SUMMARY
The E1 protein, a papillomavirus (PV)-encoded origin-binding helicase essential for PV DNA replication, is post-translationally modified by sumoylation. As this modification is essential for the nuclear accumulation of the bovine PV E1 (BPV E1), factors modulating the sumoylation of E1 could ultimately alter the outcome of a papillomavirus infection. Therefore, we systematically tested the known sumoylation enhancing factors (E3 SUMO ligases), namely RanBP2 and PIAS family proteins, to determine their ability to bind to E1 and stimulate its sumoylation, using in vitro assays. We found that RanBP2 bound to BPV E1 but failed to bind to the E1 from a human PV (HPV11 E1), and lacked any sumoylation enhancing activity for both BPV E1 and HPV11 E1. In contrast, proteins of the PIAS family (except for PIASy) bound to both BPV E1 and HPV11 E1 and stimulated their sumoylation, with PIASxβ (Miz1) exerting the largest stimulatory effect. The structural integrity of the RING finger domain of the PIAS proteins was required for their E3 SUMO ligase activity on PV E1 sumoylation, but was dispensable for their PV E1 binding activity. Furthermore, the sumoylation enhancing activity exerted by the PIAS proteins on BPV E1 was more pronounced than on HPV11 E1, and appeared to favor SUMO1 versus SUMO2 as the SUMO modifier. Altogether, this study identifies PIAS proteins as possible modulators of PV E1 sumoylation during papillomavirus infections.
Keywords: Papillomavirus, E1, BPV E1, HPV11 E1, sumoylation, E3 ligase, RanBP2, PIAS, Miz1
INTRODUCTION
Papillomaviruses (PVs1) are the etiological agents of warts in humans and animals, are the primary risk factor associated with cervical cancer, and are associated with anogenital cancers and other pathologies (1). PVs replicate their small genome (approximately 8000 bp) episomally in the nucleus of the infected cell. This ability is conferred by the E1 protein, a viral-encoded protein that recognizes the viral origin of replication in conjunction with the E2 viral protein (2), recruits host cell replication proteins to the origin (3–5), and initiates DNA replication via its helicase activity (6). Thus, the E1 and the E2 proteins are the only viral-encoded proteins required for viral DNA replication, with the rest of the replication machinery being provided by the host (7).
At present, there are at least 80 distinct genotypes of human PVs (HPVs), and the E1 ORF is the second most conserved ORF among the different types (1). A comparison between the predicted HPV E1s and the well-characterized bovine papillomavirus type 1 (BPV) E1 reveals several shared physical features, such as an acidic isoelectric point (6.48 for BPV E1 and an average of 5.44 for the HPV E1s), a similar molecular weight (68 kD for BPV E1 and an average of 72 for HPV E1s), and a similar tripartite organization (an N-terminal region of undefined activity, a variable-length spacer, and a larger C-terminal region with relatedness to ATPases and helicases) (8). BPV E1 and HPV E1s also share post-translational modification by phosphorylation at multiple serine and threonine residues, mediated by several kinases including cyclin E-cdc2 kinase (9–11), protein kinases A and C (12), and casein kinase II (13,14). In spite of these similarities, BPV E1 and HPV E1s also exhibit subtle differences in oligomerization properties, DNA binding affinity, and host protein interactions (8).
Besides phosphorylation, E1 proteins are also post-translationally modified by a process known as sumoylation (15,16). Sumoylation consists of the covalent attachment of a small ubiquitin-related protein moiety (SUMO) to a substrate protein via an iso-peptide bond formed between the carboxyl group of the C terminal glycine in SUMO and the epsilon amino group of a lysine residue in the substrate protein (17). Sumoylation involves four enzymatic steps: 1) proteolytic cleavage of the last 4 amino acid residues in SUMO to expose the internal di-glycine motif required for conjugation, 2) activation of SUMO via a thioester bond formed between the terminal carboxyl group of SUMO and Cys 173 in the Sae2 subunit of the heterodimeric SUMO activating enzyme Sae2/Sae1, 3) transfer of SUMO to the conjugating enzyme Ubc9 (also via a thioester linkage involving Cys 93 in Ubc9), and 4) final conjugation of SUMO to the target substrate protein (17). The second step (activation) requires the presence of ATP. Thus, sumoylation can be recapitulated in vitro by incubating the substrate with a mixture of purified Sae2/Sae1, Ubc9, and a truncated SUMO protein (lacking the last 4 amino acid residues), in the presence of ATP (18,19). Recently, several groups have presented evidence that, although no other enzymatic activity is required for sumoylation in vitro, there are proteins that enhance the sumoylation of specific substrates both in vitro and in vivo (20–26). This enhancing effect resembles the effect mediated by the E3 ligases in the ubiquitination pathway. Thus, these sumoylation-enhancers have been dubbed as E3 SUMO ligases (23). At present, two major types of SUMO E3 ligases have been described: RanBP2, and proteins belonging to the PIAS family.
RanBP2 (also known as Nup358) is the largest structural component of the nuclear pore complex, where it is localized to the cytosolic extensions or fibrils of the nuclear pore (27). Depletion of this protein in Xenopus egg extracts produces no detectable import defects into the nucleus, but leads to a nuclear pore devoid of cytosolic extensions, thus showing that RanBP2 is the major component of these structures (28). RanBP2 is sumoylated, contains two Zinc fingers, four RanGTP binding domains, a domain containing two internal repeat motifs, and a series of loosely spaced FG repeats thought to play a relevant role in nuclear traffic by providing docking sites for nuclear traffic factors (transportins)(for a schematic representation, see reference 23). Besides binding RanGTP and transportins in conjunction with transport complexes, RanBP2 also binds RanGDP and RanGAP, the GTPase activating protein for RanGTP. This last interaction depends on the sumoylation of RanGAP (29,30). The region in RanBP2 responsible for its interaction with sumoylated RanGAP has been mapped to the domain containing the two internal repeat motifs. This region also contains the requirements needed for SUMO1 modification of RanBP2 and binding to Ubc9 (29). Recently, a smaller version of this region, lacking the FG repeat (referred to as RanBP2ΔFG), was shown to enhance its own sumoylation and stimulate the sumoylation of Sp100 (23). Subsequent studies have shown that RanBP2ΔFG also acts as a SUMO E3 ligase for other substrates, including HDAC4 (25) and Mdm2 (31).
PIAS proteins were initially characterized by their ability to inhibit STAT-mediated signaling pathways (hence their name, Protein Inhibitors of Activated STAT) (32), but yeast two-hybrid analyses also demonstrated that PIAS1 was an interacting partner for p53 (33) and SUMO1 (21), thus leading to the prediction that PIAS1 could act as a SUMO E3 ligase for p53. This was tested and confirmed by Kahyo et. al.(21). Simultaneously, Johnson and Gupta described that the yeast PIAS homologues, Siz1 and Siz2, promote sumoylation of the septins (the major sumoylation target in yeast), thus further supporting PIAS proteins as SUMO E3 ligases (20). PIAS proteins are characterized by the presence of an N-terminal SAP (SAF-A/B, Acinus, PIAS) domain, a central RING finger domain where two consensus cysteine residues for a Zinc finger domain seem to have been replaced by serine and aspartic acid, and a C-terminal acidic domain. The SAP domain in PIASy mediates the binding of this protein to LEF1 (22), and may play a role in the interaction with other sumoylation substrates (34). The RING finger domain has been determined to be structurally required for the SUMO E3 ligase of the PIAS proteins, as mutations disrupting its integrity preclude the enhancing activity mediated by PIAS proteins (21,24,26,35,36). However, such mutations exert little or no effect on the ability of PIAS proteins to interact with the sumoylation substrates for which this has been tested (21). The current list of substrates for the SUMO E3 ligase activity of the members of the PIAS family includes p53 (21,24), GRIP1 (26), AR (26,36), LEF1 (22), c-Jun (24,26), c-Myb (37), Mdm2 (31), IRF-1 (35), C/EBPalpha (38), Sp3 (39), and the yeast Septins (20).
BPV E1 contains a Leu-Lys motif at positions 420 and 421 that is critical for Ubc9 binding (15), and mutations affecting this motif drastically impair the ability of BPV E1 to bind to Ubc9 and become sumoylated (15,16). BPV E1 is sumoylated at a single residue, lysine 514, and replacing this residue by arginine abolishes BPV E1 sumoylation (16). Importantly, all the mutations affecting BPV E1 sumoylation impair the intranuclear accumulation of BPV E1, leading to a mostly cytoplasmic distribution of this otherwise nuclear protein (16). Therefore, sumoylation of BPV E1 is essential for its nuclear accumulation, which in turn is required for the replicative functions of E1 (16). Thus, factors regulating E1 sumoylation may ultimately regulate and alter the outcome of a BPV infection, and more generally, of any PV infection as other papillomavirus E1 proteins are also sumoylated (16). Consequently, we sought to determine if the currently known SUMO E3 ligases exerted any effect on PV E1 sumoylation using an in vitro sumoylation system. We found that all of the PIAS family proteins tested, except PIASy, were able to specifically interact with both BPV E1 and HPV11 E1, and enhance their sumoylation, with PIASxβ (Miz1) producing the largest sumoylation enhancing effect. Such enhancing activity was dependant upon the integrity of the RING finger domain, although the disruption of this domain had no effect on the PV E1-binding activity of the PIAS proteins. RanBP2 exhibited significant binding activity only toward BPV E1, but failed to enhance its sumoylation. The enhancing effect mediated by the PIAS proteins on BPV E1 was more pronounced than on HPV11 E1, thus suggesting slight differences in the way the sumoylation of these proteins is regulated. Furthermore, the enhancing effect of PIASxβ on PV E1 sumoylation was greatly reduced when SUMO2 was used as the modifier, thus indicating that the E3 SUMO ligase activity exerted by PIAS proteins on PV E1 favors their SUMO1 modification. Altogether, this study supports PIAS proteins as possible modulators of PV E1 sumoylation during papillomavirus infections.
EXPERIMENTAL PROCEDURES
Expression plasmids, Protein expression and purification
The PIAS1 expression plasmid (21) was provided by Dr. Hideyo Yasuda. The pGEX4T3-ARIP3, pGEX4T3-ARIP3(W383A), and pGEX5X1-Miz1 plasmids for the expression of PIASxα (ARIP3), the RING finger domain mutant of PIASxα (ARIP3[W383A]), and PIASxβ (Miz1), respectively (26,40) were provided by Dr. Jorma J. Palvimo. The pGEX2T-mPIASy plasmid for the expression of PIASy (22) was provided by Dr. Rudolf Grosschedl. The pGEX3X plasmid for the expression of RanBP2ΔFG (23) was provided by Dr. Frauke Melchior. All of the above SUMO E3 ligases were expressed as GST-fusion proteins and purified by affinity chromatography on glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech, Piscataway, NJ) using a modified version of a previously described method (41). Briefly, Escherichia coli BL21 bacteria containing the appropriate plasmid were grown in 250 ml of liquid media at 37°C up to an optical density of 1.0 (wave length: 600 nm), incubated on ice for 4 min, induced with 0.2 mM isopropyl-thio-β-D-galactoside, and incubated at room temperature for 4 h. The cells were collected by centrifugation, resuspended in 1× PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) containing 5 mM DTT and 1/100 volume of Protease Inhibitor cocktail (SIGMA-Aldrich Corp., St. Louis, MO), treated with lysozyme at a final concentration of 0.1 mg/ml, lysed by two passages through a prechilled French press cell at 16,000 lb/in2, and sonicated briefly. The resulting extract was clarified at 12,000 g for 15 min, and the supernatant was incubated with the glutathione-Sepharose 4B beads for 2 h at 4°C. The beads were washed with 20 bead volumes of 1× PBS, 10 bead volumes of 1× PBS containing 750 mM NaCl, and another 10 bead volumes of 1× PBS, and bound proteins were eluted with 20 mM reduced glutathione, 100 mM Tris pH 8.0, 120 mM NaCl. The eluted proteins were dialyzed overnight against 1× TBS (50 mM Tris pH 7.4, 150 mM NaCl) supplemented with 10% Glycerol, aliquoted into 20–50 μl aliquots, and stored at −70°C. The purified proteins were checked by SDS-PAGE followed by Coomasie blue staining or western blotting performed using goat anti-GST antibodies (Amersham Pharmacia Biotech) and peroxidase-conjugated rabbit anti-goat IgG secondary antibodies (SIGMA-Aldrich Corp.), developed by chemoluminescence using the Western Lightning chemoluminescence reagent (PerkinElmer Life Sciences, Inc., Boston, MA). Protein concentrations were determined by a Bradford assay. Sae2/Sae1 were co-expressed and co-purified as above from the GST-Sae2/Sae1 expression plasmid (42), provided by Dr. Ronald T. Hay, but the proteins were eluted off the beads by Thrombin digestion. Ubc9 was expressed using a previously described pGEX-5X-1-based expression plasmid (15), and purified as above but the elution step was executed by Factor Xa digestion. GST-SUMO2 was expressed from a pGEX expression plasmid provided by Dr. Hisato Saitoh and purified as above. Human SUMO1 was cloned into plasmid pRSET (Invitrogen Corp., Carlsbad, CA) from a plasmid provided by Dr. Joana Desterro, and expressed and purified in E. coli BL21(DE3) bacteria as above, with the modifications indicated below. First, the cells were resuspended in a 1× PBS containing 500 mM NaCl and 5 mM Imidazole; second, a Ni2+-NTA-Agarose resin (QIAGEN Inc., Valencia, CA) was used for the purification; and third, the washes and the elution were performed by using a 1× PBS buffer containing increasing concentrations of Imidazole (50 mM for the final wash, 500 mM for the elution).
Plasmids and procedures for in vitro protein expression
pRSET constructs containing either full length or truncated forms of BPV E1 were described in a previous study (15). Plasmid pCR3-HPV11-E1, containing the full length HPV11 E1 (43), was provided by Dr. Jacques Archambault. Plasmid HA-Sp100A/pSG5, coding for a HA-tagged Sp100 (44), was provided by Dr. Jacob S. Seeler and was used as a control to verify the E3 SUMO ligase activity of the purified GST-RanBP2ΔFG. All the above constructs were used in coupled in vitro transcription and translation reactions to produce 35S-labeled proteins. Briefly, approximately 1–2 μg of plasmid DNA were mixed with 32 μci of Redivue L-(35S)-Methionine (Amersham Pharmacia Biotech) and 25 μl of the TNT® T7 Quick Coupled transcription/translation system (Promega Corp., Madison, WI), and incubated at 30°C for 90 min. Upon incubation, the samples were flash-frozen at −70°C or used directly in pulldown experiments or in vitro sumoylation assays.
Pulldown experiments
2, 6, or 12 μg of the appropriate purified GST-fusion protein were incubated with 20 μl of glutathione-Sepharose 4B beads and 3 μl of the 35S-labeled in vitro translated protein, in a 1× TBS buffer supplemented with 1% Tween 20 and 5 mM MgCl2 (1× TTBS-MgCl2), in a final volume of 500 μl. The incubations were performed at 4°C in a circular rotator for 3 h. Then, the samples were centrifuged at 5,000 g for 30 sec, the supernatant was discarded, and the beads were resuspended in 1 ml of ice cold 1× TTBS-MgCl2. This procedure was repeated three additional times to wash any unbound proteins away from the beads. After the final wash, the supernatant was discarded and the beads were resuspended in 25 μl of 4× SDS-PAGE sample buffer, incubated at 95°C for 3 min, and the resulting mixture was loaded on an SDS-PAGE gel. The proteins in the gel were blotted onto Immobilon™ (Millipore Corp., Bedford, MA), and the membrane was developed by autoradiography. Quantitative analysis of the resulting profile of 35S-labeled proteins on the membrane was performed by phosphordensitometry analysis using a Storm™ 860 laser scanning system and ImageQuant® analysis software (both from Molecular Dynamics Inc., Amersham Pharmacia Biotech).
In vitro sumoylation assays
In vitro sumoylation assays were carried out mostly as described (16), except that the partially purified SUMO-activating enzyme Sae2/Sae1 derived from NIH 3T3 cells previously used was replaced by a bacterially-expressed affinity purified Sae2/Sae1. Briefly, 2 μl of 35S-labeled in vitro translated BPV E1 or HPV11 E1 were incubated with or without 1 μg of Sae2/Sae1, 280 ng of Ubc9, 1.5 μg of SUMO1 or GST-SUMO2, and the indicated amounts of the purified E3 SUMO ligases. All the in vitro sumoylation reactions were performed in a buffer containing 50 mM Tris pH 8.0, 5 mM MgCl2, 5 mM ATP, and 0.5 mM DTT, in a final volume of 25 μl, at 30°C for 90 min. The reactions were stopped by adding 9 μl of 4× SDS-PAGE sample buffer. Then, the sample were incubated at 95°C for 3 min, loaded on an SDS-PAGE gel, and processed and quantified as described for the pulldown experiments.
RESULTS
Sumoylation of the bovine papillomavirus E1 protein regulates E1 function by controlling its access to the nuclear regions where viral DNA replication takes place (16); therefore, factors that regulate the sumoylation of this viral protein may ultimately affect viral replication. Recently, two major types of proteins exhibiting features of an E3 ligase for sumoylation have been characterized: the PIAS family and the nucleoporin RanBP2. Each one of these E3 SUMO-ligases appear to exhibit a specific range of protein targets. We sought to investigate if either of these E3 SUMO ligases enhanced the sumoylation of the bovine and human papillomavirus E1 proteins.
PIAS proteins interact with both BPV E1 and HPV11 E1
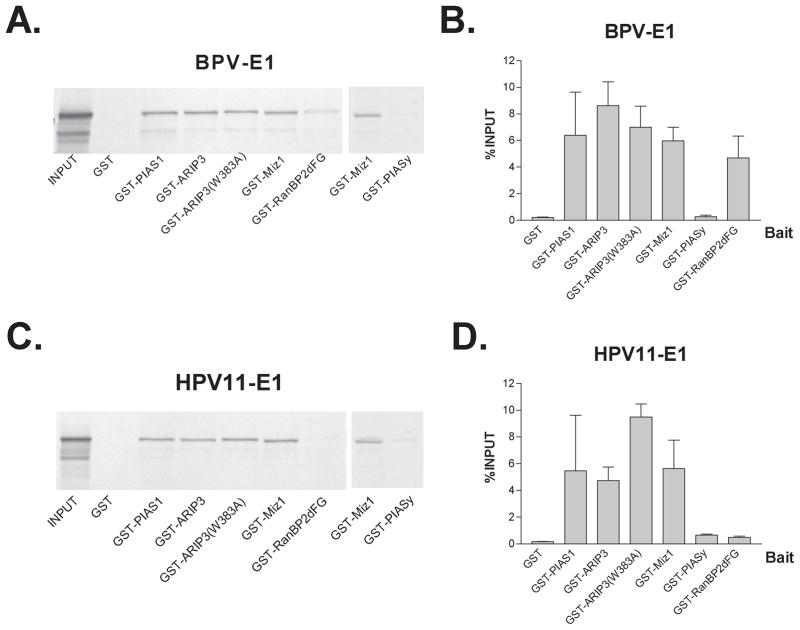
First, we examined the ability of bovine papillomavirus E1 (BPV E1) to interact with either of the known E3 SUMO ligases. To this end, we performed in vitro translations of BPV E1 in the presence of 35S-methionine and tested the ability of the in vitro translated BPV E1 to bind in pulldown experiments to a purified member of the PIAS family, PIAS1, expressed as a glutathione-S-transferase (GST) fusion protein. Since the SUMO conjugase, Ubc9, could potentially stabilize the interaction between BPV E1 and PIAS1, we also performed pulldown experiments supplemented with purified recombinant Ubc9. BPV E1 did not bind to GST alone (fig. 1, lanes 2–5), but bound to GST-PIAS1 in a protein-concentration dependent manner (fig. 1, lanes 6–8); such binding did not seem to be enhanced or compromised by Ubc9, as judged by the intensity of the BPV E1 signals obtained (fig. 1, compare lanes 8 and 9). Similar pulldown experiments were performed to determine if RanBP2 interacted with BPV E1, using in vitro translated BPV E1 and a GST fusion protein corresponding to the region in RanBP2 reported to contain the necessary determinants for SUMO modification and E3 ligase activity (GST-RanBP2ΔFG)(23). As for PIAS1, BPV E1 bound to GST-RanBP2ΔFG in a protein-concentration dependent manner (fig. 1, lanes 10–12), and Ubc9 did not seem to alter the binding of BPV E1 to GST-RanBP2ΔFG (fig. 1, compare lanes 12 and 13). An unrelated in vitro translated protein control (luciferase) did not bind to GST-PIAS1 or to GST-RanBP2ΔFG (fig. 1, lanes 15–25), thus indicating that the binding was not due to an intrinsic unspecific stickiness of the GST fusion proteins tested.
Fig. 1. BPV E1 interacts with GST-PIAS1 and GST-RanBP2ΔFG.
2, 6, or 12 μg of either GST-PIAS1 or GST-RanBP2ΔFG were incubated with 20 μl of glutathione-Sepharose 4B beads and 3 μl of 35S-labeled in vitro translated BPV E1 or Luciferase. Bound proteins were resolved on a 8% SDS-PAGE gel and analyzed by autoradiography. i represents 50% of the in vitro translation protein mixture used as initial material (input). Underlined samples were supplemented with 10 μg of Ubc9. Numbers to the left indicate molecular weight markers (in kDa).
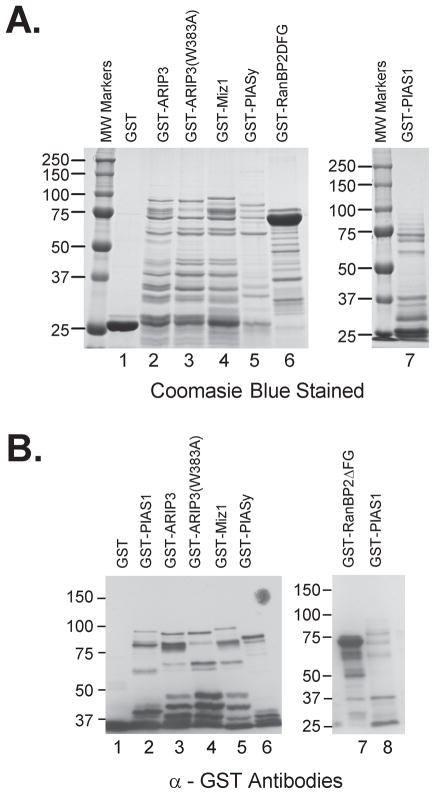
In mammals, the PIAS family comprises five different PIAS proteins, namely PIAS1, the two alternative splicing forms of PIASx (PIASxα/ARIP3 and PIASxβ/Miz1), PIASy, and PIAS 3. The binding of BPV E1 to one of the members of the PIAS family suggested the possibility that BPV E1 could interact with other members of the PIAS family. To test this possibility, we affinity purified GST-fusions of other members of the PIAS family, performed pulldown experiments with in vitro translated BPV E1 and each of the purified PIAS proteins, and quantitated the data by phosphordensitometry. The purified GST fusion proteins used for this and all subsequent experiments were evaluated by SDS-PAGE followed by Coomasie blue or immunoblotting using α-GST antibodies. Notably, all the purified GST-PIAS proteins displayed complex patterns of protein bands when resolved by SDS-PAGE (fig. 2, a and b), hence indicating that they were obtained largely as mixtures of truncated forms which contained very little of their respective full size protein. In contrast, GST-RanBP2ΔFG appeared predominantly as a single band of the expected molecular weight, comparatively exhibiting a very limited abundance of truncated forms (fig. 2a lane 6, and fig. 2b lane 7). All of the tested members of the PIAS family bound to BPV E1, except for PIASy which exhibited binding levels similar to those observed for GST (fig. 3, a and b). Likewise, a GST-ARIP3 fusion protein containing a single amino acid replacement involving a Trp residue located in the middle of the RING finger domain (GST-ARIP3[W383A]) exhibited BPV E1 binding activity similar to that of the wild type GST-ARIP3 (fig. 3, a and b). Since the above mutation is known to disrupt the structure of the RING finger domain (26), this indicates that the RING finger domain is not essential for the binding of BPV E1 to GST-ARIP3. In these experiments the amount of BPV E1 retained by GST-RanBP2ΔFG was slightly lower than that retained by any of the PIAS proteins (except for PIASy)(fig. 3, a and b).
Fig. 2. The GST-PIAS proteins were purified mostly as truncated forms of the fusion proteins.
A. 10 μg of each purified GST-fusion protein were resolved on a 10% SDS-PAGE gel and stained by Coomasie blue. B. 2 μg of each purified GST-fusion protein were resolved on a 7.5% SDS-PAGE gel, transferred to an Immobilon membrane, and immunoblotted with anti-GST antibodies. Numbers to the left indicate molecular weight markers (in kDa).
Fig. 3. Interactions between PV E1 proteins and the known SUMO-E3 ligases.
12 μg of each purified GST-fusion protein were incubated with 20 μl of glutathione-Sepharose 4B beads and 3 μl of 35S-labeled in vitro translated BPV E1 or HPV11 E1. Bound proteins were resolved on a 10% SDS-PAGE gel and analyzed by phosphordensitometry. A and C. Autoradiographs showing the binding obtained in representative experiments using BPV E1 and HPV11 E1, respectively. B and D. Graphic representation of the data obtained in two or three experiments performed with BPV E1 and HPV11 E1, respectively. The binding of the PV E1 proteins to the different GST-fusion proteins is represented as percentage of the initial material (% input). Error bars presented in this figure and throughout the paper represent standard error.
A previous study indicated that, in addition to BPV E1, the E1 proteins from one cutaneous and one mucosal human PV were also sumoylated, consistent with a general role for sumoylation in PV E1 function (16). To determine if the known E3 SUMO ligases could also bind to the E1 protein from a human papillomavirus, we performed similar pulldown experiments using in vitro translated E1 from human papillomavirus 11 (HPV11 E1). As observed for BPV E1, all of the members of the PIAS family tested bound to HPV11 E1, with the exception of PIASy; however, unlike BPV E1, HPV11 E1 did not bind to GST-RanBP2ΔFG (fig. 3, c and d).
A large central portion of the BPV E1 protein is responsible for its interaction with PIAS proteins
Next, we aimed to map the region in BPV E1 responsible for its interaction with the members of the PIAS family and with RanBP2ΔFG, as several specific protein activities have been associated with well-defined subregions within E1 (8). To this end, constructs spanning subregions of E1 (represented in figure 4e) were in vitro translated in the presence of 35S-methionine and used in pulldown experiments performed with GST, GST-PIAS1, GST-Miz1, or GST-RanBP2 ΔFG as baits. As shown in figure 4 (a and b), constructs spanning exclusively the C-terminal (amino acid residues 312–605) or the N-terminal (residues 1–312) halves of BPV E1 exhibited limited binding to GST-PIAS1. However, a construct spanning the N-terminal half of BPV E1 but lacking the first 120 amino acid residues (121–311, which corresponds to the DNA binding domain), and a construct spanning the N-terminal half and part of the C terminus of BPV E1 (1–458), exhibited similar or more abundant binding to GST-PIAS1 than the full-length BPV E1 protein (fig. 4, a and b). Similarly to the binding observed to GST-PIAS1, constructs spanning exclusively the N-terminal region (constructs 1–100 and 1–312) or the C-terminal half (312–605) of BPV E1, exhibited very little binding to GST-Miz1, while a construct spanning the N-terminal half and part of the C-terminus (1–458) exhibited abundant binding to GST-Miz1 (fig 4, c and d). Notably, the construct spanning the DNA binding domain did not appear to bind efficiently to GST-Miz1, unlike as observed for GST-PIAS1 (compare in fig. 4 panels b and d). Thus, the construct spanning residues 1–458 of BPV E1 exhibited the most consistent binding to the PIAS proteins tested. Altogether, these data indicate that amino acid sequences contained within a large central region of BPV E1 spanning residues 121–458 are involved in binding to PIAS proteins. The slight differences observed in BPV E1 binding between GST-PIAS1 and GST-Miz1 may indicate minor differences in the way small subregions within this large central core of BPV E1 contribute to the binding of the full-length protein. Alternatively, such differences could be partially due to the quality of the purified proteins used as baits, as all the purified PIAS proteins were obtained as partially truncated products, as previously indicated (fig. 2).
Fig. 4. Mapping of the region in BPV E1 responsible for its binding to SUMO-E3 ligases.
12 μg of either GST-PIAS1, GST-Miz1, or GST-RanBP2ΔFG were incubated with 20 μl of glutathione-Sepharose 4B beads and 3 μl of 35S-labeled in vitro translated proteins representing subregions within BPV E1. Bound proteins were resolved on a 10% SDS-PAGE gel and analyzed by phosphordensitometry. i: 50% of the input material; 1: GST; 2: GST-PIAS family protein; 3: GST-RanBP2ΔFG. The numbers above the line indicate the amino acid residues in the primary structure of BPV E1 covered by the in vitro translated protein. A and C. Autoradiographs showing the binding obtained in representative experiments performed with GST-PIAS1 and GST-RanBP2ΔFG, and GST-Miz1 and GST-RanBP2ΔFG, respectively. B, D, and F. Graphic representation of the data obtained in 2 different experiments performed with GST-PIAS1, GST-Miz1, and GST-RanBP2ΔFG, respectively. E. Diagram of the regions in BPV E1 covered by the different constructs used in this series of experiments. Numbers indicate amino acid residues within BPV E1. K represents Lys 514, the lysine residue previously identified as the major sumoylation site in BPV E1 (16). U represents the LK motif at positions 420 and 421, a motif previously identified as critical for interaction with Ubc9 (15).
The results with GST-RanBP2ΔFG were not definitive as all of the tested subregions within BPV E1, except for the construct spanning residues 1–458, bound GST-RanBP2ΔFG either as efficiently or even more efficiently than the full-length protein, although the construct spanning amino acid residues 121–311 consistently exhibited the most abundant binding (fig. 4, a, c, and e). Thus, the data obtained suggest the possible presence of multiple regions within BPV E1 that may mediate its binding to RanBP2ΔFG, though some regions may not actually be accessible for binding to RanBP2ΔFG in the full-length E1 protein.
The in vitro sumoylation of PV E1 is enhanced by PIAS1
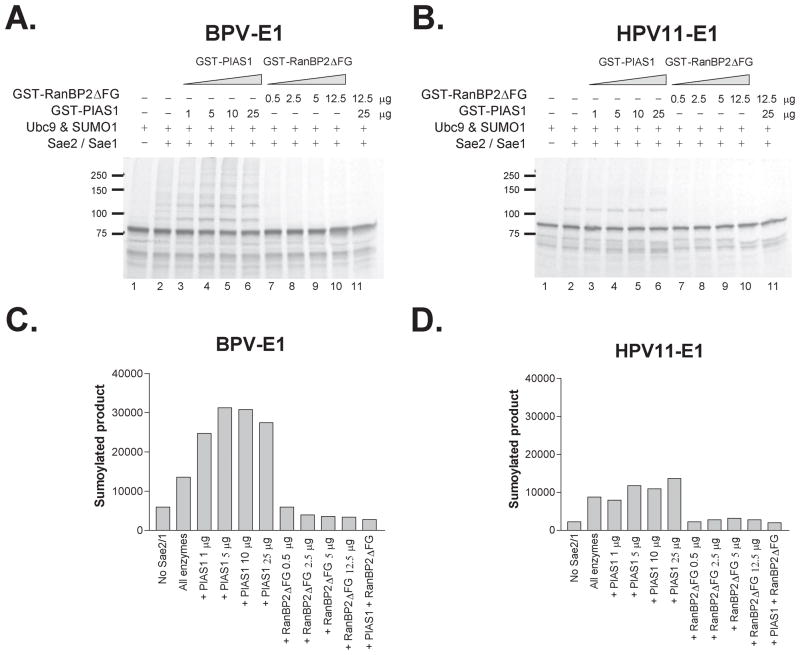
The ability of BPV E1 to establish protein interactions with the two previously characterized E3-SUMO ligases, RanBP2ΔFG and PIAS proteins, suggested that these proteins could potentially act as E3 ligases for BPV E1 sumoylation. To test this hypothesis, we developed an in vitro sumoylation assay using purified E. coli-expressed sumoylation enzymes as previously reported (42), and performed sumoylation assays of in vitro translated BPV E1 with and without each E3-ligase. The in vitro sumoylation reactions were resolved in SDS-PAGE gels, transferred to immobilon membranes, and analyzed by autoradiography and phosphordensitometry. Since RanBP2ΔFG stimulates its own sumoylation (23), we performed preliminary GST-RanBP2ΔFG sumoylation assays to test the activity of our purified sumoylation enzymes; such assays indicated that our purified sumoylation components were highly active (data not shown). The in vitro sumoylation assays performed with BPV E1 showed the appearance of high molecular forms of E1 in the presence of Ubc9 plus Sae2/Sae1 (fig. 5a). The molecular weights of the novel forms of E1 were consistent with the addition of one, two, or several SUMO molecules to E1. The apparent concentration of such high molecular weight forms increased in the presence of GST-PIAS1, and continued to increase as increasing amounts of GST-PIAS1 were added to the reaction (fig. 5a). Phosphordensitometric quantification of the predominant sumoylated form of BPV E1 indicated that GST-PIAS1 reached a maximum stimulatory effect of 2–3 fold on BPV E1 sumoylation at 5 μg per reaction, and that such stimulatory effect decreased slightly at higher concentrations (fig. 5c). In sharp contrast, the addition of GST-RanBP2ΔFG at concentrations slightly lower than those used for GST-PIAS1 seemed to exert an inhibitory effect on BPV E1 sumoylation, leading to a decrease in the amount of BPV E1 sumoylated products; such decrease became more prominent as the amount of GST-RanBP2ΔFG increased (fig. 5, a and c). This apparent inhibitory activity of GST-RanBP2 ΔFG on BPV E1 sumoylation prevailed over the stimulatory activity mediated by GST-PIAS1 when the two E3 ligases were combined in the same in vitro sumoylation reaction (fig. 5a, lane 11).
Fig. 5. The in vitro sumoylation of PV E1 is enhanced by GST-PIAS1 but not by GST-RanBP2ΔFG.
2 μl of 35S-labeled in vitro translated BPV E1 or HPV11 E1 were incubated with (+) or without (-) 1 μg of Sae2/Sae1, 1.5 μg of SUMO1, 280 ng of Ubc9, and the indicated amounts of purified GST-PIAS1 or GST-RanBP2ΔFG. Upon incubation, the samples were resolved on an 8% SDS-PAGE gel and analyzed by phosphordensitometry. A and B. Autoradiographs showing the sumoylation profile obtained for BPV E1 and HPV11 E1, respectively. Numbers to the left indicate molecular weight markers (in kDa). C and D. Graphic representation (in arbitrary units) of the data obtained by phosphordensitometry measurement of the major sumoylated product obtained for BPV E1 and HPV11 E1, respectively.
To determine if GST-PIAS1 and GST-RanBP2ΔFG exerted similar effects on the sumoylation of other PV E1 proteins, we performed similar in vitro sumoylation assays using HPV11 E1. Similarly as observed for BPV E1, the addition of GST-PIAS1 appeared to have a slight stimulatory effect on HPV11 E1 sumoylation, as increasing amounts of GST-PIAS1 led to larger amounts of sumoylated HPV11 E1 (fig. 5b). However, while such stimulatory effect appeared to continue to increase up to a concentration of 25μg of GST-PIAS1 per reaction, it did not appear to be as dramatic as the one observed for BPV E1 sumoylation (compare figs. 5c and d). GST-RanBP2ΔFG appeared to exert an inhibitory effect on HPV11 E1 sumoylation (fig. 5, b and d), which prevailed over the stimulatory activity of GST-PIAS1 (fig. 5b, lane 11), as previously seen for BPV E1.
Immunoblotting analyses of the in vitro sumoylation reactions performed using anti-SUMO1 antibodies indicated the presence of large amounts of sumoylated GST-RanBP2ΔFG in the in vitro sumoylation reactions performed with GST-RanBP2ΔFG, thus suggesting that the apparent inhibitory effect of GST-RanBP2ΔFG on BPV E1 sumoylation was due to competition for free SUMO1 (data not shown). As very small amounts of RanBP2ΔFG are sufficient to produce the E3 ligase activity mediated by RanBP2ΔFG (in the range of 1–5 ng per reaction), and larger amounts of RanBP2ΔFG are inhibitory for the sumoylation of Sp100 (a known substrate for the E3 ligase activity of RanBP2ΔFG)(23), we performed additional in vitro sumoylation assays of BPV E1 using smaller amounts of GST-RanBP2ΔFG (1 to 20 ng per reaction). Under these conditions GST-RanBP2ΔFG still lacked a stimulatory effect on BPV E1 sumoylation, although it was able to stimulate the sumoylation of Sp100 (data not shown). However, the inhibitory effect on E1 sumoylation observed at higher concentrations GST-RanBP2ΔFG was now absent, consistent with a competitive inhibition mechanism.
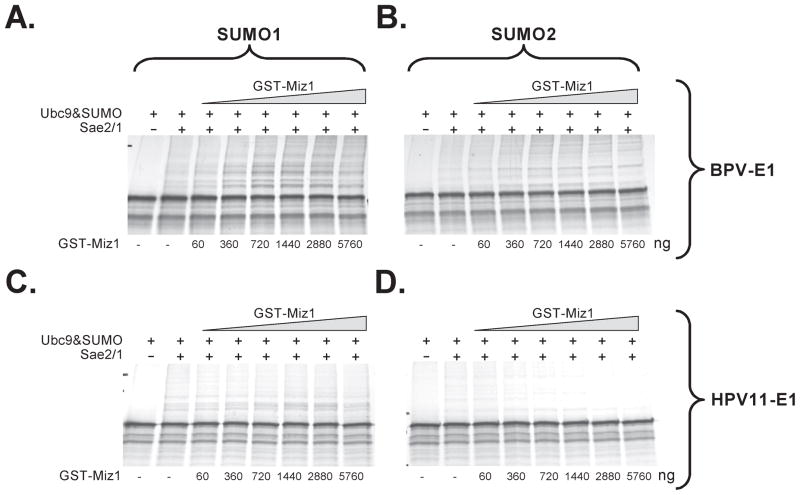
Miz1 (PIASxβ) is the PIAS family member protein that exerts the highest stimulatory activity on PV E1 sumoylation
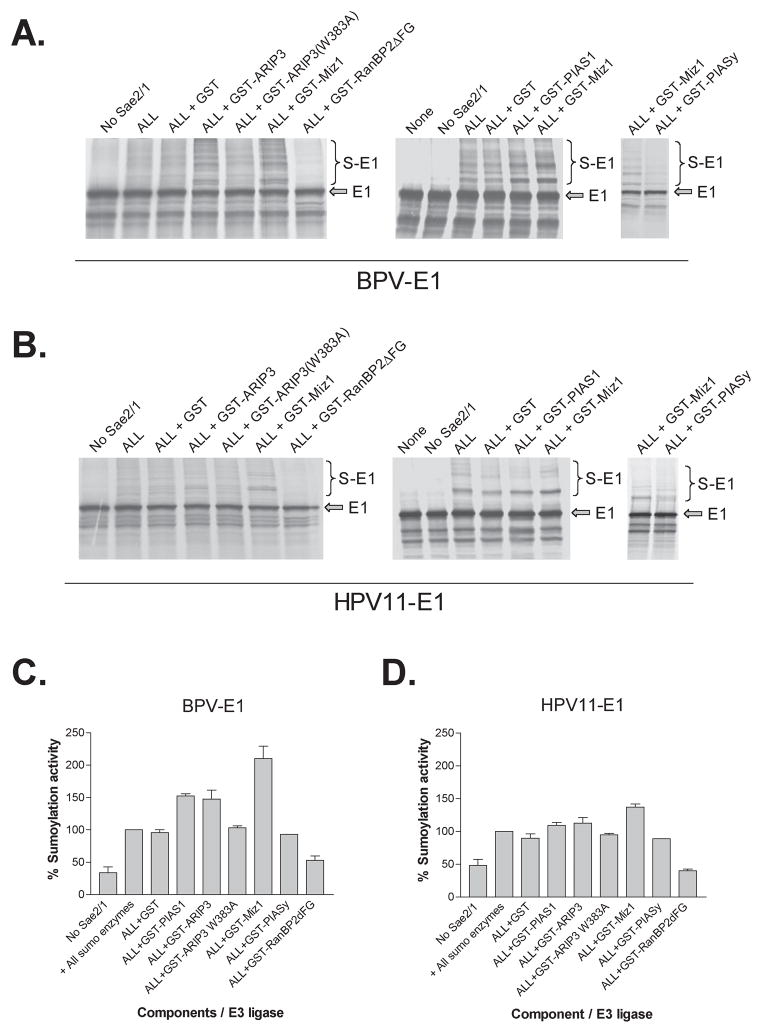
To evaluate if the different members of the PIAS family differed in their ability to stimulate the sumoylation of BPV E1, we performed additional sets of in vitro sumoylation reactions supplemented with the different purified GST-PIAS proteins at the concentration estimated to be optimal for GST-PIAS1. GST-RanBP2ΔFG was also evaluated in these assays, at a concentration of 20 ng per reaction. Large differences among the members of the PIAS family were observed in their ability to stimulate BPV E1 sumoylation. GST-PIASy did not exert any stimulatory activity on BPV E1 sumoylation, in agreement with the inability of this PIAS protein to interact with BPV E1 (fig. 6a). In contrast, GST-PIAS1, GST-ARIP3, and GST-Miz1 exerted a noticeable stimulatory activity on BPV E1 sumoylation, increasing the relative intensity of some of the sumoylation products observed, thus producing both qualitative and quantitative changes in the sumoylation patterns (fig. 6a). Out of the 3 members of the PIAS family that stimulated BPV E1 sumoylation, GST-Miz1 exerted the highest stimulatory activity on BPV E1 sumoylation, leading to a 2.3 fold increase in the apparent concentration of the most predominant form of sumoylated BPV E1, while GST-PIAS1 and GST-ARIP3 appeared to exert similar stimulatory activities, leading to a 1.5 fold increase in sumoylated BPV E1, as determined by phosphordensitometry (fig. 6c). Studies by Kotaja, et. al. (26), had indicated that the integrity of the RING finger domain was essential for the SUMO-E3 ligase activity exerted by the members of the PIAS family, as a single amino acid substitution known to disrupt this domain (i.e., a W to A substitution in position 383 of ARIP3) leads to inactivation of the sumoylation stimulatory activity of ARIP3. Importantly, no significant stimulatory activity was observed for the RING finger domain mutant GST-ARIP3(W383A), thus indicating that the RING finger domain is also required for the stimulatory activity mediated by GST-ARIP3 (fig. 6 a and c). As expected, no stimulatory activity on BPV E1 sumoylation was observed for GST-RanBP2ΔFG (fig. 6, a and c).
Fig. 6. Miz1 (PIASxβ) is the PIAS family member protein that exerts the highest stimulatory activity on PV E1 sumoylation.
2 μl of 35S-labeled in vitro translated BPV E1 or HPV11 E1 were incubated with buffer only (None), SUMO1 and Ubc9 (No Sae2/1), SUMO1+Sae2/1+Ubc9 (ALL), or SUMO1+Sae2/1+Ubc9 supplemented with 2 μg (PIAS proteins) or 20 ng (RanBP2ΔFG) of the indicated purified GST-fusion proteins. The amounts used per reaction of the different sumoylation components were 1 μg of Sae2/Sae1, 280 ng of Ubc9, and 1.5 μg of SUMO1. Upon incubation, the samples were resolved on 8 or 10% SDS-PAGE gels and analyzed by phosphordensitometry. A and B. Autoradiographs showing the sumoylation profile obtained for BPV E1 and HPV11 E1, respectively, in the presence of the different GST-fusion proteins in representative experiments. The unmodified E1 (arrow) and the sumoylated products (S-E1, bracket) are indicated. C and D. Graphic representation of the data obtained by phosphordensitometry quantification of the major sumoylated product obtained for BPV E1 and HPV11 E1, respectively, in 2 or 3 experiments. The data is expressed as percentage sumoylation activity as determined by assigning 100% sumoylation activity to the reaction performed in the presence of all the sumoylation components (Sae2/Sae1+Ubc9+SUMO1).
Similar experiments performed with HPV11 E1 showed that the effects mediated by the different PIAS proteins on HPV11 E1 sumoylation had the same profile as those observed on BPV E1 sumoylation, although the stimulatory effects were less pronounced. For instance, GST-Miz1 exerted the highest stimulatory activity on HPV11 E1 sumoylation, but led only to a 1.5 fold increase in HPV11 E1 sumoylation as compared to the un-stimulated reactions. Similarly, no stimulatory activity on HPV11 E1 sumoylation was observed for PIASy, the RING finger domain mutant GST-ARIP3(W383A), and GST-RanBP2ΔFG (fig. 6, b and d).
Miz1 (PIASxβ) stimulates the SUMO1 and SUMO2 sumoylation of BPV E1
E3 ligases are thought to enhance the transfer of SUMO from the E2 conjugating enzyme (Ubc9) to the substrate while providing specificity to the reaction. To determine if the stimulatory activity on PV-E1 sumoylation observed for the PIAS proteins was exclusive for SUMO1 sumoylation, we performed in vitro sumoylation assays using either BPV E1 or HPV11 E1 (in vitro translated), SUMO1 or SUMO2, and different concentrations of GST-Miz1 (the PIAS family member that exhibited the highest stimulatory activity on PV-E1 SUMO1 sumoylation). As shown in figure 7 (a and b), GST-Miz1 appeared to be able to stimulate BPV E1 sumoylation with both SUMO1 and SUMO2, reaching a maximum stimulatory activity at approximately the same concentration for both SUMO modifiers. However, the apparent yield of sumoylated BPV E1 was noticeably higher when SUMO1 was used as the modifier, thus suggesting that SUMO2 was a poor modifier for BPV E1 sumoylation. A more dramatic bias against SUMO2 sumoylation was observed for HPV11 E1. While GST-Miz1 clearly stimulated the SUMO1 sumoylation of HPV11 E1 (fig. 7c), no significant SUMO2 sumoylation was observed for HPV11 E1 even in the presence of GST-Miz1 (fig. 7d).
Fig. 7. Miz1 (PIASxβ) stimulates the SUMO2 sumoylation of BPV E1 but not of HPV11 E1.
1.5 μl of 35S-labeled in vitro translated BPV E1 or HPV11 E1 were incubated with (+) or without (−) 1μg of Sae2/Sae1, 280 ng of Ubc9 and 2 μg of either SUMO1 or GST-SUMO2, and the indicated amounts of purified GST-Miz1. Upon incubation, the samples were resolved on an 8% SDS-PAGE gel and analyzed by autoradiography. A and B. Sumoylation of BPV E1 with SUMO1 and GST-SUMO2, respectively. C and D. Sumoylation of HPV11 E1 with SUMO1 and GST-SUMO2, respectively.
DISCUSSION
PV E1 plays a crucial role during the natural history of papillomavirus infections by allowing the episomal replication of the viral DNA in the host cell via its origin-binding and helicase activity. Previous studies by our group showed that BPV E1 is modified by sumoylation and that this post-translational processing is essential for the nuclear accumulation of BVP E1 (15,16). As nuclear accumulation of BPV E1 is a prerequisite for replication function, the biological activity of BPV E1 is coupled to its ability to be sumoylated. Thus, sumoylation can regulate BPV E1 function, and factors that stimulate or restrict the sumoylation of BPV E1 may alter the outcome of a papillomavirus infection.
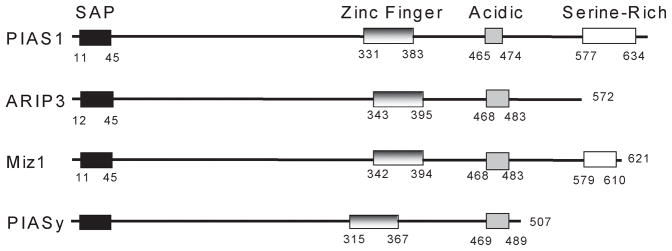
In this study we sought to investigate the ability of the known SUMO E3 ligases to stimulate BPV E1 and HPV11 E1 sumoylation, using in vitro approaches. Although the precise mechanism of action of the currently identified SUMO E3 ligases is still unknown, it is certain that binding between the sumoylation target and the E3 ligase is required for the sumoylation enhancing activity to occur. Thus, our first goal was to determine if E1 could interact with any of the known SUMO E3 ligases. We found that BPV E1 and HPV11 E1 are able to interact with all of the members of the PIAS family tested, except for PIASy, and that such interaction was not dependant on the structural integrity of the RING finger domain; furthermore, we found that BPV E1 (but not HPV11 E1) was able to interact with the other known SUMO E3 ligase, RanBP2. We also determined that a large N-terminal region within BPV E1 is responsible for its interaction with the PIAS proteins. This region of E1 defined for PIAS binding at least partially overlaps the Ubc9 interaction region (15), though the actual interaction sites for PIAS and Ubc9 must be distinct since addition of Ubc9 had no effect on E1-PIAS binding (Fig. 1). Nonetheless, the apparent proximity of the PIAS and Ubc9 binding regions on E1 is consistent with the possible formation of an E1-Ubc9-PIAS complex during the sumoylation process. In addition, although we did not map the BPV E1 binding region within the PIAS proteins, a direct comparison of the tertiary structure of PIASy with that of the other members of the PIAS family used in this study suggests that the C-terminal end of the PIAS proteins might be critical for their binding to PV E1. The variation in PIAS family members is most extensive at the C-terminus with PIASy being the smallest at 507 amino acids (fig. 8). ARIP3 with an additional 65 amino acids at its C-terminus binds E1 well, implicating this small region as critical for the interaction, and suggesting that the longer C-terminal regions of PIAS1 and Miz1 containing a serine-rich motif are not essential for E1 interaction.
Fig. 8. Schematic representation of the primary structure of the PIAS family protein members used in this study.
Numbers indicate the amino acid residues limiting the different domains identified within the primary structure of the PIAS proteins. SAP: SAF-A/B, Acinus, PIAS domain. Notice that the differences in length observed among the different PIAS proteins are all due to variations in the C-terminal end of the proteins.
Next, we tested the ability of the PIAS proteins and RanBP2 to stimulate PV E1 sumoylation, as previous reports show that binding of a given substrate to an E3 ligase does not imply per se that the ligase will enhance the sumoylation of the substrate (for instance, PIAS3 and PIASxβ interact with the human Androgen Receptor but do not enhance its sumoylation)(36). Even though the complex profile of sumoylated forms observed for BPV E1 and HPV11 E1 complicated the quantification of the stimulatory activity exerted by the different E3 ligases tested, we found that RanBP2 did not exhibit SUMO ligase activity on either BPV or HPV E1, although it bound to BPV E1; in contrast, all the PIAS proteins that exhibited PV E1 binding activity were able to enhance the in vitro sumoylation of PV E1. These results were based on the intensity of the major sumoylated form observed on each set of reactions, to allow the cross-examination of all the samples in the same experiment and the direct comparison of different experiments. However, this method does not take into account the additive effect that would be obtained if several of the bands corresponding to sumoylated forms of E1 were used for the quantification. Thus, the data obtained probably represents an underestimation of the sumoylation enhancing effect mediated by the ligases tested. Consequently, even though the apparent sumoylation enhancements mediated by the different SUMO E3 ligases tested in this study were not always quantitatively striking, we consider that the differences observed in the protein profiles obtained upon sumoylation in the presence of some of the PIAS proteins were qualitatively significant and reflected the contribution of the ligase activity. The meaning of these qualitative changes remains to be determined.
Even with the above limitations, members of the PIAS family enhanced the in vitro sumoylation of both BPV and HPV E1 proteins, consistent with SUMO ligase activity. Miz1 was the most active on both HPV and BPV E1, with a 2–3 fold stimulation of BPV E1 sumoylation. Additionally, we also observed that the integrity of the RING finger domain, while not required for E1 binding, was essential for the PV E1 sumoylation enhancing activity displayed by the PIAS proteins. This requirement for an intact RING finger domain for the SUMO E3 ligase activity displayed by the PIAS proteins, observed here and in several other reports (21,24,26,35,36), is attributable to the fact that the RING finger domain mediates the interaction between PIAS proteins and Ubc9 (36). Furthermore, the differences in the PV E1 sumoylation enhancing abilities displayed by the different members of the PIAS family did not correlate with the E1 binding activity as PIAS1, ARIP3, and Miz1 bound E1 equally well, yet Miz1 was consistently more active in stimulating sumoylation. Such differences further support a role for the C-terminal region of the PIAS proteins in their PV E1 sumoylation enhancing activity, as PIASxα (ARIP3) and PIASxβ (Miz1) only differ from each other at the C-terminal end (fig. 8) yet exhibit substantial differences in their ability to enhance the sumoylation of BPV E1. Interestingly, even though the SUMO E3 ligase activity exerted by the PIAS proteins on BPV E1 sumoylation was not restricted to SUMO1 sumoylation, as it also enhanced the SUMO2 sumoylation of BPV E1, the enhancing effect observed on SUMO2 sumoylation was notably lower. Furthermore, PIAS proteins failed to enhance the SUMO2 sumoylation of HPV11 E1. Altogether, the differences observed among the PIAS proteins in their ability to stimulate PV E1 sumoylation, and the differences observed in the type of SUMO that is preferentially used as a modifier upon PIAS stimulation, indicate a certain degree of specificity in the regulation of PV E1 sumoylation by PIAS proteins. However, as there may still be additional unknown SUMO ligases, it is possible that these other E3 proteins may exert a much more profound enhancing effect on PV E1 sumoylation. Alternatively, as several post-translational modifications affecting PV E1 have been identified, including phosphorylation (10,12–14) and ubiquitination (45), it is possible that one of these (or both) may regulate PV E1 sumoylation, further enhancing or decreasing PV E1 sumoylation. This possibility is supported by recent reports showing that phosphorylation of Heat Shock Factor 1 enhances its sumoylation (46,47). Future studies will try to establish the role of PIAS proteins in E1 sumoylation in vivo, identify additional SUMO ligases, and evaluate how the different post-translation modifications of PV E1 interact together in the cellular environment to regulate the function of this protein.
Sumoylation appears to be predominantly a nuclear event as most SUMO substrates are nuclear proteins, and sumoylation of several nuclear proteins has been shown to require intact nuclear import capacity since mutants lacking a functional nuclear localization sequence (NLS) remain unmodified (25,48). Recently, evidence was presented (31) that sumoylation can be a biphasic process with addition of SUMO both during nuclear import (utilizing the nuclear pore-associated RanBP2 E3 ligase activity) and subsequently within the nucleus via PIAS proteins which are exclusively confined to the cell nucleus (22,26,49,50). Even though the details of nuclear trafficking by PV E1 proteins remains mostly uncharacterized, BPV E1 protein resides primarily in the nucleus and possess a typical nuclear localization sequence (NLS) (9,51), suggesting that sumoylation of E1 proteins will be a nuclear or perinuclear event. The lack of PV E1 sumoylation enhancing activity displayed by RanBP2 in vitro suggests that the binding observed between BPV E1 and RanBP2 may represent an interaction established by BPV E1 along its traffic to the nucleus rather than an interaction established to enhance E1 sumoylation. In contrast, as PIAS proteins exert a sumoylation enhancing effect on BPV E1, it is tempting to propose that BPV E1 traffics to the nucleus in an un-sumoylated form, and once in the nucleus it is sumoylated in conjunction with nuclear PIAS E3 ligase activity. Intranuclear sumoylation of E1 may be necessary for its retention within the nucleus by SUMO-dependent protein interactions, and/or modulation of E1 biochemical activities necessary for replicative function. Should the PIAS proteins be the primary SUMO E3 ligases for PV E1 sumoylation in vivo, it will be necessary to determine if the expression of these cellular proteins is regulated during keratinocyte differentiation, as papillomavirus viral replication is intimately associated with the cellular differentiation of their natural host.
Lastly, the profile of sumoylated forms observed for BPV E1 in this study differs substantially from the one previously reported (16). In the former studies, a single, predominant sumoylated form of BPV E1 was observed, while in the current studies a more complex array of sumoylated products formed in vitro, even in the absence of PIAS proteins. The profile obtained in the present study suggests two possible scenarios: 1) BPV E1 may contain other potential sumoylation sites that are being used under the sumoylation assay conditions used in this study, leading to its sumoylation at multiple sites, or 2) the SUMO1 added at the single sumoylation site in BPV E1 may be undergoing polysumoylation (SUMO1 chain formation). SUMO1 was initially considered unable to undergo chain formation due to the lack of a lysine displaying the consensus sumoylation sequence and no experimental data indicative of SUMO1 polysumoylation (42). However, later reports have given strong support to the idea that SUMO1 can form chains in vitro (23), and we have also observed this for sumoylation of RanBP2 in vitro (unpublished observations). Although at present we can not discard either of these scenarios, we consider that an important contributing factor to the differences observed is the use of E. coli-expressed, purified Sae2/Sae1 in this study versus the use of partially enriched Sae2/Sae1 from mammalian cell extracts in our previous reports. Hence, a higher enzymatic activity combined with a decrease in the total pool of possible sumoylation substrates could result in either of the scenarios described above. Alternatively, an unknown E3 ligase or other factor that limits the spectrum of SUMO-modifiable lysine residues in BPV E1 (thus increasing the specificity of sumoylation) may have been present in the cell extracts previously used.
Footnotes
The abbreviations used are: PVs, Papillomaviruses; BPV E1, bovine papillomavirus type 1 E1; HPV11 E1, human papillomavirus type 11 E1; PIAS, protein inhibitor of activated STAT1; STAT, signal transducers and activators of transcription; RanBP2, Ran binding protein; Ran, ras-related nuclear protein; Sae2/1, sumo activating enzyme 2 and 1; GST, glutathione S-transferase; PAGE, polyacrylamide gel electrophoresis
This work was supported by grant CA89298 from the NIH.
References
- 1.zur Hausen H. Biochim Biophys Acta. 1996;1288(2):F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 2.Ustav M, Stenlund A. Embo J. 1991;10(2):449–57. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonne-Andrea C, Santucci S, Clertant P, Tillier F. J Virol. 1995;69(4):2341–50. doi: 10.1128/jvi.69.4.2341-2350.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conger KL, Liu JS, Kuo SR, Chow LT, Wang TS. J Biol Chem. 1999;274(5):2696–705. doi: 10.1074/jbc.274.5.2696. [DOI] [PubMed] [Google Scholar]
- 5.Amin AA, Titolo S, Pelletier A, Fink D, Cordingley MG, Archambault J. Virology. 2000;272(1):137–50. doi: 10.1006/viro.2000.0328. [DOI] [PubMed] [Google Scholar]
- 6.Bonne-Andrea C, Santucci S, Clertant P. J Virol. 1995;69(5):3201–5. doi: 10.1128/jvi.69.5.3201-3205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melendy T, Sedman J, Stenlund A. J Virol. 1995;69(12):7857–67. doi: 10.1128/jvi.69.12.7857-7867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson VG, West M, Woytek K, Rangasamy D. Virus Genes. 2002;24(3):275–290. doi: 10.1023/a:1015336817836. [DOI] [PubMed] [Google Scholar]
- 9.Lentz MR, Pak D, Mohr I, Botchan MR. J Virol. 1993;67(3):1414–23. doi: 10.1128/jvi.67.3.1414-1423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cueille N, Nougarede R, Mechali F, Philippe M, Bonne-Andrea C. J Virol. 1998;72(9):7255–62. doi: 10.1128/jvi.72.9.7255-7262.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma T, Zou N, Lin BY, Chow LT, Harper JW. Proc Natl Acad Sci U S A. 1999;96(2):382–7. doi: 10.1073/pnas.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanardi TA, Stanley CM, Saville BM, Spacek SM, Lentz MR. Virology. 1997;228(1):1–10. doi: 10.1006/viro.1996.8375. [DOI] [PubMed] [Google Scholar]
- 13.McShan GD, Wilson VG. J Gen Virol. 1997;78(1):171–7. doi: 10.1099/0022-1317-78-1-171. [DOI] [PubMed] [Google Scholar]
- 14.Lentz MR. Virus Res. 2002;83(1–2):213–9. doi: 10.1016/s0168-1702(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 15.Rangasamy D, Wilson VG. J Biol Chem. 2000;275(39):30487–95. doi: 10.1074/jbc.M003898200. [DOI] [PubMed] [Google Scholar]
- 16.Rangasamy D, Woytek K, Khan SA, Wilson VG. J Biol Chem. 2000;275(48):37999–8004. doi: 10.1074/jbc.M007777200. [DOI] [PubMed] [Google Scholar]
- 17.Wilson VG, Rangasamy D. Exp Cell Res. 2001;271(1):57–65. doi: 10.1006/excr.2001.5366. [DOI] [PubMed] [Google Scholar]
- 18.Desterro JM, Rodriguez MS, Kemp GD, Hay RT. J Biol Chem. 1999;274(15):10618–24. doi: 10.1074/jbc.274.15.10618. [DOI] [PubMed] [Google Scholar]
- 19.Okuma T, Honda R, Ichikawa G, Tsumagari N, Yasuda H. Biochem Biophys Res Commun. 1999;254(3):693–8. doi: 10.1006/bbrc.1998.9995. [DOI] [PubMed] [Google Scholar]
- 20.Johnson ES, Gupta AA. Cell. 2001;106(6):735–44. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 21.Kahyo T, Nishida T, Yasuda H. Mol Cell. 2001;8(3):713–8. doi: 10.1016/s1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F, Grosschedl R. Genes Dev. 2001;15 (23):3088–103. doi: 10.1101/gad.944801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. Cell. 2002;108(1):109–20. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt D, Muller S. Proc Natl Acad Sci U S A. 2002;99(5):2872–7. doi: 10.1073/pnas.052559499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirsh O, Seeler JS, Pichler A, Gast A, Muller S, Miska E, Mathieu M, Harel-Bellan A, Kouzarides T, Melchior F, Dejean A. Embo J. 2002;21(11):2682–91. doi: 10.1093/emboj/21.11.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotaja N, Karvonen U, Janne OA, Palvimo JJ. Mol Cell Biol. 2002;22(14):5222–34. doi: 10.1128/MCB.22.14.5222-5234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Matunis MJ, Kraemer D, Blobel G, Coutavas E. J Biol Chem. 1995;270(23):14209–13. doi: 10.1074/jbc.270.23.14209. [DOI] [PubMed] [Google Scholar]
- 28.Walther TC, Pickersgill HS, Cordes VC, Goldberg MW, Allen TD, Mattaj IW, Fornerod M. J Cell Biol. 2002;158(1):63–77. doi: 10.1083/jcb.200202088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matunis MJ, Wu J, Blobel G. J Cell Biol. 1998;140(3):499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. Cell. 1997;88(1):97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi Y, Yogosawa S, Honda R, Nishida T, Yasuda H. J Biol Chem. 2002;277(51):50131–36. doi: 10.1074/jbc.M208319200. [DOI] [PubMed] [Google Scholar]
- 32.Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, Shuai K. Proc Natl Acad Sci U S A. 1998;95(18):10626–31. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher WM, Argentini M, Sierra V, Bracco L, Debussche L, Conseiller E. Oncogene. 1999;18(24):3608–16. doi: 10.1038/sj.onc.1202937. [DOI] [PubMed] [Google Scholar]
- 34.Jackson PK. Genes Dev. 2001;15(23):3053–8. doi: 10.1101/gad.955501. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa K, Yokosawa H. FEBS Lett. 2002;530(1–3):204. doi: 10.1016/s0014-5793(02)03486-5. [DOI] [PubMed] [Google Scholar]
- 36.Nishida T, Yasuda H. J Biol Chem. 2002;277(44):41311–17. doi: 10.1074/jbc.M206741200. [DOI] [PubMed] [Google Scholar]
- 37.Dahle O, Andersen TO, Nordgard O, Matre V, Del Sal G, Gabrielsen OS. Eur J Biochem. 2003;270(6):1338–48. doi: 10.1046/j.1432-1033.2003.03504.x. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian L, Benson MD, Iniguez-Lluhi JA. J Biol Chem. 2003;278(11):9134–41. doi: 10.1074/jbc.M210440200. [DOI] [PubMed] [Google Scholar]
- 39.Sapetschnig A, Rischitor G, Braun H, Doll A, Schergaut M, Melchior F, Suske G. Embo J. 2002;21(19):5206–15. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotaja N, Aittomaki S, Silvennoinen O, Palvimo JJ, Janne OA. Mol Endocrinol. 2000;14 (12):1986–2000. doi: 10.1210/mend.14.12.0569. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez A, Bazaldua-Hernandez C, West M, Woytek K, Wilson VG. J Virol. 2000;74 (1):245–53. doi: 10.1128/jvi.74.1.245-253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. J Biol Chem. 2001;276(38):35368–74. doi: 10.1074/jbc.M104214200. [DOI] [PubMed] [Google Scholar]
- 43.Titolo S, Pelletier A, Sauve F, Brault K, Wardrop E, White PW, Amin A, Cordingley MG, Archambault J. J Virol. 1999;73(7):5282–93. doi: 10.1128/jvi.73.7.5282-5293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeler JS, Marchio A, Sitterlin D, Transy C, Dejean A. Proc Natl Acad Sci U S A. 1998;95 (13):7316–21. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malcles MH, Cueille N, Mechali F, Coux O, Bonne-Andrea C. J Virol. 2002;76(22):11350–8. doi: 10.1128/JVI.76.22.11350-11358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Mol Cell Biol. 2003;23(8):2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilgarth RS, Hong Y, Park-Sarge OK, Sarge KD. Biochem Biophys Res Commun. 2003;303(1):196–200. doi: 10.1016/s0006-291x(03)00312-7. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez MS, Dargemont C, Hay RT. J Biol Chem. 2001;276(16):12654–9. doi: 10.1074/jbc.M009476200. [DOI] [PubMed] [Google Scholar]
- 49.Tussie-Luna MI, Michel B, Hakre S, Roy AL. J Biol Chem. 2002;277(45):43185–93. doi: 10.1074/jbc.M207635200. [DOI] [PubMed] [Google Scholar]
- 50.Rodel B, Tavassoli K, Karsunky H, Schmidt T, Bachmann M, Schaper F, Heinrich P, Shuai K, Elsasser HP, Moroy T. Embo J. 2000;19(21):5845–55. doi: 10.1093/emboj/19.21.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leng X, Wilson VG, Xiao XL. J Gen Virol. 1994;75(9):2463–7. doi: 10.1099/0022-1317-75-9-2463. [DOI] [PubMed] [Google Scholar]