–1620. doi: 10.1007/s00125-005-1825-0. [DOI] [PubMed] [Google Scholar]
- 39.Nishigori T, Yanagita M, Takeuchi T. Proinsulin cleaved by furin is processed to chromatographically mature insulin by carboxypeptidases in nonneuroendocrine cells. Peptides. 1996;17:789–796. doi: 10.1016/0196-9781(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 40.Shaw JA, Delday MI, Hart AW, Docherty HM, Maltin CA, Docherty K. Secretion of bioactive human insulin following plasmid-mediated gene transfer to non-neuroendocrine cell lines, primary cultures and rat skeletal muscle in vivo. J Endocrinol. 2002;172:653–672. doi: 10.1677/joe.0.1720653. [DOI] [PubMed] [Google Scholar]
- 41.Short DK, Okada S, Yamauchi K, Pessin JE. Adenovirus-mediated transfer of a modified human proinsulin gene reverses hyperglycemia in diabetic mice. Am J Physiol. 1998;275:E748–756. doi: 10.1152/ajpendo.1998.275.5.E748. [DOI] [PubMed] [Google Scholar]
- 42.Yanagita M, Nakayama K, Takeuchi T. Processing of mutated proinsulin with tetrabasic cleavage sites to bioactive insulin in the non-endocrine cell line, COS-7. FEBS Lett. 1992;311:55–59. doi: 10.1016/0014-5793(92)81366-t. [DOI] [PubMed] [Google Scholar]
- 43.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown D, Lendon E. Structure and function of sphingolipid and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17220–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 45.Lencer WI, Moe S, Rufo PA, Madara JL. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc Natl Acad Sci USA. 1995;92:10094–10098. doi: 10.1073/pnas.92.22.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badizadegan K, Wolf AA, Rodighiero C, Jobling M, Hirst TR, Holmes RK, Lencer WI. Floating cholera toxin into epithelial cells: functional association with caveolae-like detergent-insoluble membrane microdomains. Int Med Microbiol. 2000;290:403–408. doi: 10.1016/S1438-4221(00)80052-1. [DOI] [PubMed] [Google Scholar]
- 47.Feng Y, Jadhav AP, Rodighiero C, Fujinaga Y, Kirchhausen T, Lencer WI. Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the TGN but not the Golgi apparatus in Exo2-treated cells. EMBO Rep. 2004;5:596–601. doi: 10.1038/sj.embor.7400152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujinaga, Wolf AA, Rodighiero C, Wheeler H, Tsai B, Allen L, Jobling MG, Rapoport T, Holmes RK, Lencer WI. Gangliosides that associate with lipid rafts mediate transport of cholera and and related toxins from the plasma membrane to endoplasmic reticulum. Mol Biol Cell. 2003;12:4783–4793. doi: 10.1091/mbc.E03-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Granger DN. In: Essential medical physiology. 2. Johnson Leonard R., editor. Lippincott-Raven; Philadelphia: 1997. pp. 217–225. [Google Scholar]
- 50.Jepson MA, Clark MA. Studying M cells and their role in infection. Trends Microbiol. 1998;6:359–365. doi: 10.1016/s0966-842x(98)01337-7. [DOI] [PubMed] [Google Scholar]
- 51.Heath JP. Epithelial cell migration in the intestine. Cell Biol Int. 1996;20:139–146. doi: 10.1006/cbir.1996.0018. [DOI] [PubMed] [Google Scholar]
- 52.Boonyarattanakalin S, Martin SE, Dykstra SA, Peterson BR. Synthetic mimics of small Mammalian cell surface receptors. J Am Chem Soc. 2004;126:16379–16386. doi: 10.1021/ja046663o. [DOI] [PubMed] [Google Scholar]
- 53.Kong Q, Richter L, Yang YF, Arntzen, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci USA. 2001;98:11539–11544. doi: 10.1073/pnas.191617598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koya V, Moayeri M, Leppla SH, Daniell H. Plant based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson J, Koya V, Leppla S, Daniell H. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine. 2004;22:4374–4384. doi: 10.1016/j.vaccine.2004.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molina A, Daniell H, Mingo-Castel A, Veramendi J. High-yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotech J. 2004;2:141–153. doi: 10.1046/j.1467-7652.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- 57.Molina A, Veramendi J, Hervas-Stubbs S. Induction of neutralizing antibodies by a tobacco chloroplast derived vaccine based on a B cell epitope from canine parvo virus. Virology. 2005;342:266–275. doi: 10.1016/j.virol.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Millan Fernandez-San, Mingeo-Castel A, Miller M, Daniell H. A chloroplast transgenic approach to hyper-express and purify Human Serum Albumin, a protein highly susceptible to proteolytic degradation. Plant Biotech J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001;127:852–862. [PMC free article] [PubMed] [Google Scholar]
- 60.Leelavathi SV, Reddy S. Chloroplast expression of His-tagged GUS-fusions: a general strategy to overproduce and purify foreign proteins using transplastomic plants as bioreactors. Molec Breed. 2003;11:49. [Google Scholar]
- 61.Lelivelt CL, McCabe MS, Newell CA, Desnoo CB, van Dun KM, Birch-Machin I, Gray JC, Mills KH, Nugent JM. Stable plastid transformation in lettuce (Lactuca sativa L.) Plant Mol Biol. 2005;58:763–74. doi: 10.1007/s11103-005-7704-8. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S, Dhingra A, Daniell H. Plastid-expressed betainealdehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–2854. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]